Abstract
Objectives
Nucleus basalis of Meynert deep brain stimulation (NBM-DBS) has been proposed for patients with dementia. Here, we aim to assess the safety and effects of NBM-DBS in patients with Lewy body dementia (LBD), in a randomized, double-blind, crossover clinical trial.
Methods
Six patients with mild to moderate LBD (mean [SD] age, 62.2 [7.8] years) were included, operated on for bilateral NBM-DBS, and assigned to receive either active or sham NBM-DBS followed by the opposite condition for 3 months. The primary outcome was the difference in the total free recalls of the Free and Cued Selective Reminding Test (FCSRT) between active and sham NBM-DBS. Secondary outcomes were assessments of the safety and effects of NBM-DBS on cognition, motor disability, sleep, and PET imaging.
Results
There was no significant difference in the FCSRT score with active vs sham NBM-DBS. The surgical procedures were well tolerated in all patients, but we observed significant decreases in Stroop and Benton scores after electrode implantation. We observed no significant difference in other scales between active and sham NBM-DBS. With active NBM-DBS relative to baseline, phonemic fluency and motor disability significantly decreased. Lastly, the superior lingual gyrus metabolic activity significantly increased with active NBM-DBS.
Conclusions
NBM-DBS does not appear to be totally safe for patients with LBD with no evidence of cognitive benefit.
ClinicalTrials.gov Identifier
Classification of Evidence
This study provides Class II evidence that, for patients with LBD operated on for bilateral NBM-DBS, active NBM-DBS stimulation compared to sham stimulation did not significantly change selective recall scores.
Lewy body dementia (LBD) is the second neurodegenerative dementia after Alzheimer disease (AD).1 Patients with LBD have parkinsonism; visual hallucinations; dysexecutive syndrome with fluctuating attention, memory loss, and dementia; sleep disorders; and dysautonomia.1,2 LBD is related to the presence of Lewy bodies in cortical and subcortical areas, with the loss of both dopaminergic neurons in the substantia nigra pars reticulata and cholinergic neurons in the nucleus basalis of Meynert (NBM),3,4 with the loss of cholinergic neurons of the Ch1-2 region being linked to the severity of visual hallucinations.5,6 Dopaminergic medication may be introduced to reduce motor disability,1 and cholinergic medication may be used to reduce apathy, anxiety, delusions, and hallucinations7,8 and to improve attention.8 Nonpharmacologic interventions such as physical exercise and cognitive training have also been proposed with variable effects on depressive symptoms, attention, or motor signs.9,10 However, none of these treatments have demonstrated a significant clinical effect or an effect on disease progression.10
Recently, deep brain stimulation (DBS) of cognitive networks has been envisaged to improve cognitive deficit in patients with AD or Parkinson disease (PD) dementia (PDD). DBS of the fornix or of the NBM was tested in 42 and 6 patients with AD, respectively, in 2 randomized trials without significant improvement in cognition11,12 and in 6 patients with PDD NBM-DBS with no significant effect on cognition.13 These previous studies suggest, however, that DBS is feasible and well tolerated in these patients with dementia, with no severe side effects reported after surgery or with stimulation. With NBM-DBS, patients with PDD had significantly decreased motor disability and patients with AD showed no decline in cognition after 12 months of NBM-DBS, with 4 of 6 patients considered responders.12,13 These very preliminary results suggest that DBS of the NBM could represent a new therapeutic approach in patients with LBD.
The aim of the (Effects of Nucleus Basalis of Meynert Stimulation on Cognitive Disorders in Dementia With Lewy Bodies) DEMENSTIM study was to assess the safety and effects of DBS of the NBM in 6 patients with LBD in a randomized controlled trial.
Methods
Study Design and Patients
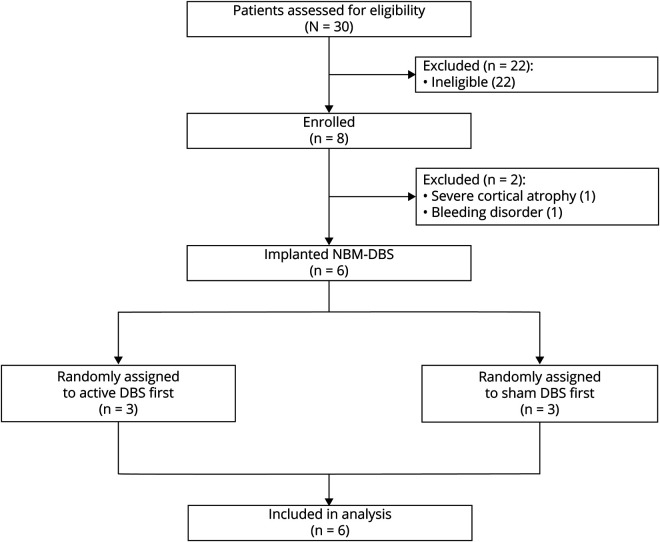
In this randomized, double-blind, crossover trial, we recruited patients from 5 university hospitals in France specialized in movement disorders and dementia. All patients were assessed and operated on at Rouen University Hospital. Patients were eligible for inclusion if they fulfilled criteria for diagnosis of probable, mild, or moderate LBD1,2; were 18 to 75 years of age; had a Mini-Mental State Examination (MMSE) score of 16 to 2614; were native French speakers; had received acetylcholinesterase inhibitors at stable dose for at least 1 month and dopaminergic drugs at stable dose for at least 3 months at the time of recruitment; had no suspicion of other causes for parkinsonism or dementia with negative CSF AD biomarkers (phospho-tau181, total-tau and β-amyloid42)15; had no history of severe psychiatric disorder; had confirmed dopaminergic denervation on dopamine transporter scan16; had no contraindications for DBS surgery; had health insurance; and agreed to participate in this study (figure 1).
Figure 1. Flowchart.
DBS = deep brain stimulation; NMB = nucleus basalis of Meynert.
Standard Protocol Approvals, Registrations, and Patient Consents
This study received approval from the local ethics committee of Rouen University (RCB 2011-A00387-34). All patients gave written informed consent. In addition, all caregivers provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. This trial was registered at ClinicalTrials.gov (NCT01340001).
