Abstract
Objective
To test the hypothesis that plasma total tau (t-tau) and neurofilament light chain (NfL) concentrations may have a differential role in the study of frontotemporal lobar degeneration syndromes (FTLD-S) and clinically diagnosed Alzheimer disease syndromes (AD-S), we determined their diagnostic and prognostic value in FTLD-S and AD-S and their sensitivity to pathologic diagnoses.
Methods
We measured plasma t-tau and NfL with the Simoa platform in 265 participants: 167 FTLD-S, 43 AD-S, and 55 healthy controls (HC), including 82 pathology-proven cases (50 FTLD-tau, 18 FTLD-TDP, 2 FTLD-FUS, and 12 AD) and 98 participants with amyloid PET. We compared cross-sectional and longitudinal biomarker concentrations between groups, their correlation with clinical measures of disease severity, progression, and survival, and cortical thickness.
Results
Plasma NfL, but not plasma t-tau, discriminated FTLD-S from HC and AD-S from HC. Both plasma NfL and t-tau were poor discriminators between FLTD-S and AD-S. In pathology-confirmed cases, plasma NfL was higher in FTLD than AD and in FTLD-TDP compared to FTLD-tau, after accounting for age and disease severity. Plasma NfL, but not plasma t-tau, predicted clinical decline and survival and correlated with regional cortical thickness in both FTLD-S and AD-S. The combination of plasma NfL with plasma t-tau did not outperform plasma NfL alone.
Conclusion
Plasma NfL is superior to plasma t-tau for the diagnosis and prediction of clinical progression of FTLD-S and AD-S.
Classification of Evidence
This study provides Class III evidence that plasma NfL has superior diagnostic and prognostic performance vs plasma t-tau in FTLD and AD.
Plasma biomarkers are powerful diagnostic and prognostic clinical tools.1 The microtubule-associated protein tau has been implicated in the pathophysiology of Alzheimer disease (AD) and frontotemporal lobar degeneration (FTLD) and can be measured in plasma with ultrasensitive immunoassays.2 Total tau (t-tau) is increased in pathology-confirmed AD and is considered a neurodegeneration biomarker in current research AD frameworks.3 The cytoskeletal protein neurofilament light (NfL) can also be measured in plasma with ultrasensitive technology. Plasma NfL increases upon neuronal injury and correlates with clinical progression and survival in FTLD syndromes (FTLD-S) and AD syndromes (AD-S).4–8
The combination of CSF t-tau and NfL discriminated between early onset AD and FTLD,9 and both analytes may be useful for the diagnosis and prognosis of FTLD.1,10–12 Whereas blood t-tau shows no diagnostic potential for AD,13 NfL in blood shows a clear increase in AD,4 and also tracks neurofibrillary tangle load and cognitive decline.14,15 Studies of plasma NfL and t-tau in pathology-confirmed FTLD and AD are scarce, and it is unknown if their concentrations are affected by comorbid AD presenting in the context of primary FTLD pathology. Here, we aimed to compare (1) the diagnostic and prognostic value of plasma t-tau and NfL in FTLD-S and AD-S; (2) their utility for the differentiation of FTLD subtypes with or without comorbid AD; and (3) their ability to track clinical and imaging measures of neurodegeneration. We hypothesize that both biomarkers would have different diagnostic sensitivities and correlations with clinical variables in FTLD and AD.
Methods
Study Participants
Participants were recruited at the University of California, San Francisco (UCSF) Memory and Aging Center from November 2011 to January 2015. A total of 267 research participants provided written informed consent and underwent neurologic, neuropsychological, and functional assessment with informant interview, and blood sampling. A subgroup of participants also underwent structural brain MRI (n = 240) and CSF sample collection (n = 181). Participants were diagnosed at a multidisciplinary consensus conference and met criteria for behavioral variant frontotemporal dementia (bvFTD),16 nonfluent/agrammatic variant of primary progressive aphasia (nfvPPA),17 semantic variant of primary progressive aphasia (svPPA),17 progressive supranuclear palsy (PSP),18 corticobasal syndrome (CBS),19 amyotrophic lateral sclerosis with frontotemporal dementia (ALS-FTD),20 or AD-S (AD type dementia and atypical variants of AD).21 Clinical diagnosis was made by clinicians blinded to fluid biomarker results. For this study, bvFTD, nfvPPA, svPPA, PSP, and CBS were grouped as FTLD-S. Participants in the healthy control group (HC) were functionally intact older adults enrolled through the Hillblom Aging Network. Plasma NfL data for 26 HC, 6 patients with AD-S, and 85 patients with FLTD-S were used elsewhere.22
Neurocognitive and Disease Staging Measures
