Abstract
Objective
To determine the incidence of epilepsy and subsequent 5-year mortality among older adults, as well as characteristics associated with mortality.
Methods
This was a retrospective cohort study of Medicare beneficiaries age 65 or above with at least 2 years enrollment before January 2009. Incident epilepsy cases were identified in 2009 using ICD-9-CM code-based algorithms; death was assessed through 2014. Cox regression models examined the association between 5-year mortality and incident epilepsy, and whether mortality differed by sociodemographic characteristics or comorbid disorders.
Results
Among the 99,990 of 33,615,037 beneficiaries who developed epilepsy, most were White (79.7%), female (57.3%), urban (80.5%), and without Medicaid (71.3%). The 5-year mortality rate for incident epilepsy was 62.8% (62,838 deaths). In multivariable models, lower mortality was associated with female sex (adjusted hazards ratio [AHR] 0.85, 95% confidence interval [CI] 0.84–0.87), Asian race (AHR 0.82, 95% CI 0.76–0.88), and Hispanic ethnicity (AHR 0.81, 95% CI 0.76–0.84). Hazard of death increased with comorbid disease burden (per 1-point increase: AHR 1.27, 95% CI 1.26–1.27) and Medicaid coinsurance (AHR 1.17, 95% CI 1.14–1.19). Incident epilepsy was particularly associated with higher mortality when diagnosed after another neurologic condition: Parkinson disease (AHR 1.29, 95% CI 1.21–1.38), multiple sclerosis (AHR 2.13, 95% CI 1.79–2.59), dementia (AHR 1.33, 95% CI 1.31–1.36), traumatic brain injury (AHR 1.55, 95% CI 1.45–1.66), and stroke/TIA (AHR 1.20, 95% CI 1.18–1.21).
Conclusions
Newly diagnosed epilepsy is associated with high 5-year mortality among Medicare beneficiaries. Future studies that parse the interplay of effects from underlying disease, race, sex, and poverty on mortality will be critical in the design of learning health care systems to reduce premature deaths.
In adults, the risk of new-onset epilepsy is greatest in patients over 70, due to multiple predisposing pathologic mechanisms, including age-related vascular, degenerative, and metabolic disorders.1,2 The older adult population is growing rapidly, and unlike all other age groups, epilepsy incidence in this group may be increasing.3,4 Consequently, the burden of epilepsy is likely to substantially increase in the coming years, and large-scale characterizations of epilepsy in older adults, including its association with mortality, are needed.
Many chronic diseases are known to have significant racial disparities in mortality.5–7 Such sociodemographic-based differences have been proposed for epilepsy-associated mortality in younger patients, but little is known about their existence among older adults.8,9 Identifying high-risk populations is essential to guide clinical decisions, because epilepsy outcomes, including mortality, are known to be improved through differential treatment choices.10–15
As such, epilepsy could be responsive to a learning health care system research framework, in which routinely collected health care data are used to inform comparative effectiveness research, track outcomes, and evaluate treatment-related adverse events. Quantification of excess mortality and associated characteristics in older patients with new-onset epilepsy would be important initial steps for the development of such programs, particularly among individuals with neurologic comorbidities as their care requires common resources. Thus, we examined 5-year mortality after epilepsy diagnosis in adults age 65 and above and its association with sociodemographic characteristics and neurologic comorbidities. To provide additional perspective, our secondary objective was to demonstrate the difference in mortality associated with new epilepsy.
Methods
Data
Medicare is the insurer for approximately 96% of the US population over age 65,16 and includes information regarding patient demographics, medications, measures of comorbidity, diagnoses, procedures, and geographic area. This study used data from multiple Medicare research identifiable files (RIFs) provided by the Centers for Medicare & Medicaid Services (CMS). These RIFs contain individual-level data that can be linked over time using a unique beneficiary identification code. We used the Master Beneficiary Summary File (MBSF) base segment files to obtain Medicare enrollment and eligibility, and determine baseline race, age, sex, postal code, Medicaid use, and date of death. The MBSF Chronic Conditions segment and Other Conditions segment both contain indicator variables for common chronic medical conditions, which are obtained using validated algorithms. These MBSF condition segments were used together with the Carrier file and Chronic Condition Warehouse, which contain diagnoses documented using ICD-9-CM to identify chronic conditions of interest in this study and identify each as prevalent in 2009 (as described below).
Study Design and Sample
We performed a retrospective cohort study of Medicare beneficiaries age 65 or above with enrollment for at least 2 years prior to January 2009. A 2-year lookback period allowed us to assess for Medicare eligibility (to assure completeness of data) and to identify and remove individuals with preexisting epilepsy.