Procedures
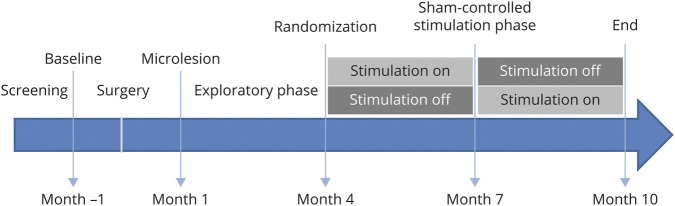
Assessments were performed at inclusion (baseline), followed 1 month later by NBM-DBS electrode implantation and 1 month after surgery by evaluation of microlesion effects (figure 2). For all patients, we targeted the CH4i subsector of the NBM located below the internal medullary lamina that separates the internal and external segments of the pallidum. We first performed preoperative nonstereotactic MRI with high-quality visualization of the neighboring structures of the CH4i, including the lateral segment of the anterior commissure, optical pathways, and amygdala, which were used as internal fiducial markers for CH4i targeting. The 2 quadripolar DBS electrodes (model 3389) and the programmable pulse generator (Activa PC, Medtronic, Minneapolis, MN) were implanted the same day under general anesthesia. All surgical procedures were performed by the same neurosurgeon (S.D.) in the Department of Neurosurgery of Rouen University Hospital. A postoperative CT scan was performed the day after surgery to check the absence of early surgical complications and to determine the definitive electrode position. For this purpose, the postoperative images were superimposed on the stereotactic preoperative MRI, and the MRI images were resliced along the anterior commissure–posterior commissure plane together with the contours of the atlas structures on 3 orthogonal standard planes (sagittal, coronal, and axial).17
Figure 2. Study Design.
One month after surgery, the stimulators were switched on, and the parameters were set for the double-blind period determined (month 1). Three different frequencies (20, 50, 100 Hz) and 2 pulse widths (60 and 90 microseconds), with intensities between 2 and 3 V, were tested each for a 2-week period (i.e., exploratory phase: month 1–4). We used the Clinician's Interview Based Impression of Change (CIBIC-Plus) to have a global impression of change.18 At month 4, active or sham NBM-DBS was applied for the following 3 months (i.e., month 4– 7), and at the end of this 3-month period, patients were switched to the opposite condition, that is, sham or active NBM-DBS, for the following 3 months (i.e., month 7–10). Lastly, a complete assessment was performed at month 10 (end of the study) (figure 2).
Randomization and Masking
Patients received first active NBM-DBS or sham NBM-DBS for 3 months, followed by the opposite condition for 3 months, in a randomized order (figure 2). We used computer-generated permutation randomization with a single block so that an equal number of patients were assigned to each group. Patients and the clinician responsible for assessments were masked to the randomization sequence, which was revealed only to the unmasked neurologist responsible for DBS programming. For this purpose, the unmasked clinician chose parameter settings according to the effects of NBM-DBS obtained during the testing period (month 1), below the side-effect threshold, and DBS parameters were not modified during the double-blind period. The same masked investigator assessed the primary outcome throughout the study.
Outcomes
The primary outcome was the difference in episodic memory performance assessed by the total free recall of the French validated version of the Free and Cued Selective Reminding Test (FCSRT)19 at the end of the active vs sham NBM-DBS periods (month 7 vs 10, figure 2).20 The FCSRT is a memory test with lower scores indicating memory impairment. In this test, the participant has to identify and learn 16 words or pictures in response to a unique category cue. Three recall trials are then performed, each preceded by 20 seconds of counting backward to prevent recall from short-term memory. The recall trials consisted of 2 parts with (1) a 2-minute period to freely recall as many items as possible (Free Immediate Recall [IR]) and (2) aurally presented category cues (Cued IR); the sum of these 2 parts were the total IR. The same procedure of recalling (freely and cued) is done after a 30-minute interval (Delayed recall [DR]) with Free DR, Cued DR, and Total DR. This test discriminates the deficits in memory encoding and storage that characterize AD relative to memory deficits observed in patients with PD21 and patients with LBD.22 The FCSRT was administered at each visit, with 2 parallel forms used to limit the test-retest learning effects.
We assessed safety by monitoring serious and nonserious adverse events (AEs). Serious AEs were defined as an untoward medical occurrence or effect that results in death, is life threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a congenital anomaly or birth defect. Acute cognitive and motor effects were assessed by comparing scores between baseline and 1 month postoperatively (month 1), and a postoperative CT scan was systematically performed in all patients after surgery to assess the occurrence of brain hemorrhage. An open interim analysis concerning the serious AEs was performed after the first 2 patients were operated on, and the occurrence of 2 consecutive intracerebral hemorrhages was considered a criterion of premature end of the study. An independent committee of experts (safety committee) was also formed and asked to review all AEs after the third patient or at the request of the principal investigator (D.M.) to decide whether the study should continue. A possible microlesion effect was also assessed by changes in cognition and motor scales 1 month after compared to 1 month before surgery, before DBS onset.
Other prespecified secondary outcome measures included differences between the end of the active vs sham NBM-DBS periods (month 7 vs 10) in the following scales: MMSE,14 Mattis Dementia Rating Scale (ranging from 0–144, with higher values indicating higher cognitive status),23 verbal fluencies (phonemic and categorical fluency), Stroop test,24 Benton visual retention test (composed of 3 sets of 10 designs),25 Wisconsin Card Sorting test (with 60 response cards, each containing 1–4 identical figures of a single color, measuring set-shifting ability),26 Rey figure, brief Frontal Assessment Battery (FAB; ranging from 0–18, with higher values indicating higher cognitive status),27 Trail Making Test (Parts A and B),28 Clinician Assessment of Fluctuation Scale and One Day Fluctuation Assessment Scale (ODFAS),29 Neuropsychiatric Interview (assessing 12 behavioral disturbances: delusions, hallucinations, dysphoria, anxiety, agitation, euphoria, disinhibition, irritability, apathy, sleep, appetite, and aberrant motor activity),30 Wechsler Intelligence Scale for Children cubes (assessing visuospatial abilities, with scores ranging from 0–62 and higher scores indicating better performance),31 praxic ability test (with 3 subsets: 5 symbolic gestures, 5 pantomime gestures, and 8 imitation gestures),32 oral picture naming test (DO80, assessing visual perception, semantic, and lexical verbal fluency),33 Epworth Sleepiness Scale (a self-administered questionnaire, ranging from 0 –24, with higher scores indicating higher sleep propensity),34 and Movement Disorders Society–Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part III (ranging from 0–132, with higher scores indicating higher motor disability).35 We also assessed the differences in these scales between baseline and 1 month after surgery except for the MMSE, FAB, MDRS, Rey figure, Trail Making Test, Wechsler Intelligence Scale for Children, and praxic ability tests and DO80.
