Abstract
Many studies tried to assess the relationship between -308G/A polymorphism of tumor necrosis factor alpha (TNF-α) gene and risk of metabolic syndrome (MS), but their results were contradictory. This meta-analysis aimed to precisely evaluate this association. A systematic literature search was performed in Pubmed database and WanFang Med Online, STATA software 14.0 was used for the meta-analysis. Eleven independent studies containing 3277 cases and 3312 controls were included in our meta-analysis. In overall analysis, significant association was found between -308G/A polymorphism of TNF-α and MS in both allele model (OR 1.47, 95% CI 1.09–1.98, P 0.013) and dominant model (OR 1.77, 95% CI 1.21–2.58, P 0.003). In the subgroup analysis, the A allele was associated with increased risk of MS in Asia group (allele model: OR 1.82 95% CI 1.31–2.53, P < 0.001; dominant model: OR 2.30, 95% CI 1.64–3.21 P < 0.001; homozygous model: OR 2.29, 95% CI 1.31–4.01, P 0.004), and decreased risk of MS in Europe group (dominant model: OR 0.83, 95% CI 0.70–0.99, P < 0.001; recessive model: OR 0.51, 95% CI 0.28–0.92, P 0.025; homozygous model: OR 0.49 95% CI 0.27–0.89, P 0.02). The A allele also appeared to linked to increased risk of MS in CDS group and IDF groups. No significant association was observed in NCEPATPIII group. Our results suggested that -308G/A of TNF-α gene was a risk factor for MS, but it may played different roles in different ethnics, further studies with larger sample size and more other ethnics should be performed to confirm our conclusions.
Subject terms: Endocrinology, Genetic association study, Medical genetics
Introduction
Metabolic syndrome (MS) is a multi-component disease characterized by the combination of a series of clinical and biochemical metabolic disorders including insulin resistance, elevated plasma triglyceride levels, decreased HDL-c, hypertension, hyperglycaemia and abdominal obesity. In 1998, the first criteria of MS was made by World Health Organization (WHO)1, since then many international organizations and expert groups, such as European Group for the study of Insulin Resistance (EGIR)2, the International Diabetes Federation (IDF)3, the National Cholesterol Education Program Adult Treatment Panel III (NCEP:ATPIII)4, the American Association of Clinical Endocrinology (AACE)5, Chinese Diabetes Society6, have defined MS using different parameters. Among those definitions, the ones made by NCEP:ATPIII and IDF were the most widely used currently. The morbidity of MS is increasing around the world, which makes MS a huge public health burden for many countries3,7–9. Many factors are involved in the development of MS. On one hand, environmental factors such as physical inactivity and improper eating habits are essential determinants for MS. On the other hand, genetic factors also play vital roles in the development of MS.
Tumor necrosis factor alpha (TNF-α) is a multifunctional pro-inflammatory cytokine with both beneficial and destructive functions for human body produced by various kinds of cells such as macrophages, fibroblasts, epithelial cells, adipocyte10. As we known, TNF-α could regulate numerous inflammatory and autoimmune processes, and participates in many life activities such as apoptosis, differentiation and cell recruitment11. Prior studies also demonstrated that TNF-α combined with other types of pro-inflammatory cytokines contributed to the development of type II diabetes, obesity and obesity-induced insulin resistance, which suggested TNF-α played as an essential role in the development of MS12–15. TNF-α gene is located at 6p21.1–21.3 chromosomal region, which is near to major histocompatibility complex. Wilson et al. firstly identified a G to A variant at 308 upstream of TNF-α gene promoter region in 199216. Subsequent studies has shown that -308G/A had great impact on the transcription activation of TNF-α gene and was relevant with the increased plasma TNF-α plasma levels17. Observational studies supported -308G/A of TNF-α gene was associated with the components of MS such as hypertension and insulin resistance18. Many studies containing different ethnic subjects tried to reveal the relationship between -308G/A of TNF-α gene and MS susceptibility, however, those results were inconsistent.
In this study, we performed a meta-analysis to assess the accurate impact of the -308G/A polymorphism of TNF-α gene promoter on the MS risk.
Methods
Literature search
A systemic literature search was performed in Pubmed and Wanfang Online database to identify all the potential studies that involved the association between -308G/A polymorphism of TNF-α gene and MS susceptibility. Following key words were used for our literature search: (“tumor necrosis factor alpha” OR “TNF-α”) AND (polymorphism OR mutation OR variant) AND (metabolic syndrome OR MetS OR MS). The language was restricted to English and Chinese, and the search process was completed on April 6th 2020.