Incident epilepsy cases were identified in a manner consistent with prior epilepsy incidence studies.17,18 Epilepsy diagnosis was determined by query of the 2009 MBSF, which contains indicator and initial diagnosis date variables for 66 chronic health conditions. The MBSF epilepsy indicator variable was based on the presence of at least 1 inpatient or 2 outpatient claims with a diagnosis code of 345.x, according to the ICD-9-CM. Previous studies support the use of this algorithm.19,20 The resulting incident epilepsy cohort formed the study sample for our primary analyses. Medicare beneficiaries meeting eligibility criteria without an incident or preexisting epilepsy diagnosis formed the comparison group for our secondary analyses.
Patient Characteristics
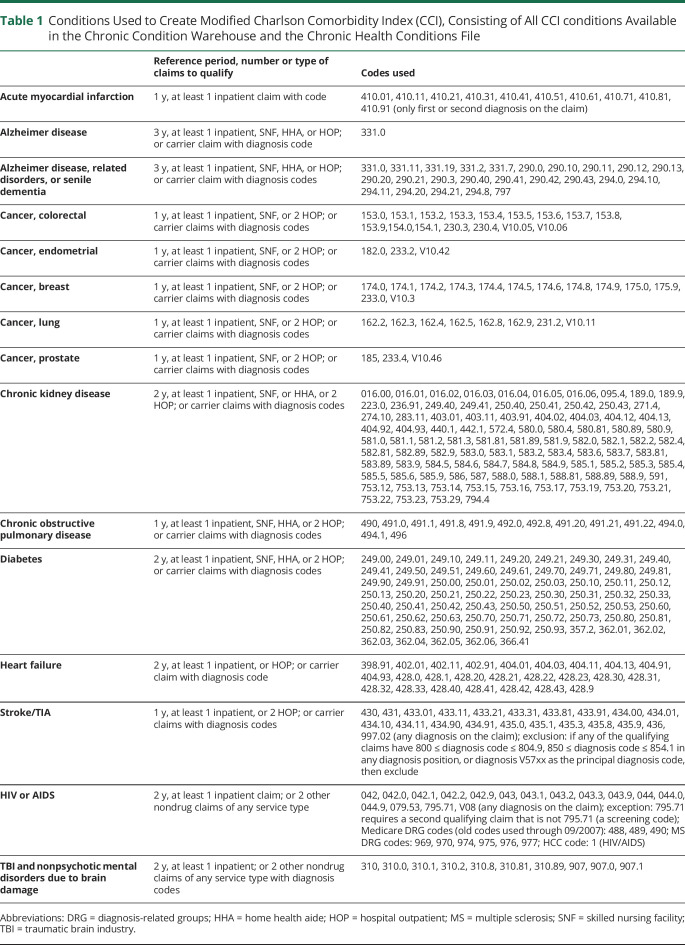
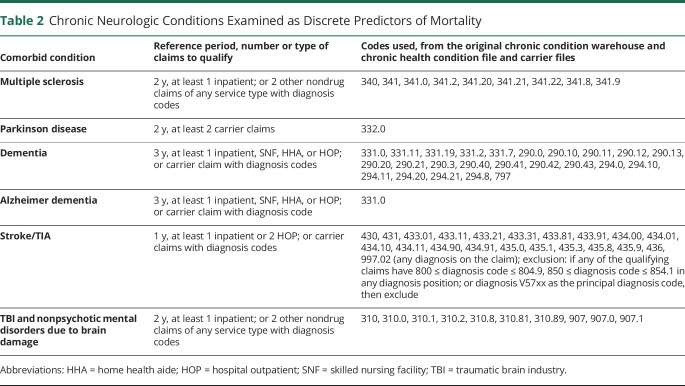
We extracted data on patient characteristics including age, sex, race, and dual Medicaid enrollment. Age in 2009 was extracted as a continuous variable, and also categorized as 65–69 years, 70–74 years, 75–79 years, 80–84 years, 85–89 years, or 90 + years. Race and ethnicity have only recently begun to be coded separately in Medicare data, therefore available demographic categorizations for our study were White, Black, Asian, Hispanic, Native North American, and other/unknown. Medicaid enrollment was used as a proxy for low income, as this is the most common way to qualify for Medicaid enrollment after meeting age criteria. Individual county of residence was merged with the US Department of Agriculture 2013 Rural Urban Continuum Code (RUCC) dataset. The RUCC is produced every 10 years, and using population density and proximity to metropolitan areas, assigns each US county to 1 of 9 categories, ranging from completely rural to completely urban. Older adults are generally at increased risk of death due to more than one chronic illness, and the Charlson Comorbidity Index (CCI) is the most widely used index to adjust for competing mortality due to comorbid disease. An age-weighted CCI score was calculated for each beneficiary, using all available indicator variables for chronic health conditions contained in the CCI.21 Finally, indicator variables for multiple sclerosis, dementia (defined as Alzheimer and other dementia types), Parkinson disease, traumatic brain injury, and stroke/TIA were derived from the Chronic Condition Warehouse and Carrier files. The extraction algorithms for all health conditions used to create the modified CCI and the chronic neurologic conditions examined as discrete predictors of mortality considered in the study are presented in tables 1 and 2, respectively.