Parameter Settings
To determine parameter settings for the double-blind crossover period, monopolar stimulation was used in each patient with stimulation applied with the 2 ventral contacts (0 or 1), with 3 different frequencies (20, 50, 100 Hz) and 2 pulse widths (60 and 90 us), with intensities between 2 and 3 V, each tested for a 2-week period (i.e., exploratory phase: month 1–4, figure 2). At the end of each 2-week period, we used chronometric tests to assess the attention abilities36 and the CIBIC-Plus to have a global impression of change with both caregiver and patient assessments.18 The combination of stimulation parameters providing the best effects on CIBIC-Plus and chronometric tests, with the fewest side effects, was chosen for the randomized crossover period.
PET Imaging
The regional metabolic rate of glucose was determined with [18F]-fluorodeoxyglucose-PET. Patients were examined at the end of the active vs sham stimulation periods to investigate the effects of NBM-DBS on brain metabolic activity (month 7 vs 10). For this purpose, patients were positioned in the PET scanner (ET/CT Biograph 16, Siemens Medical System, Knoxville, TN) with their eyes closed, low-light atmosphere, and no noise. A dose of 2 MBq/kg of fluorodeoxyglucose was injected 30 to 40 minutes (mean ± SD delay 33.4 ± 3.5 minutes) before an acquisition of 20 minutes. Patients rested in quiet surroundings with their eyes closed at least 20 minutes after injection. Follow-up scans were performed on the same tomograph as at baseline, with the same protocol. PET volumes were coregistered with their corresponding MRI volumes, segmented into gray matter and white matter probability maps, and spatially normalized to Montreal Neurological Institute space with SPM8. Individual variability was taken into account by dividing each subject voxel uptake by the mean pons uptake, yielding parametric images, obtained from a Pickatlas (fmri.wfubmc.edu/software/pickatlas) region of interest. Parametric images were smoothed with the use of an isotropic gaussian kernel of 6 mm. Patient 6 was not examined.
Statistical Analysis
In this study, we planned to recruit 6 patients because the safety of NBM-DBS in this patient population is not known and the number of patients with LBD estimated to be included in our study was considered to be low. This is a phase I study, and all efficacy outcomes are exploratory, with a Class II classification of evidence scheme. Although a primary outcome was defined, no sample size calculation based on statistical power was performed. The primary analysis compared the paired differences (delta) in the primary outcome (i.e., the FCSRT score) obtained in patients first randomized with active NBM-DBS followed by sham NBM-DBS (n = 3) and patients first randomized with sham NBM-DBS followed by active NBM-DBS (n = 3) using a Mann-Whitney test.
The safety analysis was based on the assessment of AEs without formal statistical testing and on the statistical comparison of scale measurements (48 tests) at 1 month after surgery (month 1) vs 1 month before surgery (baseline), assuming that they would show brain injuries due to surgery.
We tested differences in secondary outcome measures between active and sham NBM-DBS and between baseline and after surgery using Wilcoxon signed-rank tests. We did post hoc analyses to assess differences in the secondary outcome measures between inclusion (baseline) and active NBM-DBS using Wilcoxon signed-rank tests. We did not apply Bonferroni corrections for multiple-testing comparison, and a value of p < 0.05 was considered significant for each test. Values were described as mean and SD. Statistical analysis was performed with R statistical software (version 3.5.0, R Foundation for Statistical Computing, Vienna, Austria).
For PET data, a voxel-by-voxel comparison between active and sham NBM-DBS was performed with Wilcoxon rank tests; a value of p <0.05 was considered significant. Comparison between patients with LBD (off DBS) and a control group was also performed with Cortex ID software, which uses a 3-dimensional stereotactic surface projection to extract the peak of cortical activity on the brain surface for a set of predefined surface pixels. This activity was compared with an age-matched normal database (control group, n = 66) with a z score subtraction. A z score of >2 SD was considered significant. Statistical analyses were performed with Statistica 7.1 software.
Data Availability
All relevant data are within the article. Requests for anonymized data should be sent to D. Maltête at Rouen University Hospital, 76,000 Rouen, France.
Results
Patient Characteristics and Surgery
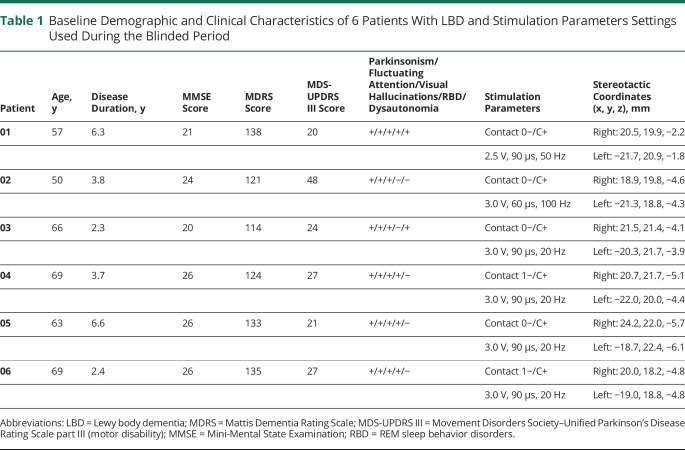
Between October 2012 and January 2017, we assessed 30 patients with LBD for eligibility, enrolling 8 in the study (figure 2). Two patients were finally excluded before surgery because of severe cortical atrophy revealed by preoperative brain MRI in 1 patient and a bleeding disorder in the other. Six patients (all male, mean [SD] age at baseline 62.2 [7.4] years, range 50–69 years, mean [SD] disease duration 4.2 [1.9] years, range 2.3–6.6 years, figure 2) were operated on for NBM-DBS electrode implantation and included in the final analysis. The demographic and clinical characteristics at baseline of each patient are displayed in table 1. All patients had a normal CSF profile (not shown), nigrostriatal depletion on dopamine transporter imaging (not shown), and a diagnosis of probable LBD according to internationally accepted consensus criteria.1 One patient (P02) had an onset at 47 years of age and a familial history of early parkinsonism and dementia in his father; genetic analysis was performed, revealing SNCA duplication. The 5 remaining patients (P01, P03, P04, P05, and P06) had no familial history of neurologic disorders; therefore, genetic testing was not recommended. All patients had a mild to moderate form of the disease with a mean (SD) MMSE score at inclusion of 23.8 (2.7) (range 20–26, table 1). All patients had received acetyl cholinesterase inhibitor medication (rivastigmine transdermal patch, 9.5 mg/d) at the highest escalated and stable dose for at least 1 month before surgery and throughout the entire duration of the protocol.
