Abstract
Background
Ceftazidime-avibactam has in vitro activity against some carbapenem-resistant gram-negative infections (GNIs), and therefore may be a useful alternative to more toxic antibiotics such as colistin. Understanding ceftazidime-avibactam uptake and usage patterns would inform hospital formularies, stewardship, and antibiotic development.
Methods
A retrospective cohort study assessed inpatient encounters in the Vizient database. Ceftazidime-avibactam and colistin administrations were categorized into presumed empiric (3 consecutive days of therapy or less with qualifying exclusions) versus targeted therapy (≥4 consecutive days of therapy) for presumed carbapenem-resistant GNIs. Quarterly percentage change (QPC) using modified Poisson regression and relative change in frequency of targeted ceftazidime-avibactam to colistin encounters was calculated. Factors associated with preferentially receiving targeted ceftazidime-avibactam versus colistin were identified using generalized estimating equations.
Results
Between 2015 quarter (q) 1 and 2017q4, ceftazidime-avibactam was administered 21 215 times across 1901 encounters. Inpatient prescriptions for ceftazidime-avibactam increased from 0.44/10 000 hospitalizations in 2015q1 to 7.7/10 000 in 2017q4 (QPC, +11%; 95% CI, 10–13%; P < .01), while conversely colistin prescriptions decreased quarterly by 5% (95% CI, 4–6%; P < .01). Ceftazidime-avibactam therapy was categorized as empiric 25% of the time, targeted 65% of the time, and indeterminate 10% of the time. Patients with chronic kidney disease were twice as likely to receive targeted ceftazidime-avibactam versus colistin (RR, 2.02; 95% CI, 1.82–2.25), whereas those on dialysis were less likely to receive ceftazidime-avibactam than colistin (RR, 0.71; 95% CI, .61–.83).
Conclusions
Since approval in 2015, ceftazidime-avibactam use has grown for presumed carbapenem-resistant GNIs, while colistin has correspondingly declined. Renal function drove the choice between ceftazidime-avibactam and colistin as targeted therapy.
Keywords: ceftazidime-avibactam, ram-negative resistance, novel beta-lactamase inhibitors, carbapenem resistance
Since US Food and Drug Administration approval in 2015, ceftazidime-avibactam utilization has increased almost 20-fold over 3 years, corresponding to a concomitant decrease in colistin utilization. Renal function appears to be the strongest predictor that a patient will receive targeted ceftazidime-avibactam versus colistin.
(See the Editorial Commentary by Satlin on pages 622–5.)
Each year, an estimated 35 000 deaths in the United States are associated with antibiotic-resistant infections [1]. Awareness of the morbidity and mortality associated with highly resistant pathogens and consequent action by professional societies, industry, along with national and global leaders have led to a revival in the dwindling antibiotic pipeline [2, 3]. In 2015, the US Food and Drug Administration (FDA) approved the novel β-lactam/β-lactamase inhibitor combination ceftazidime-avibactam for the treatment of complicated urinary tract infections and complicated intra-abdominal infections based on the results of phase II noninferiority trials, followed by hospital-associated pneumonia/ventilator-associated pneumonia in 2018 [4]. While ceftazidime has been in clinical use for years, the addition of the novel β-lactamase inhibitor avibactam restores in vitro activity against Klebsiella pneumoniae carbapenemase (KPC)– and OXA-48–producing gram-negative pathogens. Following the approval of ceftazidime-avibactam, 2 additional β-lactam/β-lactamase inhibitor combinations with activity against KPC-producing Enterobactericeae, meropenem/vaborbactam (2017) and imipenem-cilastatin/relebactam (2019), have been FDA approved. Collectively, these new drug combinations might be expected to partially displace older nephrotoxic antibiotics with limited efficacy such as colistin.
Despite FDA approval, the introduction of these agents into the clinical practice has been limited due to the paucity of data on their performance in the treatment of infections against which they are most needed, high cost, limited availability of in-house in vitro testing capabilities, overlapping spectra of activity, and remaining effective older, less expensive therapies [5]. Understanding uptake characteristics, as well as patterns of use at the patient and hospital level could inform stewardship programs and hospital formulary development and provide additional evidence to support clinical use. Here, real-world prescribing trends for ceftazidime-avibactam versus colistin were examined, as well as predictors of receiving ceftazidime-avibactam as opposed to colistin for presumed carbapenem-resistant gram-negative infections (GNIs).
METHODS
Study Design
A multicenter, retrospective cohort study was used to identify encounters in which ceftazidime-avibactam or colistin was prescribed between 2015 and 2017 at hospitals that contribute pharmacy data to the Vizient clinical database/resource manager (CDB/RM). The CDB/RM contains billing records from a large collaboration of academic and nonacademic medical centers. We hypothesized that ceftazidime-avibactam is primarily being utilized for the treatment of carbapenem-resistant GNIs based on common stewardship practices [6]. Patients receiving colistin were chosen as the comparator group since this drug is commonly also used for patients with carbapenem-resistant GNIs. Prior studies have shown that, within this database, 4 or more consecutive days of colistin (or death while receiving colistin) has an 82% positive-predictive value for carbapenem-resistant GNIs [7]. Encounters with International Classification of Diseases, 9th or 10th revision (ICD-9, ICD-10), diagnosis codes for cystic fibrosis (CF) were excluded. Based on prior work, patients with CF often receive inhaled colistin (for which there is no inhaled ceftazidime-avibactam equivalent) to reduce colonization burden, which is poorly discernible from intravenous administration in administrative datasets, and represent an overall different risk set [7, 8].
Empiric and Targeted Therapy Definitions and Analysis
The first administration within each encounter of ceftazidime-avibactam and/or colistin was identified and used to categorize encounters into empiric, targeted, or indeterminate therapy. Empiric therapy was defined as 3 or fewer consecutive days of therapy excluding as indeterminate those transferred from an outside hospital who received the first dose within 3 days and those transferred out of a participating hospital (including due to death) on day 3 to avoid misclassifying a targeted therapy patient as empiric. Targeted therapy was defined as 4 or more consecutive days of therapy. Although the absence of microbiology precluded a precise estimate of empiric and targeted therapy, we used these terms for scenarios that suggest high probability of empiric and targeted therapy. Baseline characteristics were compared in patients who received empiric and targeted therapy across 3 groups: (1) ceftazidime-avibactam only, (2) colistin only, and (3) both ceftazidime-avibactam and colistin. Targeted ceftazidime-avibactam encounters were assessed to determine the frequency of monotherapy versus combination therapy and, for the latter, the distribution of concomitantly prescribed agents. Concomitant antibiotics was defined as gram-negative agents prescribed with at least 4 consecutive days of overlap anytime during ceftazidime-avibactam administration. When evaluating characteristics of empiric and targeted cohorts for ceftazidime-avibactam and colistin, encounters with overlapping administrations of both drugs were evaluated independently.
