Abstract
Objective
To determine the association between the extent of diffusion restriction and T2/fluid-attenuated inversion recovery (FLAIR) injury on brain MRI and outcomes after pediatric out-of-hospital cardiac arrest (OHCA).
Methods
Diffusion restriction and T2/FLAIR injury were described according to the pediatric MRI modification of the Alberta Stroke Program Early Computed Tomography Score (modsASPECTS) for children from 2005 to 2013 who had an MRI within 14 days of OHCA. The primary outcome was unfavorable neurologic outcome defined as ≥1 change in Pediatric Cerebral Performance Category (PCPC) from baseline resulting in a hospital discharge PCPC score 3, 4, 5, or 6. Patients with unfavorable outcomes were further categorized into alive with PCPC 3–5, dead due to withdrawal of life-sustaining therapies for poor neurologic prognosis (WLST-neuro), or dead by neurologic criteria.
Results
We evaluated MRI scans from 77 patients (median age 2.21 [interquartile range 0.44, 13.07] years) performed 4 (2, 6) days postarrest. Patients with unfavorable outcomes had more extensive diffusion restriction (median 7 [4, 10.3] vs 0 [0, 0] regions, p < 0.001) and T2/FLAIR injury (5.5 [2.3, 8.2] vs 0 [0, 0.75] regions, p < 0.001) compared to patients with favorable outcomes. Area under the receiver operating characteristic curve for the extent of diffusion restriction and unfavorable outcome was 0.96 (95% confidence interval [CI] 0.91, 0.99) and 0.92 (95% CI 0.85, 0.97) for T2/FLAIR injury. There was no difference in extent of diffusion restriction between patients who were alive with an unfavorable outcome and patients who died from WLST-neuro (p = 0.11).
Conclusions
More extensive diffusion restriction and T2/FLAIR injury on the modsASPECTS score within the first 14 days after pediatric cardiac arrest was associated with unfavorable outcomes at hospital discharge.
Nearly 8,000 children experience an out-of-hospital cardiac arrest (OHCA) each year in the United States.1 Survival rates to hospital discharge after an OHCA range from 5% to 20% depending in part on patient age and initial cardiac rhythm.1–4 The majority of patients die as a consequence of hypoxic-ischemic brain injury (HIBI) after either being declared dead by neurologic criteria or undergoing withdrawal of life-sustaining therapies (WLSTs) due to poor neurologic prognosis.5 Survivors often have neurocognitive and neurobehavioral deficits that can impact their quality of life.6–14 Long-term morbidity and mortality are related to the degree of neurologic functioning at hospital discharge.15,16
Neuroprognostication after cardiac arrest care may influence care decisions, including withdrawal of technological support. This is a dynamic process that primarily involves integration of a patient's prearrest and arrest characteristics, neurologic examination, EEG, and neuroimaging studies.17–20 Neuroimaging, and diffusion brain MRI in particular, can assess the degree of HIBI postcardiac arrest.21 A few small pediatric studies have shown an association between poor neurologic outcome and burden of injury on both conventional and diffusion MRI.22–25 However, all had small sample sizes and none of these studies used a predefined scoring system to determine extent of injury within individual brain regions.
We aimed to quantitatively assess diffusion restriction and T2/fluid-attenuated inversion recovery (FLAIR) injury in children after OHCA and evaluate the association between the extent of MRI injury and neurologic outcomes. We hypothesized that children with unfavorable outcomes would have more extensive diffusion restriction and T2/FLAIR injury compared to patients with favorable outcomes.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Children's Hospital of Philadelphia Institutional Review Board. Consent was not required and this cohort represents a consecutive sample of patients.
Design and Setting
This was a retrospective observational study using a cardiac arrest database of patients admitted to the pediatric intensive care unit (PICU) at the Children's Hospital of Philadelphia for postcardiac arrest care after an OHCA between June 2005 and May 2013.
Patient Population and Clinical Care
We included patients ≤18 years of age if they experienced an OHCA with cardiopulmonary resuscitation (CPR), received postcardiac arrest care in the PICU, and had a clinical MRI performed within 14 days. We excluded patients with concomitant traumatic brain injury, brain tumor, or a baseline Pediatric Cerebral Performance Category (PCPC) score of 4 or 5. We excluded patients with a baseline PCPC 4 or 5 as these patients typically have baseline T2/FLAIR abnormalities on their MRI indicative of prior injury, which could affect the interpretation and impact of cardiac arrest–related signal abnormality on their postcardiac arrest pediatric MRI modification of the Alberta Stroke Program Early Computed Tomography Score (modASPECTS). Abstracted data included patient demographics, cardiac arrest characteristics, and details about postcardiac arrest care. Postcardiac arrest care was determined by the clinical team. Patients were monitored on continuous EEG to identify electrographic seizures. Some patients received therapeutic hypothermia to 33.0°C as part of routine care or a randomized controlled trial (THAPCA-OH [Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Pediatric Patients–Out of Hospital]; unique identifier: NCT00878644).26
Injury Scoring on Brain MRI Scans
Brain MRI scans were performed at the discretion of the clinical team. MRIs were performed on either a 1.5T or 3T Siemens scanner (Erlangen, Germany). Standard imaging sequences varied over the study period, but all studies included T1, T2, FLAIR, diffusion-weighted, and susceptibility-weighted images. For this study, a pediatric neuroradiologist blinded to the clinical interpretation and patient case details scored both diffusion-weighted and T2/FLAIR images according to the semiquantitative modASPECTS, with additional areas such as the hippocampus and brainstem, which can be injured in HIBI.27,28 ModASPECTS assessed 69 unique brain regions in order to provide a granular evaluation of the injury (figure 1). Each branch of the anterior, middle, and posterior cerebral artery vascular distributions was scored separately for cortical and corresponding white matter injury, and the left and right structures of the cerebral hemispheres were scored separately. Diffusion restriction was considered present if diffusion-weighted signal was hyperintense and the corresponding apparent diffusion coefficient signal was hypointense. The possible score range for modASPECTS was 0–69.
