Abstract
Objective
To characterize muscle involvement and evaluate disease severity in patients with GNE myopathy using skeletal muscle MRI and proton magnetic resonance spectroscopy (1H-MRS).
Methods
Skeletal muscle imaging of the lower extremities was performed in 31 patients with genetically confirmed GNE myopathy, including T1-weighted and short tau inversion recovery (STIR) images, T1 and T2 mapping, and 1H-MRS. Measures evaluated included longitudinal relaxation time (T1), transverse relaxation time (T2), and 1H-MRS fat fraction (FF). Thigh muscle volume was correlated with relevant measures of strength, function, and patient-reported outcomes.
Results
The cohort was representative of a wide range of disease progression. Contractile thigh muscle volume ranged from 5.51% to 62.95% and correlated with thigh strength (r = 0.91), the 6-minute walk test (r = 0.82), the adult myopathy assessment tool (r = 0.83), the activities-specific balance confidence scale (r = 0.65), and the inclusion body myositis functional rating scale (r = 0.62). Four stages of muscle involvement were distinguished by qualitative (T1W and STIR images) and quantitative methods: stage I: unaffected muscle (T1 = 1,033 ± 74.2 ms, T2 = 40.0 ± 1.9 ms, FF = 7.4 ± 3.5%); stage II: STIR hyperintense muscle with minimal or no fat infiltration (T1 = 1,305 ± 147 ms, T2 = 50.2 ± 3.5 ms, FF = 27.6 ± 12.7%); stage III: fat infiltration and STIR hyperintensity (T1 = 1,209 ± 348 ms, T2 = 73.3 ± 12.6 ms, FF = 57.5 ± 10.6%); and stage IV: complete fat replacement (T1 = 318 ± 39.9 ms, T2 = 114 ± 21.2 ms, FF = 85.6 ± 4.2%). 1H-MRS showed a significant decrease in intramyocellular lipid and trimethylamines between stage I and II, suggesting altered muscle metabolism at early stages.
Conclusion
MRI biomarkers can monitor muscle involvement and determine disease severity noninvasively in patients with GNE myopathy.
ClinicalTrials.gov Identifier
GNE myopathy is a rare degenerative muscle disease characterized by onset in young adults with anterior tibialis weakness and slow disease progression following a sequential pattern of skeletal muscle involvement, as previously described.1 The disease is caused by pathogenic variants in the GNE gene and, consequently, deficiency of the rate-limiting enzyme in sialic acid biosynthesis, UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE).2–4 Muscle histopathology shows fiber size variation, lack of inflammation, autophagic rimmed vacuoles, and amyloid deposits.5–8 There is no approved therapy for GNE myopathy, and although promising therapies have been identified, the slow progression and lack of biomarkers complicate the development of clinical trials.9
Muscle MRI and proton magnetic resonance spectroscopy (1H-MRS) have been used to characterize skeletal muscle noninvasively, and quantitative MRI measures such as longitudinal relaxation time (T1), transverse relaxation time (T2), fat fraction (FF), and muscle volume have been evaluated as biomarkers in a variety of muscle diseases.10–15 Although therapeutic interventions are unlikely to be effective in the late stages of muscle involvement, where fat replacement is the final result of many muscle diseases, early muscle alterations are more likely to reflect treatment response and provide insights into disease-specific mechanisms.
In this study, we aimed to characterize early to late stages of muscle involvement in GNE myopathy using qualitative and quantitative muscle MRI to determine whether T1, T2, and FF are relevant biomarkers of muscle involvement and to evaluate whether thigh muscle volume is a valid measure of disease severity in GNE myopathy by correlation with strength, function, and patient-reported outcomes (PROs).
Methods
Study Population
MRI and 1H-MRS were performed in 31 patients as part of an observational single-center study. Eligibility for this study required confirmation of the diagnosis by identification of biallelic pathogenic variants in the GNE gene and avoidance of potential therapies while participating in the study. Confirmation of a negative pregnancy test in women of childbearing age was obtained before MRI.
Standard Protocol Approvals, Registrations, and Patient Consents
The clinical protocol, “A Natural History Study of Patients with GNE Myopathy” (NIH study: 11HG0218; ClinicalTrials.gov Identifier: NCT01417533), was approved by the National Human Genome Research Institute Institutional Review Board, and all patients gave written informed consent prior to participation in this study.
MRI and 1H-MRS Acquisition
Muscle imaging was performed using a single 3T whole-body MRI system (Verio, Siemens Medical Systems, Erlangen, Germany) using flexible phased array body-matrix coils. Axial images of the pelvic and leg muscles were acquired by T1-weighted (T1W) fast spin-echo and short tau inversion recovery (STIR) sequences with the following measures: T1W sequence, repetition time (TR)/echo time (TE) = 600/20 ms, echo train length = 4, slice thickness/gap = 8 mm/20%, resolution = 1.4 × 1.0 mm, and 1 average; STIR sequence: TR/TE/inversion time = 5,600/82/220 ms, echo train length = 15, slice thickness/gap = 8 mm/20%, resolution = 1.2 × 1.0 mm, and 1 average.
T1 and T2 maps and 1H-MRS were acquired from regions of interest identified using T1W and STIR images as localizers as follows: (I) no abnormality identified on both T1W and STIR images; (II) minimal or no fat infiltration on T1W and increased signal intensity on STIR images; (III) fat infiltration on T1W and increased signal intensity on STIR images; (IV) complete fat replacement. T1 mapping was performed using the shortened modified Look-Locker inversion (MOLLI) recovery sequence, with each single-shot acquisition repeatedly acquired every 2 seconds after the inversion-recovery pulse.16 T2 mapping was measured by 3 T2-prepared single-shot T2-weighted images.17 Both T1 and T2 relaxation time mapping were research sequences provided by Siemens with online reconstruction. As a result, T1 and T2-maps were available for viewing following image acquisition.