Participants underwent a comprehensive neuropsychological battery at the time of plasma sampling. Four major cognitive domains were covered as previously described23: memory (delayed recall of the California Verbal Learning Test–short form and Benson Figure Test), executive functioning (Digit Span backward, Trail-Making Test part B, Stroop Color-Word card subtask, and Letter Fluency), language (Category Fluency and Boston Naming Test), and visuospatial functioning (Number Location from the Visual Object Space and Perception battery and Benson Figure copy test). To obtain composite scores for each cognitive domain, we used the means and SDs of the control group to convert raw cognitive scores into Z scores. Subsequently, the patient's Z scores were averaged within each cognitive domain. The Mini-Mental State Examination (MMSE)24 was used as a general measure of global cognition, and the Clinical Dementia Rating plus National Alzheimer's Coordinating Center FTLD sum of boxes (CDR+NACC/FTLD-SB) score was used as a measure of disease severity.25
Plasma and CSF Biomarkers
Plasma and CSF collections were performed according to the Alzheimer's Disease Neuroimaging Initiative protocol.5 Plasma NfL and plasma t-tau concentrations were determined with commercially available ultrasensitive Single molecule array technology using an HD-1 analyzer (Quanterix, Billerica, MA), by board-certified laboratory technicians blinded to clinical data, as previously described.5 CSF concentrations of both t-tau and phosphorylated tau (p-tau181) were measured with the INNO-BIA AlzBio3 platform (Fujirebio, Gent, Belgium). CSF NfL concentrations were measured using the UmanDiagnostics (Umeå, Sweden) ELISA kit (NF-Light kit), as previously described.26
Amyloid PET
Ninety-eight participants had available brain amyloid PET data (n = 71 with [11C]Pittsburgh compound B, n = 27 with [18F]Florbetapir) within 6 months of plasma sampling. PET scans were read as positive or negative, as previously described and validated against neuropathology.27
MRI Acquisition and Preprocessing
A total of 240 participants (160 FTLD-S, 29 AD-S, and 51 HC) underwent MRI at the time of plasma sampling (mean time from plasma sampling to scan 1 month, with a maximum time between plasma sampling to MRI of 6 months). MRIs were acquired on a 3T Siemens Tim Trio system equipped with a 12-channel head coil. Fifteen MRIs were excluded from final neuroimaging analyses: 8 because of low image quality (i.e., significant movement artifact) or preprocessing errors and 7 because they were performed in a different MRI scanner. The remaining 225 MRIs (160 FTLD-S, 29 AD-S, and 51 HC) were processed with the CAT12 toolbox (version 1450)28 within SPM12 (version 7487, running in MATLAB r2019b)29 to gather cortical thickness estimates, as previously described.28 Briefly, the CAT12 toolbox uses tissue segmentation to estimate the white matter distance, and it then projects the local maxima (which is equal to cortical thickness) to other gray matter voxels by using a neighbor relationship described by the white matter distance. Previous studies have shown that projection-based thickness allows the handling of partial volume information, sulcal blurring, and sulcal asymmetries without explicit sulcus reconstruction.28 Topologic correction, spherical mapping, and spherical registration were performed to obtain vertex-wise cortical thickness. Finally, surface maps were smoothed using a 15 mm full width at half maximum for group comparisons and correlations with plasma biomarkers.
Genetic Analysis
Genetic screening was conducted for mutations known to cause autosomal dominant FTLD or AD (MAPT, C9orf72, GRN, TARDBP, FUS, PSEN1, PSEN2, and APP) at the Coppola Lab at the University of California, Los Angeles.30
Neuropathologic Assessment
Neuropathologic assessments performed at UCSF followed previously described procedures.30 Participants were classified into FTLD major molecular classes (tau, TDP-43, or FUS) and subtypes31 or AD.32 AD pathology was classified according to the National Institute on Aging–Alzheimer's Association guidelines for the likelihood of AD pathology as low, intermediate, or high.33 For secondary analyses, we considered that participants with either a positive amyloid PET or at least comorbid AD (as defined by at least intermediate likelihood of AD pathology) had an increased certainty of underlying AD, either as a primary or contributing pathology, in both FTLD-S and AD-S groups.
Statistical Analysis
Data were explored for normality using the Shapiro-Wilk test. Fluid biomarker concentrations were log-transformed using the natural log to fulfill the normal distribution assumption. Between-group differences were determined with analysis of variance or t test for continuous variables (with Bonferroni correction for multiple comparisons) and the χ2 for dichotomous or categorical data.