Table 1.
Conditions Used to Create Modified Charlson Comorbidity Index (CCI), Consisting of All CCI conditions Available in the Chronic Condition Warehouse and the Chronic Health Conditions File
Table 2.
Chronic Neurologic Conditions Examined as Discrete Predictors of Mortality
Outcomes
The primary outcome was mortality, measured through December 31, 2014. CMS receives death information from a number of sources (including Medicare claims data from the Medicare Common Working File, online date of death edits submitted by family members, benefit information used to administer the Medicare program collected from the Railroad Retirement Board, and the Social Security Administration), and 99% of the death reports are validated.22
Five-year mortality was compared between beneficiaries with no epilepsy and those with incident epilepsy, adjusting for sociodemographic characteristics and comorbidities. We also examined whether a prior diagnosis of Parkinson disease, multiple sclerosis, dementia, traumatic brain injury, or stroke/TIA affected mortality among newly diagnosed persons with epilepsy. Among individuals with incident epilepsy, we examined whether mortality varied according to sociodemographic and residential characteristics.
Statistical Analyses
Demographic characteristics were compared using χ2 tests for categorical variables. To examine patient characteristics associated with mortality, we built multivariable Cox regression models to estimate the risk of death for each characteristic. Time to death was measured in months from the date of epilepsy diagnosis through December 31, 2014. Surviving