Abstract
Objective
To determine the association between hypertensive disorders of pregnancy (HDP) and cognitive impairment 15 years after pregnancy, we measured cognitive performance in 115 women with a history of HDP and in 481 women with a previous normotensive pregnancy.
Methods
This was a nested cohort study embedded in a population-based prospective cohort from early pregnancy onwards. Cognitive function was assessed with cognitive tests 15 years after the index pregnancy (median 14.7 years, 90% range [13.9–16.1]). Cognitive performance was measured in different cognitive domains: executive function, processing speed, verbal memory, motor function, and visuospatial ability. A global cognition factor (g-factor) was derived from principal component analysis.
Results
Of the women with HDP, 80 (69.6%) had gestational hypertension (GH) and 35 (30.4%) had preeclampsia. Women with HDP had a lower g-factor than women with a previous normotensive pregnancy (mean −0.22, 90% range [−2.06−1.29]). HDP was negatively associated with the 15-word learning test: immediate recall (−0.25, 95% CI [−0.44 to −0.06]) and delayed recall (−0.30, 95% CI [−0.50 to −0.10]). Women with GH perform significantly worse on their 15-word learning test than women with a previous normotensive pregnancy.
Conclusion
A history of HDP is independently associated with poorer working memory and verbal learning 15 years after pregnancy. This association is mainly driven by women with GH. Clinicians and women who experienced HDP should be aware of this risk.
Cardiovascular disease (CVD) and cerebrovascular disease including dementia, which share common risk factors, remain among the leading causes of death and disability globally.1–3 Although women and men share many risk factors, there are important sex differences in the effect of risk factors such as hypertension on cerebrovascular disease.4,5 In addition, a number of cardiovascular risk factors are specific to women. Several of these factors are specifically related to pregnancy and its complications, such as hypertensive disorders of pregnancy (HDP).6,7 Women who have experienced HDP, in particular gestational hypertension (GH) or preeclampsia, are advised to make lifestyle changes to reduce their risk of later-life disease.8–10 Recent studies show a possible association of preeclampsia with cognitive impairment and even dementia,11,12 leading some to question whether cognitive impairment or dementia should be included as a potentially preventable outcome in CVD guidelines, rather than exclusively focusing on CVD risk reduction in women with previous HDP.13
Previous studies have been limited by their retrospective design, small study population, follow-up time, and focus specifically on only women with a history of preeclampsia, with sometimes no validation of this diagnosis.13–15 However, women with GH also may be at risk for CVD later in life.16 In addition, earlier studies are often limited by the use of well-validated and uniform neurocognitive tests to assess cognitive domains.15
In this prospective study, we aimed to determine the association of HDP, including preeclampsia and GH, with cognitive impairment years after pregnancy, hypothesizing that women with a history of HDP have impaired cognition years after pregnancy. In addition, we examined this association in a subgroup, including only women with GH.
Methods
Design and Study Population
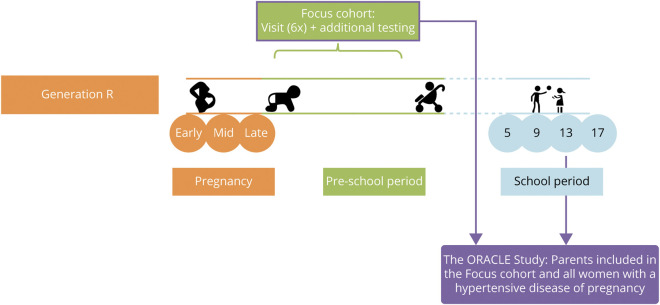
This study was performed as a substudy (termed ORACLE), embedded within the Generation R Study, an ongoing population-based prospective birth cohort study. In this study, all pregnant women living in a defined region in Rotterdam with a delivery date between April 2002 and January 2006 were invited to participate. A total of 9,778 mothers were included from early pregnancy onwards. The design of the ORACLE Study and Generation R Study has recently been published.17 The ORACLE Study started in 2017 as a dedicated research visit for the parents to conduct extensive measures on brain health, including neuroimaging and cognitive testing. An additional goal was to study HDP in relation to brain health, as Generation R has extensive prenatal data. The medical ethical committee (MEC) of the Erasmus Medical Center in Rotterdam, the Netherlands, approved the study (MEC 2015-749 NL55105.078.15). All participants signed a written informed consent. For the present study, we included a total of 596 women: 481 women from a subgroup of the Generation R cohort already participating with additional detailed testing from the beginning of Generation in 2002 (the focus subcohort) as well as the entire group of women with a history of HDP still participating in the cohort 15 years after pregnancy (figure 1).17 Due to logistic reasons and the MEC approval, women with HDP were invited to the research center a little later than women with a previous normotensive pregnancy. File 1 contains a Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for the current study.