1H-MRS was performed using a single-voxel point-resolved spectroscopy sequence (PRESS) (TR/TE = 2000/35 ms). The PRESS sequence was applied twice in muscle locations with little or fat infiltration, as seen on T1W (locations I and II), one with water suppression (32–48 averages), and another without water suppression (16 averages). For muscle locations with moderate fat replacement (location III and IV), no water-suppressed spectra (32 averages) were acquired.
1H-MRS Analyses
All 1H-MRS data were fitted and quantified using the Magnetic Resonance User Interface (jMRUI version 6.0) software based on the Advanced Method for Accurate, Robust, and Efficient Spectral fitting (AMARES) algorithm.18 The fitting procedure was done with the assumption of Gaussian distribution, except for the water peak in which Gaussian and Lorentzian line shapes were used to ensure a better fit. Prior knowledge was incorporated into the fitting algorithm as previously described.19 Peaks identified included intramyocellular lipid (IMCL at ∼1.3 ppm), extramyocellular lipid (EMCL at ∼1.5 ppm), allylic lipids (∼2.2 ppm), creatine (3.03 and 3.9 ppm), trimethylamines (TMA at 3.2 ppm), glycerol (∼5.2 ppm), and olefinic lipids (∼5.5 ppm). Total creatine was calculated from both creatine resonances (3.03 and 3.8 ppm). Overall lipid content was determined from all the spectral peaks representing lipids. 1H-MRS FF was determined using water and lipid content [100×lipid/(water + lipid)], as previously described.20,21
Volumetric Quantification of Thigh Tissue Composition
Because the involvement of lower extremity muscles varies at different stages of the disease, volumetric quantification of the entire thigh was used to determine the extent of muscle involvement in patients at different stages of disease progression using the same measure. Volumetric quantification of thigh tissue composition was performed using an automated sequence of image processing algorithms to classify tissues and identify fascia lata in the thighs, as previously reported.22 First, the 2 thighs were automatically segmented from the background, and each thigh was processed separately. Due to the inhomogeneity artifacts in MRI, a nonparametric nonuniform intensity normalization algorithm was employed for bias field correction.23 An anisotropic filter was then applied to enhance the image. After the image was preprocessed and the thigh was segmented, muscle regions were separated from adipose tissue regions using an unsupervised pixel classification method using a fuzzy c-means algorithm.24 To further separate the intramuscular fat (IMF) from the subcutaneous adipose tissue, the fascia lata between muscle and fat were robustly identified as the boundary by fitting a piecewise smooth Bernstein polynomial. The above procedures were conducted on axial slices and iterated through the entire thigh region. The volume of each tissue is expressed as the percentage of the volume of the thigh in each patient.
Strength, Functional, and PROs
Strength, functional, and PRO measures were performed during the same visit as the muscle MRI. Muscle strength was evaluated using the Quantitative Muscle Assessment (Aeverl Medical, Gainesville, GA), a sensitive and reliable measure of strength.25 Lower extremity strength was calculated as the sum of ankle dorsiflexion, knee flexion, knee extension, hip abduction, and hip extension. Thigh strength was calculated as the sum of knee flexion, knee extension, and hip extension. Strength is shown as percent of predicted for age, sex, and body mass index (BMI), based on the National Isometric Muscle Strength Database Consortium.26 Physical function was evaluated by the 6-minute walk test (6MWT) and the Adult Myopathy Assessment Tool (AMAT).27 PROs included the Inclusion Body Myositis Functional Rating Scale (IBMFRS) and the Activities-Specific Balance Confidence (ABC) Scale.28,29 Three patients elected not to complete the PRO questionnaires.
Statistical Analysis
Statistical analyses were performed using JMP software (SAS Institute, Cary, NC). Data are presented as mean ± SD and statistical significance assigned at p < 0.05. Missing data were excluded from the analysis. Correlations between muscle volume and clinical outcomes were assessed using Pearson correlation tests. Tukey-Kramer honestly significant difference test was used to perform mean comparisons for variables by stage and muscle type.
Data Availability
The data have been shared with the Rare Disease Cures Accelerator–Data and Analytics Platform (RDCA-DAP), and is available at the request of investigators for purposes such as replicating procedures and results.