Figure 1. Modified Alberta Stroke Program Early Computed Tomography Score (ASPECTS) Scoring System.
Modified ASPECTS scoring system showing all 69 brain regions and the 23 condensed regions analyzed in this study. ACA = anterior cerebral artery vascular distribution; MCA = middle cerebral artery vascular distribution; PCA = posterior cerebral artery vascular distribution; WM = white matter.
For analysis, we simplified these 69 brain regions into 23 condensed regions based on vascular or anatomic distributions because HIBI due to cardiac arrest is a global injury (figures 1 and 2). For each of these 23 condensed regions, a maximum injury score of up to 1 point was possible for both diffusion restriction and T2/FLAIR injury. The amount of injury in each condensed region was proportional to the injury in the original brain regions. For example, the 12 left middle cerebral artery (MCA) regions were simplified into 1 condensed region, thus each region contributed 1/12 of the injury score, leading to a total of 1 point for the condensed region (e.g., if 6 of the 12 left MCA regions had diffusion restriction, the condensed left MCA region received 0.5 points). The analysis evaluated the association between both the extent of injury in condensed regions (possible score range 0–23, including proportions) and the absolute presence or absence of injury in each of the 23 condensed regions (possible score range 0–23) with outcome.
Figure 2. Representative Regions on the Modified Alberta Stroke Program Early Computed Tomography Score (ASPECTS) Scoring System Depicted on Sample MRI (Left Hemisphere).
The percentage of patients with a favorable (top row right hemisphere) or unfavorable (bottom row right hemisphere) outcome that had at least one brain region with diffusion restriction for each of the 23 condensed regions. All 69 of the modASPECTS regions are detailed in figure 1. A1-A2 = anterior cerebral artery cortical and subcortical vascular territory; C = caudate; CP = cerebral peduncle; D = dentate; G = globus pallidus; H = hippocampus; I = insula; IC = internal capsule; M1-M6 = middle cerebral artery cortical and subcortical vascular territory; P = putamen; P1-P2 = posterior cerebral artery cortical and subcortical vascular territory; Po = pons; T = thalamus.
Pearson, polychoric, and tetrachoric correlations were performed to determine coherence in degree of injury between corresponding right and left hemispheric regions. The correlations between hemispheres were all above >0.9. Therefore, given the expected homogenous injury from HIBI after cardiac arrest, right and left regions were averaged for a secondary analysis of 13 regions (10 supratentorial and 3 brainstem) comparing injury across brain regions (figure 1).
Clinical Outcomes
The primary outcome was unfavorable neurologic outcome at hospital discharge. Neurologic outcome was based on the PCPC score.29,30 The PCPC is a 6-point scale of global neurologic function: (1) normal; (2) mild disability; (3) moderate disability; (4) severe disability; (5) coma and vegetative state; (6) death. Baseline PCPC scores were retrospectively assigned by 2 raters via medical record review based on neurologic function prior to the patient's OHCA. Discrepancies were resolved by consensus. Favorable outcome was defined as no change in PCPC score from baseline to discharge or a change in PCPC score from 1 to 2. Unfavorable outcome was defined as a change in PCPC ≥1 from baseline that resulted in a hospital discharge PCPC score of 3, 4, 5, or 6.30 Patients with an unfavorable outcome were further subclassified as (1) alive with PCPC 3–5 or PCPC change ≥1 from baseline; (2) dead from WLST due to poor neurologic prognosis (WLST-neuro); (3) dead by neurologic criteria (DNC); or (4) dead from withdrawal or limitation of life-sustaining therapies due to rearrest or refractory cardiopulmonary failure (WLST-cardio). Patients could only be counted once per unfavorable outcome subclassification. Secondary outcomes included length of PICU and hospital stay, placement of tracheostomy or gastrostomy tubes, and disposition.
