Abstract
Introduction
Understanding how hospital staff members (HSMs), including healthcare workers, acquired severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the first wave can guide the control measures in the current second wave in Europe.
Methods
From March 5 to May 10, 2020, the Raymond-Poincaré Hospital held a weekday consultation for HSMs for PCR testing. HSMs were requested to complete a questionnaire on their potential exposure to SARS-CoV-2.
Results
Of 200 HSMs screened, 70 tested positive for SARS-CoV-2. Ninety-nine HSMs completed the questionnaire of whom 28 tested positive for SARS-CoV-2. In the multivariable analysis, age of ≥44 years (aOR = 5.2, 95% CI [1.4–22.5]) and not systematically using a facemask when caring for a patient (aOR = 13.9, 95% CI [1.8–293.0]) were significantly associated with SARS-CoV-2 infection. Working in a COVID-19-dedicated ward (aOR = 0.7, 95% CI [0.2–3.2]) was not significantly associated with infection. Community-related exposure in and outside the hospital, hospital meetings without facemasks (aOR = 21.3, 95% CI [4.5–143.9]) and private gatherings (aOR = 10, 95% CI [1.3–91.0]) were significantly associated with infection.
Conclusions
Our results support the effectiveness of barrier precautions and highlight in-hospital infections not related to patient care and infections related to exposure in the community. Protecting HSMs against COVID-19 is crucial in fighting the second wave of the epidemic.
Keywords: COVID-19, Healthcare workers, Personal protective equipment, Control measures, SARS-CoV-2
Introduction
Healthcare workers (HCWs) are deemed to be at high risk of exposure to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with a potential risk of transmission to vulnerable patients (Keeley et al., 2020, Galmiche et al., 2020). In Japan, Furuse et al. have shown that 30% of clusters identified in reported Coronavirus Disease 2019 (COVID-19) cases are linked to healthcare facilities (Furuse et al., 2020). Hospitals have initiated infection control measures to protect HCWs, ensure workforce availability, and decrease in-hospital transmission risk.
The Assistance Publique–Hôpitaux de Paris (AP-HP) university hospitals group is the largest public hospital system in Europe with 39 centres and 100,000 employees serving 8.3 million patients through a 20 000-bed capacity. In France, when the first patients infected with SARS-CoV-2 arrived from China and Italy in February 2020, AP-HP decided to prioritise testing COVID-19 for individuals suffering from unknown and severe pneumonia and organise a screening consultation for symptomatic HCWs. It was estimated that approximately 500 HCWs had been already infected at the AP-HP before the first lockdown in mid-March 2020. Along with standard precautions, droplet precautions for routine care and using personal protective equipment (PPE) such as wearing a medical mask, medical glasses or a face shield and overcoat, were implemented (Société française d’Hygiène Hospitalière, 2020). Wearing an N95 mask when conducting aerosol-generating procedures (e.g. endotracheal intubation, airway suctioning) was recommended soon after by the Haut Conseil de la Santé Publique (Haut Conseil de la Santé Publique, 2020). Hospital staff members (HSMs) may have different in-hospital exposures according to their occupational roles (clinical or administrative task), their assignment wards (e.g. first-line emergency, critical care, medical technology departments), the type of care provided, in-hospital meetings attended and their access to PPE. The level of in-hospital exposure may vary according to the phases of the epidemic. HSMs are also at risk from general community transmission through contact with an infected person outside the hospital (e.g. relatives, colleagues or friends, passengers in public transportation).
A better understanding of how HCWs were infected during the first wave can guide the implementation of preventive measures to reduce the future risk of infection, especially during the current second wave in Europe.
Our objectives were to characterise exposure types and explore factors associated with the acquisition of SARS-CoV-2 infection among Raymond Poincaré’s Hospital staff, a tertiary care hospital in the greater Paris area belonging to the AP-HP.
Methods
Setting
Raymond Poincaré Hospital, a 386-bed AP-HP hospital located in the surrounds of Paris, is a second-line centre in epidemic risk management and pandemics and was appointed as one of the referral centres for the management of COVID-19 patients by the Health Ministry. Due to its pavilion-like structure, one of its buildings, comprising a 42-bed physical medicine and rehabilitation department on the first floor, a 26-bed infectious disease department on the second floor and a 15-bed intensive care unit (ICU) on the third floor, was dedicated to COVID-19 patients. Soon after the first COVID-19 patient was admitted to the hospital, the infectious disease department opened a weekday consultation dedicated to the HSMs. Initially, in March, only symptomatic HSMs had access to COVID-19 diagnosis; from July, SARS-CoV-2 screening was extended to HSMs who had been in contact with a confirmed COVID-19 case. HSMs were considered symptomatic if they reported any of the following symptoms before the visit: fatigue, headache, cough, myalgia, sore throat, fever, diarrhoea, anosmia or shortness of breath. A real-time reverse transcriptase-polymerase chain reaction (RT-PCR) amplifying the betacoronavirus E gene and the SARS-CoV-2 RdRp gene was performed to detect the SARS-CoV-2 genome from a nasopharyngeal swab collected by a trained nurse. The physician in charge of the consultation communicated results to the HSM within 12–24 h, by phone, if positive, by email if negative.