Results
Patient Characteristics
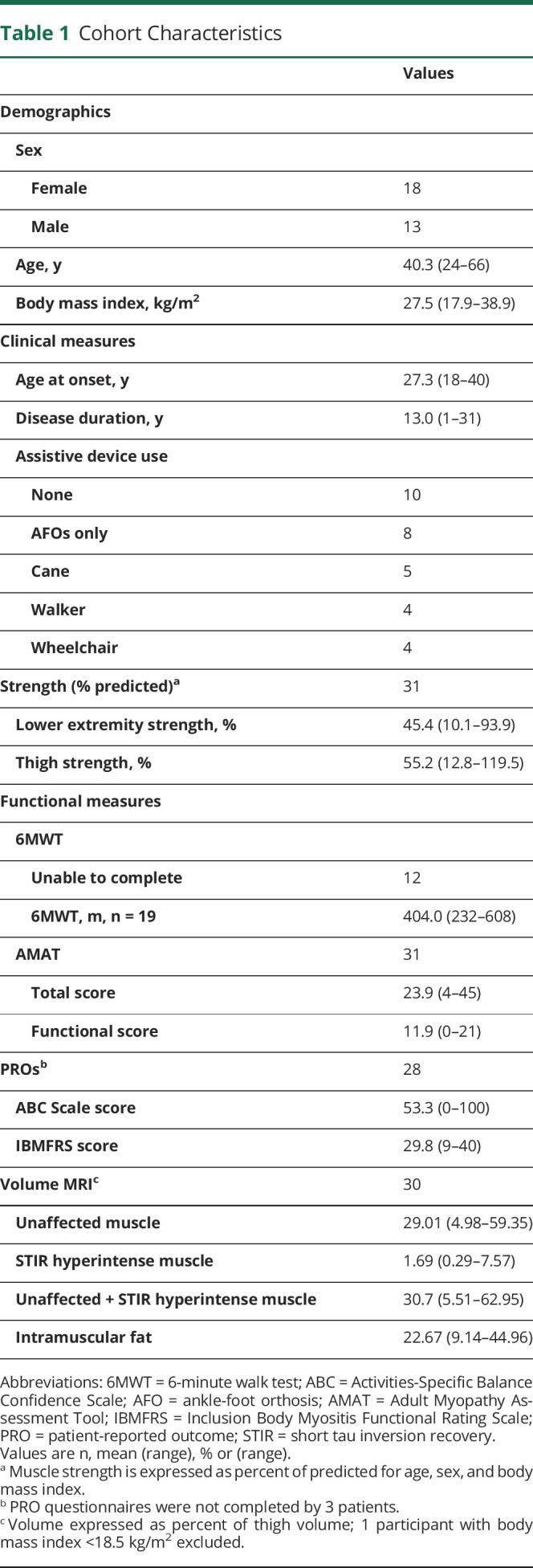
We evaluated 31 patients with genetically confirmed GNE myopathy using MRI and 1H-MRS. The cohort represented a wide range of disease progression, as determined by a variety of clinical, strength, functional, and PRO measures (table 1). At the time of evaluation, the mean age was 40 years (range 24–66), and disease duration ranged from 1 to 31 years since disease onset. Quantitative lower extremity strength ranged from 10% to 94% of predicted. Of the 31 patients in the cohort, 19 (61.3%) were able to complete the 6MWT with a mean distance walked of 404 meters (range 232–608). Of the patients who could not complete the 6MWT, 4 were nonambulatory, and 8 did not have enough balance to complete the test (table 1).
Table 1.
Cohort Characteristics
Qualitative MRI Findings
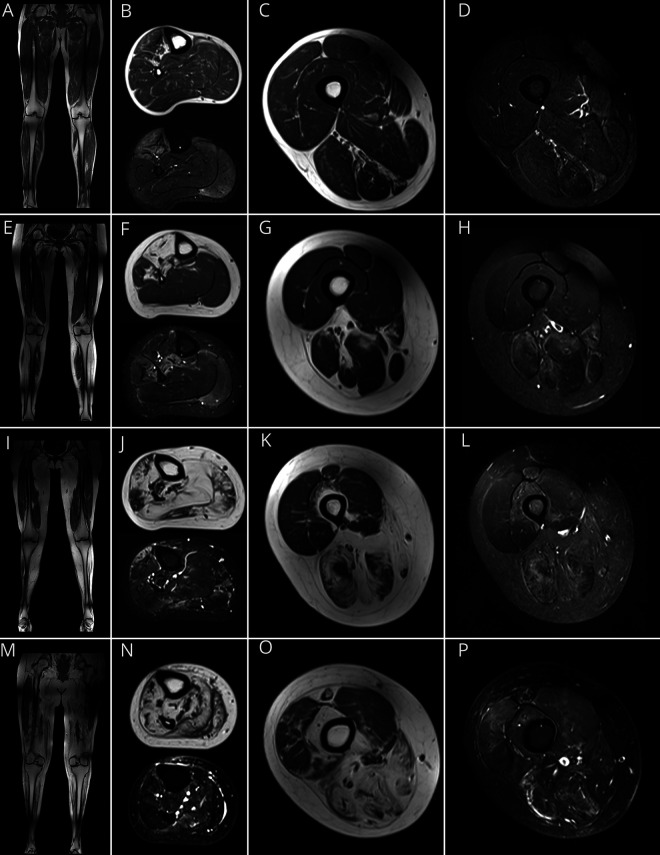
T1W and STIR images were used to identify fat replacement and active involvement of affected muscles, respectively. In patients with early disease, STIR hyperintensity of lower leg muscles could be the only visible abnormality (figure 1, A–D), while patients with advanced disease had fat replacement of the majority of lower leg muscles (figure 1, M–P). The pattern of distribution of muscle involvement changed among patients with varying degrees of disease progression due to the sequential involvement of lower extremity muscles in GNE myopathy. The anterior tibialis and toe dorsiflexors were the first muscles involved, as evidenced by STIR hyperintensity (figure 1B), followed by fat replacement (figure 1F). In general, muscle involvement proceeded from the anterior lower leg to the posterior lower leg and posterior thigh. The quadriceps muscles were affected in patients with advanced disease, with initial involvement of the rectus femoris and the vastus intermedius muscles (figures 1, K, L, O, and P).
Figure 1. MRI Findings in GNE Myopathy.
Representative T1-weighted (T1W) coronal and axial MRI of lower legs and thighs and corresponding axial short tau inversion recovery (STIR) images in patients with GNE myopathy. (A–D) MRI of a patient early in the disease progression (lower extremity [LE] strength: 94%) shows (A) minimal changes on T1W images and (B) STIR hyperintensity of the anterior tibialis, extensor digitorum longus (EDL), extensor hallucis longus (EHL), and gastrocnemius medialis muscles, and minimal changes in the thigh muscles (C and D). (E–H) A patient with intermediate disease progression (LE strength: 73%) showing 4 stages of muscle involvement identified by MRI: stage I: muscles with no visible abnormalities on T1W or STIR soleus (F) and quadriceps (G) muscles; stage II: STIR hyperintense muscle with minimal or no fat infiltration: gastrocnemius medialis (F) and muscles of the medial and posterior compartments of the thigh (G and H); stage III: muscles with fat infiltration and STIR hyperintensity: tibialis posterior and peroneus brevis (F); stage IV: complete fat replacement: anterior tibialis, EHL, and EDL muscles (F). (I–L) Patient with more advanced disease progression (LE strength: 39%); MRI shows the majority of lower leg muscles at stage IV (J), and involvement of the medial and posterior thigh muscles (K, L). In the anterior thigh compartment, there is STIR hyperintensity of the rectus femoris and vastus intermedius (stage II), with no visible abnormalities of the vastus lateralis (L). (M–P) Patient with advanced disease progression (LE strength: 16%); most of the lower extremity muscles were at stage IV (M, N, O), except for portions of the vastus lateralis and medialis, which show STIR hyperintensity (O, P).