Inclusion criteria
The following inclusion criteria should be met when the studies were included in this meta-analysis: (1) studies should examine the association between -308G/A polymorphism of TNF-α gene and MS susceptibility; (2) studies should be case–control designed or cohort designed; (3) studies should provide enough data to calculate the odds ratios (ORs) and the corresponding 95% confidence intervals (CIs).
Data extraction and quality assessment
Two authors independently extracted the following information from each study: the first authors, the years of publication, the demographic information of each study, the numbers of case and control groups, and the frequencies of the three types of genotypes in both cases and controls. Newcastle–Ottawa quality assessment scale was used for the quality assessment of each research19.
Statistical analysis
We calculated the pooled ORs and corresponding 95%CIs to assess the strength of the association between -308G/A polymorphism of TNF-α gene and MS susceptibility. Pooled ORs and 95% CIs were performed under four genetic models: allele model (A vs.G), dominant model (AA + GA vs. GG), recessive model (AA vs. GG + GA), and homozygous model (AA vs. GG), respectively. We used random effect models to calculate all the pooled ORs and corresponding 95%CIs. The Z tests were used to assess the significance of the ORs. Hardy–Weinberg equilibrium (HWE) was tested using Chi-square test in the control groups. Heterogeneity between the studies included in this meta-analysis was evaluated using χ2 based Q tests and I-square (I2) tests. We defined low, moderate, and high degrees of heterogeneity when the values of I2 were 25%, 50%, and 75%, respectively. We carried out sensitive analysis to assess the impact of each study on the pooled ORs and 95% CIs. The Begg’s and Egger’s tests were used to evaluate the publication bias. All tests were two-sided, and a value < 0.05 was considered as statistically significant. STATA software (version 14.0; State Corporation, College Station, TX, USA) was used to perform all the statistical tests in this meta-analysis.
Results
Study characteristics
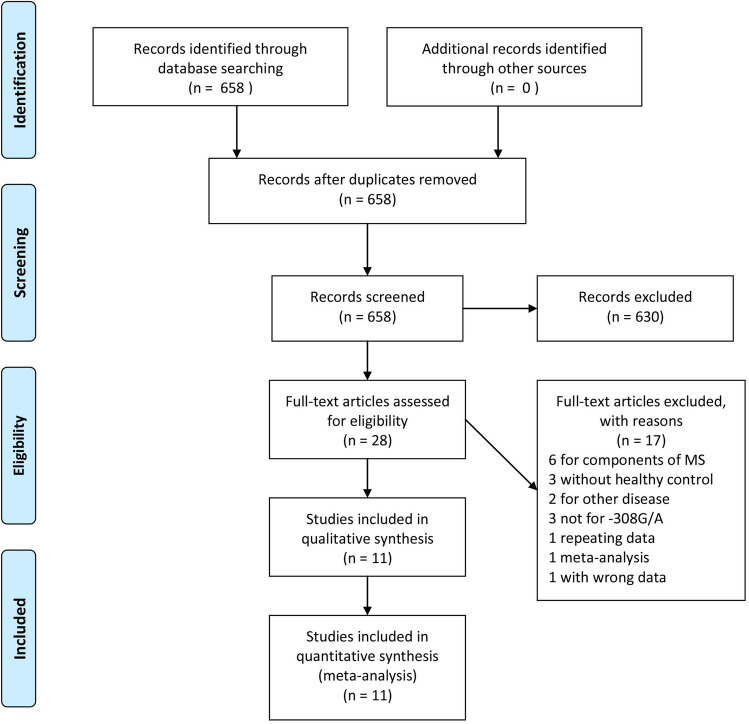
Depending on the literature search strategy mentioned above, 658 articles were initially identified in the Pubmed database and Wanfang Med Online. The sift process was shown in Fig. 1. Briefly, 630 articles were excluded after reviewing their titles and abstracts. Of the remaining 28 articles, 17 ones were excluded for the following reasons: six studies were performed for the association between -308G/A polymorphism of TNF-α gene and the components of MS, three studies did not have healthy controls, three studies were for other polymorphism sties of TNF-α gene, two studies were for other disorders, one study with repeating data, one study was meta-analysis, one study with wrong data. Finally, eleven studies containing 3277 cases and 3312 controls were included in our meta-analysis. Eight of the eleven studies were published in English, three ones were in Chinese. The publication time of the eleven studies ranged from 2005 to 2019. There are three types of diagnostic criteria were used for the diagnosis of MS, five studies diagnosed MS using the National Cholesterol Education Program Adult Treatment Panel III (NCEPATP III) definition, four studies adopted International Diabetes Federation (IDF) definition, two studies recruit their subjects using the definition made by Chinese Diabetes Society (CDS). Four different kinds of genotyping methods existed, the numbers of studies used restriction fragment length polymorphism (RFLP) was eight. Golden Gate Assay, Taqman probe, melting curves were respectively used in the remaining three studies. All the studies included in our studies were in HWE (P > 0.05). The general characteristics of the eleven studies and the frequencies of different genotypes extracted from each study were shown in the Table 1.