Table 1.
Baseline Demographic and Clinical Characteristics of 6 Patients With LBD and Stimulation Parameters Settings Used During the Blinded Period
Electrodes Location and Parameter Settings for NBM-DBS
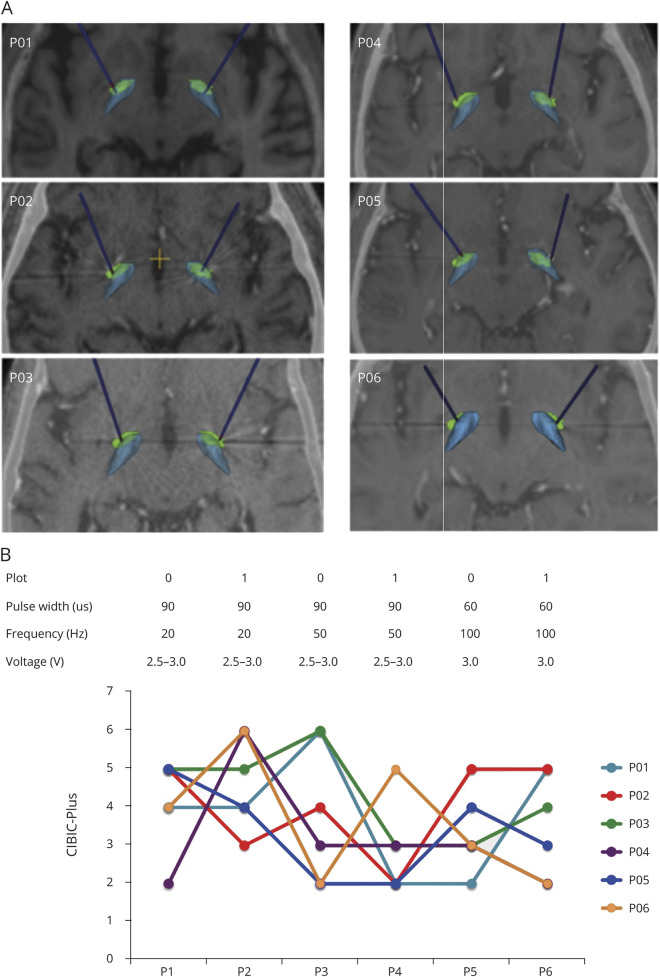
All electrodes were implanted within the NBM (figure 3A). For the active DBS period, monopolar stimulation was applied bilaterally in all patients, with 20-Hz frequency in 4 of 6 patients (P03, P04, P05 and P06), 50 Hz in 1 patient (P01) and 100 Hz in 1 patient (P02), with pulse width of 60 or 90 microseconds and intensity of 2.5 to 3.0 V (table 1 and figure 3B). Three patients (P01, P04, and P06) were randomized to have sham first followed by active NBM-DBS, and 3 patients (P02, P03 and P05) to have active first followed by sham NBM-DBS.
Figure 3. Localization of DBS Electrodes and Differences in the CIBIC-Plus Score During NBM-DBS Parameter Setting Testing.
(A) Position of electrodes for each individual participant along the long axis of each electrode within the nucleus basalis of Meynert (NBM; green) with the internal globus pallidus located dorsally (blue). The 3-dimensional superior views after fusion with the 3-dimensional MRI images are presented, the axial positions of which are shown. All electrodes penetrate the NBM. (B) Graph represents the individual scores for the Clinician's Interview Based Impression of Change (CIBIC-Plus) assessed at the end of each 2-week period with the different parameter settings used (top panel). Higher score indicates clinical impression of improvement. DBS = deep brain stimulation.
Safety and Tolerability of Surgery
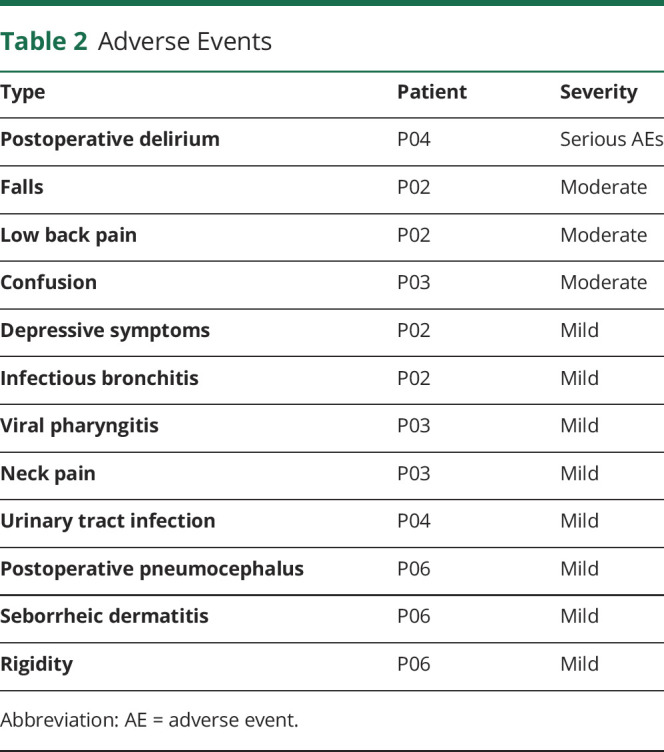
The surgical procedures were well tolerated in all patients with no serious AE related to surgery. One patient (P04) presented postoperative delirium associated with urinary tract infection treated by ofloxacine (200 mg twice a day) for 7 days, considered a serious AE. We recorded 11 nonserious AEs in 4 patients (table 2). We observed no adverse effect induced by NBM-DBS either during the stimulation testing period (month 1–4) or during the randomized crossover period (month 4–7).