Thigh Muscle Volume Correlated With Clinical Measures of Disease Severity
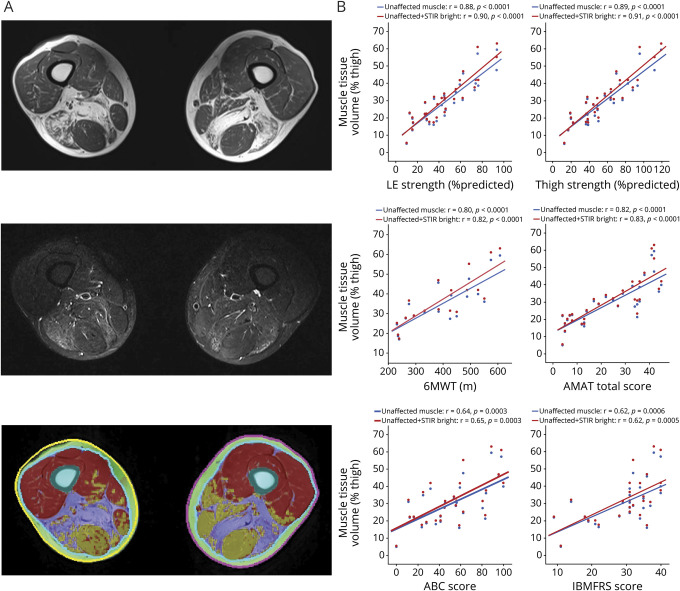
The extent of muscle involvement in the thigh was evaluated by quantifying the volume of unaffected muscle, STIR hyperintense muscle, and IMF (table 1 and figure 2A). Significant correlations were found between the volume of unaffected muscle and thigh strength (r = 0.89, p < 0.0001), lower extremity strength (r = 0.88, p < 0.0001), distance on the 6MWT (r = 0.80, p < 0.0001), and the AMAT total score (r = 0.82, p < 0.0001). The correlations of these same measures with PROs were lower. The combined volume of unaffected and STIR hyperintense muscle correlated better than unaffected muscle only with all the clinical measures, including thigh strength (r = 0.91, p < 0.0001), lower extremity strength (r = 0.90, p < 0.0001), distance on the 6MWT (r = 0.82, p < 0.0001), the AMAT total score (r = 0.83, p < 0.0001), the IBMFRS (r = 0.62, p = 0.0005), and the ABC Scale (r = 0.65, p = 0.0003) (figure 2B). This suggested that both unaffected and STIR hyperintense muscle contribute to the generation of strength and function. On the other hand, addition of IMF decreased correlations with all clinical measures. Unaffected muscle volume was also correlated with patient age (r = −0.40, p = 0.0270) and disease duration (r = −0.50, p = 0.0048).
Figure 2. Thigh Muscle Volume.
(A) Sample axial slice of the thigh including T1-weighted (top), short tau inversion recovery (STIR) (center), and volumetric thigh tissue composition (bottom) showing regions of unaffected muscle (red), STIR hyperintense muscle (yellow), and intramuscular fat (IMF) (purple). (B) Correlations between the volume of unaffected muscle (blue) and the combined volume of unaffected and STIR hyperintense muscle (red) in the thigh are shown. The correlation coefficients decrease when IMF volume is included (not shown): thigh strength (r = 0.38), lower extremity (LE) strength (r = 0.37), Inclusion Body Myositis Functional Rating Scale (IBMFRS) (r = 0.14), 6-minute walk test (6MWT) (r = 0.32), Adult Myopathy Assessment Tool (AMAT) total score (r = 0.28), and Activities-Specific Balance Confidence Scale (ABC) score (r = 0.30).
Pathologic and Metabolic Changes With Progressive Muscle Involvement
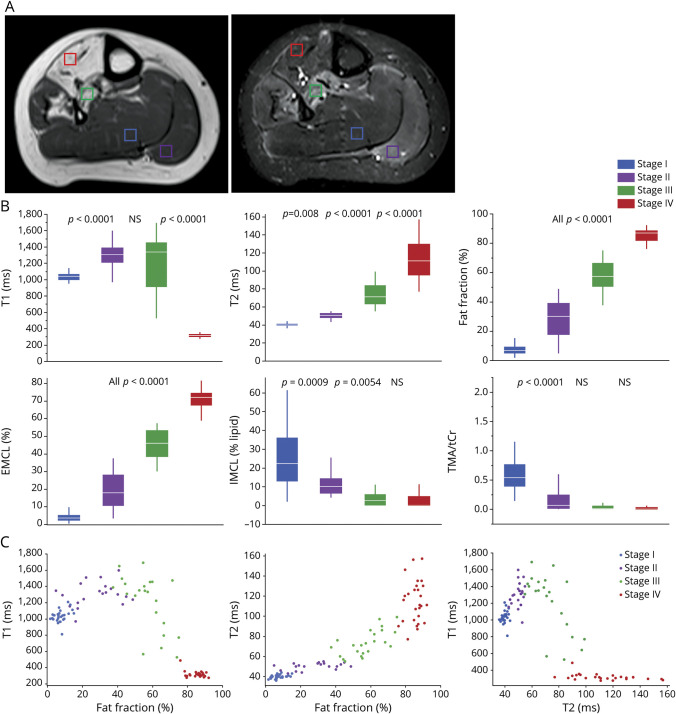
When evaluating individual muscles, 4 stages of muscle involvement were identified based on T1W and STIR images (figure 3A): stage I: muscle with no visible abnormalities on T1W or STIR images; stage II: STIR hyperintense muscle with minimal or no fat infiltration; stage III: muscle with fat infiltration and STIR hyperintensity; stage IV: complete fat replacement. In 16 of the patients, all 4 stages were represented in the lower extremities. To characterize these stages of muscle tissue involvement, suitable regions of interest representative of each stage were identified in each patient, whenever present, as shown by voxels on figure 3A.