Figure 1. Flowchart Showing Inclusion and Exclusion Criteria17.
Women were invited 3 times in pregnancy and returned to the research center with their children 5 years, 9 years, and 13 years after pregnancy. Currently, participants will be invited 17 years after pregnancy. Additional detailed measurements of fetal and postnatal growth and development have been conducted in a randomly selected subgroup of Dutch children (n = 1,232, known as the Focus cohort) and their parents at 32 weeks of gestational age and the postnatal ages of 1.5, 6, 14, 24, 36, and 48 months.
Hypertensive Disorders of Pregnancy
Women who experienced GH and preeclampsia in their index pregnancy were classified as HDP. All diagnoses were cross-validated retrospectively by obstetric records that were obtained from midwife and hospital registries. The criteria for HDP were defined by the criteria that applied at the time of study inclusion of Generation R. This was according to the statement from the International Society for the Study of Hypertension in Pregnancy of 2001. Therefore, GH was defined as development of systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg without proteinuria after 20 weeks of gestation in previously normotensive women. Preeclampsia was defined as a new onset of hypertension with SBP ≥140 mm Hg or DBP ≥90 mm Hg and proteinuria (≥300 mg/d) at or after 20 weeks of gestational age. SBP and DBP were measured multiple times in pregnancy (median in early pregnancy 12.9 weeks of gestation, 90% range [10.9–16.9 weeks]) using a validated Omron 907 automated digital oscillometric sphygmomanometer (Omron Healthcare Europe B.V., Hoofddorp, the Netherlands).
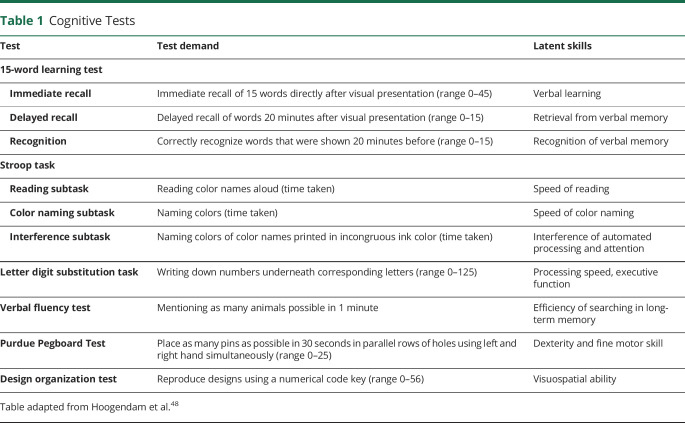
Cognitive Tests
We used validated cognitive tests. All participants were tested individually and testing was conducted by trained examiners. The cognitive test battery included 15-word learning test (15WLT), Stroop task, letter-digit substitution task, verbal fluency test, Purdue Pegboard, and design organization test. Test demands of these cognitive tests and latent skills measured are presented in table 1. No difference in participation rates for the 15WLT, Stroop task, letter digit substitution task, and verbal fluency test were found between women with HDP and a previous normotensive pregnancy. Due to logistic reasons, the Purdue Pegboard test and design organization test were started later in the ORACLE Study. Therefore, women with HDP participated more often in the Purdue Pegboard test and design organization test (table 2).
Table 1.