Figure 1.
Flow chart of research and selection process.
Table 1.
General characteristics of studies included in the meta-analysis.
| Author | Year | Language | Country | Diagnostic criteria | Source of control | Sample size (case/control) | Method | Genotype | HWE | NOS | Ref | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||||||||||||||
| G | A | GG | GA | AA | G | A | GG | GA | AA | |||||||||||
| Bu | 2014 | English | China | IDF | HB | 200/200 | RFLP | 351 | 49 | 162 | 27 | 11 | 384 | 16 | 188 | 8 | 4 | Yes | 7 | 20 |
| Uzma | 2019 | English | Pakistan | IDF | HB | 224/200 | RFLP | 301 | 147 | 100 | 101 | 23 | 280 | 120 | 102 | 76 | 22 | Yes | 8 | 21 |
| Vani | 2012 | English | India | NCEPATP III | HB | 269/272 | RFLP | 429 | 109 | 169 | 91 | 9 | 502 | 42 | 230 | 42 | 0 | Yes | 6 | 22 |
| Zhao | 2013 | Chinese | China | NCEPATP III | HB | 600/600 | Taqman | 1083 | 117 | 488 | 107 | 5 | 1142 | 58 | 543 | 56 | 1 | Yes | 7 | 23 |
| Catherine | 2010 | English | France | NCEPATP III | PB | 877/877 | Golden Gate Assay | 1549 | 205 | 683 | 183 | 11 | 1494 | 260 | 635 | 224 | 18 | Yes | 6 | 24 |
| Ranbir | 2012 | English | India | NCEPATP III | HB | 245/321 | RFLP | 236 | 254 | 11 | 214 | 20 | 343 | 299 | 56 | 231 | 34 | Yes | 8 | 25 |
| Aline | 2005 | English | France | NCEPATP III | PB | 601/594 | RFLP | 496 | 79 | 198 | 73 | 3 | 1409 | 269 | 599 | 211 | 29 | Yes | 7 | 26 |
| Gong | 2016 | Chinese | China | CDS | HB | 119/60 | RFLP | 204 | 34 | 89 | 26 | 4 | 111 | 9 | 51 | 9 | 0 | Yes | 7 | 27 |
| Fan | 2019 | Chinese | China | CDS | HB | 48/60 | RFLP | 81 | 15 | 35 | 11 | 2 | 113 | 7 | 53 | 7 | 0 | Yes | 8 | 28 |
| Seyed | 2015 | English | Iran | IDF | HB | 94/128 | RFLP | 122 | 66 | 41 | 40 | 20 | 168 | 88 | 60 | 48 | 20 | Yes | 7 | 29 |
| Szkup | 2018 | English | Poland | IDF | PB | 118/298 | Melting curves | 201 | 35 | 85 | 31 | 2 | 515 | 81 | 226 | 63 | 9 | Yes | 8 | 30 |
HB hospital based, PB population based, IDF the International Diabetes Federation, NCEPATP III the National Cholesterol Education Program Adult Treatment Panel III, HWE Hardy–Weinberg Equilibrium, CDS Chinese Diabetes Society, RFLP restriction fragment length polymorphism, NOS Newcastle–Ottawa Score.