Figure 3. MRI Stages of Muscle Involvement.
(A) Muscle regions representative of stages of muscle involvement as seen on T1-weighted (T1W) and short tau inversion recovery (STIR) images of the lower leg: stage I: soleus muscle with no visible abnormalities on T1W or STIR images (blue voxel); stage II: gastrocnemius muscle showing STIR hyperintensity muscle and no fat infiltration (purple voxel); stage III: posterior tibialis muscle with fat infiltration and STIR hyperintensity (green voxel); stage IV: complete fat replacement of the anterior tibialis (red voxel). (B) Comparison of different MRI and proton magnetic resonance spectroscopy measures by stage. Boxes represent mean, whiskers show SD. p Values of the difference between contiguous stages are shown. (C) T1 and T2 plotted as a function of fat fraction and T1 plotted as a function of T2 are shown for each stage of muscle involvement. Cr = total creatine; EMCL = extramyocellular lipid; IMCL = intramyocellular lipid; TMA = trimethylamines.
A total of 99 lower extremity muscles were characterized using T1 and T2 maps and 1H-MRS. Distinct pathologic and metabolic changes were seen at each stage of muscle involvement (figure 3B). T2 and FF values increased progressively at each stage. On the other hand, T1 values increased initially, but started to decrease in stage III (table 2). IMCL, EMCL, allylic, and olefinic lipids were distinguished by 1H-MRS in the majority of muscles evaluated. EMCL lipids accounted for the majority of the increase in FF as muscle involvement progressed. On the other hand, IMCL, which represent the metabolically active lipid pool, decreased from stages I to III (figure 3B). Allylic and olefinic lipids increased proportionately to the overall lipid content (not shown). Other metabolic changes identified using 1H-MRS included a significant decrease in TMA (p < 0.001) between stage I and II (figure 3B) and the appearance of glycerol at stage IV (data not shown). Overall, each stage of muscle involvement could be clearly distinguished based on T1, T2, and FF (figure 3C).
Table 2.
Characteristics of MRI Stages of Muscle Involvement
Analyses were performed to evaluate whether muscle type was a confounding factor underlying changes in MRI measures. Quadriceps muscles accounted for the majority (86%) of stage I muscles evaluated, while there was a more even distribution of muscle groups in other stages. All pairs Tukey-Kramer analyses showed no significant differences in T1, T2, FF, EMCL, or TMA between muscle types (all p > 0.05), except for an outlier stage IV gastrocnemius muscle, which had a significantly higher T1 value of 487 ms (p < 0.0001), compared to other muscles. The semitendinosus, gastrocnemius, and adductor muscles had significantly higher IMCL values than other muscles in stage II (p > 0.05), but no other significant differences were found at other stages or other muscles (data not shown).
Discussion
Magnetic resonance and proton spectroscopy provided useful, noninvasive tools to evaluate patients with GNE myopathy. Clinically, qualitative MRI findings provide information on muscles that are actively affected (STIR hyperintensity) and replaced by fat as seen on STIR and T1W images, respectively. Our observations were consistent with previous reports of the imaging pattern of lower extremity muscles in GNE myopathy.30,31 The early involvement of the anterior tibialis and toe dorsiflexors is well known and corresponds to the presenting symptoms of foot drop in patients with GNE myopathy.1,9 As previously reported, the quadriceps muscles are relatively spared but eventually become affected, with the rectus femoris involved first and the vastus lateralis last (figure 1O).30 While the MRI pattern of muscle involvement can be used for diagnostic purposes in muscle diseases,32 the imaging pattern in GNE myopathy is not static because muscles are affected sequentially as the disease progresses (figure 1).1
In degenerative muscle diseases, muscle MRI can be used to evaluate the degree of muscle involvement. Here, we classified lower extremity muscles in GNE myopathy based on 4 visually distinct MRI stages of muscle involvement identified using T1W and STIR imaging. As seen in figure 3A, muscle groups at different MRI stages of muscle involvement can be seen in the same patient, since the disease progresses to sequentially involve different muscle groups. The identification of the MRI stage of muscle involvement can be used clinically to facilitate the selection of muscle biopsies, whenever needed, such as in the diagnosis of patients with typical clinical characteristics but with novel GNE variants or variants of unknown significance identified by genetic testing. Biopsies of a muscle that is either unaffected (stage I) or already replaced by fat (stage IV) are likely uninformative and may confuse the diagnosis in these cases.7 Furthermore, stage IV muscles are likely not amenable to treatment. In contrast, muscles with active disease as signaled by STIR hyperintensity (stage II and III) are likely to yield positive histopathologic findings and benefit the most from rehabilitation or potential treatment interventions.
The pathologic and in vivo metabolic characteristics were defined at each stage of muscle involvement (table 2 and figure 3). Stage I muscle with no visible alterations on T1W and STIR images had an FF <10% and T2 values of 40 ms, which are comparable to values seen in unaffected control muscles.33 T1 values (mean 1,033; range 810–1,207 ms) measured at this stage were lower than previously reported in skeletal muscle of healthy volunteers obtained with various methods, including an approach similar to ours (3T, Look-Locker method).33–35 Whereas it is well established that T1 decreases as muscle tissue is replaced by fat,36 the decreased T1 at this stage could not be explained by differences in the fat content (figure 3). Nor can it be explained by differences in muscle type, which had no effect on T1 in our study. In fact, other studies have shown that quadriceps muscles, which accounted for the majority of stage I muscles we evaluated, have been reported to have a higher T1 than other lower extremity muscles.37 However, we did not measure T1 in nondisease controls and our definition of unaffected muscle only refers to muscle that has no noticeable abnormalities on T1W and STIR images. Another caveat is that our results are based on MRI variables measured in different muscle groups, rather than in the same muscle across patients, which was unfeasible due to the slow progression of the disease. However, analyses performed to evaluate whether muscle type was a confounding factor underlying changes in MRI measures showed no significant differences in T1, T2, or FF between muscle types.