Cognitive Tests
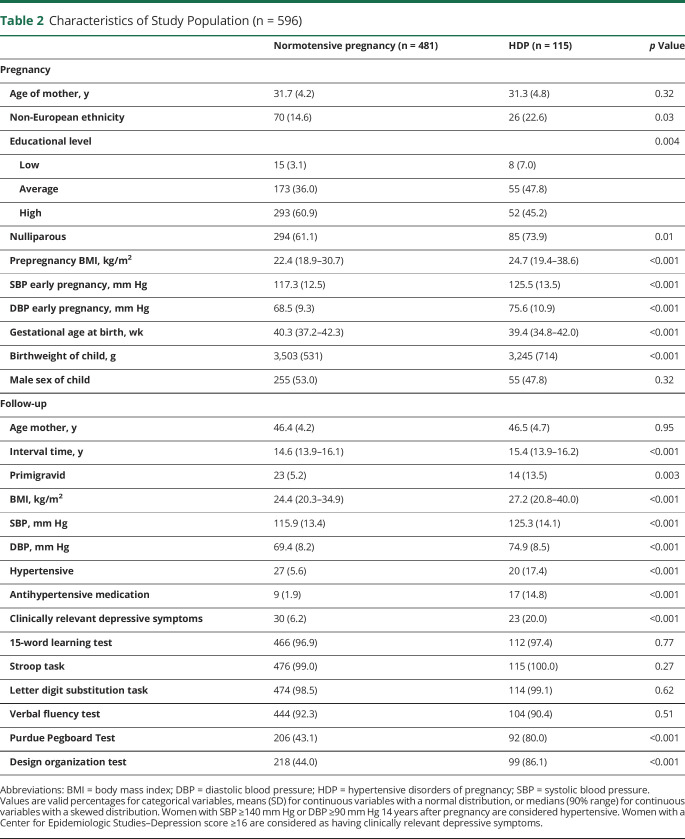
Table 2.
Characteristics of Study Population (n = 596)
Covariates
Information on ethnicity, level of education, and prepregnancy body mass index (BMI) was obtained through questionnaires in pregnancy. We categorized ethnicity into European and non-European ethnicity. Prepregnancy BMI was highly correlated with BMI measured at study enrollment (Pearson correlation coefficient 0.97 [p value <0.001]), and therefore prepregnancy BMI was used in the analyses. All women were invited to a standardized visit at the research center 14 years after pregnancy (median 14.1 years, 90% range [13.6–15.7 years]) where blood pressure was measured with the validated automatic sphygmomanometer Datascope Accutorr Plus (Paramus, NJ). The interval time between blood pressure measurement and cognitive testing was median 0.5 years, 90% range 0.1−1.9 years. Blood pressure was measured 2 times over a 60-second interval of which the mean value was used for analyses. Depressive symptoms at the time of cognitive testing were evaluated with the Center for Epidemiologic Studies–Depression (CES-D) scale and a cutoff of 16 was used to detect participants with clinically relevant depressive symptoms.
Statistical Analyses
Differences in baseline characteristics between women with a normotensive pregnancy and HDP were analyzed using Student t test for normally distributed data, Mann-Whitney U test for continuous variables with a skewed distribution, and χ2 test for categorical data. Cognitive test results of women with a previous normotensive pregnancy were compared to test results of women with HDP by a Mann-Whitney U test. Stroop task scores were right-skewed and subsequently log-transformed to obtain a normal distribution. To facilitate comparison of effect sizes with other tests, all test scores were standardized by converting them to SD scores (SDS) and Stroop task scores were multiplied by −1. Because different studies often use different cognitive test batteries, it is difficult to compare findings of separate test scores across studies. In contrast, global cognition factor (g-factor) scores of cognitive function tests derived from different cognitive test batteries are often very similar and typically explain approximately 50% of all variance in the cognitive tests. The g-factor is identified as the first unrotated component of a principal component analysis that incorporates tasks from all available cognitive function tests. Although Stroop and 15WLT comprised several tasks, we only used data from the most complicated task of each to prevent highly correlated tasks distorting factor loadings in the principal component analysis.
Linear regression analyses were performed to investigate the association of HDP with cognitive test scores. The crude model was not adjusted for confounders. The confounder model adjusted for ethnicity and educational level and pre-pregnancy BMI.
Because we wanted to examine the effect of GH besides preeclampsia, we performed an additional sensitivity analysis excluding women with preeclampsia. In this study, we had very little missing data. Only 0.2% of women had missing information on ethnicity, 1.8% on educational level, and 6.8% on blood pressure 15 years after pregnancy. Missing values of the covariates were imputed through multiple imputation procedures. Data were imputed according to the Markov Chain Monte Carlo method. Data were analyzed in each set separately, and pooled estimates from the 5 imputed datasets were used to report the effect estimates and their 95% confidence intervals. For the multiple imputation procedure, we performed 10 iterations. In all analyses, a p value < 0.05 was considered statistically significant. All analyses were performed using the Statistical Package of Social Sciences version 25.0 for Windows (SPSS Inc. Chicago, IL).
Standard Protocol Approvals, Registrations, and Patient Consents
The study has been approved by the MEC of the Erasmus Medical Centre in Rotterdam (MEC 198.782/2001/31, MEC-2007-413, and MEC 2015-749). Written consent was obtained from all participants. Consent for publication was not applicable.