We calculated correlations between plasma biomarkers, age at plasma sampling, and CDR+NACC/FTLD-SB with Pearson correlation coefficient in all clinical groups. Then, we studied the correlation between neuropsychology testing variables and plasma biomarkers with partial correlations adjusting for age and education, to account for the well-known effect of these variables on cognitive performance. We also explored the correlation between plasma and CSF concentrations of NfL and t-tau with Pearson correlation coefficient. In addition to main clinical group comparisons (namely, FTLD-S, AD-S, and HC), we also performed secondary analyses comparing plasma biomarkers between FTLD subtypes in cases with pathologic confirmation. To explore the relationship between AD pathophysiology and plasma biomarkers, we compared participants with and without increased certainty of underlying AD (as defined in the neuropathologic assessment section). We assessed the clinical utility of plasma biomarkers by calculating the areas under the receiver operator characteristic curve (AUC) for the differentiation between FTLD-S, AD-S, and HC. To study longitudinal changes in plasma biomarkers, we used linear mixed-effects models controlling for age, sex, CDR+NACC/FTLD-SB, and time between samples in the subset of participants with 2 plasma samples (n = 123, mean time between samples = 1.2 ± 0.4 years). We also used linear mixed-effects analyses controlling for age, sex, and baseline disease severity (as measured by the CDR+NACC/FTLD-SB) to predict longitudinal change as measured by the CDR+NACC/FTLD-SB score at year 1 and year 2 after baseline. To account for phenotypic heterogeneity, we designed additional models for each sub phenotype in both FTLD-S (bvFTD, SD, PSP, CBS, and ALS-FTD) and AD-S groups (amnestic and nonamnestic presentation). We used a compound symmetry covariance matrix in all linear-mixed models, and we included random intercepts to account for the effect of baseline values. A term for biomarker by time interaction was used to study the association between the baseline biomarker level and the outcome slope (e.g., CDR+NACC/FTLD-SB) over time.
Between-group cortical thickness comparisons and correlations between cortical thickness and plasma biomarkers were performed in SPM12. For between-group comparison of cortical thickness, age and sex were introduced as covariates in 2 multiple regression models comparing cortical thickness between FTLD-S and healthy controls and AD-S and healthy controls. In these analyses, a significant statistical threshold of p < 0.05, false discovery rate–corrected, was considered using an extent threshold of the expected vertices per cluster. Correlation of regional cortical thickness maps with plasma t-tau and NfL concentrations was performed in FTLD-S and AD-S groups using multiple regressions with individual plasma biomarker levels as the variable of interest, and age and sex as covariates. For correlation analyses, t-maps were transformed to correlation coefficient maps with CAT12 and a threshold of uncorrected p < 0.001 was set to detect moderate correlation coefficients.
Survival was calculated from the date of blood draw until death. Patients alive at analysis were censored at that date. For survival analyses, we first evaluated the association of age at diagnosis, sex, and disease severity at symptom onset with survival in FTLD-S and AD-S. In FTLD-S, we controlled for the clinical phenotype at plasma sampling. We applied Cox regression analyses to estimate survival, controlling for age at diagnosis, sex, disease severity at symptom onset, and primary clinical phenotype (only in the FTLD-S group). We next introduced plasma biomarkers in the Cox regression models to test if plasma biomarkers were independent predictors of survival. Of note, we checked that the assumption of proportionality of hazards was fulfilled.
Statistical significance for all tests was set at 5% (α = 0.05), and all statistical tests were 2-sided. All analyses were performed using SPSS 25 (IBM Corp., Armonk, NY).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the UCSF Institutional Review Board and was conducted following the Declaration of Helsinki. All participants gave their written informed consent to participate in the study.
Data Availability
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
Results
Sample Composition and Demographics
From an initial sample of 304 participants with available plasma t-tau measurements, we excluded 29 participants with preclinical FTLD (asymptomatic mutation carriers), 9 participants with a clinical or pathologic diagnosis of Lewy body disease, and 1 participant with primary psychiatric disease. The final sample included 265 participants: 167 FTLD-S (43 bvFTD, 28 nfvPPA, 18 svPPA, 36 PSP, 32 CBS, and 10 ALS-FTD), 43 AD-S, and 55 HC. Age at plasma sampling, education, MMSE, and CDR+NACC/FTLD-SB was similar in FTLD-S and AD-S. The HC group, however, was younger than both disease groups (table 1). Supplementary information on sample characteristics is shown in supplementary table e-1 and supplementary table e-2 (10.5061/dryad.12jm63xvv).
Table 1.