Demographics, comorbidities, and site of infection were classified based on ICD-9 and ICD-10 codes (Supplementary Table 1). All Patient Refined Diagnosis-Related Groups (APR-DRGs) severity-of-illness (SOI) and risk-of-mortality (ROM) assignments, procedure codes, and medication administrations were used to evaluate illness severity and treatment characteristics (Supplementary Table 1).
Statistical Methods
Baseline patient characteristics are presented as frequencies with associated percentages or medians with their associated confidence intervals (CIs) and interquartile ranges (IQRs). Modified Poisson regression was used to calculate the quarterly percentage change (QPC) in the frequency of ceftazidime-avibactam and colistin encounters. To determine patient characteristics associated with receiving ceftazidime-avibactam versus colistin-based regimens for the treatment of carbapenem-resistant GNIs, we also used generalized estimating equations (GEEs) with an exchangeable correlation structure for robust variation estimation allowing for the inclusion of patient- and hospital-level variables to account for unknown correlations that may affect prescribing patterns. The variance inflation factor was used to check for multicollinearity; no substantial collinearity was found between variables. Patients who received ceftazidime-avibactam and colistin concomitantly were excluded from the risk factor model and were categorized based on the first prescription per encounter. Clinically relevant encounter-level variables included age, sex, comorbid conditions (tracheostomy, congestive heart failure, diabetes, metastatic cancer, dialysis, neutropenia, and chronic kidney disease), septic shock, APR-DRG SOI score, site of infection, and admission quarter (Supplementary Table 1). Hospital characteristics included hospital region, bed capacity, and teaching status. Logistic regression with GEEs was used to determine unadjusted and adjusted odds of mortality for empiric and targeted ceftazidime-avibactam to colistin and ceftazidime-avibactam monotherapy to combination therapy. All statistical analysis was done using R software version 3.5.1.
RESULTS
Encounter and Hospital Trends
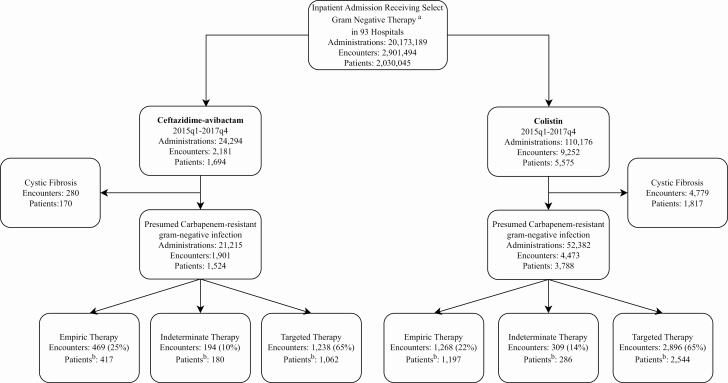
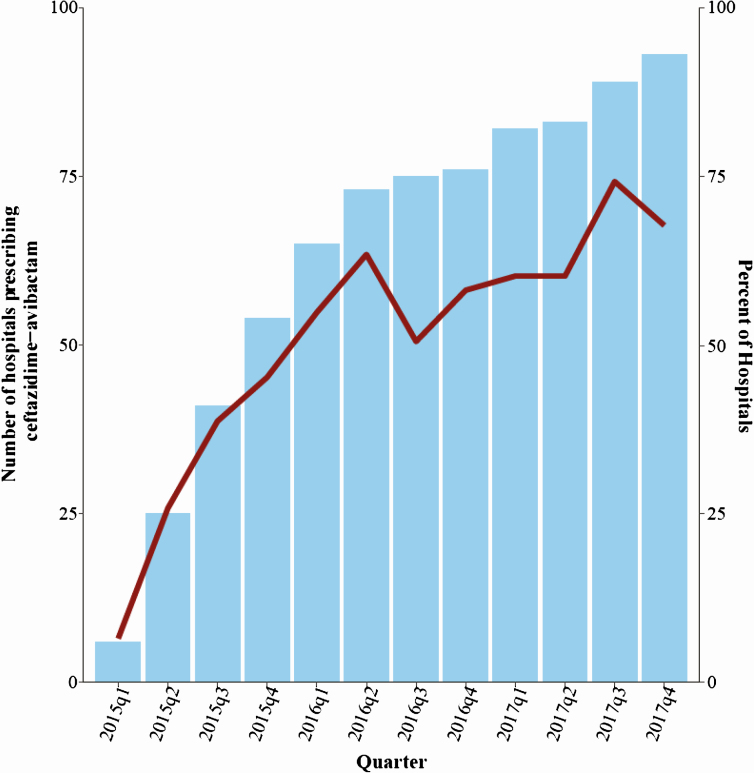
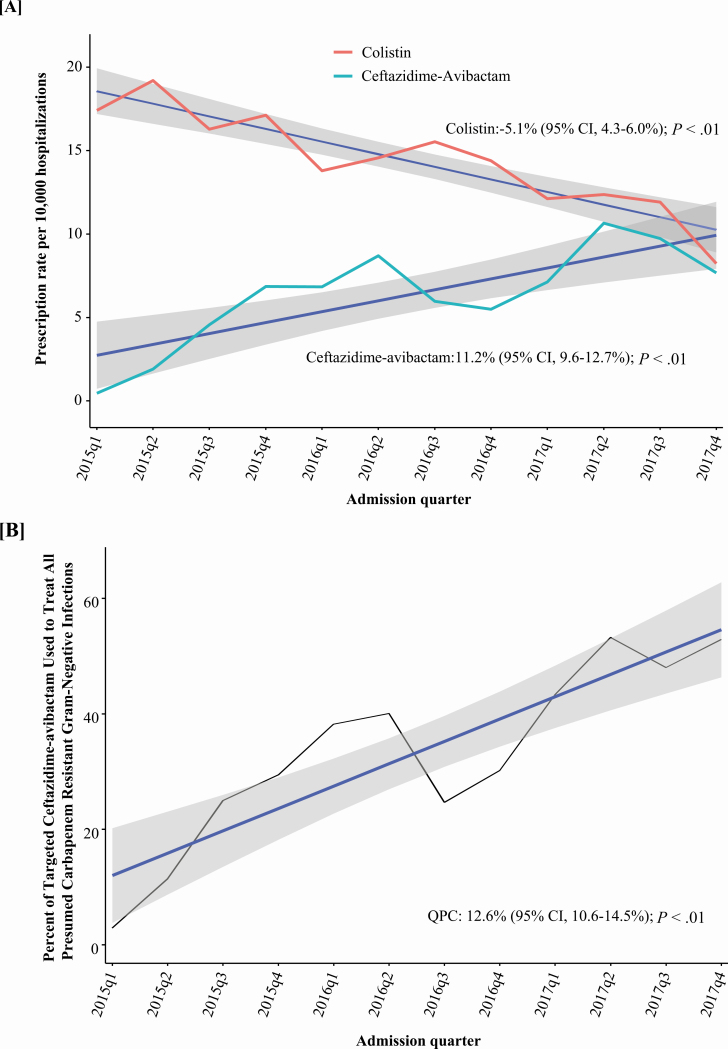
Between 2015 quarter (q) 1 and 2017q4, 93 of 210 hospitals in the CDB/RM reported ceftazidime-avibactam use. After excluding patients with a diagnosis of CF, ceftazidime-avibactam was administered 21 215 times across 1901 encounters among 1524 patients potentially for suspected or known carbapenem-resistant GNIs (Figure 1). There was a progressive increase in the number of hospitals that prescribed ceftazidime-avibactam from 5 (2.4%) out of 210 to 93 (44%) over 3 years (Figure 2). The frequency of ceftazidime-avibactam prescriptions increased from 0.44 of 10 000 hospitalizations in 2015q1 to 7.7 of 10 000 in 2017q4, representing a QPC of +11% (95% CI, 10–13%; P < .01). Conversely, the prescription rate for colistin decreased from 17.4 of 10 000 hospitalizations to 8.2 of 10 000 hospitalizations, a QPC of −5% (95% CI, 4–6%; P < .01) across the 3-year study period (Figure 3A).