Data Availability
Data requests can be made to the secretary office of Generation R (secretariaat.genr@erasmusmc.nl). To be able to share data from the Generation R Study, a data transfer agreement needs to be completed.
Results
We included 596 women: 481 women (80.7%) had a previous normotensive pregnancy and 115 women (18.0%) had HDP. Of the women with HDP, 80 (69.6%) had GH and 35 (30.4%) had preeclampsia. Of the 35 women with preeclampsia, almost all developed preeclampsia at term, n = 33 (94.3%), as defined as an onset >34 weeks of gestation. Women with HDP were more often of non-European descent and had a lower level of education, a higher prepregnancy BMI, and higher SBP and DBP at early pregnancy. Women were on average 46.4 (SD 4.3) years of age at time of cognitive testing and the pregnancy–cognitive test interval time had a median of 14.7 years (90% range 13.9–16.1) (table 2). Approximately 0.5 years (90% range 0.1−1.9 years) prior to the cognitive testing assessment, 68 (11.4%) women were classified as hypertensive. Of these 68 women, 47 were hypertensive and 26 used antihypertensive medication at the research center. Women with a history of HDP had a higher proportion of hypertension (30.4%) than women with a previous normotensive pregnancy (6.9%). At the time of cognitive testing, 20% of the women with HDP had clinically depressive symptoms (table 2).
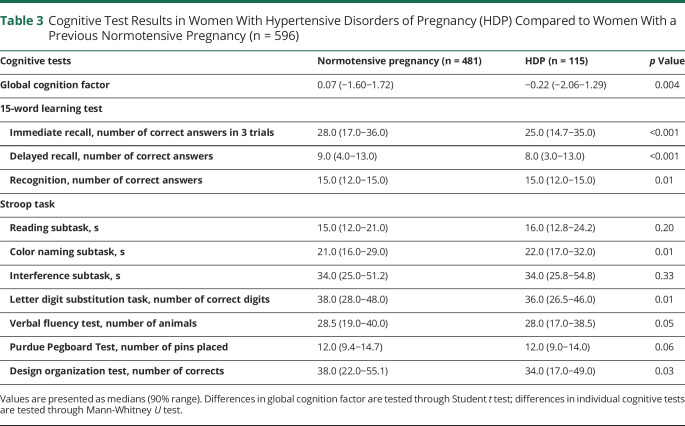
Table 3 shows that women with HDP had a lower g-factor compared to women with a previous normotensive pregnancy. Women with HDP performed worse on the 15WLT immediate recall subtask, 15WLT delayed recall subtask, 15WLT recognition subtask, Stroop color naming task, letter digit substitution task, and design organization test than women with a previous normotensive pregnancy.
Table 3.
Cognitive Test Results in Women With Hypertensive Disorders of Pregnancy (HDP) Compared to Women With a Previous Normotensive Pregnancy (n = 596)
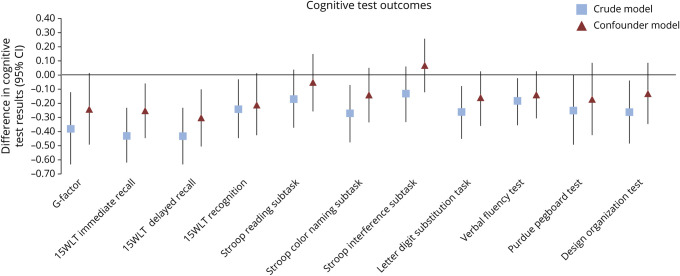
Figure 2 shows the association between HDP and cognitive test outcomes presented in SDS. Women with HDP performed worse on the immediate recall and delayed recall subtask of the 15WLT compared to women with a previous normotensive pregnancy. These associations remained significant after adjustment for confounders: immediate recall (−0.25, 95% CI −0.44 to −0.06), delayed recall (−0.30, 95% CI −0.50 to −0.10). HDP was negatively associated with the g-factor, recognition subtask of the 15WLT, Stroop color naming subtask, letter digit substitution task, verbal fluency test, Purdue Pegboard test, and design organization test. These negative associations attenuated to nonsignificant levels after adjustment for ethnicity, educational level and prepregnancy BMI.
Figure 2. Associations of Women With Hypertensive Disorders of Pregnancy Compared to Women With a Previous Normotensive Pregnancy (n = 596).