cases were censored at the end of the observation period. Model variables were initially selected a priori based on clinical knowledge, review of existing literature, and variable availability. Individuals with more than one preexisting neurologic disease indicator were excluded from that analysis. The statistical analyses for our study were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the University of Pennsylvania's institutional review board and was subject to a data use agreement as per CMS policy.22
Data Availability
US Medicare data are publicly available for purchase through an application process overseen by the Research Data Assistance Center.22
Results
Demographics
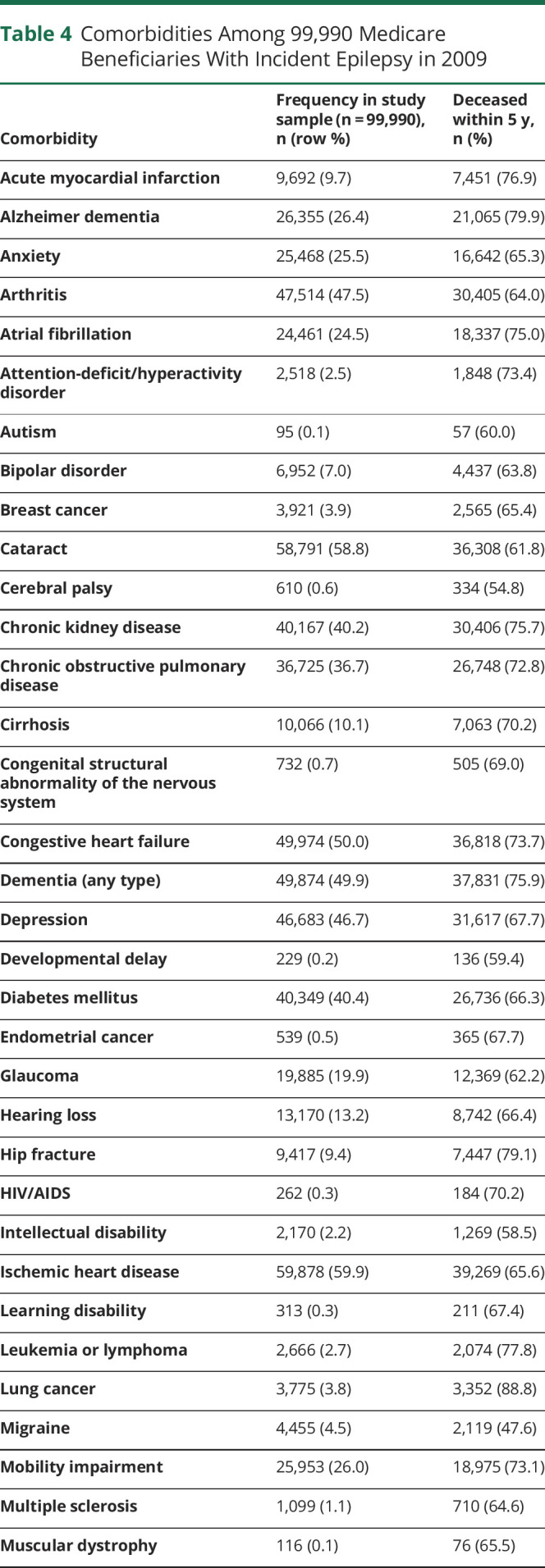
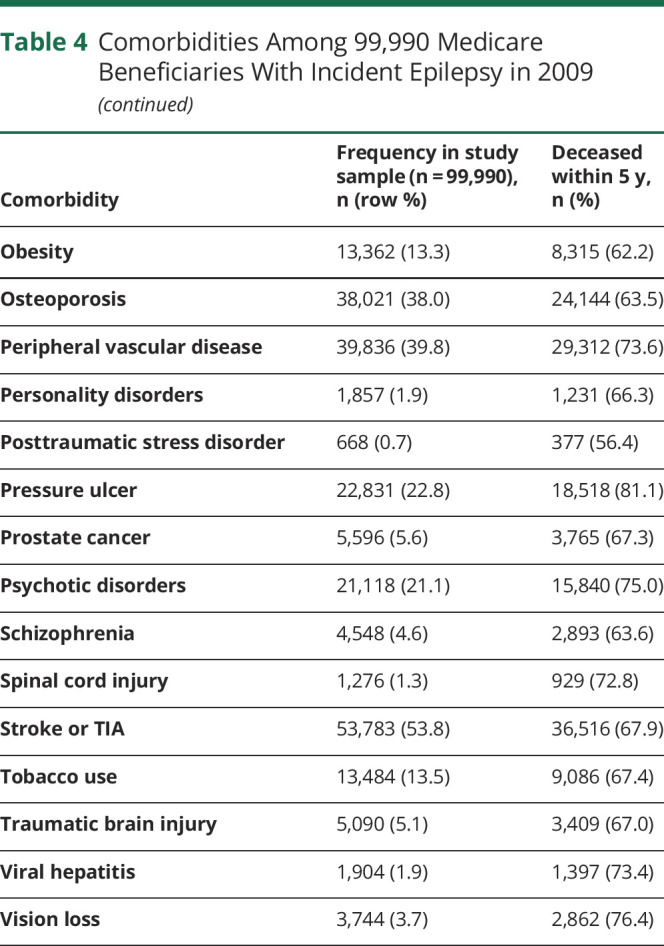
We identified a total of 33,615,037 people, ages 65 and above, who had been enrolled in Medicare for at least 2 years on January 1, 2009. Of these, 99,990 (0.3%) were diagnosed with epilepsy in 2009. Table 3 displays characteristics of individuals who were newly diagnosed with epilepsy in our older adult sample. Patients with incident epilepsy were majority white (79.7%), female (57.3%), and urban residing (80.5%), and did not have Medicaid (71.3%). The mean age-weighted modified CCI score was 5.98 (95% confidence interval [CI] 5.97–5.99). The most common comorbidities were ischemic heart disease (59.9%), cataract (58.8%), stroke/TIA (53.8%), congestive heart failure (50.0%), and dementia (49.9%) (table 4).
Table 3.
Characteristics of Medicare Beneficiaries With Incident Epilepsy in 2009
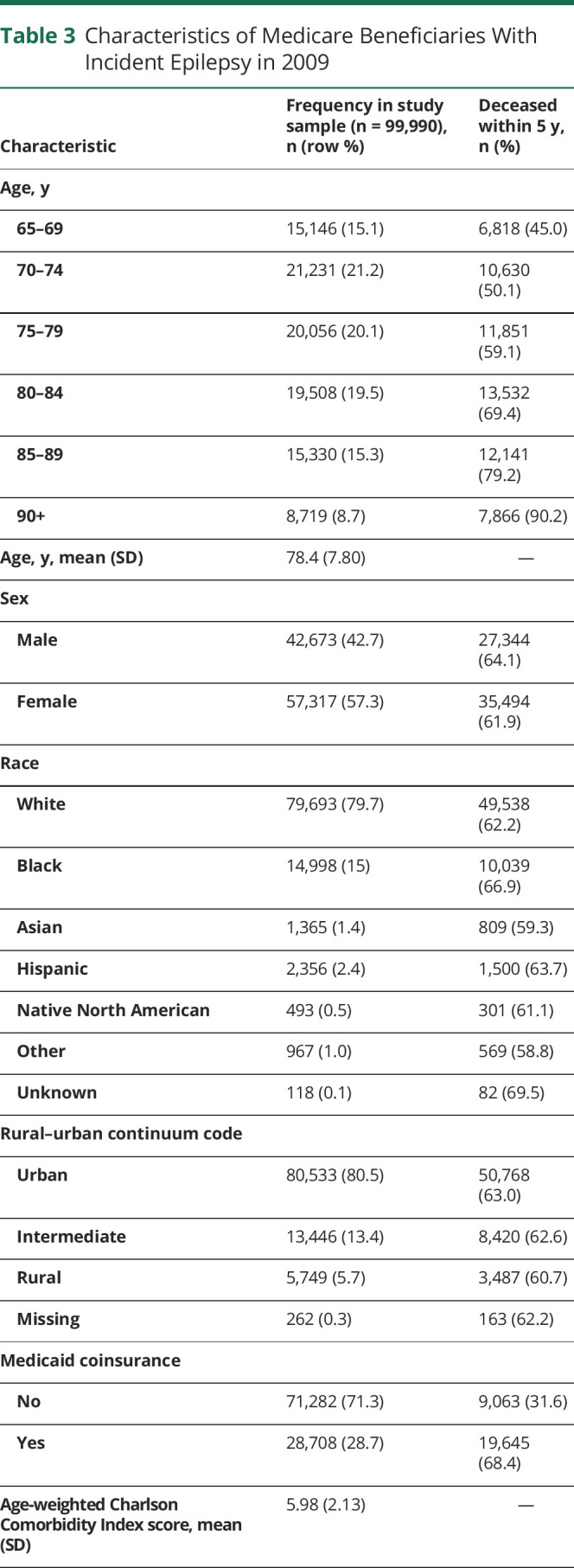
Table 4.