Statistical Analysis
Descriptive statistics are reported as frequencies and percentages for categorical variables and median with interquartile range (IQR) for continuous variables. χ2 and Fisher exact tests were used to test associations between categorical variables and categorical outcomes (unfavorable vs favorable outcome), and Wilcoxon rank sum for continuous variables across the 2 outcome groups.
To examine whether the extent of brain injury on MRI was associated with cardiac arrest characteristics, we reported the median (IQR) extent of brain injury across each of the categorical arrest characteristics groups and tested each association using a Wilcoxon test. We tested the relationship between the extent of injury in each brain region and the occurrence of an unfavorable outcome using Fisher exact tests. We could not examine how a set of regions was associated with the likelihood of an unfavorable outcome in a multivariable setting due to the small sample size and high degree of collinearity between pairs of regions. To test whether the extent of diffusion restriction or T2/FLAIR injury was correlated between pairs of the 13 condensed regions, we ran pairwise Pearson, polychoric, and tetrachoric correlations (depending on the distributions of the regional brain injury variables). To determine how well the extent of brain injury on MRI predicted outcome, we ran 2 univariable logistic regressions using the extent of diffusion restriction (or T2/FLAIR injury) as the predictor and occurrence of an unfavorable outcome as the outcome. From the logistic regression, we obtained the area under the receiver operating characteristic (AUROC) curve and calculated bootstrapped 95% confidence intervals (CIs) from 2,000 replications.
Missing data were minimal and present only in the following 3 variables: admit pH (3%), admit lactate (4%), and CPR time (13%). All statistical tests were 2-sided. A Bonferroni correction was applied to account for multiple comparisons with threshold for determining statistical significance of 0.00028. In tables, p values below 0.00028 were indicated to retain statistical significance after adjusting for multiple comparisons. All analyses were run using SAS software (version 9.4) and R software.
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
Patient Population
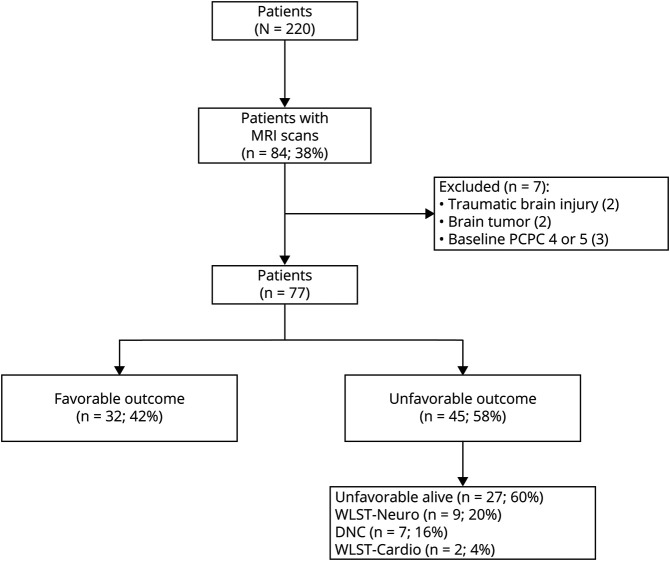
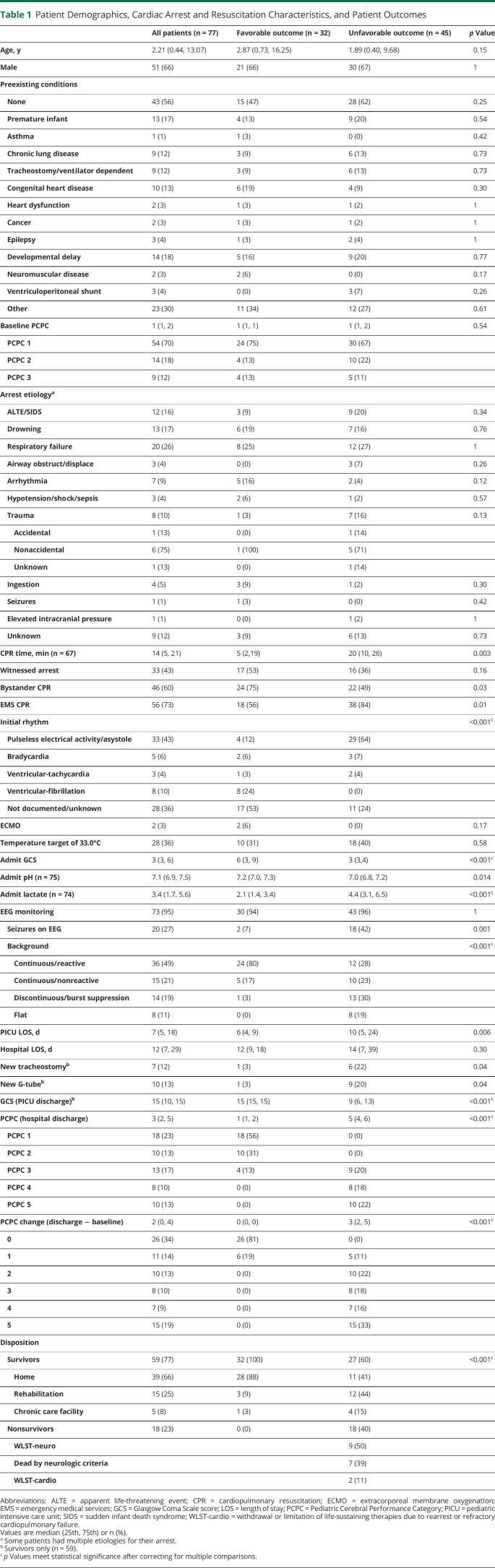
Eighty-four patients were eligible and 7 were excluded (figure 3). Seventy-seven patients were analyzable (median age 2.21 years [IQR 0.44–13.07] and 66% male). Forty-three (56%) children had no preexisting medical conditions and 55 (71%) had a baseline PCPC of 1 (table 1). The most common arrest etiologies were respiratory failure (26%), drowning (17%), apparent life-threatening event, or sudden infant death syndrome (16%). Of the patients with an initial documented rhythm, pulseless electrical activity (PEA) and asystole were the most common (67%), and 36% were undocumented. Twenty-eight (36%) patients were managed with targeted temperature management to 33.0°C, 7 of whom were enrolled in a clinical trial of hypothermia after OHCA.26 Two (3%) patients were treated with extracorporeal membrane oxygenation (ECMO).