Stage II and III muscles had the common characteristic of STIR hyperintensity, and therefore were considered to be actively diseased muscles. STIR hyperintensity precedes fat infiltration and is clinically used to facilitate the identification of active muscle involvement. However, unlike other muscle diseases in which muscle edema and inflammation have been postulated as the underlying cause for STIR hyperintensity, this has not been shown in GNE myopathy, in which muscle inflammation is typically absent.7,38 Active muscle involvement in GNE myopathy is characterized by the presence of rimmed vacuoles that correspond to autophagosomes,6 amyloid deposits, mitochondrial dysfunction, cell stress, and early apoptotic markers.4,8,39,40 In our study, the main changes between stage I to II muscles, in which STIR hyperintensity becomes evident, involved an increase in T1, T2, and FF, and a decrease in TMA and IMCL (figure 3B), suggesting ongoing physiologic and metabolic changes in actively involved muscles. The decrease in TMA and IMCL, which represent the trimethyl ammonium groups of carnitine and choline, and the metabolically active lipid pool, respectively, are suggestive of altered muscle metabolism and mitochondrial dysfunction. At this point it is unclear which underlying mechanisms are responsible for increased T1 and STIR hyperintensity of actively diseased muscles in GNE myopathy. Future studies could evaluate the role of rimmed vacuoles, apoptosis, and amyloid with muscle biopsies or with novel imaging agents, such as 18F-florbetapir to detect muscle amyloid.41
During stage III, the heterogenous distribution of T1 values may result from the competition between the substances prolonging T1 in stage II, and the progressive accumulation of fat, which is characterized by a short T1. The change in the trajectory of T1 during stage III may represent the point at which muscle damage becomes irreversible, which appears to correspond to the intersection of T1 < 1,000 and T2 > 70 ms (figure 3C). Stage IV areas correspond to atrophic muscles replaced by fat, with FF of ∼85%, short T1 values (∼315 ms), and long T2 (∼115 ms), which are similar to subcutaneous fat values.36
Whereas the MOLLI sequence can be used as a fast and powerful tool for quantitative MRI, the limitations of this technique under the influence of fat and off-resonance had been addressed.42,43 We speculate T2 mapping would likely have some degree of dependence on FF. The values listed in table 2 could be system and protocol dependent (MRI field strength, pulse sequences, acquisition parameters, and so forth). Generalization of these values to other studies should take these technical aspects into consideration.
Overall, quantitative MRI measures changed significantly with each stage of muscle involvement, and each MRI stage could be differentiated based on T1, T2, and FF values (table 2 and figure 3C), all of which have been studied extensively as MRI biomarkers in other muscle diseases.10,11,13,14,21,44–46 A longitudinal study of 10 patients with GNE myopathy found no significant change in the FF of specific muscle groups over 1 year using the Dixon method,47 suggesting more robust MRI biomarkers are needed for clinical trials. In this study, measured FF is based on 1H-MRS,20,21 which has become the gold standard in clinical research, particularly in longitudinal studies, where serial biopsies can be impractical and unethical. Furthermore, it allowed us to show that lipid accumulation is primarily driven by EMCL, while there is a decrease in the metabolically active IMCL. However, FF is unlikely to be a responsive outcome measure in clinical trials, as potential therapies are unlikely to reverse fatty infiltration. Instead, measures of active muscle involvement, such as T1 and T2, are likely to be more responsive.
Finally, we found that MRI-measured thigh muscle volume correlated with a variety of relevant clinical measures of disease severity, showing it is a valid measure of disease severity in patients with GNE myopathy. The volumetric quantification was performed using a robust automated method that has been previously shown to have high reliability with manual measurements of muscle (R2 = 0.99) and IMF (R2 = 0.96).22 The entire thigh muscle volume was assessed instead of specific muscle groups because the distribution of affected thigh muscles varies at different stages of the disease. This allowed us to determine the extent of muscle involvement in patients from early to advanced disease, including muscles at different stages of involvement using the same measure. Expressing muscle volume as a percentage of the volume of the thigh in each patient allowed us to normalize the muscle volume within each patient, rather than comparing it to age, and BMI-matched controls. However, this method may overestimate muscle volume in patients who are underweight (BMI <18.5 kg/m2), as the percent of thigh volume includes all thigh tissues, including subcutaneous fat and bone. The volume of unaffected muscle determined by this method correlated strongly with measures of strength and function, and to a lesser extent with PROs; the latter was expected since the tested PROs are less sensitive measures of disease progression in GNE myopathy,1 and the IBMFRS includes several upper extremity function items. The correlations between thigh muscle volume and measures of strength and function provided an understanding of the clinical significance of different stages of muscle involvement as identified by MRI and supported the validity of this method to determine disease severity in GNE myopathy. The correlations increased further when including STIR hyperintense muscle (figure 2B), showing that it contributes to the generation of strength and function in affected muscles; therefore, we propose that STIR hyperintense muscle should be included in the estimation of contractile muscle volume. In contrast, correlations decreased significantly when including IMF. Based on this, it can be assumed that stage I and stage II muscles are the main contributors to the generation of strength and function in patients with GNE myopathy. The lower (although significant) correlations with age and disease duration are not surprising, since the onset and rate of disease progression varies widely among patients with GNE myopathy.9
One deficiency of our study was not evaluating the volume of lower leg muscles since there was limited experience using the automated volumetric quantification method for this purpose. A recent study found strong correlations between the 6MWT and the FFs of the fibular and the extensor muscle groups of the lower leg muscles, which include the anterior tibialis and extensor digitorum muscles.47 The same study evaluated the contractile cross-sectional area of individual muscle groups and found no significant correlations between the 6MWT and the contractile cross-sectional areas of the quadriceps and the hamstring muscle groups in patients with GNE myopathy.47 Our findings suggest that the entire muscle volume of the thigh may be a better outcome measure than individual muscle groups when evaluating patients at different stages of the disease.