Meta-analysis
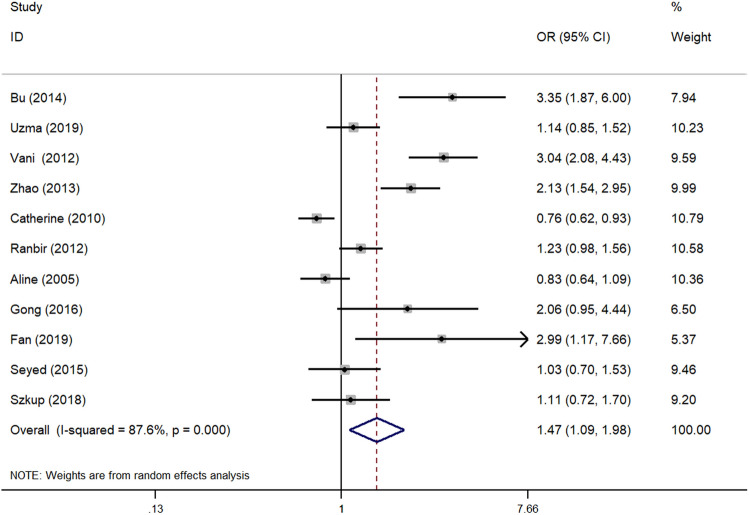
We evaluated the association between -308G/A polymorphism of TNF-α gene and MS susceptibility in four genetic models. Overall, significant association was found in both allele model (OR 1.47, 95% CI 1.09–1.98, P 0.013) (Fig. 2) and dominant model (OR 1.77, 95% CI 1.21–2.58, P 0.003). No significant association was observed in recessive model (OR 1.08, 95% CI 0.67–1.74, P 0.744) and homozygous model (OR 1.50, 95% CI 0.84–2.70, P 0.174).
Figure 2.
Forest plot of the association between -308G/A polymorphism of TNF-α gene promoter and MS risk under allele model. OR odds ratio, CI confidence interval.
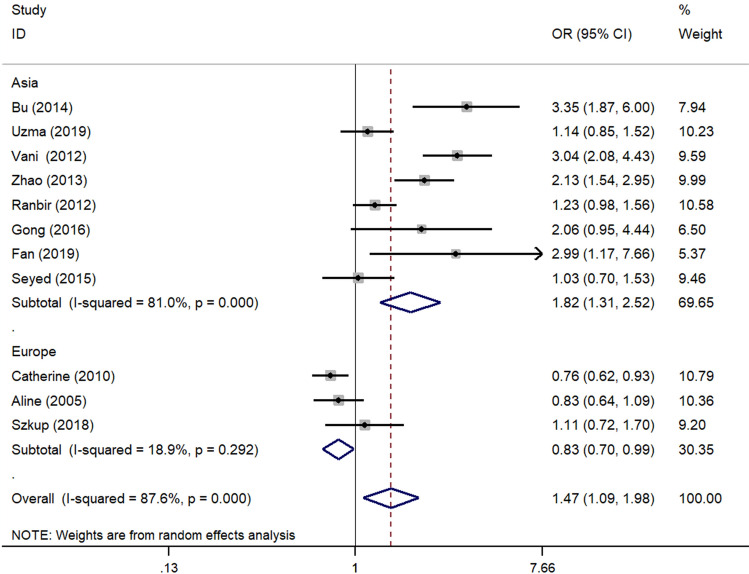
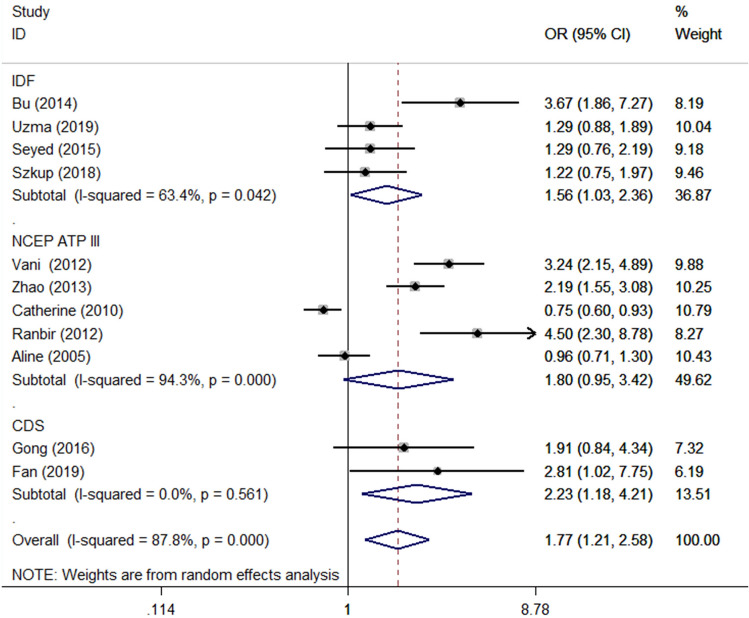
In the subgroup analysis performed by the regions of the studies, A allele showed increased risk of MS in Asia group (allele model: OR 1.82 95% CI 1.31–2.53, P < 0.001; dominant model: OR 2.30, 95% CI 1.64–3.21 P < 0.001; homozygous model: OR 2.29, 95% CI 1.31–4.01, P 0.004). Inversely, the A allele showed decreased risk of MS in Europe group (dominant model: OR 0.83, 95% CI 0.70–0.99, P < 0.001; recessive model: OR 0.51, 95% CI 0.28–0.92, P 0.025; homozygous model: OR 0.49 95% CI 0.27–0.89, P 0.02) (Fig. 3). When subgroup analysis was stratified by diagnostic criteria, the A allele showed increased risk of MS in CDS group (allele model: OR 2.39, 95% CI 1.31–4.34, P 0.004; dominant model: OR 2.23, 95% CI 1.18–4.21, P 0.014) and IDF groups (dominant model: OR 1.56, 95% CI 1.03–2.36, P 0.038) (Fig. 4). No significant association was observed in NCEP ATP III group. When stratified by the source of control, the results were the same as the ones stratified by regions of the studies (Fig. 4; Table 2).