Data collection
After receiving information on the study from the physician, HSMs were requested to complete a questionnaire while waiting for the nasopharyngeal swab to be performed or shortly after they received their result. The questionnaire collected data on their professional category (e.g. nurse, physician, nursing assistant, support staff, administrative employee), their symptoms and date of onset, and exposures in the 2 weeks before the onset of symptoms. Exposures covered in-hospital activities (e.g. working in a “COVID-19 dedicated unit”, specific at-risk procedures, use of PPE, meetings) and potential outside exposures including transportation (e.g. public transport, car-sharing), private gatherings, contact with a suspected COVID-19 case, and the number of children in the household.
Statistical analysis
Participants’ characteristics were described as median (interquartile range, IQR) and numbers (proportions) for continuous and categorical variables, respectively. In the analysis of associations between characteristics/exposures and SARS-CoV-2 PCR status, univariate odds ratios (OR) and 95% CI were estimated using logistic regression. Variables with P values ≤ 0.2 in the univariate analysis or variables having a specific interest (ward type variable) were further assessed in 2 backward stepwise multivariable logistic regression models. In the first model, restricted to HCWs, we explored exposures related to the context of care. In the second model, including all the participants (i.e. administrative employees and HCWs), we explored “community” related exposures occurring in and outside the hospital (such as “meals with coworkers” and presence of “child under 15 years old at home”). They are referred to as “community-related exposures in-and-out hospital” later in the text. Gender was included in both models. Results are presented using adjusted odds ratios (aOR) with 95% CI.
In univariate and multivariable analysis, continuous variables were expressed as dichotomous variables using the median. The variable “systematic use of facemasks” was obtained from 2 variables describing the wearing of the surgical mask and the N95 mask. Only the HCWs who stated that they had always worn a surgical mask or an N95 mask were considered to have systematically used a facemask.
Statistical analyses were carried out using the R studio open-source software. A P value of ≤0.05 was considered statistically significant.
Missing data handling
Several independent variables of interest in the dataset had missing values. As the models detailed above are a complete-case analysis, we performed the following additional analysis. For each variable of interest, with more than 5% missing values (i.e. “systematic use of facemask” and “procedure involving patient airways” in the HCW model and “meetings” and “private social gathering” in the HSM model), we first compared the proportion of missing values in the PCR positive and negative groups (Fisher exact test) to evaluate a potential information bias. We then performed a sensitivity analysis by estimating ORs, with all missing values imputed either to exposure or non-exposure, and evaluated the potential impact on the direction and strength of association and statistical significance (Supplementary material 2 and 3).
Additional data from other sources
We also present additional aggregated data from the occupational health department: number of infected hospital staff by date of onset of symptoms from March to May 2020 and the cumulative daily number of patients hospitalised for COVID-19.
Ethics
The Ethical Review Committee of the GH University Paris-Saclay (Polethis) (CER-Paris-Saclay-2020-048) approved the study. All participants received written and oral information on the study and signed a non-opposition statement.
Results
Of the 200 HSMs examined at the COVID-19 consultation from March 5 to May 10, 99 (49.5%) completed the questionnaire. More than two-thirds were female, and the median age was 44 (Table 1 ). Approximately 9/10 HSMs were HCWs (n = 86) of which, 33 (38.4%) were nurses, and 52 (60.5%) worked in a COVID-19 dedicated ward, including Medicine or ICU. The systematic use of a facemask when taking care of patients was reported by 70/77 (90.1%) HCWs who answered this question.
Table 1.
Characteristics of the hospital staff members who participate to the study.
| Characteristics | N = 99 |
|---|---|
| n (%) | |
| Sex | |
| Male | 27 (27.3) |
| Female | 72 (72.7) |
| Age, Median (Iqr) | 44 (34–55) |
| Age groups | |
| <44 ans | 49 (49.5) |
| >44 ans | 50 (50.5) |
| Hospital staff professional category | |
| Healthcare worker | 86 (86.9) |
| Physiciana | 18 |
| Nurseb | 33 |
| Nursing assistant | 14 |
| Support staffc | 21 |
| Administrative employee | 13 (13.1) |
| Unit | |
| COVID-19 wards | 52 (52.5) |
| Medical COVID-19 ward | 31 |
| ICU | 21 |
| COVID-19 free wards | 34 (34.3) |
| General management | 13 (13.1) |
| Training session in hygiened | 53 (55.2) |
| Flu vaccination 2019–2020e | 36 (37.1) |
| Use of personal protective equipmentf | |
| Surgical masks (n = 61) | 45 (73.8) |
| N95 masks (n = 58) | 22 (55) |
| Overcoats (n = 70) | 24 (34.3) |
| Goggles (n = 70) | 19 (27.1) |
| Systematic use of maskg | 70 (90.9) |
| Individual risk factor for severityh | 14 (15.2) |
| At least one COVID-19 symptom | 87 (87.9) |
| Fatigue | 70 (80.5) |
| Headache | 56 (64.4) |
| Cough | 46 (52.9) |
| Myalgia | 44 (50.6) |
| Sore throat | 40 (46.0) |
| Fever | 35 (40.2) |
| Diarrhea | 28 (32.2) |
| Anosmia | 27 (31.0) |
| Shortness of breath | 15 (17.2) |
| COVID-19 PCR status | |
| Positive | 28 (28.3) |
| Negative | 71 (71.2) |
Also include residents and medical students.