Table 2.
Adverse Events
One month after surgery relative to baseline, we observed no significant difference in the total free recall of FCSRT scores (table 3) but a decrease of >2 points in 4 of 6 patients (P01, P03, P04, and P06, figure 4A). We also found a significant increase of completion time in denominating, reading, and interference of the Stroop test and a decrease in the Benton score, with no other significant differences (table 3), and a trend to lower MDS-UPDRS III scores after surgery, with in particular a decrease of 22, 12, and 7 points in P02, P05, and P06, respectively (table 3 and figure 4B).
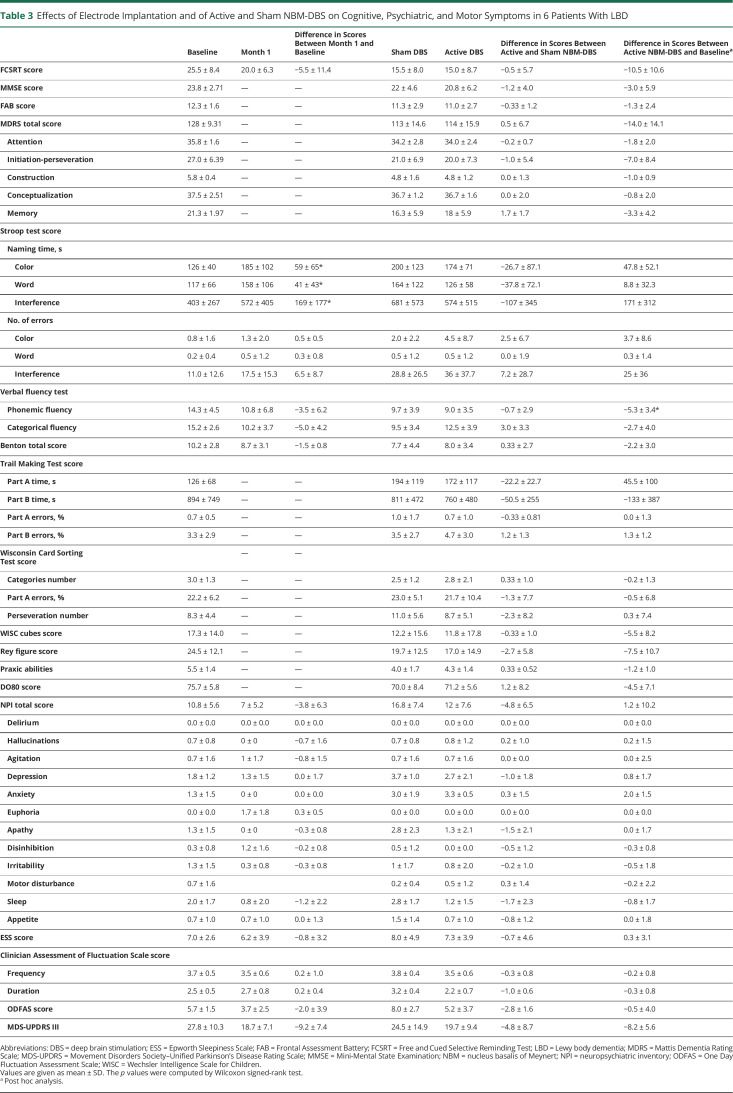
Table 3.
Effects of Electrode Implantation and of Active and Sham NBM-DBS on Cognitive, Psychiatric, and Motor Symptoms in 6 Patients With LBD
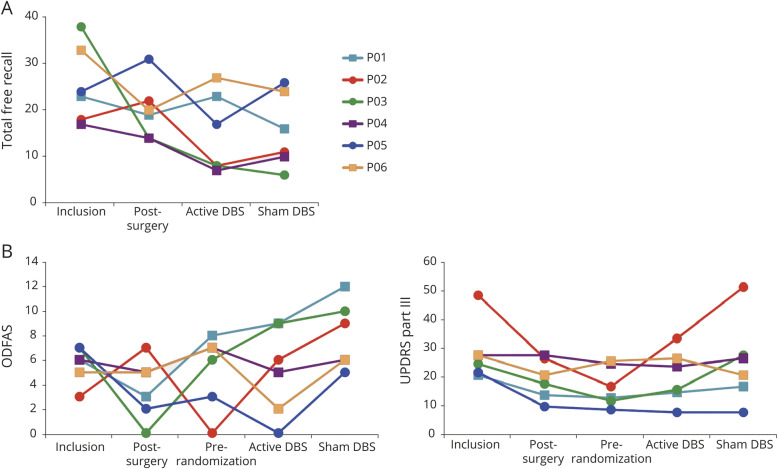
Figure 4. Effects of NBM-DBS on FCSRT Scores, Cognitive Fluctuations, Parkinsonian Motor Disability Scores, and Brain Metabolic Activity in Patients With LBD.
Graph represents the individual scores for (A) the 3 free recalls at inclusion (baseline assessment), after surgery (post-surgery), and after active nucleus basalis of Meynert deep brain stimulation (NBM-DBS) and sham NBM-DBS and (B) cognitive fluctuations (One Day Fluctuation Assessment Scale [ODFAS] score, top panel) and parkinsonian motor disability (Unified Parkinson's Disease Rating Scale [UPDRS] part III, bottom panel) assessed before surgery (Inclusion), after surgery (Post-surgery at month 1), at the beginning of the randomized double-blind period (Pre-randomization), and after 3 months with active NBM-DBS (active DBS) and without (sham DBS). Patients first randomized to active NBM-DBS are represented with squares; patients first randomized to sham NBM-DBS are represented with circles. (C) Regions of greater brain metabolic activity with active relative to sham NBM-DBS in patients with Lewy body dementia (LBD). FCSRT = Free and Cued Selective Reminding Test.