Figure 3. CONSORT (Consolidated Standards of Reporting Trials) Diagram.
CONSORT diagram showing patient numbers (%) in each outcome category. DNC = death by neurologic criteria; PCPC = Pediatric Cerebral Performance Category; WLST-cardio = withdrawal of life-sustaining therapies due to rearrest or cardiopulmonary failure; WLST-neuro = withdrawal of life-sustaining therapies due to poor neurologic prognosis.
Table 1.
Patient Demographics, Cardiac Arrest and Resuscitation Characteristics, and Patient Outcomes
Overall, 32 (42%) patients had a favorable outcome and 45 (58%) had an unfavorable outcome. Patients with an unfavorable outcome had longer durations of CPR (20 [10, 26] vs 5 [2.3, 19.5] minutes, p = 0.003), higher initial lactate (4.4 [3.1, 6.5] vs 2.1 [1.4, 3.4], p < 0.001), and lower pH levels (7.0 [6.8, 7.2] vs 7.2 [7.0, 7.3], p = 0.014) than patients with favorable outcomes. A greater percentage of patients with unfavorable outcomes had seizures on EEG (42% vs 7%, p = 0.001). In patients with unfavorable outcomes, the EEG background was more often continuous and nonreactive (23% vs 17%), discontinuous or burst suppressed (30% vs 3%), or flat (19% vs 0%) (p < 0.001). In contrast, the EEG background of patients with a favorable outcome was more often continuous and reactive (80% vs 28%, p < 0.001). A greater percentage of patients with unfavorable outcomes were discharged with a new tracheostomy (22% vs 3%, p = 0.04) or gastrostomy tube (20% vs 3%, p = 0.001). Patients with unfavorable outcomes had longer PICU lengths of stay (10 [5, 24] vs 6 [4, 9] days, p = 0.006). Sixty-six percent of survivors were discharged home.
Patients who survived their cardiac arrest but did not receive an MRI scan were similar to those who had an MRI: median age 3.8 (1, 9) years, 70% male, 58% baseline PCPC = 1, CPR duration 19 (5, 35) minutes, admit pH 7.14 (7.0, 7.3), admission Glasgow Coma Scale score 3 (3, 6.5), 4 treated with ECMO, and 8 patients had targeted temperature management to 33.0°C. Patients who did not have an MRI had higher admit lactate (7.6 [2.4, 10.4]). A greater percentage of patients who did not have an MRI scan died (60% vs 23%). Patients who did not receive an MRI had a median PICU length of stay 2 (1, 4) days and a hospital length of stay 3 (1, 5) days.
Brain MRI Scans
Forty-eight (62%) brain MRI scans were performed on a 1.5T and 29 (38%) on a 3T magnet. MRI scans were performed at a median of 4 (2, 6) days after cardiac arrest. There was no difference in time to MRI between patients with favorable and unfavorable outcomes (4 [2, 6] vs 3 [2, 6] days, p = 0.57).
Extent of Injury on Brain MRI Scans (23 Condensed Regions)
Patients with unfavorable outcomes had more extensive diffusion restriction (median 7 [4, 10.3] vs 0 [0, 0] regions, p < 0.001) and T2/FLAIR injury (5.5 [2.3, 8.2] vs 0 [0, 0.75] regions, p < 0.001) (figure 4A) and a greater number of condensed brain regions with diffusion restriction (median 6 [4, 8] vs 0 [0, 0], p < 0.001) and T2/FLAIR injury (5 [3, 7] vs 0 [0, 1], p < 0.001) than patients with favorable outcomes. A greater percentage of patients with an unfavorable outcome had at least one brain region with diffusion restriction (98% vs 13%, p < 0.001) or T2/FLAIR injury (96% vs 31%, p < 0.001, figure 2) compared to patients with a favorable outcome. AUROC for the extent of diffusion restriction and unfavorable outcome was 0.96 (95% CI 0.91, 0.99) and 0.92 (95% CI 0.85, 0.97) for T2/FLAIR injury (figure 5). In addition, patients who survived with unfavorable outcomes (i.e., PCPC 3–5) had a greater extent of diffusion restriction (4.8 [1.3, 8.3] vs 0 [0, 0] regions, p < 0.001) and T2/FLAIR injury (3.3 [1, 6] vs 0 [0, 0.75] regions, p < 0.001) compared to survivors with favorable outcomes.