Cognitive test results are converted to SD scores. Values are regression coefficients reflecting the difference in cognitive test result with 95% confidence interval (CI) derived from multiple linear regression analyses. Crude value is unadjusted. The confounder model is adjusted for ethnicity, educational level, and prepregnacy body mass index. 15WLT = 15-word learning test; G-factor = global cognition factor.
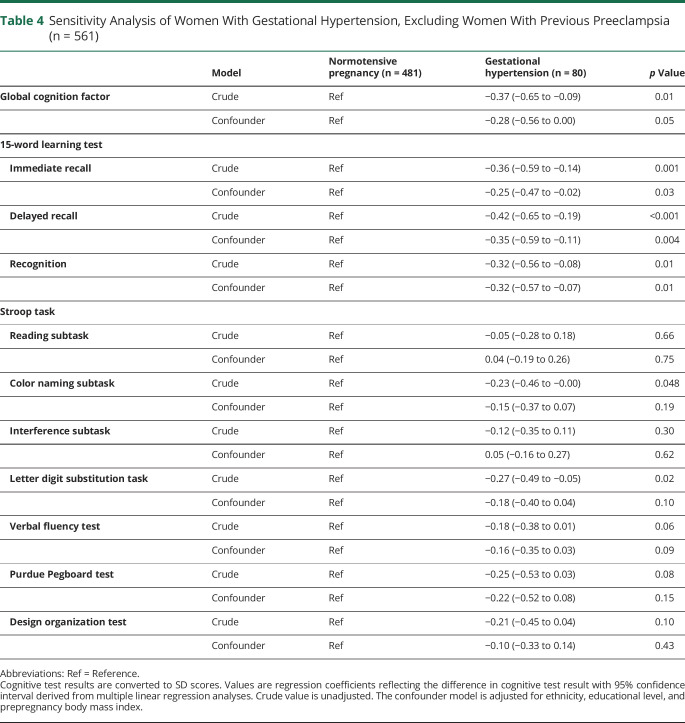
Table 4 shows the results of a sensitivity analysis excluding women with preeclampsia (n = 35) from our analyses. Women with GH performed worse on all 3 subtasks of the 15WLT compared to women with a previous normotensive pregnancy. These associations remained significant after adjustment for confounders.
Table 4.
Sensitivity Analysis of Women With Gestational Hypertension, Excluding Women With Previous Preeclampsia (n = 561)
Discussion
With this prospective-cohort study of relatively young women 15 years after pregnancy, we show that women with a history of HDP have reduced objective cognitive function. This association is mainly driven by women with GH. Women with a history of HDP have more problems in working memory and verbal learning than women with a previous normotensive pregnancy. This impairment is independent of ethnicity, educational level, and prepregnancy BMI.
The hypothesis of this study is partly based on the possible association between HDP and dementia.11,18 Dementia may result from vascular injury by 2 mechanisms.19 The first is dementia as a result of a “strategic infarct” due to a clinically diagnosed stroke. The second mechanism is through microvascular brain injury due to cerebral small vessel disease, identified by characteristic radiographic findings (such as white matter hyperintensities, silent lacunar infarcts, and microhemorrhages) in a patient with cognitive impairment but without a clinical history of stroke.20,21 Radiographic changes associated with cerebral small vessel disease have been seen in women up to 15 years after experiencing preeclampsia.22,23 However, the cognitive problems that are found in patients with vascular dementia are the greatest in speed, praxis, executive function, and visual memory, and are less prominent in verbal learning.24 This is different from our results because we show a difference in working memory and verbal learning. Problems in verbal learning are more in agreement with impairment seen in patients with Alzheimer disease.25 However, because vascular dementia is a heterogeneous syndrome, a cognitive profile overlapping vascular dementia and Alzheimer disease should be considered.