Also include physiotherapists.
Include:pharmacy and laboratory staff, cleaning staff, health manager, hospital porter, speech therapists, ergo therapists, daycare staff, logisticians.
Missing data: n = 3.
Missing data: n = 2.
Only healthcare workers are included; the total exceeds 100% because the same respondent could use several equipment. Only staff working in COVID-19 wards are included for N95 mask.
“Yes” is when the mask (surgical or N95) is always worn. Any other case is coded as “no”.
Presence of at least one comorbidity associated with a high of risk of severe COVID-19 form according to the national authorities (Société française d’Hygiène Hospitalière, 2020). Missing data: n = 7.
Among the 87 (87.9%) symptomatic HSMs, the most frequently reported symptoms were fatigue (80.5%), headache (64.4%) and cough (52.9%). Fever and anosmia were reported by 40.2% and 31.0% of HSMs, respectively. In total, 28 (28.3%) HSMs had a positive SARS-CoV-2 PCR, all reported at least 1 symptom, with fever, cough and anosmia being the most frequent symptoms (each n = 18; 64.3%) and dyspnoea reported by 5 (17.9%) of them.
In the multivariable analysis among HCWs (Table 2 ), being aged ≥44 (aOR = 5.2, 95% CI [1.4–22.5]) and not systematically using a facemask when caring for a patient (aOR = 13.9, 95% CI [1.8–293.0]) were significantly associated with SARS-CoV-2 infection. Working in a ward dedicated to COVID-19 patients (aOR = 0.7, 95% CI [0.2–3.2]) and performing procedures involving patient airways (aOR = 2.6, 95% CI [0.6–12.5]) were not significantly associated with SARS-CoV-2 infection (Supplementary material 1).
Table 2.
Frequency of exposures related to care according to SARS-CoV-2 PCR status, univariate and multivariable analysis. Healthcare workers, March–April 2020.
| PCR result |
Univariate OR | p | Adjusted ORa | ||
|---|---|---|---|---|---|
| N = 86 |
(95% CI) | (95% CI) | |||
| Positive: 27 | Negative: 59 | ||||
| n (%) | n (%) | ||||
| Age | |||||
| <44 | 9 (33.3) | 35 (59.3) | ref | ref | |
| >44 | 18 (66.7) | 24 (40.7) | 2.9 (1.2–7.9) | 0.03 | 5.2 (1.4–22.5) |
| Gender | |||||
| Female | 22 (81.5) | 41 (69.5) | ref | ref | |
| Male | 5 (18.5) | 18 (30.5) | 0.5 (0.2–1.5) | 0.2 | 1.5 (0.3–6.3) |
| Systematic use of mask | |||||
| Yes | 14 (70.0) | 49 (98.0) | ref | ref | |
| No | 6 (30.0) | 1 (2.0) | 21.0 (3.2–414.6) | 0.007 | 13.9 (1.8–293.0) |
| Missing datab:16 | |||||
| Ward type | |||||
| COVID-19 free ward | 13 (48.1) | 21 (35.6) | ref | ref | |
| COVID-19 ward | 14 (51.9) | 38 (64.4) | 0.6 (0.2–1.5) | 0.3 | 0.7 (0.2–3.2) |
| Procedure involving patient airways | |||||
| No | 11 (50.0) | 36 (65.5) | ref | ref | |
| Yes | 11 (50.0) | 19 (34.5) | 1.9 (0.7–5.2) | 0.2 | 2.6 (0.6–12.5) |
| Missing datac: 9 | |||||
| Profession | |||||
| Physiciansd | 4 (14.8) | 14 (23.7) | 0.7 (0.2–4.3) | 0.7 | |
| Nursese | 11 (40.7) | 22 (37.3) | 1.3 (0.4–4.3) | 0.7 | |
| Nursing assistants | 6 (22.2) | 8 (13.6) | 1.9 (0.5–8.0) | 0.4 | |
| Support staff | 6 (22.2) | 15 (25.4) | ref | ||
| Access to a locker | |||||
| No | 9 (37.5) | 10 (17.9) | ref | ||
| Yes | 15 (62.5) | 46 (82.1) | 0.4 (0.1–1.1) | 0.06 | |
| Missing data:6 | |||||
| Contact with infected coworker | |||||
| No or unknown | 13 (52.0) | 36 (64.3) | ref | ||
| Yes | 12 (48.0) | 20 (35.7) | 1.7 (0.6–4.4) | 0.3 | |
| Missing data: 5 | |||||
Multivariable logistic regression analysis final model.
Fisher-test: p = 0.25.
Fisher-test: p = 0.13.
Also include residents and medical students.
Also include physiotherapist.