Figure 4. Diffusion Restriction and T2/Fluid-Attenuated Inversion Recovery (FLAIR) MRI Injury for Patients With Favorable and Unfavorable Outcomes.
Extent of diffusion restriction and T2/FLAIR MRI injury for patients with favorable and unfavorable outcomes (A) and for patients in the subcategories of unfavorable outcomes (B). Boxplots with whiskers demonstrating 5 and 95th percentiles. DNC = death by neurologic criteria; PCPC = Pediatric Cerebral Performance Category; WLST-neuro = withdrawal of life-sustaining therapies due to poor neurologic prognosis.
Figure 5. Receiver Operating Characteristic (ROC) Curves for Diffusion Restriction and T2/Fluid-Attenuated Inversion Recovery (FLAIR) Injury.
ROC curves (for diffusion restriction (A) and T2/FLAIR injury (B) to predict unfavorable outcome.
Among the 45 patients with an unfavorable outcome, there was no difference in the extent of diffusion restriction between patients who died from WLST-neuro compared to those who were alive with an unfavorable outcome (p = 0.11) and compared to those declared DNC (p = 0.072) (figure 4B). Patients declared DNC had more extensive diffusion restriction than patients who lived with an unfavorable outcome (p = 0.002). There was no difference in T2/FLAIR injury between patients who died from WLST-neuro and those declared DNC (p = 0.20). However, patients who were alive with an unfavorable outcome had less T2/FLAIR injury than patients who died from WLST-neuro (p = 0.004) or DNC (p = 0.001).
One patient with a favorable outcome (PCPC = 1) had diffusion restriction in 8.75 condensed regions, similar to patients with unfavorable outcomes. This patient was a 2-month-old infant with suspected underlying metabolic disease and refractory status epilepticus, both of which may have contributed to the large areas of diffusion restriction. The patient had a relatively small extent of T2/FLAIR injury, which was consistent with other patients with favorable outcomes.
Brain Regions Associated With Unfavorable Outcomes (13 Condensed Regions)
Patients with unfavorable outcomes had more extensive diffusion restriction compared to patients with favorable outcomes in the bilateral anterior cerebral artery (ACA), MCA, and posterior cerebral artery (PCA) vascular territories (p < 0.001), the bilateral basal ganglia, thalami, hippocampi, and brainstem (p < 0.001), and the bilateral internal capsule, insula, and cerebellum (p < 0.01). There was no difference in diffusion restriction in the cerebral peduncles or Wallerian degeneration in the corpus callosum and descending corticospinal tracts. Patients with unfavorable outcomes had more extensive T2/FLAIR injury in the bilateral ACA, MCA, and PCA vascular territories (p < 0.01) and in the bilateral basal ganglia, thalami, and brainstem (p < 0.001). The extent of T2/FLAIR injury was not greater in the internal capsules, bilateral insula, hippocampi, cerebellum, cerebral peduncles, or Wallerian degeneration in the corpus callosum and descending corticospinal tracts.
Correlations Between Extent of Injury Between Brain Regions
The extent of brain injury was highly correlated between brain regions, and correlations were similar between diffusion restriction and T2/FLAIR injury. Correlation coefficients between the ACA, MCA, and PCA regions were 0.8–0.9 (all ps < 0.001). Correlations were 0.7 between basal ganglia and thalami, 0.4–0.6 between basal ganglia/thalami and ACA/MCA/PCA regions, and 0.6–0.7 between basal ganglia/thalami and the brainstem (all ps < 0.001). In addition, correlations were 0.6–0.8 between ACA/MCA/PCA regions and Wallerian degeneration in the corpus callosum and descending cortical spinal tracts (all ps < 0.001).
Association Between Injury on Brain MRI and Clinical Cardiac Arrest Characteristics
Patients had more extensive diffusion restriction and T2/FLAIR brain injury on MRI when CPR duration was >10 minutes or they received ≥2 doses of epinephrine (table 2). More extensive MRI injury was also associated with an initial cardiac rhythm of PEA or asystole, postcardiac arrest seizures, or a postcardiac arrest EEG background that was discontinuous, burst suppressed, or flat.
Table 2.