Figure 1.
Encounter flowsheet. aList of select antibiotics in which ceftazidime-avibactam was prescribed: amikacin, aztreonam, ceftazidime, chloramphenicol, colistin, imipenem/cilastatin, minocycline, ampicillin/sulbactam, ticarcillin/clavulanate/tobramycin, ertapenem, tigecycline, fosfomycin, doripenem, ceftazidime-avibactam, ceftolozane-tazobactam, piperacillin-tazobactam, ciprofloxacin, cefepime, meropenem, levofloxacin, gentamicin. bPatients non–mutually exclusive as encounters who received concomitant ceftazidime-avibactam and colistin are not removed and they may have multiple encounters across different therapy categories and multiple encounters within a therapy category. Abbreviation: q, quarter.
Figure 2.
Cumulative hospital uptake was evaluated by quarter. Blue bars represent a summation of the number of hospitals eligible to prescribe each quarter based on prescriptions in prior quarters. In quarter 1 of 2015, there were a total of 5 hospitals that had prescribed ceftazidime-avibactam and this increased to 93 in quarter 4 of 2017. The overlaying dark-red trend line represents the percentage of hospitals that actually prescribed in each respective quarter out of the total that had prescribed at least 1 dose in prior quarters (n = 93). A total of 210 hospitals reported any pharmacy data during the study period. Abbreviation: q, quarter.
Figure 3.
Ceftazidime-avibactam utilization by quarter. A, After the removal of all encounters with diagnosis codes for cystic fibrosis quarterly rates in prescriptions for ceftazidime-avibactam and colistin were evaluated. Using Poisson regression, the number of ceftazidime-avibactam prescriptions increased by 11.2% (95% CI, 9.6–12.7%; P < .01), and over the same time period, colistin decreased by 5.1% (95% CI, 4.3–6.0%; P < .01). The red line represents colistin prescription, the light blue line represents ceftazidime-avibactam, the dark-blue lines represent trend lines, and gray shading represents CIs. B, Targeted encounters for ceftazidime-avibactam and colistin were summed to quantify the overall treatments for carbapenem-resistant gram-negative infections. The percentage of carbapenem-resistant gram-negative infections treated with ceftazidime-avibactam increased from 3% in 2015q1 to 53% in 2017q4, a QPC of 12.6% (95% CI, 10.6–14.5%). Abbreviations: CI, confidence interval; q, quarter; QPC, quarterly percentage change.
Baseline Patient and Hospital Characteristics for Ceftazidime-avibactam Encounters
Patients who received any ceftazidime-avibactam had a median age of 59.7 years (IQR, 21.3 years) and were approximately 60% were male and white of the time (Table 1). The most prevalent comorbidity was chronic kidney disease (36%), followed by congestive heart failure (28%) and diabetes mellitus (28%). The lower respiratory tract was the most common presumed site of infection at 39%. Overall, the ceftazidime-avibactam cohort was very sick, with 60% classified in the “extreme” category of admission APR-DRG SOI, and less than 1% in the “minor” category. The first administration occurred in the intensive care unit 49% of the time and the median overall length of hospital stay was 19 days, with a crude mortality of 23%. More than 50% of ceftazidime-avibactam encounters occurred at hospitals with bed capacities that were 750 or greater and were more common at teaching hospitals (94.8% of encounters) (Supplementary Table 2).
Table 1.
Baseline Patient Characteristics for Any Patient Who Received Ceftazidime-Avib actam for Presumed Carbapenem-Resistant Gram-negative Infections
| Variable | Values |
|---|---|
| Age, median (IQR), years | 59.7 (49.1–70.4) |
| Sex | |
| Male | 914 (60) |
| Female | 610 (40) |
| Race | |
| White | 924 (60) |
| African American | 347 (23) |
| Other | 196 (13) |
| Unknown | 57 (4) |
| Comorbid conditiona | |
| Congestive heart failure | 426 (28) |
| Diabetes mellitus | 425 (28) |
| Transplant | 58 (4) |
| Malignancy | 79 (5) |
| Dialysis | 171 (11) |
| Tracheostomy | 202 (13) |
| Chronic kidney disease | 544 (36) |
| Neutropenia | 82 (5) |
| Presumed site of infectionb | |
| Abdominal | 286 (19) |
| Bacteremia | 95 (6) |
| Central nervous system | 9 (0.6) |
| Central venous catheter | 126 (8) |
| Respiratory | 590 (39) |
| Skin/soft tissue | 157(10) |
| Urinary | 510 (34) |
| Unknown/other | 380 (25) |
| APR-DRG severity of Illness | |
| Minor | 11 (0.7) |
| Moderate | 104 (7) |
| Major | 475 (32) |
| Extreme | 899 (60) |
| APR-DRG risk of mortality | |
| Minor | 79 (5) |
| Moderate | 256 (17) |
| Major | 584 (39) |
| Extreme | 570 (38) |
| ICU during first dose | 460 (49) |
| Vasopressor administrationc | 633 (42) |
| Mechanical ventilation | 241 (16) |
| Overall length of stay, median (IQR), days | 19 (3.5–34.5) |
Data are shown as n (%) unless otherwise indicated. N = 1524 patients.