While the proportion of missing values did not differ between HCWs with a positive SARS-CoV-2 PCR test versus those testing negative, sensitivity analysis for the systematic use of a facemask did not show a significant impact of imputed values on the OR (Supplementary material 2). Regarding “community”-related exposures in-and-out the hospital among the HSMs, participation in meetings inside the hospital without wearing a facemask (aOR = 21.3, 95% CI [4.5–143.9]) and participation in private gatherings (aOR = 10.0, 95% CI [1.3–91.0]) were significantly associated with SARS-CoV-2 infection (Table 3 ). The proportion of missing values did not differ between HSMs with a positive SARS-CoV-2 and those with a negative test. Sensitivity analysis for these 2 variables did not show a significant impact on the ORs (Supplementary material 3).
Table 3.
Frequency of community related exposure: in-and-out hospital according to SARS-CoV-2 PCR status univariate and multivariable analysis. Hospital staff members, March-April 2020.
| PCR result |
Univariate OR | p | Adjusted ORa | ||
|---|---|---|---|---|---|
| N = 99 |
(95% CI) | (95% CI) | |||
| Positive: 28 | Negative: 71 | ||||
| n (%) | n (%) | ||||
| Age | |||||
| <44 | 9 (32.1) | 40 (56.3) | ref | ref | |
| >44 | 19 (67.9) | 31 (43.7) | 2.9 (1.2–7.9) | 0.03 | 6.7 (1.7–37.7) |
| Gender | |||||
| Female | 22 (78.6) | 50 (70.4) | ref | ref | |
| Male | 6 (21.4) | 21 (29.6) | 0.7 (0.2–1.8) | 0.4 | 0.8 (0.2–2.36) |
| Meetingsb | |||||
| None or meetings with mask | 16 (59.3) | 60 (93.8) | ref | ref | |
| Meetings without mask | 11 (40.7) | 4 (6.2) | 10.3 (3.1–41.4) | <0.001 | 21.3 (4.5–143.9) |
| Missing datac: 8 | |||||
| Child < 15 years old at home | |||||
| No | 10 (43.5) | 44 (62) | ref | ref | |
| Yes | 13 (56.5) | 27 (38) | 2.1 (0.8–5.6) | 0.1 | 3.1 (0.9–12.9) |
| Missing data: 5 | |||||
| Private social gathering | |||||
| No | 18 (78.3) | 61 (91.0) | ref | ref | |
| Yes | 5 (21.7) | 6 (9.0) | 2.8 (0.7–10.5) | 0.1 | 10.0 (1.3–91.0) |
| Missing datad: 9 | |||||
| Active smoking | |||||
| No | 25 (92.6) | 54 (76.1) | ref | ||
| Yes | 2 (7.4) | 17 (23.9) | 0.3 (0.04–1.0) | 0.08 | |
| Missing data:1 | |||||
| Meals with coworkers | |||||
| No | 5 (18.5) | 19 (28.4) | ref | ||
| Yes | 22 (81.5) | 48 (71.6) | 1.7 (0.6–5.8) | 0.3 | |
| Missing data:5 | |||||
| Suspected COVID-19 at home | |||||
| No | 22 (78.6) | 61 (87.1) | ref | ||
| Yes | 6 (21.4) | 9 (12.9) | 1.9 (0.6–5.7) | 0.3 | |
| Missing data:1 | |||||
| Type of transportation | |||||
| Personal | 23 (82.1) | 54 (77.1) | ref | ||
| Public | 5 (17.9) | 16 (22.9) | 1.4 (0.5–4.6) | 0.6 | |
| Missing data:1 | |||||
Multivariable logistic regression analysis final model.
Meetings with variable number of participants.
Fisher-test: p = 0.43.
Fisher-test: p = 0.11.
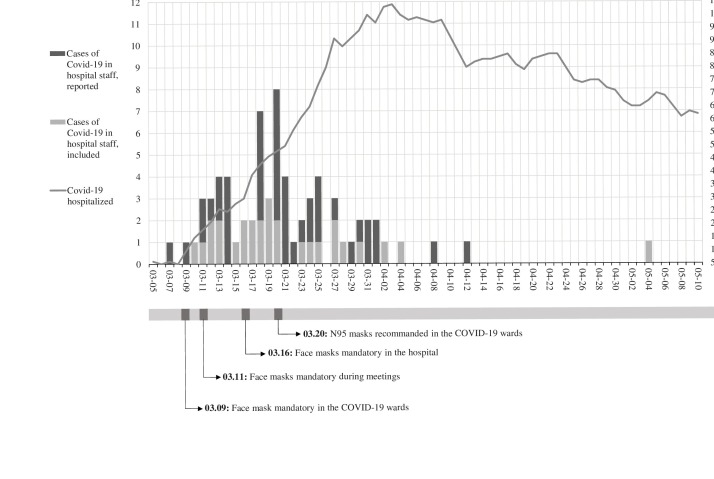
Epidemic curve (Figure 1)
Figure 1.
Cases of COVID-19 in hospital staff reported and included by date of onset of symptoms (day) and the cumulative number of patients hospitalised by date.