Together, our findings show that muscle MRI measures can be used to characterize and monitor muscle involvement and determine disease severity in patients with GNE myopathy. Longitudinal studies are needed to determine changes over time and support the use of these MRI measures in clinical trials evaluating potential therapies in patients with GNE myopathy.
Acknowledgment
The authors thank the patients with GNE myopathy and their families, Robert Evers for MRI acquisitions, and John Perreault and Christina Slota for patient care.
Glossary
- 6MWT
6-minute walk test
- ABC
Activities-Specific Balance Confidence Scale
- AMAT
Adult Myopathy Assessment Tool
- BMI
body mass index
- EMCL
extramyocellular lipid
- FF
fat fraction
- 1H-MRS
proton magnetic resonance spectroscopy
- IBMFRS
Inclusion Body Myositis Functional Rating Scale
- IMCL
intramyocellular lipid
- IMF
intramuscular fat
- MOLLI
modified look-locker inversion
- PRESS
point-resolved spectroscopy sequence
- PRO
patient-reported outcome
- STIR
short tau inversion recovery
- T1W
T1-weighted
- TE
echo time
- TMA
trimethylamines
- TR
repetition time
Appendix. Authors
Study Funding
This work was supported by the Intramural Research Programs of the NIH Clinical Center and the National Human Genome Research Institute, NIH.
Disclosures
Dr. Liu, Dr. Yao, W. Kovacs, J. Shrader, Dr. Joe, Dr. Ouwerkerk, and Dr. Mankodi report no disclosures. Dr. Gahl is a coinventor on patent PCT/US2008/006895 “N-acetyl mannosamine as a therapeutic agent.” Dr. Summers receives royalties from iCAD, Philips, ScanMed, PingAn and Translation Holdings. His laboratory received research support through a Cooperative Research and Development Agreement with PingAn and received GPU card donations from NVIDIA. Dr. Carrillo received financial support for clinical trials through a Cooperative Research and Development Agreement with Leadiant Biosciences and served as co-PI on NIAMS U01AR070498-01A1 grant to evaluate the efficacy of ManNAc in patients with GNE myopathy. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011231 for full disclosures.
References
- 1.Quintana M, Shrader J, Slota C, et al. Bayesian model of disease progression in GNE myopathy. Stat Med 2019;38:1459–1474. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg I, Avidan N, Potikha T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet 2001;29:83–87. [DOI] [PubMed] [Google Scholar]
- 3.Cho A, Christine M, Malicdan V, et al. Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy. Hum Mol Genet 2017;26:3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R, Arya R. GNE myopathy and cell apoptosis: a comparative mutation analysis. Mol Neurobiol 2016;53:3088–3101. [DOI] [PubMed] [Google Scholar]
- 5.Yan C, Ikezoe K, Nonaka I. Apoptotic muscle fiber degeneration in distal myopathy with rimmed vacuoles. Acta Neuropathol 2001;101:9–16. [DOI] [PubMed] [Google Scholar]
- 6.Malicdan MC, Noguchi S, Nishino I. Autophagy in a mouse model of distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Autophagy 2007;3:396–398. [DOI] [PubMed] [Google Scholar]
- 7.Nishino I, Carrillo-Carrasco N, Argov Z. GNE myopathy: current update and future therapy. J Neurol Neurosurg Psychiatry 2015;86:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer C, Kleinschnitz K, Wrede A, et al. Cell stress molecules in the skeletal muscle of GNE myopathy. BMC Neurol 2013;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrillo N, Malicdan MC, Huizing M. GNE myopathy: etiology, diagnosis, and therapeutic challenges. Neurotherapeutics 2018;15:900–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrow JM, Sinclair CD, Fischmann A, et al. MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 2016;15:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klickovic U, Zampedri L, Sinclair CDJ, et al. Skeletal muscle MRI differentiates SBMA and ALS and correlates with disease severity. Neurology 2019;93:e895–e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strijkers GJ, Araujo ECA, Azzabou N, et al. Exploration of new contrasts, targets, and MR imaging and spectroscopy techniques for neuromuscular disease: a workshop report of working group 3 of the biomedicine and molecular Biosciences COST action BM1304 MYO-MRI. J Neuromuscul Dis 2019;6:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ropars J, Gravot F, Ben Salem D, Rousseau F, Brochard S, Pons C. Muscle MRI: a biomarker of disease severity in Duchenne muscular dystrophy? A systematic review. Neurology 2020;94:117–133. [DOI] [PubMed] [Google Scholar]
- 14.Barnard AM, Willcocks RJ, Triplett WT, et al. MR biomarkers predict clinical function in Duchenne muscular dystrophy. Neurology 2020;94:e897–e909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heskamp L, van Nimwegen M, Ploegmakers MJ, et al. Lower extremity muscle pathology in myotonic dystrophy type 1 assessed by quantitative MRI. Neurology 2019;92:e2803–e2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piechnik SK, Ferreira VM, Dall'Armellina E, et al. Shortened modified Look-Locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med 2007;57:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 2001;31:269–286. [DOI] [PubMed] [Google Scholar]
- 19.Krssak M, Lindeboom L, Schrauwen-Hinderling V, et al. Proton magnetic resonance spectroscopy in skeletal muscle: experts' consensus recommendations. NMR Biomed 2020:e4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burakiewicz J, Sinclair CDJ, Fischer D, Walter GA, Kan HE, Hollingsworth KG. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol 2017;264:2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HK, Serai S, Lindquist D, et al. Quantitative skeletal muscle MRI: part 2, MR spectroscopy and T2 relaxation time mapping-comparison between boys with Duchenne muscular dystrophy and healthy boys. AJR Am J Roentgenol 2015;205:W216–W223. [DOI] [PubMed] [Google Scholar]