The mechanism behind the association of HDP with cognitive dysfunction remains unclear, because the pathophysiology of HDP is largely unknown. One hypothesis proposes that preeclampsia, CVD, and dementia have a shared cause and that pregnancy may be a stress test that reveals women with a poor phenotype for being at risk of developing CVD later in life.8 Women with preeclampsia have a higher incidence of hypertension, antihypertensive medication use, a greater accumulation of calcium in the coronary arteries, and thicker carotid intima–media thickness compared to previously normotensive women, all associated with CVD later in life.26–28 A second hypothesis proposes that women with preeclampsia develop vascular damage in pregnancy, which progresses into clinical vascular damage later in life.6,8 This hypothesis is supported by animal studies and small clinical studies showing neurovascular unit dysfunction in preeclampsia including neurovascular unit dysfunction and autoregulatory dysfunction, and the association of preeclampsia with altered central hemodynamics.13,29–32 Changes in the structure of central elastic arteries may manifest as increased arterial stiffness or elevated central blood pressure, even prior to clinically detectable hypertension by brachial blood pressure.13,33 In addition, increases in central blood pressure may be more influential than brachial blood pressure when evaluating the hemodynamic stress on organs.13,34 Therefore, even in the absence of a diagnosis of hypertension, it is possible that women with a history of hypertensive disorders of pregnancy could develop increased central blood pressure and subclinical arterial stiffness, contributing to the risk of vascular brain injury.13
Our results are in agreement with previous studies that show poorer learning capacity and more memory problems in women with a history of severe preeclampsia 3–8 months postpartum and more problems in working memory 6–18 months postpartum than in women with a previous normotensive pregnancy.35,36 Our results are also in agreement with a previous study of Postma et al.37 showing no difference in sustained attention or executive functioning in women with a history of eclampsia and preeclampsia 9 years after the index pregnancy compared to age-matched women with previous normotensive pregnancies.
Besides the 15WLT, HDP was not associated with other objective cognitive test measures. In a previous study, women with preeclampsia subjectively experienced more cognitive problems in daily life situations.38 An explanation for our findings may be the quiet and structured test setting in which the actual cognitive problems in complex daily life situations cannot be reproduced22,39,40 or the high variability in the test outcomes in both groups (women with a previous normotensive pregnancy and women with an HDP). Cognitive impairment in women with preeclampsia may also be subtle and may therefore only appear in complex tasks, that is, tasks that require more cognitive processes at the same time.36 Another explanation may be that the cognitive tests were assessed at a relatively young age (mean age of 46.4 years). At this age, there is a high compensational capacity of the brain.22,41 As these women age, the compensational capacity of the brain may deteriorate and they may experience more cognitive problems,41 which may result in a higher risk of cognitive impairment and dementia later in life.11
A strength of our study is that it is based on a large population-based prospective cohort study, enabling us to examine many women 15 years after their pregnancy. Other strengths include the long follow-up time and the focus on GH, whereas most studies focus on preeclampsia only. In addition, we examined a broad range of cognitive domains. Our study underlines the importance of GH, because GH was a large contributor in our HDP group. A total of 70% of all HDP women in our study experienced GH, and we found the same results after exclusion of women with preeclampsia. Our results suggest that a history of GH should be incorporated in guidelines for follow-up of women after pregnancy.13
Consistent with previous literature, our results showed that women with a history of HDP have more hypertension 14 years after pregnancy than women with a previous normotensive pregnancy. Development of chronic hypertension may potentially mediate the relationship between HDP and cognitive impairment. Therefore we underline the importance of blood pressure monitoring after an HDP, because hypertension is a major risk factor for cardiovascular and cerebrovascular disease later in life.42,43
It is known that depressive symptoms are associated with CVD and with poor cognitive function and cognitive decline.44,45 Moreover, women with a history of preeclampsia more frequently report depressive symptoms and physical and mental complaints.40,46 We did not measure these psychosocial factors prior to pregnancy or early in pregnancy. It may therefore be possible that the association of HDP with cognitive impairment is mediated by depressive symptoms. Nevertheless, it is striking that 20% of the women with HDP had clinically depressive symptoms at the time of cognitive testing. Although it is not within the scope of this study, clinicians and women with HDP should be aware of their high risk of developing clinically depressive symptoms, even up to 15 years after pregnancy. These women should be monitored closely, because depressive symptoms are associated with HDP and CVD in previous studies and it is a risk factor that may be treatable.47
There are several limitations of our study. We performed no cognitive tests prior to pregnancy or during pregnancy. Therefore, we could not investigate the effect of a pregnancy complicated by HDP on the cognitive function within one woman. Second, it was not possible to test the women in their daily life environment, which may better reflect the cognitive problems they subjectively report.39 There may be a response and selection bias, because women with HDP may refuse to participate 15 years after pregnancy due to fear of reliving the traumatic experience or due to the risk of confirmation of poor performance.12,22 HDP was defined according to the criteria that were in effect at inclusion of this study. It is possible that with the new International Society for the Study of Hypertension in Pregnancy and American College of Cardiology/American Heart Association criteria for HDP, more women would have been defined as having HDP, affecting our results. Lastly, MRI scans are not available. Future studies should include imaging to confirm the vascular statement.