Abbreviations: APR-DRG, All Patient Refined Diagnosis Related Group; ICU, intensive care unit; IQR, interquartile range.
aComorbid conditions reported on admission (see Supplementary Table 1 for list of associated International Classification of Diseases, 9th and 10th revision, codes).
bNon–mutually exclusive.
cDefined as administration of dopamine, epinephrine, norepinephrine, phenylephrine, or vasopressin ±1 day from initial ceftazidime-avibactam administration.
Ceftazidime-avibactam Prescribing Patterns
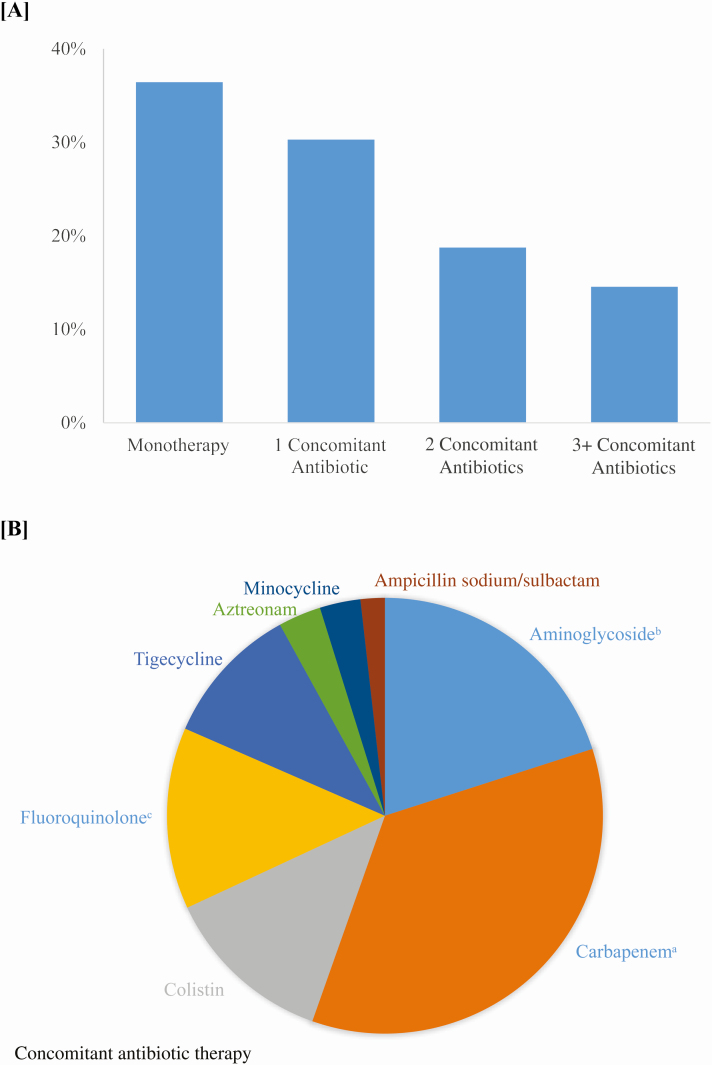
Ceftazidime-avibactam was prescribed as empiric therapy 25% of the time (469 encounters) and 65% of the time as targeted therapy (1238 encounters), whereas 10% of courses (194 encounters) were indeterminate (Figure 1, Supplementary Table 3). With a more conservative definition of empiric therapy (≤2 days), the percentage of empiric therapy decreases to 19%. There were no significant differences over time in the proportion of ceftazidime-avibactam encounters that were prescribed as empiric versus combination therapy (Supplementary Figure 1A). Targeted ceftazidime-avibactam was prescribed as monotherapy in 36% of encounters and as combination therapy in 64% of encounters (Figure 4). Although there was a significant increase in the proportion of targeted therapy that was prescribed as monotherapy over the entire study period, there was no significant increase between 2016q1 and the end of the study (Supplementary Figure 1B). Most commonly, combination therapy consisted of 1 concomitant GNI-directed antibiotic, with the most common second antibiotic class being carbapenems (38%) followed by aminoglycosides (22%) and colistin (14%). Mortality was 17% (95% CI, 12–21%) in the monotherapy group and 28% (95% CI, 25–32%) in the combination therapy group. After risk adjustment for patient- and hospital-level characteristics, patients who received ceftazidime-avibactam monotherapy had a decreased odds of mortality compared with combination therapy (odds ratio [OR], .48; 95% CI. .32–.7; P = .0002) (Supplementary Table 4). In hospitals reporting infectious disease consultation 99.8% (1236/1238) of targeted ceftazidime-avibactam encounters had an infectious disease consult.
Figure 4.
A, Percentage of targeted ceftazidime-avibactam encounters that were prescribed as monotherapy and combination therapy with concomitant antibiotics having at least 4 consecutive days of overlap with ceftazidime-avibactam therapy. Monotherapy (36%), 1 concomitant antibiotic (30%), 2 concomitant antibiotics (19%), 3+ concomitant antibiotics (15%). B, Distribution of concomitant antibiotics. Carbapenems (38%), aminoglycosides (22%), colistin (14%), fluoroquinolones (15%), tigecycline (11%), aztreonam (4%), minocycline (3%), ampicillin/sulbactam (2%). Cefepime, ceftazidime, and piperacillin-tazobactam not included as concomitant antibiotics as they are unlikely to maintain in vitro activity in carbapenem-resistant gram-negative infections. aCarbapenems (meropenem, doripenem, imipenem, ertapenem). bAminoglycosides (amikacin, gentamicin, tobramycin). cFluoroquinolones (ciprofloxacin and levofloxacin).
Comparison of Characteristics of Empiric Ceftazidime-avibactam Versus Colistin Groups
Age, sex, and race had similar distributions between groups (Table 2). Patients receiving empiric ceftazidime-avibactam were more likely to have chronic kidney disease (38% vs 5%; P < .001) or be on dialysis (17% vs 11%; P < .001) compared with patients receiving colistin, but the drugs were prescribed at similar rates in patients with acute kidney injury. A majority of patients in both the empiric ceftazidime-avibactam and empiric colistin cohorts also had APR-DRG SOI scores in the extreme category.
Table 2.