Black bars represent COVID-19 positive cases among hospital staff. Grey bars represent the COVID-19 cases who participated in the study. The sum of the black and grey bars represents the total number of COVID-19 positive cases at each date. For example: on March 20, 2020, there were 8 new cases among hospital staff members, including 2 who participated in the study. The grey line represents the cumulative number of patients hospitalised by day. Dates of several control measures implemented in the hospital are indicated below the figure.
From March 5 to May 10, 2020, 70 HSMs tested positive for SARS-CoV-2, with a higher number of cases in mid-March parallel to the increasing number of COVID-19 patients hospitalised, followed by a substantial decrease in April, although the number of patients stayed at its highest level. Following the first case among HSMs, wearing facemasks (surgical) became mandatory in the COVID-19 wards. A few days later, wearing a facemask (surgical) became mandatory during meetings and ultimately in all hospital premises. Two weeks after the first case among HSMs, N95 masks were recommended in the COVID-19 wards. The 28 HSMs who tested positive for SARS-CoV-2 during the study were evenly distributed across the epidemic curve by date of onset of symptoms.
Discussion
Reporting of SARS-CoV-2 infection, together with the investigation of potential exposures, both implemented at the very beginning of the first wave, provided insights into transmission among the HSMs of a referral hospital. The number of cases in HSMs first increased rapidly, just before the increasing number of patients hospitalised in the first 3 weeks of March 2020. After that, the number of HSM cases started to decrease before the peak of COVID-19 patients hospitalised at the beginning of April. This trend may be a response to the control measures rapidly implemented by the hospital in the second week of March, such as mandatory facemask wearing for HSMs as in other settings where the effectiveness of these measures had been reported. Suarez Garcia et al. (Suárez-García et al., 2020) described the same shift between HCW infection and the global population in Madrid (Spain), with an earlier control of the transmission of SARS-CoV-2 due to the recommendation to wear N95 masks. Wang et al. also observed a strong association between the universal wearing of facemasks in a healthcare facility and a decrease in the incidence of SARS-CoV-2 among HCWs (Wang et al., 2020).
The 28.3% of symptomatic HSMs testing positive for SARS-CoV-2 in our study is higher than the reported rate during the first wave in a study conducted in a large NHS Foundation Trust hospital in the United Kingdom during March 2020. This study by J. Keeley et al. (Keeley et al., 2020) described 18% of symptomatic HCWs testing positive for SARS-CoV-2. This discrepancy may be due to our study being in a region affected severely and early by COVID-19. However, our rate of approximately 30% is concordant with a study by the Québec Public Health Institute in Canada, where 37.4% of symptomatic HCWs tested positive for SARS-CoV-2 between February and June 2020 (De Serres et al., 2020).
Approximately 25% of individuals infected with SARS-CoV-2 in the general population are asymptomatic (Santé publique France, 2020). Therefore, the SARS-CoV-2 positivity in symptomatic HCWs does not reflect overall transmission in this population. Other studies reported variable proportions of positive SARS-CoV-2 tests among asymptomatic HCWs. At London Hospital, a study by Treible et al. (Treibel et al., 2020) found that early in the first wave, starting on March 23, 2020, the percentage of asymptomatic HCWs who tested positive peaked at 7.1% and decreased to 1.1% after 4 weeks despite the persistence of a high number of COVID-19 in-patients, but consistent with the decreasing number of new cases in London. As suggested by the authors, testing strategies in HCWs could target those presenting symptoms in low circulation phases and be extended to asymptomatic HCWs during new waves.
In our study, HCWs who reported not systematically wearing a facemask were more likely to be infected. Indeed, intermittent use of a facemask places HCWs at risk of infection. It has been acknowledged that surgical and N95 masks are effective in the prevention of infection (Bartoszko et al., 2020). However, medical masks (surgical masks and even N95 masks) could not completely block the transmission of virus droplets/aerosols even when sealed (Ueki et al., 2020), which suggests that both HCWs and patients should wear medical masks.
Our results did not show a higher risk of SARS-CoV-2 infection in HCWs practising in COVID-19 dedicated units; this could be linked to the pavilion layout of Raymond Poincaré’s hospital, with a single building dedicated to the management of COVID-19, and better use of PPE in these units. The hospital-initiated training sessions in hygiene regarding isolation precautions for 668 HSMs between January 27 and April 30, 2020 (652 h of training) may have contributed to limiting nosocomial transmission of COVID-19. Suárez-García et al. (2020), in a study where 11.1% of 1911 HCWs tested positive for SARS-CoV-2, also did not find any significant difference between COVID-19 and non-COVID-19 departments. Procedures involving patients’ airways were not associated with a higher risk in our study; this may be interpreted as a global low-risk exposure as long as N95 masks are used when performing aerosol-generating procedures (Ueki et al., 2020). We did not find any significant difference in the risk of infection between physicians and nurses.