A history of HDP is associated with impairment in working memory and verbal learning 15 years after index pregnancy. Not only women with a history of preeclampsia, but also those with GH should be monitored closely after their pregnancy. Whether early treatment of hypertension prevents cognitive decline in women with a history of HDP should be investigated in clinical trials. Both clinicians and women with a history of HDP should be aware of these patients' risk of cognitive impairment later in life.
Acknowledgment
The authors thank the participants and the general practitioners, hospitals, midwives, and the pharmacies in Rotterdam and all those concerned in the Generation R Study. The Generation R Study was conducted by the Erasmus Medical Center, Rotterdam, the Netherlands in close collaboration with the School of Law and the Faculty of Social Sciences of Erasmus University, Rotterdam, the Netherlands.
Glossary
- 15WLT
15-word learning test
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- GH
gestational hypertension
- g-factor
global cognition factor
- HDP
hypertensive disorders of pregnancy
- MEC
medical ethical committee
- SBP
systolic blood pressure
- SDS
SD scores
Appendix. Authors
Footnotes
Editorial, page 193
CME Course: NPub.org/cmelist
Study Funding
The Generation R Study was made possible by financial support from Erasmus MC, University Medical Centre Rotterdam, the Netherlands; the Netherlands Organization for Health Research and Development; the Netherlands Organization for Scientific Research; the Ministry of Health, Welfare and Sport; and the Ministry of Youth and Families. This project has received funding from the European Research Council under the European Union's Horizon 2020 research and innovation programme (project: ORACLE, grant agreement: 678543), Preeclampsia Foundation (2016 Vision Grant) and Coolsingel Foundation (2016, project number 471).
Disclosure
S. Schalekamp-Timmermans received funding from the Preeclampsia Foundation (2016 Vision Grant) and Coolsingel Foundation (2016, project number 471). E. Miller received support from the NIH National Institute of Neurologic Disorders and Stroke (K23NS107645) and the Louis V. Gerstner, Jr Foundation (Gerstner Scholars Program). M.A. Ikram received funding from the European Union's Horizon 2020 research and innovation programme (678,543, ORACLE). M. Adank, R. Hussainali, L. Oosterveer, and E. Steegers report no disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011363 for full disclosures.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics: 2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmis A, Townsend N, Gale C, et al. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
- 3.Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat Rev Cardiol 2017;14:273–293. [DOI] [PubMed] [Google Scholar]
- 4.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J 2010;18:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leening MJ, Ferket BS, Steyerberg EW, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc 2018;7:e009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams D. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol 2003;15:465–471. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management (NICE guideline NG133) [online]. Available at: nice.org.uk/guidance/ng133. [PubMed]
- 11.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ 2018;363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields JA, Garovic VD, Mielke MM, et al. Preeclampsia and cognitive impairment later in life. Am J Obstet Gynecol 2017;217:74.e1–74.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KB, Miller VM, Barnes JN. Pregnancy history, hypertension, and cognitive impairment in postmenopausal women. Curr Hypertens Rep 2019;21:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayan N, Kaur A, Elharram M, Rossi AM, Pilote L. Impact of preeclampsia on long-term cognitive function. Hypertension 2018;72:1374–1380. [DOI] [PubMed] [Google Scholar]
- 15.Elharram M, Dayan N, Kaur A, Landry T, Pilote L. Long-term cognitive impairment after preeclampsia: a systematic review and meta-analysis. Obstet Gynecol 2018;132:355–364. [DOI] [PubMed] [Google Scholar]
- 16.Riise HKR, Sulo G, Tell GS, et al. Association between gestational hypertension and risk of cardiovascular disease among 617 589 Norwegian women. J Am Heart Assoc 2018;7:e008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamballais S, Adank MC, Hussainali RF, et al. Design and overview of the Origins Of Alzheimer's Disease Across the Life Course (ORACLE) Study. Eur J Epidemiol Epub 2020 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci 2017;131:1059–1068. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction: the disregarded partner of Alzheimer's disease. Alzheimers Dement 2019;15:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw JM. William M. Feinberg Award for Excellence in Clinical Stroke: small vessel disease: a big problem, but fixable. Stroke 2018;49:1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postma IR, Bouma A, de Groot JC, Aukes AM, Aarnoudse JG, Zeeman GG. Cerebral white matter lesions, subjective cognitive failures, and objective neurocognitive functioning: a follow-up study in women after hypertensive disorders of pregnancy. J Clin Exp Neuropsychol 2016;38:585–598. [DOI] [PubMed] [Google Scholar]