Effects of NBM-DBS on Cognitive, Psychiatric, and Motor Disability Scores
We observed no significant difference in the total free recall FCSRT score between active and sham NBM-DBS (median score [interquartile range] 12.5 [7.92–23.33] and 13.5 [9.67–24.17] at the end of active and sham NBM periods, respectively; median [interquartile range] difference between the 2 stimulation conditions −0.5 [−3.5 to 3.33], p = 0.80, table 3 and figure 4A). At an individual level, 3 patients (P01, P03, P06) had increased scores with active vs sham NBM-DBS, and inversely, 3 patients (P02, P04, P05) had decreased scores (figure 4A).
With active vs sham NBM-DBS, a significantly lower ODFAS score was observed but not for other scores (table 3 and figure 4B).
At the end of the active NBM-DBS period relative to baseline, no significant difference was observed in the total free recall FCSRT score (table 3 and figure 4A), but a decrease of >2 points was seen in 5 of 6 patients. For other secondary outcomes, a significant decrease was observed in motor disability score (MDS-UPDRS III) (table 3 and figure 4B) but not in other scores.
Effects of NBM-DBS on Brain Metabolic Activity
Comparing the brain metabolic activity of the 5 patients with LBD with age-matched controls from a historical reference database revealed a significant decrease in metabolic activity in the frontal, parietal, and occipital areas (not shown). This was more pronounced in patients P01, P02, and P03. With active vs sham NBM-DBS, we observed a significant increase in metabolic activity in the superior lingual gyrus (figure 4C).
Discussion
In this phase I randomized, double-blind, crossover controlled trial of 6 patients with mild to moderate LBD, surgery for NBM-DBS was well tolerated, but we observed a trend toward cognitive worsening after electrode implantation, suggesting a possible deleterious microlesion effect. There was no significant improvement in cognitive or behavioral status after 3 months of NBM-DBS. There was increased brain occipital metabolic activity with NBM-DBS.
The surgical procedure was well tolerated in all patients, as previously reported in 2 recent trials that included 6 patients with AD37 or 6 patients with PDD.13 In our study, only 1 patient (P04) of the 6 patients presented postoperative confusion related to urinary infection, which resolved rapidly after specific medical drug treatment. In a recent randomized protocol study that reported the effects of fornix DBS in 42 patients with mild AD, none developed neurologic side effects after surgery.11 This suggests that such a surgical approach could be envisaged in these patients with mild dementia without additional risk of brain hemorrhage or postoperative confusion. However, in our patients, we observed a significant worsening of some cognitive scores after surgery (month 1) relative to baseline with conversely a significant improvement in motor disability. These opposite effects are unlikely to be explained by the natural course of the disease considering the short duration and the low amplitude of performance change. This could reflect a microlesion effect considered to reflect disruption of cells or fibers during the definitive placement of electrodes.38 In patients with PD after DBS surgery, this microlesion effect has been shown to be a good predictor of motor outcomes39 but a bad predictor of cognitive outcomes.40 Such a microlesion effect, however, was not reported in patients with PDD13 and not mentioned in patients with AD.37 This suggests that this surgery could induce an additional disruption of basal cholinergic neurons, leading to a worsening of cognitive deficit. The motor improvement found in our patients with LBD after surgery might also reflect the disruption of the overlying globus pallidus internus mimicking bilateral pallidotomy,41 also previously reported with low-frequency NBM-DBS in patients with PDD.13 Lastly, we were able to include only 20% of the patients assessed for eligibility over a 5-year period. Such a rate of inclusion was also reported for NBM-DBS in patients with PDD, including 6 of 25 patients screened over a 3-year period.13 This could indicate that such a procedure could be proposed to only a limited number of patients. However, in our study, which proposes NBM-DBS in a randomized phase I study, we used strict inclusion criteria, in particular mild cognitive decline (MMSE score >16) and age <75 years, which prevented us from fully determining its feasibility in a larger population of patients.
The absence of a significant cognitive improvement with NBM-DBS in our patients with LBD is consistent with previous trials performed in patients with dementia linked to basal forebrain cholinergic degeneration. In 2 recent crossover double-blind trials, low-frequency (20-Hz) NBM-DBS induced no significant effect on cognition outcomes in 6 patients with AD and in 6 patients with PDD.12,13 However, in our study, 3 patients seemed to have better memory performance with NBM-DBS, and 5 patients had improved fluctuations of cognitive functions with a significantly decreased ODFAS score with active vs sham NBM-DBS (table 2). This could suggest that NBM-DBS may improve cognitive fluctuations and some cognitive processes, as also previously reported in a single case study.42 The relatively short duration of the stimulated period also could have masked a potential benefit. Indeed, in patients with mild to moderate AD, cognition performance remained stable after NBM-DBS applied during 1 year, suggesting that NBM-DBS could slow disease progression in these patients.12,43 Conversely to the previous report of patients with PDD,13 we did not observe any significant reduction in hallucinations or other behavioral disorders. However, our patients with LBD had fewer severe delusions and hallucinations with a higher dose of cholinesterase inhibitors (9.5 mg/d),13 which could have influenced the effects of NBM-DBS on these signs.
In line with the clinical effects observed while testing the different parameter settings (figure 3B), we chose different frequencies of stimulation (20–100 Hz) among patients for the randomized crossover period (table 1). This difference could have influenced the effects of NBM-DBS on cognition in each patient. In anesthetized rats, unilateral 50-Hz NBM electric stimulation has been shown to increase regional cerebral blood flow in the ipsilateral frontal, parietal, and occipital cortices,44 and 100-Hz stimulation has been shown to increase acetylcholine release in the ipsilateral parietal cortex,45 suggesting that both low and high frequencies may promote increased cholinergic cortical transmission with probably complex inhibition/activation effects on NBM cholinergic neurons and GABAergic interneurons.46,47 In patients with AD, bilateral 20-Hz NBM-DBS slightly increased cerebrum metabolic activity (2.2% to 4.9%), more particularly in the parietal, temporal, and amygdalo-hippocampal regions.12,37 In patients with PDD, low-frequency NBM-DBS induced no significant difference in the resting-state brain on fMRI.13 Here, we observed significantly increased metabolic activity with NBM-DBS only in the superior lingual gyrus, with no changes in the temporal or amygdalo-hippocampal regions, thus limiting the interpretation of the underlying mechanism of NBM-DBS.