Baseline Patient-level Characteristics for Patients Who Received Empiric or Targeted Ceftazidime-avibactam and Colistin
| Empiric Therapy | Targeted Therapy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime-avibactam (n = 349) | Ceftazidime-avibactam and Colistin (n = 72) | Colistin (n = 1034) | Ceftazidime-avibactam (n = 819) | Ceftazidime-avibactam and Colistin (n = 265) | Colistin (n = 2177) | |||||||
| Variable | n | % | n | % | n | % | n | % | n | % | n | % |
| Age, median (IQR), years | 59.1 (22.9) | 59.5 (18.0) | 60.2 (23.1) | 60.5 (20.6) | 58.3 (21.9) | 59.7 (20.7) | ||||||
| Sex | ||||||||||||
| Male | 217 | 62.2 | 37 | 51.4 | 624 | 60.4 | 491 | 60.0 | 171 | 64.5 | 1336 | 61.4 |
| Female | 132 | 37.8 | 35 | 48.6 | 410 | 39.7 | 328 | 40.1 | 94 | 35.5 | 840 | 38.6 |
| Race | ||||||||||||
| White | 196 | 56.2 | 43 | 59.7 | 642 | 62.1 | 506 | 61.8 | 154 | 58.1 | 1367 | 62.8 |
| African American | 89 | 25.5 | 19 | 26.4 | 274 | 26.5 | 180 | 22.0 | 65 | 24.5 | 484 | 22.2 |
| Other | 39 | 11.2 | 8 | 11.1 | 84 | 8.1 | 64 | 7.8 | 27 | 10.2 | 213 | 9.8 |
| Asian | 13 | 3.7 | 1 | 1.4 | 19 | 1.8 | 32 | 3.9 | 10 | 3.8 | 66 | 3.0 |
| Unknown | 12 | 3.4 | 1 | 1.4 | 15 | 1.5 | 37 | 4.5 | 9 | 3.4 | 47 | 2.2 |
| Comorbid conditiona,b | ||||||||||||
| Congestive heart failure | 97 | 27.8 | 19 | 26.4 | 318 | 30.8 | 237 | 28.9 | 67 | 25.2 | 579 | 26.6 |
| Diabetes mellitus | 89 | 25.5 | 33 | 45.8 | 226 | 21.9 | 227 | 27.7 | 64 | 24.1 | 425 | 19.5 |
| Transplant | 15 | 4.3 | 3 | 4.2 | 26 | 2.5 | 39 | 4.8 | 9 | 3.4 | 295 | 13.6 |
| Mechanical ventilation | 73 | 20.9 | 11 | 15.3 | 240 | 23.2 | 116 | 14.2 | 46 | 17.4 | 569 | 26.1 |
| Malignancy | 23 | 6.6 | 2 | 2.8 | 51 | 4.9 | 34 | 4.2 | 12 | 4.5 | 54 | 2.5 |
| Dialysis | 59 | 16.9 | 15 | 20.8 | 115 | 11.1 | 68 | 8.3 | 29 | 10.9 | 178 | 8.2 |
| Tracheostomy | 36 | 10.3 | 9 | 12.5 | 193 | 18.7 | 96 | 11.7 | 59 | 22.3 | 461 | 21.2 |
| Chronic kidney disease | 132 | 37.8 | 37 | 51.4 | 51 | 4.9 | 289 | 35.2 | 86 | 32.5 | 91 | 4.2 |
| Neutropenia | 14 | 4.0 | 5 | 6.9 | 30 | 2.9 | 42 | 5.1 | 20 | 7.5 | 46 | 2.1 |
| Vasopressor administrationc | 126 | 36.1 | 33 | 45.8 | 432 | 41.8 | 320 | 39.1 | 135 | 50.9 | 1036 | 47.6 |
| Acute kidney injury | 170 | 48.7 | 47 | 65.3 | 493 | 47.7 | 442 | 54.0 | 178 | 67.2 | 1006 | 46.2 |
| Presumed site of infection | ||||||||||||
| Abdominal | 55 | 15.8 | 13 | 18.1 | 17 | 1.6 | 168 | 20.5 | 53 | 20.0 | 31 | 1.4 |
| Bacteremia | 28 | 8.0 | 2 | 2.8 | 22 | 2.1 | 66 | 8.1 | 13 | 4.1 | 45 | 2.1 |
| Central nervous system | 2 | 0.6 | 2 | 2.8 | 2 | 0.2 | 2 | 0.2 | 3 | 1.1 | 2 | 0.1 |
| Central venous catheter | 28 | 8.0 | 8 | 11.1 | 8 | 0.7 | 70 | 8.5 | 29 | 10.9 | 14 | 0.6 |
| Respiratory | 118 | 33.8 | 44 | 61.1 | 189 | 18.3 | 289 | 35.3 | 147 | 55.5 | 374 | 17.2 |
| Skin/soft tissue | 40 | 11.5 | 9 | 12.5 | 13 | 1.3 | 94 | 11.5 | 26 | 9.8 | 20 | 0.2 |
| Urinary | 127 | 36.4 | 26 | 36.1 | 230 | 22.2 | 279 | 34.1 | 100 | 37.7 | 402 | 18.5 |
| Unknown/other | 78 | 22.4 | 7 | 9.7 | 654 | 63.3 | 169 | 20.6 | 35 | 13.2 | 333 | 64.8 |
| APR-DRG severity of illness | ||||||||||||
| Minor | 4 | 1.2 | 0 | NA | 17 | 1.6 | 3 | 0.4 | 1 | 0.4 | 63 | 2.9 |
| Moderate | 31 | 8.9 | 3 | 4.2 | 71 | 6.9 | 54 | 6.7 | 11 | 4.2 | 194 | 8.9 |
| Major | 119 | 34.1 | 19 | 26.4 | 308 | 29.8 | 267 | 32.9 | 59 | 22.3 | 540 | 24.8 |
| Extreme | 176 | 50.4 | 50 | 69.4 | 638 | 61.7 | 487 | 60.1 | 194 | 73.2 | 1378 | 63.4 |
| APR-DRG risk of mortality | ||||||||||||
| Minor | 19 | 5.4 | 4 | 5.6 | 55 | 5.3 | 43 | 5.3 | 4 | 1.5 | 90 | 4.4 |
| Moderate | 63 | 18.1 | 7 | 9.7 | 148 | 14.3 | 137 | 16.9 | 36 | 13.6 | 344 | 15.8 |
| Major | 143 | 41.0 | 26 | 36.1 | 411 | 39.8 | 325 | 40.1 | 96 | 36.2 | 902 | 41.5 |
| Extreme | 105 | 30.1 | 35 | 48.6 | 420 | 40.6 | 306 | 37.7 | 129 | 48.7 | 839 | 38.6 |
| ICU during first dose | 92 | 26.4 | 26 | 36.1 | 38 | 3.7 | 232 | 47.4 | 102 | 48.6 | 272 | 12.5 |
Abbreviations: APR-DRG, All Patient Refined Diagnosis Related Group; ICU, intensive care unit; IQR, interquartile range.
aComorbid conditions reported on admission (see Supplementary Table 1 for list of associated International Classification of Diseases, 9th and 10th revision, codes).
bNon–mutually exclusive.
cDefined as administration of dopamine, epinephrine, norepinephrine, phenylephrine, or vasopressin ±1 day from initial ceftazidime-avibactam administration.