Cardiac Arrest and Resuscitation Characteristics and Extent of MRI Injury
Discussion
These data demonstrate that the extent of diffusion restriction and T2/FLAIR injury using rigorously defined MRI regions are highly associated with unfavorable neurologic outcomes following pediatric OHCA, with outstanding AUROCs of 0.96 (95% CI, 0.91, 0.99) and 0.92 (95% CI, 0.85, 0.97). Notably, there was no difference in the extent of diffusion restriction between patients who survived with an unfavorable outcome and those who died due to WLST-neuro. In addition, patients declared DNC had a similar extent of diffusion restriction and T2/FLAIR injury compared to patients who died from WLST-neuro.
Our findings demonstrate a strong association between burden of MRI injury and unfavorable neurologic outcomes following pediatric OHCA. These data support the use of MRI to provide clinically relevant prognostic information for families and health care providers. In addition, more extensive MRI injury was associated with well-established factors related to worse neurologic outcomes, including longer duration CPR, more epinephrine doses, initial rhythms of PEA and asystole, postarrest seizures, and discontinuous or flat EEG background. Several smaller studies have similarly shown associations of MRI abnormalities with unfavorable outcomes following pediatric cardiac arrest, but the AUROC in this study revealed a much stronger relationship.22–25 In contrast to previous studies, our definitions of MRI regions were much more rigorous and granular. Other studies also included both in-hospital cardiac arrests (IHCAs) and OHCAs, which added another level of potential confounding since many more children with IHCAs have preexisting brain injury and developmental disabilities.22,23,25
In contrast to previous postcardiac arrest MRI studies in children, we analyzed the extent of diffusion restriction and T2/FLAIR injury using the MRI modification of ASPECTS scoring system.22,27,28 We assigned condensed regions a score proportional to the number of modASPECTS regions with diffusion restriction or T2/FLAIR injury. This method, however, gave more weight to regions that did not require condensing (e.g., thalamus, hippocampus). We demonstrated a minimal effect of this potential biasing by achieving similar results when each condensed region was treated with equal weight. We also demonstrated strong correlations across hemispheres and between brain regions, which is consistent with the relatively homogenous and global nature of HIBI induced by cardiac arrest.
We scored and analyzed both diffusion and T2/FLAIR images since there was variability in the time from cardiac arrest to MRI and diffusion injury may have resolved for patients who underwent MRI longer after cardiac arrest. Diffusion imaging is sensitive to the cytotoxic edema that occurs after cerebral ischemia due to intracellular accumulation of water and neuronal swelling as a result of dysfunction of the ATP-dependent sodium/potassium membrane pumps. Restricted diffusion can be appreciated within 30 minutes of arterial ischemic stroke and may persist up to 7–14 days, although the timing for initial appearance of restricted diffusion is less well established and more variable for HIBI.31,32 For this reason, we excluded patients who had their MRI performed >14 days after their cardiac arrest. Abnormal T2/FLAIR signal after HIBI can be due to loss of integrity of the blood–brain barrier and associated vasogenic edema, as well as cerebral inflammation. These changes are typically appreciated hours to days after the cardiac arrest. T2/FLAIR abnormalities may also represent chronic brain injury and gliotic changes. Thus, evaluating both sequences allowed for the potential identification of a wider temporal range of injury compared to what diffusion or T2/FLAIR would have been able to capture individually. The differences we appreciated between the imaging sequences may reflect the timing between the cardiac arrest and MRI scan, severity of secondary injury, the temporal course of the inflammatory response, or the relative amount of cytotoxic vs vasogenic edema.
It may be feasible to incorporate the modASPECTS scoring system into standardized clinical reports for MRI scans performed after pediatric cardiac arrest. The scoring time varies according to the imaging performed (e.g., slice thickness, quality, conspicuity of abnormalities). It initially took our pediatric neuroradiologist approximately 30 minutes to score the 69 regions in both diffusion and T2/FLAIR images, but was quicker over time. Scoring 23 regions instead of 69 would only require 1/3 of the time and should not affect the interpretation of the results. This may facilitate clinical implementation of such a scoring system in the future.
Due to the small number of patients who had any diffusion restriction and a favorable outcome, it was not possible to determine whether isolated injury to certain regions was associated with unfavorable prognosis. Unfavorable outcome could result from either extensive injury to cortical and subcortical regions or targeted injury to select regions involved in fundamental neurologic processes like cognition or consciousness. The functional impact of a small focal injury to a critical brain region such as the thalamus, hippocampus, or brainstem would likely have more functional impact than a comparable volume of injury to a noneloquent region of cortex or subcortical white matter. Further research will be needed to distinguish the impact of the anatomical distribution from the extent of diffusion restriction on clinical outcomes after pediatric cardiac arrest.