Comorbidities Among 99,990 Medicare Beneficiaries With Incident Epilepsy in 2009
Mortality
Nearly one third (29.2%) of the 33 million qualifying beneficiaries died during the observation period. The death rate was substantially higher in the incident epilepsy subpopulation: 62.8% (n = 62,838) died within 5 years. Cox models, built using the 98.8% of the sample that had no missing data for any covariates, demonstrated that incident epilepsy (as compared to no epilepsy) was associated with increased mortality (adjusted hazards ratio [AHR] 1.30, 95% CI 1.29–1.31) (figure 1), even after adjusting for covariates.
Figure 1. Effect of New Epilepsy Diagnosis on Survival of Medicare Beneficiaries, 2009–2014.
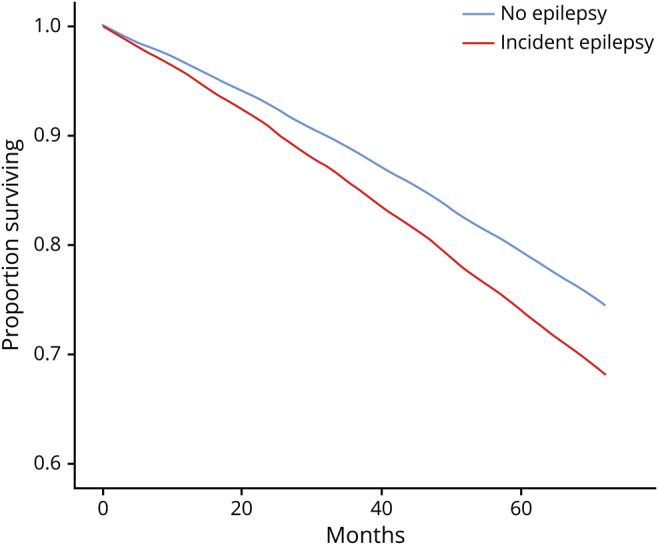
Models included sex, race, age, age-adjusted Charlson Comorbidity Index, Rural Urban Continuum Code category, and Medicaid enrollment category. MS = multiple sclerosis; PD = Parkinson disease; TBI = traumatic brain injury.
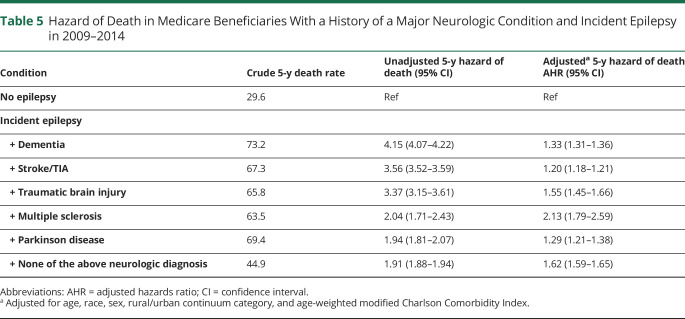
Table 5 and figure 2 display the results of a separate Cox model that stratified incident epilepsy according to whether one of the identifiable neurologic diagnoses was present prior to epilepsy. A preexisting diagnosis of Parkinson disease (AHR 1.29, 95% CI 1.21–1.38), multiple sclerosis (AHR 2.13, 95% CI 1.79–2.59), dementia (AHR 1.33, 95% CI 1.31–1.36), or traumatic brain injury (AHR 1.55, 95% CI 1.45–1.66) was associated with similar or greater increases in mortality. Epilepsy after stroke/TIA had the lowest measured increase in mortality (AHR 1.20, 95% CI 1.18–1.21) among Medicare-tracked neurologic conditions.
Table 5.
Hazard of Death in Medicare Beneficiaries With a History of a Major Neurologic Condition and Incident Epilepsy in 2009–2014
Figure 2. Underlying Neurologic Disease is Associated With Increased Mortality Among Older US Adults With Incident Epilepsy, 2009–2014.