Rates and Predictors Associated With Receipt of Targeted Ceftazidime-avibactam Versus Colistin for Presumed Carbapenem-resistant Gram-negative Infection and Mortality
Over the study period there was a shift in trends of targeted therapy for presumed carbapenem-resistant GNIs. In 2015q1, ceftazidime-avibactam–based therapy accounted for 3% of evaluated targeted treatments for presumed carbapenem-resistant GNIs, which increased to 53% in 2017q4 (Figure 3B). Factors associated with prescriptions for targeted ceftazidime-avibactam versus colistin are shown in Table 3. Encounters with chronic kidney disease (relative risk [RR], 2.02; 95% CI, 1.82–2.25) and diabetes (RR, 1.14; 95% CI, 1.04–1.26) were more likely to be prescribed targeted ceftazidime-avibactam compared with colistin, while those on dialysis were less likely to be prescribed targeted ceftazidime-avibactam (RR, 0.71; 95% CI, .61–.83) (Table 3). Targeted colistin therapy was also more common in transplant (hematologic and solid organ) patients and those on mechanical ventilation or with a tracheostomy. Infections with intra-abdominal, central venous catheters, or skin/soft tissue sites were more likely to receive targeted ceftazidime-avibactam, while for respiratory site there was no difference. As compared with patients with a “minor” APR-DRG SOI assignment, those with “moderate,” “major,” or “extreme” categories were all more likely to receive ceftazidime-avibactam regimens. Patients at hospitals in the Northeast (RR, 1.63; 95% CI, 1.46–1.83) were more likely to receive a ceftazidime-avibactam–based regimen compared with those at hospitals in the Midwest. For each successive quarter of admission, patients were 8% more likely to receive targeted therapy with ceftazidime-avibactam than colistin (RR, 1.08; 95% CI, 1.06–1.10). When evaluating targeted ceftazidime-avibactam and colistin cases after removal of overlapping colistin, ceftazidime-avibactam had a crude mortality of 22% (95% CI, 19–25%), while those receiving colistin had a crude mortality of 26% (95% CI, 24–27%) (Supplementary Table 5). Following risk adjustment, there was no significant difference in the odds of death (OR, 0.84; 95% CI, .61–1.06; P = .15) (Supplementary Table 4).
Table 3.
Risk Factor Model To Predict Variables Associated With Receiving a Targeted Ceftazidime-Avibactam Versus Colistin-based Regimen
| Variable | Relative Risk of Receiving Ceftazidime-Avibactam Versus Colistina (95% CI) |
|---|---|
| Age (years) | 1.01 (.96–1.06) |
| Sex (male) | 1.05 (.96–1.15) |
| APR-ARG SOIb | |
| Moderate | 2.07 (1.12–3.81) |
| Major | 2.00 (1.09–3.66) |
| Extreme | 1.75 (.95–3.22) |
| Septic shock | 1.05 (.95–1.15) |
| Mechanical ventilation | 0.80 (.70–.90) |
| Vasopressor administration | 1.11 (1.00–1.23) |
| Comorbid conditionsc | |
| Elixhauser comorbidity index | 0.96 (.94–.98) |
| Congestive heart failure | 1.00 (.90–1.11) |
| Chronic kidney disease | 2.02 (1.82–2.25) |
| Diabetes | 1.14 (1.04–1.26) |
| Transplant | 0.72 (.59–.89) |
| Dialysis | 0.71 (.61–.83) |
| Metastatic cancer | 1.18 (.96–1.47) |
| Neutropenia | 1.16 (.94–1.43) |
| Tracheostomy | 0.67 (.58–.78) |
| Presumed site of infectiond | |
| Abdominal | 1.68 (1.51–1.87) |
| Bacteremia | 1.01 (.88–1.17) |
| Central nervous system | 1.65 (.81–3.37) |
| Central venous catheter | 1.44 (1.27–1.64) |
| Respiratory | 0.94 (.85–1.04) |
| Skin/soft tissue | 1.50 (1.35–1.68) |
| Urine | 0.93 (.84–1.04) |
| Unknown source | 0.4 (.34–.47) |
| Hospital regione | |
| Northeast | 1.63 (1.46–1.83) |
| South | 0.90 (.78–1.04) |
| West | 1.10 (.94–1.29) |
| Hospital teaching status | 0.93 (.70–1.25) |
| Hospital bed capacityf | |
| 250–499 | 0.92 (.65–1.31) |
| 500–749 | 1.27 (.90–1.80) |
| ≥750 | 1.21 (.85–1.71) |
| Admission quarterg | 1.08 (1.06–1.10) |
Abbreviations: APR-DRG, All Patient Refined Diagnosis Related Group; CI, confidence interval; IQR, interquartile range; SOI, severity of illness.
aRelative risk >1 favors ceftazidime-avibactam and <1 favors colistin.
bAPR-DRG SOI compared with minor.
cComorbid conditions reported on admission.
dSite of infection not mutually exclusive.
eCompared with Midwest.
fBed capacity compared with <250.
gReported as a continuous variable.
DISCUSSION
Our study represents the first pharmaco-epidemiologic assessment of uptake and utilization for the novel β-lactam/β-lactamase inhibitor ceftazidime-avibactam at US medical centers since its US regulatory approval in 2015. We observed a steady increase from 0.44 prescriptions per 10 000 encounters in 2015q1 to 7.7 per 10 000 encounters in 2017q4, an 11% growth in use per quarter. This was accompanied by a 5% decrease in colistin use per quarter. Over 3 years, this translates to a dramatic change in the relative proportion of ceftazidime-avibactam to colistin prescriptions from 1:38 in 2015q1 to nearly 1:1 in 2017q4 for presumed carbapenem-resistant GNIs.