In the model of all HSMs, our study shows that attending meetings without facemasks is a high risk for SARS-CoV-2 infection. It has been previously highlighted that there is an increased potential risk of transmission during situations where HCWs are not wearing a facemask, such as meetings or lunch breaks in small spaces (Belingheri et al., 2020). Richterman et al. emphasise that adequate and well-ventilated dedicated spaces must be provided for breaks from daily work activities and mealtimes for HCWs to minimise contact and reduce the risk of transmission (Richterman et al., 2020). In a national French survey among HCWs diagnosed with COVID-19, approximately two-thirds reported participating in meetings without facemasks before March 20, 2020, and 20% after that (Olivier et al., 2020). Our work emphasises that meetings are a situation at risk for transmission, but we did not find that sharing meals with coworkers was associated with a high risk of SARS-CoV-2 infection. Although this could be due to the limited sample size, it could be partly explained by instructions given in our hospital to avoid having meals with colleagues, especially in small rooms.
We did not find any association with exposures in the community, such as using public transportation or contact with a suspected case at home. The testing policy for contact-cases was implemented in July 2020 and therefore did not affect the HSMs in our study; this may have impacted how they answered the question on contacts with COVID-19 cases and the possibility for the study to find an association. However, private social gatherings were associated with SARS-CoV-2 infection, underlying that prevention outside the hospital is also essential, as previously described in the ComCor study (Galmiche et al., 2020).
Our study has several limitations. First, we mainly studied symptomatic HSMs, which may partly explain why being aged ≥44 was independently associated with infection since older individuals are more likely to develop symptoms of COVID-19 (Davies et al., 2020). It has also been reported that HCWs are uncommonly asymptomatic, ranging from 1.6% to 3% of the individuals tested (Rivett et al., 2020, Lombardi et al., 2020).
Second, our study only evaluated the HSMs who completed the questionnaire (50%), contributing to limited sample size. Therefore, our study was underpowered and unlikely to find exposures not strongly associated with SARS-CoV-2 infection. Third, some symptomatic HSMs may have been classified wrongly as SARS-CoV-2 negative due to an early nasopharyngeal PCR testing with up to 30% false-negatives reported (Woloshin et al., 2020), therefore potentially underestimating the OR.
Fourth, due to the study’s design, memorisation bias cannot be ruled out, with HSMs testing positive for SARS-CoV-2 more likely to remember potential exposures than those testing negative. However, a substantial proportion of the participants completed the questionnaire before knowing their PCR status.
Nevertheless, to our knowledge, this work is one of the first to explore the risk factors associated with HSMs infection due to SARS-CoV-2. Our results support the effectiveness of PPE and underline that in-hospital transmission not related to patient care may occur and that some infections may be related to exposures in the community. Therefore, better protecting HCWs against COVID-19 includes limiting the number of face-to-face meetings and wearing facemasks during those meetings. In addition to the timely screening of symptomatic HSMs, or those with known contact with a case of SARS-CoV-2 infection, systematic screening of asymptomatic HSMs after potential out-of-work exposures, including holidays, could be suggested. Those recommendations are necessary until the ongoing COVID-19 vaccination campaign demonstrates efficacy to block the transmission of SARS-CoV-2 between individuals.
Funding
The authors have no financial relationships relevant to this article to disclose.
Conflicts of interest
BD has received consulting fees or travel grants from ViiV Healthcare and Gilead Sc. PdT has received consulting fees or travel grants from ViiV Healthcare, M.S.D and Gilead Sc. The remaining authors have no specific conflict of interest.
Ethics approval
The Ethical Rpeview Committee of the GH University Paris-Saclay (Polethis) (CER-Paris-Saclay-2020-048) approved the study.
Consent to participate
All participants received written and oral information on the study and signed a non-opposition statement.
Consent for publication
All participants signed a non-opposition statement. Data were anonymised for publication.
Availability of data and material
Data are available to any reader directly upon reasonable request.
Code availability
Not applicable.
Authors’ contribution
BD, PDT, SG, ED, SL, and IR conceptualised and designed the study, carried out the initial analyses, coordinated and supervised data collection, drafted the initial manuscript, and reviewed the manuscript.
BD, ER, PDT, DA designed the data collection instruments, collected data and reviewed and revised the manuscript. PDT, SB, MDR, HM, VP, JLH, LN, BT, CL participated in patient enrolment.