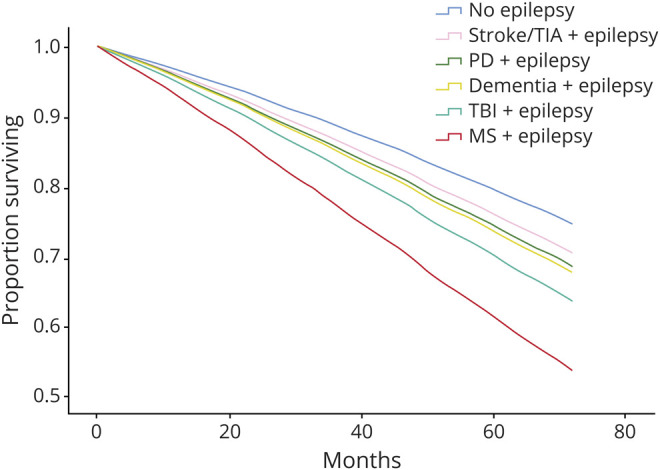
Models included neurologic disease category, sex, race, age, age-adjusted Charlson Comorbidity Index, Rural Urban Continuum Code category, and Medicaid enrollment category.
Analyses of the sample restricted to the nearly 1,000 persons with incident epilepsy found that demographic characteristics were modestly associated with mortality. In our unadjusted Cox models, female sex (hazard ratio [HR] 0.94, 95% CI 0.92–0.95) and Black race (HR 1.14, 95% CI 1.12–1.17) were associated with statistically significant differences in mortality; however, after adjustment for age, comorbid disease burden, poverty, and geographic location, only the HR for female sex (AHR 0.85, 95% CI 0.84–0.87) remained significant. Asian race (AHR 0.82, 95% CI 0.76–0.88) and Hispanic ethnicity (AHR 0.81, 95% CI 0.76–0.84) were associated with lower risk of death, but only in the adjusted model. Comorbid disease burden, on the contrary, was consistently associated with increased mortality (per 1-point increase: unadjusted HR 1.31, 95% CI 1.31–1.32, AHR 1.27, 95% CI 1.26–1.27). Medicaid coinsurance was also persistently associated with increased mortality (unadjusted HR 1.21, 95% CI 1.19–1.23, AHR 1.17, 95% CI 1.14–1.19). In unadjusted Cox models, residing in counties classified as intermediate by the RUCC was associated with significantly lower morality compared to residing in counties classified as urban (HR 0.94, 95% CI 0.91–0.97); however, after adjustment, the relationship was partially reversed, with intermediate counties being associated with increased mortality (AHR 1.06, 95% CI 1.03–1.08). There was no such difference in mortality after epilepsy diagnosis observed when comparing rural and urban counties.
Discussion
In this study, we found a 5-year mortality rate of 62.8% in Medicare beneficiaries age 65 and above with new-onset epilepsy. The national mortality rate in older US patients with epilepsy has not been previously described. Although comparing persons with epilepsy to those without was not the primary focus of this study, the 62.8% 5-year mortality rate is notable because although it is less than the mortality associated with neurologic emergencies such as stroke or status epilepticus, it is more than double the 5-year mortality rate in the overall Medicare population from which our sample was drawn.23 Previous studies of mortality in epilepsy have not commonly focused on older adults, and instead often highlight the excess mortality seen due to epilepsy and associated disorders in childhood.24 Our study shows that death in older adults with incident epilepsy is common. This result, together with the changing demographics of an aging society, illustrates the need to better understand contributors to mortality in epilepsy in a mature population.
Underlining these changing demographics, we identified 99,990 incident epilepsy cases in 2009. This is notably increased from the average of around 60,000 incident epilepsy cases per year found in the 2001–2005 Medicare population (the last years this population was studied in totality).17 There is little interval data on epilepsy incidence in the older adult population, but, in agreement with our findings, 2 recent studies of the Medicare population in Arizona also showed an increase in epilepsy incidence within the past decade.25,26 There are multiple likely reasons for this ostensible difference in 1-year incident sample size, including (1) changes to epilepsy diagnosis coding due to evolving clinical recognition or alterations in billing practices; (2) growth in the aging adult population, as suggested by census data27; or (3) increasing numbers of individuals surviving with conditions known to increase the risk of secondary epilepsy, including cardiovascular and cerebrovascular disease, metabolic derangement, and neurodegeneration.28–30
Defining the variance in the outcomes and burden of epilepsy in the Medicare population is critical because of the known association between outpatient epilepsy care quality and health outcomes across epilepsy subtypes.10,11 Importantly, unlike what has been found in other chronic diseases, we found little evidence of health inequity in terms of increased mortality among minorities or women.31,32 Rather, after correcting for lower income (via Medicaid coenrollment) and comorbidity burden, female sex, Hispanic ethnicity, and Asian race appear to be inversely associated with death. One possible explanation relates to the underlying mechanisms for epilepsy: it is possible that women and some minorities develop epilepsy through less malignant pathologic mechanisms or have a more benign epilepsy. Although seemingly reassuring at first consideration, this potential explanation calls into question whether such benign epilepsy syndromes are more easily preventable. If so, our data provide early evidence of a preponderance of preventable, nonlethal epilepsy syndromes among older women and minorities.