- 22.Yao J, Kovacs W, Hsieh N, Liu C-Y, Summers RM. Holistic Segmentation of Intermuscular Adipose Tissues on Thigh MRI. Cham: Springer International Publishing; 2017: 737–745. [Google Scholar]
- 23.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010;29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Positano V, Christiansen T, Santarelli MF, Ringgaard S, Landini L, Gastaldelli A. Accurate segmentation of subcutaneous and intermuscular adipose tissue from MR images of the thigh. J Magn Reson Imaging 2009;29:677–684. [DOI] [PubMed] [Google Scholar]
- 25.Visser J, Mans E, de Visser M, et al. Comparison of maximal voluntary isometric contraction and hand-held dynamometry in measuring muscle strength of patients with progressive lower motor neuron syndrome. Neuromuscul Disord 2003;13:744–750. [DOI] [PubMed] [Google Scholar]
- 26.Muscular NIMS. Weakness assessment: use of normal isometric strength data: The National Isometric Muscle Strength (NIMS) Database Consortium. Arch Phys Med Rehabil 1996;77:1251–1255. [DOI] [PubMed] [Google Scholar]
- 27.Harris-Love MO, Fernandez-Rhodes L, Joe G, et al. Assessing function and endurance in adults with spinal and bulbar muscular atrophy: validity of the adult myopathy assessment tool. Rehabil Res Pract 2014;2014:873872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L, Muscle Study G. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve 2008;37:473–476. [DOI] [PubMed] [Google Scholar]
- 29.Powell LE, Myers AM. The Activities-Specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995;50A:M28–M34. [DOI] [PubMed] [Google Scholar]
- 30.Tasca G, Ricci E, Monforte M, et al. Muscle imaging findings in GNE myopathy. J Neurol 2012;259:1358–1365. [DOI] [PubMed] [Google Scholar]
- 31.Bugiardini E, Morrow JM, Shah S, et al. The diagnostic value of MRI pattern recognition in distal myopathies. Front Neurol 2018;9:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein A, Jungbluth H, Clement E, et al. Muscle magnetic resonance imaging in congenital myopathies due to ryanodine receptor type 1 gene mutations. Arch Neurol 2011;68:1171–1179. [DOI] [PubMed] [Google Scholar]
- 33.Li K, Dortch RD, Welch EB, et al. Multi-parametric MRI characterization of healthy human thigh muscles at 3.0 T—relaxation, magnetization transfer, fat/water, and diffusion tensor imaging. NMR Biomed 2014;27:1070–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol 2004;183:343–351. [DOI] [PubMed] [Google Scholar]
- 35.Morrow JM, Sinclair CD, Fischmann A, et al. Reproducibility, and age, body-weight and gender dependency of candidate skeletal muscle MRI outcome measures in healthy volunteers. Eur Radiol 2014;24:1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marty B, Coppa B, Carlier PG. Monitoring skeletal muscle chronic fatty degenerations with fast T1-mapping. Eur Radiol 2018;28:4662–4668. [DOI] [PubMed] [Google Scholar]
- 37.Marty B, Carlier PG. Physiological and pathological skeletal muscle T1 changes quantified using a fast inversion-recovery radial NMR imaging sequence. Sci Rep 2019;9:6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argov Z, Yarom R. "Rimmed vacuole myopathy" sparing the quadriceps: a unique disorder in Iranian Jews. J Neurol Sci 1984;64:33–43. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka I, Noguchi S, Nishino I. Distal myopathy with rimmed vacuoles and hereditary inclusion body myopathy. Curr Neurol Neurosci Rep 2005;5:61–65. [DOI] [PubMed] [Google Scholar]
- 40.Malicdan MC, Noguchi S, Nonaka I, Hayashi YK, Nishino I. A Gne knockout mouse expressing human V572L mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 2007;16:115–128. [DOI] [PubMed] [Google Scholar]
- 41.Lilleker JB, Hodgson R, Roberts M, et al. [18F]Florbetapir positron emission tomography: identification of muscle amyloid in inclusion body myositis and differentiation from polymyositis. Ann Rheum Dis 2019;78:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larmour S, Chow K, Kellman P, Thompson RB. Characterization of T1 bias in skeletal muscle from fat in MOLLI and SASHA pulse sequences: quantitative fat-fraction imaging with T1 mapping. Magn Reson Med 2017;77:237–249. [DOI] [PubMed] [Google Scholar]
- 43.Kellman P, Bandettini WP, Mancini C, Hammer-Hansen S, Hansen MS, Arai AE. Characterization of myocardial T1-mapping bias caused by intramyocardial fat in inversion recovery and saturation recovery techniques. J Cardiovasc Magn Reson 2015;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patten C, Meyer RA, Fleckenstein JL. T2 mapping of muscle. Semin Musculoskelet Radiol 2003;7:297–305. [DOI] [PubMed] [Google Scholar]
- 45.Arpan I, Forbes SC, Lott DJ, et al. T(2) mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5-15-year-old boys with Duchenne muscular dystrophy. NMR Biomed 2013;26:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaeta M, Messina S, Mileto A, et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments: preliminary experience. Skeletal Radiol 2012;41:955–961. [DOI] [PubMed] [Google Scholar]
- 47.Gidaro T, Reyngoudt H, Le Louer J, et al. Quantitative nuclear magnetic resonance imaging detects subclinical changes over 1 year in skeletal muscle of GNE myopathy. J Neurol 2020;267:228–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data have been shared with the Rare Disease Cures Accelerator–Data and Analytics Platform (RDCA-DAP), and is available at the request of investigators for purposes such as replicating procedures and results.