While our data demonstrate a simultaneous decrease in the prescribing of colistin and an increase in ceftazidime-avibactam, we found ongoing high usage rates of colistin at the end of the study period. This mirrors the aggregate vial sales reported from the IQVIA National Sales Perspective Database in which colistin accounted for 245 894 days of therapy in 2017 while ceftazidime-avibactam only accounted for 64 833 days [5, 9]. However, the decrease in colistin prescribing seen in this study is greater than previously reported. We hypothesize that this may have been because prior reports included patients with CF (53% of colistin encounters in our study had a diagnosis of CF) who are sometimes treated for organisms other than carbapenem-resistant Enterobacteriaceae with inhaled colistin. Highlighting this fact, in 2017, IQVIA vial data show the ratio of sales of ceftazidime-avibactam to colistin was 0.26. On the other hand, in our study, the ratio of administrations of ceftazidime-avibactam to colistin in 2017 was 0.41 when including patients with CF and 1.01 after removing patients with CF.
Notably, complete replacement of colistin by ceftazidime-avibactam would be inappropriate for carbapenem-resistant GNIs for which it lacks activity, such as Acinetobacter baumannii and metallo-β-lactamase producers. Nonetheless, other considerations may also impact its use, including limited access and experience with ceftazidime-avibactam, prescribing volume, lack of immediate reliable ceftazidime-avibactam susceptibility testing, and most importantly, limited safety and efficacy data in patients with carbapenem-resistant infections for whom this drug should be reserved [10]. Poorly defined safety concerns include the phase III study comparing meropenem to ceftazidime-avibactam for treatment of complicated intra-abdominal infections, which showed a numerical increase in mortality in patients with moderate renal impairment (>30 to ≤50 mL/min) treated with ceftazidime-avibactam, and the phase III study for nosocomial pneumonia demonstrating excess serious adverse events in the ceftazidime-avibactam arm [11, 12]. Furthermore, only 1 clinical trial of ceftazidime-avibactam has been published in patients with antibiotic-resistant infections, specifically ceftazidime-nonsusceptible urinary tract infections. This small study demonstrated a higher clinical cure rate with ceftazidime-avibactam compared with the best available therapy, but did not assess death “related to infection” or all-cause mortality [13]. Further evidence of efficacy is limited to a small number of observational studies that suggest superior outcomes in patients treated with ceftazidime-avibactam compared with the best available therapy for carbapenem-resistant GNIs; however, these studies have a high risk of unmeasured confounding [14–17].
While awaiting further prospective and innovative clinical trial data on safety and efficacy in patients with highly resistant, gram-negative pathogens, our study identifies specific scenarios where prescribers are choosing ceftazidime-avibactam over colistin-based regimens [18]. The strongest predictor of receiving ceftazidime-avibactam was chronic kidney disease, while those patients already on dialysis are much more likely to receive colistin. This is interesting given evidence suggesting less efficacy with ceftazidime-avibactam in patients with renal insufficiency noted in the prescribing information for the drug [19]. One explanatory hypothesis for this observation is that prescribers are reluctant to prescribe a known nephrotoxic antibiotic to patients with reduced kidney function, but a lower threshold in those whose kidneys are irrecoverable. Furthermore, we note that patients who received targeted ceftazidime-avibactam compared with colistin were more often coded for acute kidney injury, again suggesting possible attempts to avoid nephrotoxic agents in patients with kidney dysfunction (Table 2). Because coding encompasses the entire hospitalization, it was not possible to relate the timing of acute kidney injury to whether it occurred before or after drug administration, therefore limiting any conclusions about toxicity. Finally, patients who have a history of prior transplant are more likely to receive targeted colistin therapy as compared with ceftazidime-avibactam, potentially reflecting the lack of data in this population, as history of heart/lung transplant and immunosuppression were exclusion criteria from the phase III randomized controlled trial of ceftazidime-avibactam for nosocomial pneumonia [12].
Some practitioners and literature recommend treatment of carbapenem-resistant GNIs with combination therapy; however, some recent randomized controlled trials suggest no advantage over monotherapy [20, 21]. Colistin is the antibiotic most commonly included in combination regimens and is often the backbone of such therapy [22]. Combination therapy has not been sufficiently evaluated since the introduction of novel β-lactam/β-lactamase agents with activity against carbapenem-resistant GNIs. Notably, in our study, the majority (64%) of targeted therapy with ceftazidime-avibactam occurred in the form of combination therapy. It remains unclear whether this finding represents providers’ belief in the value of combination therapy or simply their reluctance to rely on ceftazidime-avibactam alone. Furthermore, the finding of increased mortality associated with combination must be interpreted with caution and evaluated further as risk adjustment is limited and SOI may drive the decision of combination therapy in some patients.
Despite the introduction of several antibiotics in recent years there remains a need for the development of drugs with novel mechanisms of action and improved efficacy [23]. However, there is concern that the antibiotic pipeline lacks innovation [2]. Compounding the urgency of this problem is the emergence of resistance to ceftazidime-avibactam [24, 25]. Additionally, an epidemiological shift in intensive care units has been reported between KPC and Verona Integron mediated β-lactamase (VIM)-producing organisms following the introduction of ceftazidime-avibactam [26]. Optimally deploying a new drug under the guidance of antibiotic stewardship remains a challenging and an evolving endeavor. Importantly, providers are relying heavily on infectious disease specialists to guide them in the use of ceftazidime-avibactam, as seen in our study where nearly all targeted ceftazidime-avibactam use involved their oversight. This trend is likely to continue as other novel antibiotics enter clinical care and represents an important leadership role for infectious disease consultants [27].
While this study provides insight into real-world prescribing patterns along with hospital- and patient-level uptake, several limitations warrant mention. Our study was not designed to make precise estimates of empiric and targeted therapy and these estimates are further limited as microbiology data are not available in this dataset and site of infection relies on coding. We have created algorithms that account for outside hospital transfer and discharge to help discriminate between empiric and targeted therapy. Although we have performed prior studies to validate the colistin algorithm used in this study to identify highly resistant GNIs, uncertainty remains, particularly revolving around the treatment of highly resistant A. baumannii complex and metallo-β-lactamase producers for which colistin would be the preferred agent over ceftazidime-avibactam [7, 28]. Furthermore, there are case reports of ceftazidime-avibactam having been used for less common organisms such as Burkholderia spp., with most of these case reports in patients with CF [29–32]. Mortality data in our study are adjusted but there is a lack of microbiological, physiologic, and laboratory data. This along with inability to adjust for confounding by indication limits the conclusions that can be drawn regarding the comparative effectiveness or safety of ceftazidime-avibactam versus colistin and ceftazidime-avibactam monotherapy versus combination therapy. However, our study’s purpose was not to compare effectiveness but solely to describe the populations being treated with 1 agent versus the other. Last, the use of polymyxin-B could not be quantified due to previously reported limitations in the way polymyxin-B is mapped in the Vizient electronic clinical database [8].