There appears to be geographic variation in mortality—after accounting for race, sex, age, and Medicaid enrollment, patients living in urban counties appear to have lower mortality than those living in intermediately rural/suburban counties, but not completely rural areas. Possible reasons for this urban-associated decrease in mortality are not clear from these data, but could relate to specialist availability and other health care resources that would be predicted to be more plentiful in urban locations, or differential health-seeking behaviors of suburban-dwelling individuals.33,34 This study provides the baseline data for future interventional studies of health care delivery, quality, and survival after an epilepsy diagnosis, which we hope will identify scalable process improvements that achieve better outcomes for persons with epilepsy, regardless of where they reside.
Incident epilepsy was associated with higher mortality when compared to mortality in a general Medicare population, as can be reasonably expected. Having a second, prior neurologic diagnosis was uniformly associated with increased mortality in the 5 years following epilepsy diagnosis. Stroke, an episodic disorder associated with the development of seizures or epilepsy, had lower HRs than neurodegenerative disorders (Parkinson disease and dementia). This observation most likely reflects the higher risk of death conferred by neurodegenerative disease. Whereas the focus of this study was on developing the population-level data to inform policy, epidemiologic surveillance, and health care system design, the high mortality in the dementia subgroup reaffirms clinical knowledge that seizures are typically a late manifestation of neurodegenerative disease and a marker of poor prognosis.
This study is unique in that it uses a national sample of US adults age 65 and above to describe 5-year mortality in incident epilepsy and provide evidence that poverty and comorbid disease drives death in this population. This study, however, has several possible limitations. First, our study assessed the Medicare population during 2009–2014, and may not reflect the current experience. Administrative claims data contain codes produced for billing and documentation purposes, and so do not allow for a detailed examination of socioeconomic background, health care attitudes, medication adherence, functional status, or disease severity. This is a significant consideration as all these factors would be expected to affect mortality and are important in developing effective interventions to improve clinical outcomes. We used validated ICD-9-CM codes for epilepsy, but despite being widely used in Medicare analyses, these codes have not been specifically validated in this population. There is a known lag-time between epilepsy onset and diagnosis35,36; our results, therefore, likely represent mortality further into the disease state than 5 years.
A learning health care system is one in which research (science, informatics) and care (delivery, incentives, and culture) are aligned for continuous improvement.37 Initial knowledge to fuel these health systems requires careful epidemiologic studies that can then be effectively translated prospectively into appropriate real-time tools at a patient, clinician, health system, or policy level. We assessed a large cohort of Medicare beneficiaries and present national data on mortality in older patients with new-onset epilepsy in the United States. Our data represent the afferent limb of a learning health system approach to epilepsy in the older adult population, as an initial step toward structural improvements in the delivery of epilepsy care. Future studies will use Medicare claims linked to electronic medical records to parse the interplay between race, sex, poverty, and comorbid disease on mortality and to determine the comparative effectiveness of newer epilepsy treatments in elderly patients with epilepsy. Together with these initial data, these epidemiologic studies will inform the development of interdisciplinary real-time, practice-changing interventions to reduce premature deaths and improve outcomes in older adults with epilepsy.
Glossary
- AHR
adjusted hazards ratio
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MBSF
Master Beneficiary Summary File
- RIF
research identifiable file
- RUCC
Rural Urban Continuum Code
Appendix. Authors
Study Funding
The first author was supported by the Mirowski Family Fund as well as the Pharmacoepidemiology Training Grant (T32-NS-061779) and the Department of Neurology at the University of Pennsylvania.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Cloyd J, Hauser W, Towne A, et al. Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res 2006;68:39–48. [DOI] [PubMed] [Google Scholar]
- 2.Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet 2019;393:689–701. [DOI] [PubMed] [Google Scholar]
- 3.Sillanpää M, Lastunen S, Helenius H, Schmidt D. Regional differences and secular trends in the incidence of epilepsy in Finland: a nationwide 23-year registry study. Epilepsia 2011;52:1857–1867. [DOI] [PubMed] [Google Scholar]
- 4.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States Population Estimates and Projections Current Population Reports. 2014. Available at: www.census.gov/population. Accessed March 4, 2019. [Google Scholar]
- 5.Benjamins MR, Hirschtick JL, Hunt BR, Hughes MM, Hunter B. Racial disparities in heart disease mortality in the 50 largest U.S. Cities. J Racial Ethn Heal Disparities 2017;4:967–975. [DOI] [PubMed] [Google Scholar]