SG, ED, IR and AT were in charge of the statistical analyses and contributed to the final version of the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Appendix A
List of Collaborators
*COVID-19 RPC Team
Department of Intensive Care
Djillali Annane, MD, PhD (1,2,5)
Xavier Ambrosi, MD (4)
Suzanne Amthor, MD (1)
Rania Bounab, MD (1,2)
Ryme Chentouh, MD (1)
Bernard Clair, MD (1)
Abdallah Fayssoil, MD (1,2,5)
Diane Friedman, MD (1)
Nicholas Heming, MD, PhD (1,2,5)
Virginie Maxime, MD, (1)
Pierre Moine, MD, PhD (1,2,5)
Myriam Niel Duriez, MD (1)
David Orlikowski, MD, PhD (1,2,5,8)
Francesca Santi, MD (1,2)
Pharmacy
Frédérique Bouchand, PharmD (1)
Muriel Farcy-Afif, PharmD (1)
Hugues Michelon, PharmD, MSc (1)
Maryvonne Villart, PharmD (1)
Research Staff
Isabelle Bossard (8)
Tiphaine Barbarin Nicolier (1)
Stanislas Grassin Delyle, MCUPH (2,3,5)
Elodie Lamy (2,5)
Camille Roquencourt, MD (5)
Gabriel Saffroy (2)
Etienne Thevenot (5)
Department of Intensive Care Interns
Baptiste Abbar (1)
Steven Bennington (1)
Juliah Dray (1)
Pierre Gay (1)
Elias Kochbati (1)
Majistor Luxman (1)
Myriam Moucachen (1)
Alice Pascault (1)
Juan Tamayo (1)
Justine Zini (1)
Department of Anesthesia, Perioperative Care, and Pain
Marie Boutros, MD (1)
Anne Lyse Bron, MD (11)
Denys Coester, MD (12)
Etiennette Defouchecour, MD (11)
Brigitte Dosne Blachier, MD (11)
Léa Guichard, MD (1)
Damien Hamon Pietrin, MD, PhD (1)
Hakim Khiter, MD (1)
Valéria Martinez, MD, PhD (1,2,6)
Simone Meuleye, MD (1)
Suzanne Reysz, MD (1)
Sebastien Schitter, MD (1)
Chawki Trabelsi, MD (1)
Pediatric Critical Care Unit
Helge Amthor, MD, PhD (1,2,7)
Jean Bergounioux MD (1,2,5)
Maud Guillon, MD (1)
Amal Omar, MD (1)
Laboratory of Physiology
Frédéric Lofaso, MD, PhD (1,2,7,10)
Helene Prigent, MD, PhD (1,2,7,10)
Department of Rehabilitation and Physical Medicine
Djamel Bensmail, MD, PhD (1,2,7,10)
Pierre Denys, MD, PhD (1,2,7,10)
Charles Joussain, MD, PhD (1)
Lauren Kagane, MD (1)
Thibaut Lansaman, MD (1)
Hélène Le Liepvre, MD (1)
Antoine Leotard, MD, MS (1)
Jonathan Levy, MD, MS (1,2,7,10)
Claire Malot, MD (1)
Julie Paquereau, MD (1)
Celia Rech, MD (1)
Department of Rehabilitation Interns
Florence Angioni (1)
Elsa Chkron (1)
Céline Karabulut (1)
Jérôme Lemoine (1)
Noémie Trystram (1)
Julien Vibert (1)
Department of Infectious Diseases
Pascal Crenn, MD, PhD (1,2,7)
Benjamin Davido, MD, MS (1)
Stéphanie Landowski, MD (1)
Christian Perronne, MD, PhD (1,2)
Véronique Perronne, MD (1)
Pierre de Truchis, MD, MS (1)
Department of Infectious Diseases Interns
Marc Hobeika (1)
Louis Jacob (1)
Nicolas Kiavue (1)
Aymeric Lanore (1)
Aurélie Le Gal (1)
Julia Nguyen Van Thang (1)
Department of Microbiology and Innovative Biomarkers Platform
Coralie Favier (1)
Jean Louis Gaillard, MD, PhD (1,2,5)
Elyanne Gault, MD, PhD (1,2,5)
Jean-Louis Herrmann, PharmD, PhD (1,2,5)
Christine Lawrence, PharmD (1)
Virginie Lebidois, PharmD (1)
Latifa Noussair, MD (1)
Martin Rottman, MD, PhD (1,2,5)
Anne-Laure Roux, PharmD, PhD (1,2,5)
Sophie Tocqueville (1)
Marie-Anne Welti, MD, PhD (1,2,5)
And the nonmedical staff of the Department
Department of Laboratory Medicine and Pharmacology
Jean Claude Alvarez, MD, PhD (1,2,5)
Mehdi Djebrani, PharmD (1)
Pierre-Alexandre Emmanuelli (1)
Firas Jabbour, PharmD (1)
Lotfi Lahjomri, MD (1)
Mathilde Parent, MD (1)
And the nonmedical staff of the Department
Department of Radiology
Amine Ammar, MD (1)
Najete Berradja, MD (1)
Robert-Yves Carlier, MD, MS (1,2,7,14)
Annaelle Chetrit, MD (1,2)
Caroline Diffre, MD (1,2)
Myriam Edjlali, MD, PhD (1,15)
Zaki El Baz, MD (1,14)
Adrien Felter, MD (1)
Catherine Girardot, MD (1,13)
Ahmed Mekki, MD, MS (1,2)
Dominique Mompoint, MD (1)
Dominique Safa, MD (1)
Tristan Thiry, MD (1)
Department of Radiology Interns
Margot Armani (1)
Olivier de Barry (1)
Antoine Kirchner (1)
Jeffery Zhou (1)
Department of Forensic Medicine
Geoffroy Lorin de La Grandmaison MD, PhD (1)
Department of Forensic Medicine Intern
Kevin Mahe (1)
Affiliations
Hôpital Raymond Poincaré, GHU APHP, Université Paris Saclay, Garches, France.
Faculté Simone Veil Santé, Université Versailles Saint Quentin en Yvelines, Université Paris Saclay, Montigny-le-Bretonneux, France.
Hôpital Foch, Suresnes, France.