Retrospective studies evaluating the prognostic accuracy of MRI are often compounded by the self-fulfilling prophecy—the results used to determine treatment decisions, such as withdrawal of life-sustaining therapies, prove the hypothesis that studies are evaluating.33 Therefore, we compared the extent of diffusion restriction between patients who survived with unfavorable outcomes and those who died from WLST-neuro, and found no difference. This suggests that if life-sustaining technology was not withdrawn, these patients would probably have survived with substantial neurologic disability. Depending on the timing of MRI postcardiac arrest, it may be feasible to combine diffusion and T2/FLAIR imaging to improve prognostic accuracy, as patients who died from WLST-neuro had greater T2/FLAIR injury than those who survived with unfavorable outcomes. It is possible that physicians preferentially recommended WLST for patients with a greater extent of brain injury on MRI. Despite this possibility, we still demonstrated a greater extent of MRI brain injury in patients who survived with unfavorable outcomes compared to those with favorable outcomes.
We found no difference in the extent of MRI injury between patients who died from WLST-neuro and those declared DNC. Patients who died from WLST-neuro had variable degrees of brain injury on MRI. Some patients may have met clinical DNC criteria but had WLST prior to formal DNC testing. Families often choose WLST based on poor neurologic prognosis as communicated by the primary PICU or consulting neurology teams. However, this decision may have been influenced by other factors including the patient's chronic medical conditions and baseline neurologic capabilities, degree of other organ system injury, and the family's perception of the child's potential quality of life.
There are several limitations to this study. Due to sample size, we were unable to perform multivariable analyses to control for clinical cardiac arrest characteristics and EEG background or seizure burden when evaluating the association between the burden of MRI injury and outcomes. More severely abnormal EEG background is associated with unfavorable outcomes after cardiac arrest,34 but there was one patient in our cohort with a favorable outcome who had a discontinuous/burst suppression EEG pattern, yet had normal diffusion and T2/FLAIR MRI scans. This suggests that MRI injury patterns may be able to identify patients who are more likely to have a favorable outcome despite other evidence suggesting otherwise.
This study only included patients with OHCA and may not be generalizable to patients with IHCA who may have less severe HIBI and different MRI injury patterns since survival with favorable neurologic outcome is more common following IHCA.35 The timing of obtaining MRI scans was not standardized and may have impacted the extent of diffusion restriction and T2/FLAIR injury reported. Diffusion restriction can occur for reasons other than HIBI including status epilepticus or active metabolic disease. We only included patients who had clinically obtained MRIs, thus patients with a reassuring neurologic examination after resuscitation, as well as patients with devastating neurologic injury, may have died without receiving an MRI. In addition, we were unable to evaluate the association of modASPECTS scores with routine clinical reports available to bedside clinicians due to variability in the reporting of details related to hypoxic-ischemic injury severity and location. Lastly, favorable categorization may be overestimated due to limitations of the PCPC in infants. However, the International Liaison Committee on Resuscitation and the Utstein criteria recommend PCPC at discharge for determination of neurologic outcome following pediatric cardiac arrest.30,36 To standardize the application of PCPC to infants and young children, we used a correlation table between age-specific Denver II developmental milestones and PCPC used in the Virtual Pediatric Systems database.
Children with unfavorable outcomes at hospital discharge after OHCA had more extensive diffusion restriction and T2/FLAIR injury compared to patients with favorable outcomes. There was no difference in amount of diffusion restriction between patients who survived with unfavorable outcome compared to those who died from WLST due to poor neurologic prognosis. Further research in larger cohorts is needed to elucidate the extent and location of MRI injury that may distinguish between certain neurocognitive or neurobehavioral phenotypes and the degree to which MRI improves the accuracy of neuroprognostication after pediatric cardiac arrest.
Glossary
- ACA
anterior cerebral artery
- AUROC
area under the receiver operating characteristic
- CI
confidence interval
- CPR
cardiopulmonary resuscitation
- DNC
dead by neurologic criteria
- ECMO
extracorporeal membrane oxygenation
- FLAIR
fluid-attenuated inversion recovery
- HIBI
hypoxic-ischemic brain injury
- IHCA
in-hospital cardiac arrest
- IQR
interquartile range
- MCA
middle cerebral artery
- modASPECTS
pediatric MRI modification of the Alberta Stroke Program Early Computed Tomography Score
- OHCA
out-of-hospital cardiac arrest
- PCA
posterior cerebral artery
- PCPC
Pediatric Cerebral Performance Category
- PEA
pulseless electrical activity
- PICU
pediatric intensive care unit
- WLST
withdrawal of life-sustaining therapy
- WLST-neuro
withdrawal of life-sustaining therapy due to poor neurologic prognosis
Appendix. Authors


Study Funding
Department of Anesthesiology and Critical Care, Children's Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics: 2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Fink EL, Prince DK, Kaltman JR, et al. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation 2016;107:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitta M, Iwami T, Kitamura T, et al. Age-specific differences in outcomes after out-of-hospital cardiac arrests. Pediatrics 2011;128:e812–e820. [DOI] [PubMed] [Google Scholar]