In conclusion, ceftazidime-avibactam utilization is increasing, while there are concomitant decreases in the utilization of colistin for the treatment of carbapenem-resistant GNIs. A preference for ceftazidime-avibactam appeared to be driven by concern for colistin nephrotoxicity in patients with chronic kidney disease and/or diabetes who do not require dialysis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The opinions expressed in this article are those of the authors and do not represent any position or policy of the National Institutes of Health, the US Department of Health and Human Services, or the US government.
Financial support. This work was supported in part by the Intramural Research Program of the National Institutes of Health Clinical Center and the National Institute of Allergy and Infectious Diseases and the National Cancer Institute (contract number HHSN261200800001E).
Potential conflicts of interest. M. K. reports personal fees from UpToDate, outside the submitted work. J. H. P. reports consulting fees from Arrevus, Corbus, DaVolterra, Eicos, Eli Lilly, Gilead, MedImmune, Microbion, Otsuka, Roche, Romark, and Shinogi, outside the submitted work. C. R. reports royalties from UpToDate, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control. Antibiotic resistance threats in the United States 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf [Google Scholar]
- 2. Theuretzbacher U, Gottwalt S, Beyer P, et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect Dis 2019; 19:e40–50. [DOI] [PubMed] [Google Scholar]
- 3.Ardal C, Findlay D, Savic M, et al. DRIVE-AB report. Revitalizing the antibiotic pipeline, 2018. Available at: http://drive-ab.eu/wp-content/uploads/2018/01/DRIVE-AB-Final-Report-Jan2018.pdf [Google Scholar]
- 4. Falcone M, Paterson D. Spotlight on ceftazidime/avibactam: a new option for MDR gram-negative infections. J Antimicrob Chemother 2016; 71:2713–22. [DOI] [PubMed] [Google Scholar]
- 5. Carr A, Stringer J.. Antibiotic R&D update 20. 2019. Needham Biotechnology. Available at: https://needham.bluematrix.com/sellside/EmailDocViewer?encrypt=06ec2018-46a9-47e8-9e52-c0be3e6a0c9c&mime=pdf&co=Needham&id=tmaloney@needhamco.com&source=libraryView [Google Scholar]
- 6. Montravers P, Bassetti M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr Opin Infect Dis 2018; 31:587–93. [DOI] [PubMed] [Google Scholar]
- 7. Kadri SS, Hohmann SF, Orav EJ, et al. Tracking colistin-treated patients to monitor the incidence and outcome of carbapenem-resistant gram-negative infections. Clin Infect Dis 2015; 60:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadri SS, Strich JR, Swihart BJ, et al. Attributable mortality from extensively drug-resistant gram-negative infections using propensity-matched tracer antibiotic algorithms. Am J Infect Control 2019; 47:1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaPlante K, Cusumano J, Tillotson G. Colistin for the treatment of multidrug-resistant infections. Lancet Infect Dis 2018; 18:1174–5. [DOI] [PubMed] [Google Scholar]
- 10. Lubloy A Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res 2014; 14:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62:1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 2018; 18:285–95. [DOI] [PubMed] [Google Scholar]
- 13. Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16:661–73. [DOI] [PubMed] [Google Scholar]
- 14. Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 2019; 68: 355–64. [DOI] [PubMed] [Google Scholar]
- 15. Castón JJ, Lacort-Peralta I, Martín-Dávila P, et al. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis 2017; 59:118–23. [DOI] [PubMed] [Google Scholar]
- 16. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Duin D, Lok JJ, Earley M, et al. ; Antibacterial Resistance Leadership Group Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lanini S, Ioannidis JPA, Vairo F, et al. Non-inferiority versus superiority trial design for new antibiotics in an era of high antimicrobial resistance: the case for post-marketing, adaptive randomised controlled trials. Lancet Infect Dis 2019; 19:e444–51. [DOI] [PubMed] [Google Scholar]
- 19. Food and Drug Administration. Highlights of prescribing information. Ceftazidime-avibactam package insert. 2015. Allergen. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206494s005,s006lbl.pdf [Google Scholar]
- 20. Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18:391–400. [DOI] [PubMed] [Google Scholar]
- 21. Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–50. [DOI] [PubMed] [Google Scholar]
- 22. Cheng IL, Chen YH, Lai CC, Tang HJ. Intravenous colistin monotherapy versus combination therapy against carbapenem-resistant gram-negative bacteria infections: meta-analysis of randomized controlled trials. J Clin Med 2018; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talbot GH, Jezek A, Murray BE, et al. The Infectious Diseases Society of America’s 10 x ‘20 initiative (Ten New Systemic Antibacterial Agents FDA-approved by 2020): Is 20 x ‘20 a possibility? Clin Infect Dis 2019; 56:1685–94. [DOI] [PubMed] [Google Scholar]
- 24. Humphries RM, Yang S, Hemarajata P, et al. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 2015; 59:6605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papadimitriou-Olivgeris M, Bartzavali C, Lambropoulou A, et al. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J Antimicrob Chemother 2019; 74:2051–4. [DOI] [PubMed] [Google Scholar]
- 27. Walensky RP, Del Rio C, Armstrong WS. Charting the future of infectious disease: anticipating and addressing the supply and demand mismatch. Clin Infect Dis 2017; 64:1299–301. [DOI] [PubMed] [Google Scholar]
- 28. Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 2019; 68:519–24. [DOI] [PubMed] [Google Scholar]
- 29. Tamma PD, Fan Y, Bergman Y, et al. Successful treatment of persistent burkholderia cepacia complex bacteremia with ceftazidime-avibactam. Antimicrob Agents Chemother 2018; 62:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spoletini G, Etherington C, Shaw N, et al. Use of ceftazidime/avibactam for the treatment of MDR Pseudomonas aeruginosa and Burkholderia cepacia complex infections in cystic fibrosis: a case series. J Antimicrob Chemother 2019; 74:1425–9. [DOI] [PubMed] [Google Scholar]
- 31. Barlow G, Morice A. Successful treatment of resistant Burkholderia multivorans infection in a patient with cystic fibrosis using ceftazidime/avibactam plus aztreonam. J Antimicrob Chemother 2018; 73:2270–1. [DOI] [PubMed] [Google Scholar]
- 32. Los-Arcos I, Len O, Martín-Gómez MT, et al. Lung transplantation in two cystic fibrosis patients infected with previously pandrug-resistant Burkholderia cepacia complex treated with ceftazidime-avibactam. Infection 2019; 47:289–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.