Several limitations of this study need to be pointed out. First, this study was designed as a phase I study and aimed to assess the safety of NBM-DBS with a small number of patients and was underpowered for any efficacy analysis. Heterogeneity in disease severity and lesion distribution between participants also may have potentially influenced the cognitive effects of NBM-DBS, as well as the quite low cognitive deficit of our patients (MMSE score >19). Second, the choice of the optimal parameters during the exploratory phase was not based on the improvement of the main outcome measure (FCSRT scores) but on the CIBIC-Plus score. We cannot exclude the fact that the assessment of this global score may first reflect the improvement in motor disability, not in cognition per se. Lastly, patients 01, 03, and 06 had deteriorated memory performance just after NBM implantation, and the cognitive improvement observed later with NBM-DBS in these patients could reflect only the disappearance of the microlesion effect. However, we chose to begin the randomized period 4 months after surgery, a delay considered sufficient to disentangle NBM-DBS effect from microlesion effect. Finally, these limitations indicate that our results preclude any definite conclusion on the potential therapeutic interest of NBM-DBS for these patients.
Our results obtained in a small group of patients suggest that NBM-DBS might not be totally safe in patients with LBD, with its potential therapeutic effects probably limited in the early stage of dementia. Further randomized controlled studies are needed that include a larger number of patients, perhaps also with more severe cognitive deficit and with a longer duration of active DBS to explore the tolerance and efficacy of NBM-DBS in patients with LBD, as well as long-term follow-up studies using specific biological and imaging approaches to examine NBM-DBS effects on neuronal degeneration.
Acknowledgment
The authors acknowledge the patients who participated in this research with dedication. The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript; Eric Bardinet, ICM CENIR, for his help in neuroimaging analysis; and Dorothée Pouliquen, Neurology Department, Rouen University Hospital, for neuropsychological assessment.
Glossary
- AD
Alzheimer disease
- AE
adverse event
- CIBIC
Clinician's Interview Based Impression of Change
- DBS
deep brain stimulation
- DEMENSTIM
Effects of Nucleus Basalis of Meynert Stimulation on Cognitive Disorders in Dementia With Lewy Bodies
- DR
delayed recall
- FAB
Frontal Assessment Battery
- FCSRT
Free and Cued Selective Reminding Test
- IR
immediate recall
- LBD
Lewy body dementia
- MDS-UPDRS
Movement Disorders Society–Unified Parkinson's Disease Rating Scale
- MMSE
Mini-Mental State Examination
- NBM
nucleus basalis of Meynert
- ODFAS
One Day Fluctuation Assessment Scale
- PD
Parkinson disease
- PDD
PD dementia
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was funded by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique). Medtronic provided additional funding for the study.
Disclosure
Prof David Maltête has received honoraria from Elivie, Abbvie, and Roche and travel grant from Orkyn. Dr. David Wallon, Dr. Julie Bourilhon, and Dr. Romain Lefaucheur report no disclosures. Dr. Teodor Danaila has received personal fees for scientific expertise from Abbvie. Prof Stéphane Thobois has received research grant from France Parkinson and FRM; honoraria from Boston Scientific and Aguettant; and travel grant from Abbvie, Elivie, and Aguettant. Prof Luc Defebvre has received research grant from France Parkinson; honoraria from Abbvie, UCB, and Orkyn; and travel grant from Abbvie. Prof Kathy Dujardin reports no disclosures. Prof Jean-Luc Houeto has received fees for lectures and consultancies from Medtronic. Prof Olivier Godefroy has served on scientific advisory boards and speaker for Novartis, CSL-Behring, Biogen, Gensyme, Lilly, Bristol-Myers Squibb, Boerhinger-Engelheim, Covidien, Teva, and AstraZeneca and received funding travel and meetings from Novartis, Lilly, Genzyme, AstraZeneca, Biogen, Teva, Pfizer, CSL-Berhing, GSK, Bristol-Myers Squibb, Boerhinger-Engelheim, and Covidien. Prof Pierre Krystkowiak has received personal fees for scientific expertise or lectures from Elivie, Homeperf, Aguettant, and Abbvie. Prof Olivier Martinaud, Dr. André Gillibert, Dr. Mathieu Chastan, Prof Pierre Vera, and Prof Didier Hannequin report no disclosures. Prof Marie-Laure Welter has received research grant from France Parkinson; personal fees for scientific expertise from Genious Healthcare and Boston Scientific; and travel grant from Allergan. Prof Stéphane Derrey has received personal fees for consultancy from Medtronic and Zimmer-Biomet Industries. Go to Neurology.org/N for full disclosures.