Figure 3.
Subgroup analysis stratified by regions under allele model. OR odds ratio, CI confidence interval.
Figure 4.
Subgroup analysis stratified by criteria under dominant model. OR odds ratio, CI confidence interval. IDF the International Diabetes Federation, NCEPATP III the National Cholesterol Education Program Adult Treatment Panel III; CDS Chinese Diabetes Society.
Table 2.
The association between -308G/A polymorphism of TNF-α gene and MS susceptibility.
| Group | No. of studies | Case | Control | Pool OR | 95% CI | P | I2 | Ph | Begg' test | Egger' test |
|---|---|---|---|---|---|---|---|---|---|---|
| Allele model (A vs. G) | ||||||||||
| Overall | 11 | 3277 | 3312 | 1.47 | 1.09–1.98 | 0.013 | 80.93% | < 0.001 | 0.061 | 0.032 |
| Region | ||||||||||
| Asia | 8 | 1799 | 1841 | 1.82 | 1.31–2.53 | < 0.001 | 81.00% | < 0.001 | ||
| Europe | 3 | 1696 | 1796 | 0.83 | 0.70–0.99 | 0.033 | 18.90% | 0.0048 | ||
| Diagnostic criteria | ||||||||||
| IDF | 4 | 636 | 826 | 1.37 | 0.90–2.01 | 0.138 | 76.30% | 0.006 | ||
| NCEP ATP III | 5 | 2592 | 2664 | 1.36 | 0.85–2.18 | 0.2 | 93.50% | < 0.001 | ||
| CDS | 2 | 167 | 120 | 2.39 | 1.31–4.34 | 0.004 | 0.00% | 0.546 | ||
| Dominant model (AA + GA vs GG) | ||||||||||
| Overall | 11 | 3277 | 3312 | 1.77 | 1.21–2.58 | 0.003 | 82.19% | < 0.001 | 0.161 | 0.017 |
| Region | ||||||||||
| Asia | 8 | 1799 | 1841 | 2.3 | 1.64–3.21 | < 0.001 | 67.70% | 0.003 | ||
| Europe | 3 | 1696 | 1796 | 0.9 | 0.69–1.17 | 0.426 | 51.80% | 0.126 | ||
| Diagnostic criteria | ||||||||||
| IDF | 4 | 636 | 826 | 1.56 | 1.03–2.36 | 0.038 | 63.40% | 0.042 | ||
| NCEP ATP III | 5 | 2592 | 2664 | 1.8 | 0.95–3.42 | 0.72 | 94.30% | < 0.001 | ||
| CDS | 2 | 167 | 120 | 2.23 | 1.18–4.21 | 0.014 | 0.00% | 0.561 | ||
| Recessive model (AA vs GG + GA) | ||||||||||
| Overall | 11 | 3277 | 3312 | 1.08 | 0.67–1.74 | 0.744 | 49.40% | 0.032 | 0.161 | 0.077 |
| Region | ||||||||||
| Asia | 8 | 1799 | 1841 | 1.52 | 0.87–2.67 | 0.14 | 47.30% | 0.066 | ||
| Europe | 3 | 1696 | 1796 | 0.51 | 0.28–0.92 | 0.025 | 0.00% | 0.636 | ||
| Diagnostic criteria | ||||||||||
| IDF | 4 | 636 | 826 | 1.19 | 0.73–1.95 | 0.48 | 22.10% | 0.278 | ||
| NCEP ATP III | 5 | 2592 | 2664 | 0.91 | 0.39–2.11 | 0.821 | 63.10% | 0.028 | ||
| CDS | 2 | 167 | 120 | 5.5 | 0.66–45.81 | 0.115 | 0.00% | 0.028 | ||
| Homozygous model (AA vs GG) | ||||||||||
| Overall | 11 | 3277 | 3312 | 1.5 | 0.84–2.70 | 0.174 | 61.00% | 0.004 | 0.533 | 0.169 |
| Region | ||||||||||
| Asia | 8 | 1799 | 1841 | 2.29 | 1.31–4.01 | 0.004 | 37.20% | 0.132 | ||
| Europe | 3 | 1696 | 1796 | 0.49 | 0.27–0.89 | 0.02 | 0.00% | 0.686 | ||
| Diagnostic criteria | ||||||||||
| IDF | 4 | 636 | 826 | 1.34 | 0.81–2.21 | 0.256 | 19.70% | 0.291 | ||
| NCEP ATP III | 5 | 2592 | 2664 | 1.65 | 0.48–5.75 | 0.428 | 79.50% | 0.001 | ||
| CDS | 2 | 167 | 120 | 6.2 | 0.74–51.78 | 0.092 | 0.00% | 0.862 | ||
IDF the International Diabetes Federation, NCEPATP III the National Cholesterol Education Program Adult Treatment Panel III, CDS Chinese Diabetes Society, OR odds ratio, CI confidence interval.