- 4.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation 2009;119:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Pont-Thibodeau G, Fry M, Kirschen M, et al. Timing and modes of death after pediatric out-of-hospital cardiac arrest resuscitation. Resuscitation 2018;133:160–166. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein FS, Slomine BS, Christensen J, et al. Functional outcome trajectories after out-of-hospital pediatric cardiac arrest. Crit Care Med 2016;44:e1165–e1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slomine BS, Silverstein FS, Christensen JR, et al. Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics 2016;137:e20153412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meert KL, Telford R, Holubkov R, et al. Pediatric out-of-hospital cardiac arrest characteristics and their association with survival and neurobehavioral outcome. Pediatr Crit Care Med 2016;17:e543–e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meert KL, Slomine BS, Christensen JR, et al. Family burden after out-of-hospital cardiac arrest in children. Pediatr Crit Care Med 2016;17:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slomine BS, Silverstein FS, Page K, et al. Relationships between three and twelve month outcomes in children enrolled in the therapeutic hypothermia after pediatric cardiac arrest trials. Resuscitation 2019;139:329–336. [DOI] [PubMed] [Google Scholar]
- 11.Slomine BS, Silverstein FS, Christensen JR, et al. Neuropsychological outcomes of children 1 year after pediatric cardiac arrest: secondary analysis of 2 randomized clinical trials. JAMA Neurol 2018;75:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichord R, Silverstein FS, Slomine BS, et al. Neurologic outcomes in pediatric cardiac arrest survivors enrolled in the THAPCA trials. Neurology 2018;91:e123–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zellem L, Utens EM, Madderom M, et al. Cardiac arrest in infants, children, and adolescents: long-term emotional and behavioral functioning. Eur J Pediatr 2016;175:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zellem L, Utens EM, Legerstee JS, et al. Cardiac arrest in children: long-term health status and health-related quality of life. Pediatr Crit Care Med 2015;16:693–702. [DOI] [PubMed] [Google Scholar]
- 15.Michiels E, Quan L, Dumas F, Rea T. Long-term neurologic outcomes following paediatric out-of-hospital cardiac arrest. Resuscitation 2016;102:122–126. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Herce J, Garcia C, Dominguez P, et al. Outcome of out-of-hospital cardiorespiratory arrest in children. Pediatr Emerg Care 2005;21:807–815. [DOI] [PubMed] [Google Scholar]
- 17.Geocadin RG, Callaway CW, Fink EL, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation 2019;140:e517–e542. [DOI] [PubMed] [Google Scholar]
- 18.de Caen AR, Berg MD, Chameides L, et al. Part 12: pediatric advanced life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S526–S542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric post-cardiac arrest care: a scientific statement from the American Heart Association. Circulation 2019;140:e194–e233. [DOI] [PubMed] [Google Scholar]
- 20.Kirschen MP, Topjian AA, Hammond R, Illes J, Abend NS. Neuroprognostication after pediatric cardiac arrest. Pediatr Neurol 2014;51:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez Soto C, Dragoi L, Heyn CC, et al. Imaging for neuroprognostication after cardiac arrest: systematic review and meta-analysis. Neurocrit Care 2019;32:206–216. [DOI] [PubMed] [Google Scholar]
- 22.Fink EL, Panigrahy A, Clark RS, et al. Regional brain injury on conventional and diffusion weighted MRI is associated with outcome after pediatric cardiac arrest. Neurocrit Care 2013;19:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchester LC, Lee V, Schmithorst V, Kochanek PM, Panigrahy A, Fink EL. Global and regional derangements of cerebral blood flow and diffusion magnetic resonance imaging after pediatric cardiac arrest. J Pediatr 2016;169:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oualha M, Gatterre P, Boddaert N, et al. Early diffusion-weighted magnetic resonance imaging in children after cardiac arrest may provide valuable prognostic information on clinical outcome. Intens Care Med 2013;39:1306–1312. [DOI] [PubMed] [Google Scholar]
- 25.Yacoub M, Birchansky B, Mlynash M, et al. The prognostic value of quantitative diffusion-weighted MRI after pediatric cardiopulmonary arrest. Resuscitation 2019;135:103–109. [DOI] [PubMed] [Google Scholar]
- 26.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med 2015;372:1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wusthoff CJ, Kessler SK, Vossough A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics 2011;127:e1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beslow LA, Vossough A, Dahmoush HM, et al. Modified pediatric ASPECTS correlates with infarct volume in childhood arterial ischemic stroke. Front Neurol 2012;3:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68–74. [DOI] [PubMed] [Google Scholar]
- 30.Nolan JP, Berg RA, Andersen LW, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry template for in-hospital cardiac arrest: a consensus report from a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Circulation 2019;140:e746–e757. [DOI] [PubMed] [Google Scholar]
- 31.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217:331–345. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues K, Grant PE. Diffusion-weighted imaging in neonates. Neuroimaging Clin N Am 2011;21:127–151, viii. [DOI] [PubMed] [Google Scholar]
- 33.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation 2014;85:1779–1789. [DOI] [PubMed] [Google Scholar]
- 34.Topjian AA, Sanchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early electroencephalographic background features predict outcomes in children resuscitated from cardiac arrest. Pediatr Crit Care Med 2016;17:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med 2016;44:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a task force of the international Liaison Committee on resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation 2015;132:1286–1300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.