Centre Hospitalier Universitaire de Nantes, Nantes, France.
Université de Versailles Saint-Quentin-en-Yvelines/INSERM, Laboratory of Infection & Inflammation–U-1173, Montigny-le-Bretonneux, France.
Université de Versailles Saint-Quentin-en-Yvelines/INSERM, Centre d’Evaluation et de Traitement de la Douleur–U-987, Boulogne-Billancourt, France.
Université de Versailles Saint-Quentin-en-Yvelines/INSERM, Handicap Neuromusculaire–U-1179, Montigny-le-Bretonneux, France.
Centre d’Investigation Clinique, Garches, France.
Commissariat à l’Energie Atomique, CEA Paris Saclay, Gif-sur-Yvette, France.
Fondation Garches, Garches, France.
Clinique Jouvenet, Ramsay Santé, Paris, France.
Clinique de la Muette, Ramsay Santé, Paris, France.
Polyclinique Mantaise, Mantes-La-Jolie, France.
Centre Hospitalier Intercommunal Poissy/Saint-Germain, GHT Yvelines Nord, Poissy, France.
IMA-BRAIN/INSERM–UMR-1266, DHU-Neurovasc, Centre Hospitalier Sainte-Anne, Paris, France.
Acknowledgements
The authors would like to thank the nurses and the nursing assistants involved in the screening process: Michel Collin, Carla Amorim, Nadege Letilly, Sylvie Leconte, Nathalie Megret, Elena Belaunde, Sadia Abella, Celine Delhoum De Castro, Isabelle Lecrivain, Aurore Esteves, Morgane Le Minoux, Marie Annie Linard and Felixia Mourequito. The authors are grateful to Helene Jacques and Ludovic Ringot for the recruitment of healthcare workers during the epidemic.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2021.02.055.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomised trials. Influenza Other Respir Viruses. 2020;14(4):365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belingheri M., Paladino M.E., Riva M.A. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect. 2020;105(2):353. doi: 10.1016/j.jhin.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N., Klepac P., Liu Y., Prem K., Jit M., Eggo R. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- De Serres G., Carazo S., Lorcy A., Villeneuve J., Laliberté D., Martin R. Institut national de santé publique du Québec; 2020. Enquête épidémiologique sur les travailleurs de la santé atteints par la COVID-19 au printemps 2020; p. 76. [Google Scholar]
- Furuse Y., Sando E., Tsuchiya N., Miyahara R., Yasuda I., K.Ko Y. Clusters of coronavirus disease in communities, Japan, January–April 2020. Emerg Infect Dis. 2020;26(9):2176–2179. doi: 10.3201/eid2609.202272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche S., Charmet T., Schaeffer L., Paireau J., Grant R., Cheny O. Institut Pasteur; 2020. ComCor study on places of infection with SARS-CoV-2: where are French people catching the virus? url: https://www.pasteur.fr/en/press-area/press-documents/comcor-study-places-infection-sars-cov-2-where-are-french-people-catching-virus (last visited:02/08/2021) [Google Scholar]
- Haut Conseil de la Santé Publique . 2020. Coronavirus SARS-CoV-2: Rationalisation de l’utilisation des masques respiratoires pour les professionnels de santé en période épidémique. [Google Scholar]
- Keeley A.J., Evans C., Colton H., Ankcorn M., Cope A., State A. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Eurosurveillance. 2020;25(14):1. doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A., Consonni D., Carugno M., Bozzi G., Mangioni D., Muscatello A. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect. 2020;26(10):1413.e9–1413.e13. doi: 10.1016/j.cmi.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier C., Bouvet E., Abiteboul D., Lolom I., Pellissier G., Delarocque-Astagneau E. Contexte de contamination des professionnels de santé par la COVID-19: résultats préliminaires. Médecine Mal Infect. 2020;50(6):S71. [Google Scholar]
- Richterman A., Meyerowitz E.A., Cevik M. Hospital-acquired SARS-CoV-2 infection. JAMA. 2020;(November) doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- Rivett L., Sridhar S., Sparkes D., Routledge M., Jones Nick K., Forrest S. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santé publique France . 2020. Part des formes asymptomatiques et transmission du SARS-CoV-2 en phase pré-symptomatique. Synthèse rapide COVID-19; p. 7. [Google Scholar]
- Société française d’Hygiène Hospitalière . 2020. Avis Relatif Aux Indications Du Port Des Masques Chirurgicaux et Des Appareils de de Protection Respiratoire de Type FFP2 Pour Les Professionnels de Santé. [Google Scholar]
- Suárez-García I., Martínez de Aramayona López M.J., Sáez Vicente A., Lobo Abascal P. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect. 2020;106(2):357–363. doi: 10.1016/j.jhin.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treibel T.A., Manisty C., Burton M., McKnight A., Lambourne J., Augusto Jao B. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(10237):1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki H., Furusawa Y., Iwatsuki-Horimoto K., Imai M., Kabata H., Nishimura H. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere. 2020;5(5) doi: 10.1128/mSphere.00637-20. Imperiale MJ, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324(7):703. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available to any reader directly upon reasonable request.