Results of tests for heterogeneity were also shown in Table 2. High degree heterogeneity was shown in allele model (I2 80.93%, Ph < 0.001) and dominant model (I2 82.19%, Ph < 0.001). Low and moderate degree heterogeneity was found in recessive model (I2 49.94%, Ph 0.032) homozygous model (I2 61.00%, Ph 0.004), respectively. Sensitive analysis was performed by omitting one study at a time and then the ORs and 95% CI of the remaining studies were recalculated. In this study, the pooled ORs and the 95% CI calculated after excluding single study did not show violent fluctuation compared with the value of overall analysis, suggesting the results of our study were stable. Publication bias analysis was performed using Begg’s and Egger’s tests in all the four genetic models. As shown in Table 2, all the P values were > 0.05 except the Egger’s test in dominant model (P = 0.017).
Discussion
Prior studies have revealed chronic inflammation may be one of most important promoting factors for the development of MS31–35. TNF-α secreted by M1 pro-inflammatory adipose tissue macrophages is one of the most important pro-inflammatory mediators in various organs of human body and could initiate the NF-κB and JNK pathways involved in the insulin resistance and the apoptosis of the β cells within the pancreatic islets36–41, which suggested TNF-α may play a central role in the development of MS. Many case–control studies tried to assess the relationship between -308G/A polymorphism of TNF-α gene promoter and the risk of MS, however, their results were inconsistent and with low statistic power due to the small sample sizes of those studies.
In this study, we performed a meta-analysis to evaluate the relationship between -308G/A polymorphism of TNF-α gene promoter and MS risk. Eleven studies containing 3277 cases and 3312 controls were included totally. Our results revealed that the A allele increased 48%, 77% risk for MS in allele model and dominant model in overall study, respectively. In the subgroup analysis, it was of great interests for us to find opposite conclusions in the Asia and Europe subgroup. The A allele distinctly increased MS susceptibilities in allele model (OR 1.82, 95% CI 1.31–2.53, P < 0.001), dominant model (OR 2.30, 95% CI 1.64–3.21, P < 0.001), and homozygous model (OR 2.29, 95% CI 1.31–4.01, P 0.004) in Asia subgroups, while in Europe subgroup the A allele seemed to play a protective role for MS in allele model (OR 0.83, 95% CI 0.70–0.99, P 0.033), recessive model(OR 0.51, 95% CI 0.28–0.92, P 0.025) and homozygous model (OR 0.49, 95% CI 0.27–0.89, P 0.02). Our results showed the complexities for MS once again. Why did the same variant play antipodal roles of MS in different countries? MS is a kind of complex and polygenetic disorder. Different ethnic group may have entirely different genetic background. Silvia et al. performed a meta-analysis to assess the relationship between -308G/A polymorphism and the risk of MS components18. They found ethnicity was also an important factor to influence relationship between -308G/A polymorphism and hypertension, T2DM and obesity. Our study further demonstrated that -308G/A polymorphism could play different roles in the development of MS in different ethnicities on the base of Silvia et al. Some issues should be discussed when we explained this result: firstly, the three studies for Europe included were population-based, while the eight studies from Asia were hospital-based, whether the different methods to include the control populations by each study influence the results of the two subgroups still need to be consulted, even if the minor allele frequencies of A was near to each other between the two the control groups (0.173 in Asia group and 0.151 in Europe group). Secondly, as another risk factor, age also played vital roles in the development of MS, another meta-analysis performed by us revealed the rs9939609 of FTO gene was associated with MS in Chinese adults but not in Chinese children and adolescents42, that means time is needed in the interaction between genetic factors and environment. The subgroup analysis stratified in different age groups could not be performed in our study. Thirdly, in our study, only three studies in Europe were included the sample size was still limited, further studies with larger sample sizes especially from Europe should perform to confirm our conclusions.