- 6.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dyke M, Greer S, Odom E, et al. Heart disease death rates among blacks and whites aged ≥35 years: United States, 1968–2015. MMWR Surveill Summ 2018;67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenlund SF, Croft JB, Kobau R. Epilepsy by the numbers: epilepsy deaths by age, race/ethnicity, and gender in the United States significantly increased from 2005 to 2014. Epilepsy Behav 2017;69:28–30. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DA, Malek AM, Wagner JL, Wannamaker BB, Selassie AW. Mortality in people with epilepsy: a statewide retrospective cohort study. Epilepsy Res 2016;122:7–14. [DOI] [PubMed] [Google Scholar]
- 10.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 11.Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology 2016;86:1938–1944. [DOI] [PubMed] [Google Scholar]
- 12.Granbichler CA, Oberaigner W, Kuchukhidze G, et al. Decrease in mortality of adult epilepsy patients since 1980: lessons learned from a hospital-based cohort. Eur J Neurol 2017;24:667–672. [DOI] [PubMed] [Google Scholar]
- 13.Vossler DG, Weingarten M, Gidal BE, American Epilepsy Society Treatments Committee. Summary of antiepileptic drugs available in the United States of America: working toward a world without epilepsy. Epilepsy Curr 2018;18(4 suppl 1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrell MJ; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–1304. [DOI] [PubMed] [Google Scholar]
- 15.Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015;84:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdem E, Korda H, Haffer SC, Sennett C. Medicare claims data as public use files: a new tool for public health surveillance. J Public Health Manag Pract 2014;20:445–452. [DOI] [PubMed] [Google Scholar]
- 17.Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology 2012;78:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H, Pack A, Elkind MS, Longstreth WT, Ton TG, Onchiri F.. Predictors of incident epilepsy in older adults: the Cardiovascular Health Study. Neurology 2017;88:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu K, Wang M, Jaakkimainen RL, et al. Assessing the validity of using administrative data to identify patients with epilepsy. Epilepsia 2014;55:335. [DOI] [PubMed] [Google Scholar]
- 20.Jette N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia 2010;51:62–69. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 22.ResDAC. Research Data Assistance Center. Available at: www.resdac.org/. Accessed February 13, 2019. [Google Scholar]
- 23.Lichtman JH, Jones SB, Leifheit-Limson EC, Wang Y, Goldstein LB. 30-Day mortality and readmission after hemorrhagic stroke among Medicare beneficiaries in Joint Commission Primary Stroke Center-certified and noncertified hospitals. Stroke 2011;42:3387–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurman DJ, Logroscino G, Beghi E, et al. The burden of premature mortality of epilepsy in high-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia 2017;58:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ip Q, Malone DC, Chong J, Harris RB, Labiner DM. An update on the prevalence and incidence of epilepsy among older adults. Epilepsy Res 2018;139:107–112. [DOI] [PubMed] [Google Scholar]
- 26.Tang DH, Malone DC, Warholak TL, et al. Prevalence and incidence of epilepsy in an elderly and low-income population in the United States. J Clin Neurol 2015;11:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The Population 65 Years and Older in the United States: 2016 American Community Survey Reports. 2018. Available at: www.census.gov/acs. Accessed February 14, 2019. [Google Scholar]
- 28.Hannerz H, Nielsen ML. Life expectancies among survivors of acute cerebrovascular disease. Stroke 2001;32:1739–1744. [DOI] [PubMed] [Google Scholar]
- 29.Pandya A, Gaziano TA, Weinstein MC, Cutler D. More Americans living longer with cardiovascular disease will increase costs while lowering quality of life. Health Aff 2013;32:1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell SP, Saraf AA. Epidemiology of multimorbidity in older adults with cardiovascular disease. Clin Geriatr Med 2016;32:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000-2010. Front Public Heal 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frierson GM, Howard EN, DeFina LE, Powell-Wiley TM, Willis BL. Effect of race and socioeconomic status on cardiovascular risk factor burden: the Cooper Center Longitudinal Study. Ethn Dis 2013;23:35–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Weisgrau S. Issues in rural health: access, hospitals, and reform. Health Care Financ Rev 1995;17:1–14. [PMC free article] [PubMed] [Google Scholar]
- 34.Cossman J, James W, Wolf JK. The differential effects of rural health care access on race-specific mortality. SSM Popul Heal 2017;3:618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Juárez IE, Funes B, Moreno-Castellanos JC, et al. A comparison of waiting times for assessment and epilepsy surgery between a Canadian and a Mexican referral center. Epilepsia Open 2017;2:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE Study. Epilepsia 2001;42:464–475. [DOI] [PubMed] [Google Scholar]
- 37.Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2012. Available at: books.google.com/books?hl=en&lr=&id=_wUw6XCFqGwC&oi=fnd&pg=PR1&ots=0Knyr4ngrr&sig=JRuhkBa5YRRAaYQar1gTPG4UZZc#v=onepage&q&f=false. Accessed February 25, 2019. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
US Medicare data are publicly available for purchase through an application process overseen by the Research Data Assistance Center.22