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippa CF, Smith TW, Perry E. Dementia with Lewy bodies: choline acetyltransferase parallels nucleus basalis pathology. J Neural Transm 1999;106:525–535. [DOI] [PubMed] [Google Scholar]
- 4.Fujishiro H, Umegaki H, Isojima D, Akatsu H, Iguchi A, Kosaka K. Depletion of cholinergic neurons in the nucleus of the medial septum and the vertical limb of the diagonal band in dementia with Lewy bodies. Acta Neuropathol 2006;111:109–114. [DOI] [PubMed] [Google Scholar]
- 5.Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 2009;73:273–278. [DOI] [PubMed] [Google Scholar]
- 6.Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010;74:885–892. [DOI] [PubMed] [Google Scholar]
- 7.McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000;356:2031–2036. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin M, Rowan E, Edwards K, McKeith I. Cholinesterase inhibitors in dementia with Lewy bodies: a comparative analysis. Int J Geriatr Psychiatry 2007;22:890–895. [DOI] [PubMed] [Google Scholar]
- 9.Morrin H, Fang T, Servant D, Aarsland D, Rajkumar AP. Systematic review of the efficacy of non-pharmacological interventions in people with Lewy body dementia. Int Psychogeriatr 2018;30:395–407. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JP, McKeith IG, Burn DJ, et al. New evidence on the management of Lewy body dementia. Lancet Neurol 2020;19:157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano AM, Fosdick L, Chakravarty MM, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis 2016;54:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn J, Hardenacke K, Lenartz D, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's dementia. Mol Psychiatry 2015;20:353–360. [DOI] [PubMed] [Google Scholar]
- 13.Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral deep brain stimulation of the nucleus basalis of Meynert for Parkinson disease dementia: a randomized clinical trial. JAMA Neurol 2018;75:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE). Psychopharmacol Bull 1988;24:689–692. [PubMed] [Google Scholar]
- 15.Schade S, Mollenhauer B. Biomarkers in biological fluids for dementia with Lewy bodies. Alzheimers Res Ther 2014;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6:305–313. [DOI] [PubMed] [Google Scholar]
- 17.Bardinet E, Bhattacharjee M, Dormont D, et al. A three-dimensional histological atlas of the human basal ganglia, II: atlas deformation strategy and evaluation in deep brain stimulation for Parkinson disease. J Neurosurg 2009;110:208–219. [DOI] [PubMed] [Google Scholar]
- 18.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study: clinical global impression of change: the Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(suppl 2):S22–S32. [DOI] [PubMed] [Google Scholar]
- 19.Van der Linden M, Juillerat AC. Neuropsychological rehabilitation in early stage Alzheimer's disease: principles, methods and perspectives. Rev Neurol 2004;160:S64–S70. [DOI] [PubMed] [Google Scholar]
- 20.Grober E, Gitlin HL, Bang S, Buschke H. Implicit and explicit memory in young, old, and demented adults. J Clin Exp Neuropsychol 1992;14:298–316. [DOI] [PubMed] [Google Scholar]
- 21.Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer's, Huntington's, and Parkinson's diseases. Arch Neurol 1993;50:374–379. [DOI] [PubMed] [Google Scholar]
- 22.Mormont E, Laurier-Grymonprez L, Baisset-Mouly C, Pasquier F. The profile of memory disturbance in early Lewy body dementia differs from that in Alzheimer's disease [in French]. Rev Neurol 2003;159:762–766. [PubMed] [Google Scholar]
- 23.Mattis S. Dementia Rating Scale. Psychological Assessment Resources:Odessa, FL; 1988. [Google Scholar]
- 24.Stroop JR. Studies of interference in seria verb reactions. J Exp Psyschol 1935;18:643–662. [Google Scholar]
- 25.Benton AL. A visual retention test for clinical use. Arch Neurol Psychiatry 1945;54:212–216. [DOI] [PubMed] [Google Scholar]
- 26.Pillon B. Neuropsychological assessment for management of patients with deep brain stimulation. Mov Disord 2002;17(suppl 3):S116–S122. [DOI] [PubMed] [Google Scholar]
- 27.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000;55:1621–1626. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Ski 1958;8:271–276. [Google Scholar]
- 29.Walker MP, Ayre GA, Cummings JL, et al. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale: two methods to assess fluctuating confusion in dementia. Br J Psychiatry 2000;177:252–256. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton JM, Salmon DP, Galasko D, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology 2008;22:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahieux-Laurent F, Fabre C, Galbrun E, Dubrulle A, Moroni C; Groupe de reflexion sur les praxies du CMRR Ile-de-France Sud. Validation of a brief screening scale evaluating praxic abilities for use in memory clinics: evaluation in 419 controls, 127 mild cognitive impairment and 320 demented patients [in French]. Rev Neurol 2009;165:560–567. [DOI] [PubMed] [Google Scholar]
- 33.Deloche G, Hannequin D. DO 80 : test de dénomination orale d’images. In: Les Éditions du Centre de Psychologie Appliquée. Paris; 1997. [Google Scholar]
- 34.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep 1997;20:844–849. [DOI] [PubMed] [Google Scholar]
- 35.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 36.Bailon O, Roussel M, Boucart M, Krystkowiak P, Godefroy O. Psychomotor slowing in mild cognitive impairment, Alzheimer's disease and Lewy body dementia: mechanisms and diagnostic value. Dement Geriatr Cogn Disord 2010;29:388–396. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn J, Hardenacke K, Shubina E, et al. Deep brain stimulation of the nucleus basalis of Meynert in early stage of Alzheimer's dementia. Brain Stimul 2015;8:838–839. [DOI] [PubMed] [Google Scholar]
- 38.Maltete D, Chastan N, Derrey S, et al. Microsubthalamotomy effect at day 3: screening for determinants. Mov Disord 2009;24:286–289. [DOI] [PubMed] [Google Scholar]
- 39.Maltete D, Derrey S, Chastan N, et al. Microsubthalamotomy: an immediate predictor of long-term subthalamic stimulation efficacy in Parkinson disease. Mov Disord 2008;23:1047–1050. [DOI] [PubMed] [Google Scholar]
- 40.Le Goff F, Derrey S, Lefaucheur R, et al. Decline in verbal fluency after subthalamic nucleus deep brain stimulation in Parkinson's disease: a microlesion effect of the electrode trajectory? J Park Dis 2015;5:95–104. [DOI] [PubMed] [Google Scholar]
- 41.Cersosimo MG, Raina GB, Benarroch EE, Piedimonte F, Aleman GG, Micheli FE. Micro lesion effect of the globus pallidus internus and outcome with deep brain stimulation in patients with Parkinson disease and dystonia. Mov Disord 2009;24:1488–1493. [DOI] [PubMed] [Google Scholar]
- 42.Freund HJ, Kuhn J, Lenartz D, et al. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol 2009;66:781–785. [DOI] [PubMed] [Google Scholar]
- 43.Hardenacke K, Hashemiyoon R, Visser-Vandewalle V, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's dementia: potential predictors of cognitive change and results of a long-term follow-up in eight patients. Brain Stimul 2016;9:799–800. [DOI] [PubMed] [Google Scholar]
- 44.Adachi T, Biesold D, Inanami O, Sato A. Stimulation of the nucleus basalis of Meynert and substantia innominata produces widespread increases in cerebral blood flow in the frontal, parietal and occipital cortices. Brain Res 1990;514:163–166. [DOI] [PubMed] [Google Scholar]
- 45.Rasmusson DD, Clow K, Szerb JC. Frequency-dependent increase in cortical acetylcholine release evoked by stimulation of the nucleus basalis magnocellularis in the rat. Brain Res 1992;594:150–154. [DOI] [PubMed] [Google Scholar]
- 46.Parent A, Carpenter MB.. Carpenter's Human Neuroanatomy. Baltimore: Williams & Wilkins, 1996. [Google Scholar]
- 47.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 2004;115:1239–1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the article. Requests for anonymized data should be sent to D. Maltête at Rouen University Hospital, 76,000 Rouen, France.