The heterogeneity of meta-analysis usually comes from three aspects: clinical heterogeneity, statistical heterogeneity, and methodological heterogeneity. During the overall analysis, high degrees of heterogeneity was found in the allele model (I2 = 80.93%, P < 0.001) and dominant model (I2 = 82.19%, P < 0.001), while the heterogeneity in recessive model and homozygous model was low (I2 = 49.40%, P 0.032) and moderate (I2 = 61.00%, P 0.004). The results from overall analysis indicated that the different statistical methods could be a source of heterogeneity. In the subgroup analysis, we found the levels of heterogeneity were dramatically decreased in the Asia and Europe subgroups in all the four genetic models, suggesting that the ethnic may be an important source of heterogeneity. In the subgroup analysis stratified by the diagnostic criteria, lower levels of heterogeneity were found in IDF subgroups and no heterogeneity was found in CDS subgroup, the main heterogeneity came from the NCEP ATP III subgroups, which means diagnostic criteria should be another important source of heterogeneity.
Begg’s and Egger’s tests were performed to assess the publication bias tests, A P value 0.017 was found in the Egger’s test in dominant model. Although there were no statistical significance was found in the other Begg’s and Egger’s tests, potential publication bias may exist in our study. Because only eleven studies were included in our meta-analysis, limited number of studies may also contributed to the results of the tests for publication bias.
Our study still had several limitations should be mentioned: firstly, because we searched the articles only in Chinese and English, researches published in other languages were ignored, that may result in bias; secondly, other risk factors of MS such as physical inactivity, excessive food intake were not be considered in our meta-analysis; thirdly, the total sample size still limited in our research, and only studies from Asia and Europe were included, further studies with larger sample size and more other ethnics should be performed to confirm our conclusions.
To our knowledge, this is the first meta-analysis to assess the relationship between the -308G/A polymorphism of TNF-α gene promoter and the susceptibility of the MS. Our results suggested -308G/A polymorphism was significantly associated with increased MS susceptibility in overall analysis, in the further analysis a completely apposite result was found in the Asia and Europe subgroup. Our results illuminated the complex impact of -308G/A polymorphism of TNF-α gene promoter played on the MS susceptibility.
Acknowledgements
This work was supported by Grants from the Natural Science Foundation of Hubei Province (no. 2019CFB405), Hubei Provincial Health and Family Planning Commission (no. WJ2019Q054).
Author contributions
D.W., L.H. designed this study; D.W. and X.Z. searched databases and collected full-text papers; L.H, and X.Z. extracted data; D.W. analyzed the data and wrote the manuscript; L.H. and X.Z. reviewed the manuscript. All of the authors have read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liqun He, Email: Liqunhe0902@163.com.
Xiaotian Zhang, Email: xtzhang@mail.bnu.edu.cn.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diab. Med. J. Brit. Diab. Assoc. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diab. Med. J. Brit. Diab. Assoc. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet P, Shaw J, Grxoup, I. D. F. E. T. F. C The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, E. & Treatment of High Blood Cholesterol in, A Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn D, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocrine Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 6.Cooperative group of metabolic syndrome research, D. B., Chinese Medical Association Recommendations of the Chinese Diabetes Society on metabolic syndrome. Chin. J. Diabetes Mellitus. 2004;12:156–161. [Google Scholar]
- 7.Ekelund U, et al. Prevalence and correlates of the metabolic syndrome in a population-based sample of European youth. Am. J. Clin. Nutr. 2009;89:90–96. doi: 10.3945/ajcn.2008.26649. [DOI] [PubMed] [Google Scholar]