- 23.Siepmann T, Boardman H, Bilderbeck A, et al. Long-term cerebral white and gray matter changes after preeclampsia. Neurology 2017;88:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 2004;62:912–919. [DOI] [PubMed] [Google Scholar]
- 25.Peters F, Collette F, Degueldre C, Sterpenich V, Majerus S, Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study. Brain 2009;132:1833–1846. [DOI] [PubMed] [Google Scholar]
- 26.Garovic VD, Milic NM, Weissgerber TL, et al. Carotid artery intima-media thickness and subclinical atherosclerosis in women with remote histories of preeclampsia: results from a Rochester Epidemiology Project–based study and meta-analysis. Mayo Clin Proc 2017;92:1328–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White WM, Mielke MM, Araoz PA, et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol 2016;214:519 e511–519 e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benschop L, Schalekamp-Timmermans S, Schelling SJC, Steegers EAP, Roeters van Lennep JE. Early pregnancy cardiovascular health and subclinical atherosclerosis. J Am Heart Assoc 2019;8:e011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T, Richard Jennings J, Kim SG. Regional cerebral blood flow and arterial blood volume and their reactivity to hypercapnia in hypertensive and normotensive rats. J Cereb Blood Flow Metab 2014;34:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Shen Q, Huang S, et al. Cerebral angiography, blood flow and vascular reactivity in progressive hypertension. Neuroimage 2015;111:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones-Muhammad M, Warrington JP. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci 2019;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller EC. Preeclampsia and cerebrovascular disease. Hypertension 2019;74:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols W, O'Rourke M. McDonalds's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 5th ed. London: Hodder Arnold Publishing; 2005. [Google Scholar]
- 34.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension 2016;67:183–190. [DOI] [PubMed] [Google Scholar]
- 35.Brusse I, Duvekot J, Jongerling J, Steegers E, De Koning I. Impaired maternal cognitive functioning after pregnancies complicated by severe pre-eclampsia: a pilot case-control study. Acta Obstet Gynecol Scand 2008;87:408–412. [DOI] [PubMed] [Google Scholar]
- 36.Baecke M, Spaanderman ME, van der Werf SP. Cognitive function after pre-eclampsia: an explorative study. J Psychosom Obstet Gynaecol 2009;30:58–64. [DOI] [PubMed] [Google Scholar]
- 37.Postma IR, Wessel I, Aarnoudse JG, Zeeman GG. Neurocognitive functioning in women with a history of eclampsia: executive functioning and sustained attention. Am J Perinatol 2010;27:685–690. [DOI] [PubMed] [Google Scholar]
- 38.Postma IR, Groen H, Easterling TR, et al. The brain study: cognition, quality of life and social functioning following preeclampsia; an observational study. Pregnancy Hypertens 2013;3:227–234. [DOI] [PubMed] [Google Scholar]
- 39.Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol 2007;197:365.e361–365.e366. [DOI] [PubMed] [Google Scholar]
- 40.Postma IR, Bouma A, Ankersmit IF, Zeeman GG. Neurocognitive functioning following preeclampsia and eclampsia: a long-term follow-up study. Am J Obstet Gynecol 2014;211:37.e31–37.e39. [DOI] [PubMed] [Google Scholar]
- 41.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson A, Andersch B, Hansson L. Prediction of later hypertension following a hypertensive pregnancy. J Hypertens Suppl 1983;1:94–96. [PubMed] [Google Scholar]
- 43.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart 1997;77:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry 1999;56:425–430. [DOI] [PubMed] [Google Scholar]
- 45.Dhar AK, Barton DA. Depression and the link with cardiovascular disease. Front Psychiatry 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roes EM, Raijmakers MT, Schoonenberg M, Wanner N, Peters WH, Steegers EA. Physical well-being in women with a history of severe preeclampsia. J Matern Fetal Neonatal Med 2005;18:39–45. [DOI] [PubMed] [Google Scholar]
- 47.Roberts L, Davis GK, Homer CSE. Depression, anxiety, and post-traumatic stress disorder following a hypertensive disorder of pregnancy: a narrative literature review. Front Cardiovasc Med 2019;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA. Patterns of cognitive function in aging: the Rotterdam Study. Eur J Epidemiol 2014;29:133–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data requests can be made to the secretary office of Generation R (secretariaat.genr@erasmusmc.nl). To be able to share data from the Generation R Study, a data transfer agreement needs to be completed.