- 8.Steinberger J, et al. Progress and challenges in metabolic syndrome in children and adolescents: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 10.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: Insights from therapeutic interventions. J. Am. Coll. Cardiol. 2005;46:1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers S. Role of tumour necrosis factor (TNF) in host defence against tuberculosis: Implications for immunotherapies targeting TNF. Ann. Rheum. Dis. 2003;62(Suppl 2):37–42. doi: 10.1136/ard.62.suppl_2.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada D, et al. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheumatol. 2016;68:1392–1402. doi: 10.1002/art.39561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Rocha AF, et al. Tumor necrosis factor alpha abolished the suppressive effect of insulin on hepatic glucose production and glycogenolysis stimulated by cAMP. Pharmacol. Rep. 2014;66:380–385. doi: 10.1016/j.pharep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Kabayama K, et al. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: Involvement of ganglioside GM3. Glycobiology. 2005;15:21–29. doi: 10.1093/glycob/cwh135. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum. Mol. Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 17.Abraham LJ, Kroeger KM. Impact of the -308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: Relevance to disease. J. Leukoc. Biol. 1999;66:562–566. doi: 10.1002/jlb.66.4.562. [DOI] [PubMed] [Google Scholar]
- 18.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes. Res. 2005;13:2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Bu DY, et al. Association of polymorphisms in stress-related TNFalpha and NPY genes with the metabolic syndrome in Han and Hui ethnic groups. Asian Pac. J. Cancer Prev. 2014;15:5895–5900. doi: 10.7314/apjcp.2014.15.14.5895. [DOI] [PubMed] [Google Scholar]
- 21.Zafar U, Khaliq S, Ahmad HU, Lone KP. Serum profile of cytokines and their genetic variants in metabolic syndrome and healthy subjects: A comparative study. Biosci. Rep. 2019 doi: 10.1042/BSR20181202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta V, et al. Association of TNF-alpha promoter gene G-308A polymorphism with metabolic syndrome, insulin resistance, serum TNF-alpha and leptin levels in Indian adult women. Cytokine. 2012;57:32–36. doi: 10.1016/j.cyto.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhao, C. Association of Hsp70-1 Gene -110, +190 and TNF-α Gene -308 Polymorphisms with Metabolic Syndrome. Master's Thesis of Ningxia Medical University (2013).
- 24.Phillips CM, et al. Additive effect of polymorphisms in the IL-6, LTA, and TNF-{alpha} genes and plasma fatty acid level modulate risk for the metabolic syndrome and its components. J. Clin. Endocrinol. Metab. 2010;95:1386–1394. doi: 10.1210/jc.2009-1081. [DOI] [PubMed] [Google Scholar]
- 25.Sobti RC, Kler R, Sharma YP, Talwar KK, Singh N. Risk of obesity and type 2 diabetes with tumor necrosis factor-alpha 308G/A gene polymorphism in metabolic syndrome and coronary artery disease subjects. Mol. Cell. Biochem. 2012;360:1–7. doi: 10.1007/s11010-011-0917-z. [DOI] [PubMed] [Google Scholar]
- 26.Meirhaeghe A, Cottel D, Amouyel P, Dallongeville J. Lack of association between certain candidate gene polymorphisms and the metabolic syndrome. Mol. Genet. Metab. 2005;86:293–299. doi: 10.1016/j.ymgme.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Gong X, Ma Q, Sun Z. Correlation analysis of TNFα mRNA expression gene polymorphism in patients with metabolic syndrome and asthma. Guizhou Med. J. 2016;40:25–27. [Google Scholar]
- 28.Jia, S., Zhang, W., Ji, X., Li, J. & Wang, H. Association of TNF-alpha gene G-308A polymorphism and mRNA expression with metabolic syndrome in asthmatic patients. Chin. J. Immunol.29, 960–968. 10.3969/j.issn.1000-484X.2013.09.016 (2013).
- 29.Mirhafez SR, et al. Association of tumor necrosis factor-alpha promoter G-308A gene polymorphism with increased triglyceride level of subjects with metabolic syndrome. Gene. 2015;568:81–84. doi: 10.1016/j.gene.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Szkup M, Chelmecka E, Lubkowska A, Owczarek AJ, Grochans E. The influence of the TNFalpha rs1800629 polymorphism on some inflammatory biomarkers in 45–60-year-old women with metabolic syndrome. Aging. 2018;10:2935–2943. doi: 10.18632/aging.101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 32.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson AL. Regulation of GLUT4 and insulin-dependent glucose flux. ISRN Mol. Biol. 2012;2012:856987. doi: 10.5402/2012/856987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46:1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 40.Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013;114:525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 41.Akash MS, Shen Q, Rehman K, Chen S. Interleukin-1 receptor antagonist: A new therapy for type 2 diabetes mellitus. J. Pharm. Sci. 2012;101:1647–1658. doi: 10.1002/jps.23057. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Wu Z, Zhou J, Zhang X. Rs9939609 polymorphism of the fat mass and obesity-associated (FTO) gene and metabolic syndrome susceptibility in the Chinese population: A meta-analysis. Endocrine. 2020 doi: 10.1007/s12020-020-02280-x. [DOI] [PubMed] [Google Scholar]