Sample Characteristics
Relationship Between Plasma Biomarkers, Age, and Clinical Measures
There were no correlations between plasma t-tau and NfL in the total sample (r = −0.035, p = 0.58) or within any clinical group (r = −0.02, p = 0.70; r = 0.02, p = 0.88; and r = −0.05, p = 0.71 for FTLD-S, AD-S, and HC, respectively). Age at plasma sampling did not correlate with plasma t-tau concentrations in any clinical group (r = 0.13, p = 0.08; r = 0.11, p = 0.44; and r = −0.05, p = 0.67 for FTLD-S, AD-S, and HC, respectively). In contrast, age and plasma NfL were moderately correlated in AD-S and HC (r = 0.51 and r = 0.63, respectively, all p < 0.001), but not in FTLD-S (r = 0.02, p = 0.79). In the whole sample, plasma NfL correlated with MMSE (r = −0.26, p < 0.001), the language and executive cognitive composites (r = −0.22 and r = −0.22, respectively, all p < 0.001), and CDR+NACC/FTLD-SB (r = 0.41, p < 0.001) scores. When we restricted the analyses to each clinical group, however, only CDR+NACC/FTLD-SB correlated with plasma NfL in AD-S (r = 0.52, p = 0.003).
Relationship Between Plasma and CSF Biomarkers
Plasma and CSF t-tau did not correlate with each other in the general sample or in any clinical group (general sample r = 0.01, p = 0.19; FTLD-S r = 0.09, p = 0.34; AD-S r = −0.17, p = 0.41; and HC r = −0.14, p = 0.36). Conversely, plasma NfL and CSF NfL concentrations were strongly correlated in all clinical groups (r = 0.82, r = 0.63, r = 0.64, and r = 0.66, in the general sample, FTLD-S, AD-S, and HC, respectively, all p < 0.001).
Differences in Plasma t-Tau and NfL Concentrations by Clinical Group
There were differences in plasma NfL, but not t-tau, concentrations among FTLD-S, AD-S, and controls (figure 1, A and B). Plasma NfL concentrations in FTLD-S (50.2 ± 31 pg/mL) were higher than in AD-S and HC (28.5 ± 11 pg/mL and 12.1 ± 4 pg/mL, p < 0.001 and p < 0.001, respectively). Plasma NfL concentrations were also higher in AD-S than HC (p < 0.001). As shown in figure 1, C and D, within the FTLD-S group, the ALS-FTD subgroup showed low plasma t-tau (1.6 ± 0.9 pg/mL) and high plasma NfL concentrations (99.1 ± 46 pg/mL), compared to other clinical phenotypes. All the FTLD-S subgroups had higher plasma NfL concentrations than AD-S. Of note, participants in the FTLD-S group without a mutation had similar plasma NfL concentrations (46.1 ± 23 pg/mL) compared to GRN (88.1 ± 60 pg/mL, p = 0.43) and MAPT (30.1 ± 17 pg/mL, p = 0.89) mutation carriers, but lower than those with a C9orf72 repeat expansion (88.1 ± 57, p = 0.049). Plasma t-tau concentrations, however, did not differ between mutation carriers and sporadic FTLD-S. Of note, we observed almost identical differences in plasma biomarkers between clinical groups after excluding ALS-FTD participants and participants with FTLD-related mutations (supplementary table e-3, 10.5061/dryad.12jm63xvv).
Figure 1. Group Differences in Plasma Total Tau (t-Tau) and Neurofilament Light (NfL) Concentrations.
Group differences in the plasma levels of t-tau (A) and NfL (B) between the main clinical groups. Group differences in the plasma levels of t-tau (C) and NfL (D) between frontotemporal lobar degeneration syndromes (FTLD-S) subgroups, the Alzheimer disease syndromes (AD-S) group, and healthy controls. Differences in the plasma levels of t-tau (E) and NfL (F) between major neuropathologic subtypes. In (E, F), participants with C9orf72 (n = 11) or GRN (n = 7) mutations were included in the FTLD-TDP group (n = 27), while participants with a MAPT mutation (n = 4) were included in the FTLD-tau group (n = 52). The Alzheimer disease (AD) group in (E, F) included all AD-S with pathologic confirmation of AD or a positive amyloid PET (n = 30). *p < 0.001, Bonferroni post hoc test. a: Inferior to all other groups (p < 0.05, Bonferroni post hoc test) expect nonfluent/agrammatic variant of primary progressive aphasia (nfvPPA) (p = 0.08). b: Superior to all other groups (p < 0.05, Bonferroni post hoc test). c: Inferior to all other groups (p < 0.05, Bonferroni post hoc test). ns: no statistically significant differences between groups (p > 0.05). bvFTD = behavioral variant frontotemporal dementia; CBS = corticobasal syndrome; FTD-ALS = frontotemporal dementia with amyotrophic lateral sclerosis; FTLD = frontotemporal lobar degeneration; HC = healthy control; PSP = progressive supranuclear palsy; svPPA = semantic variant of primary progressive aphasia.
Plasma t-Tau and NfL in Pathologically Confirmed FTLD
We refer to FTLD-tau and FTLD-TDP cases as those with autopsy-proven diagnosis, or either a FTLD-TDP mutation (C9orf72 and GRN) or a FTLD-tau mutation (MAPT). Of note, 9 of the pathology-proven FTLD-TDP (6 C9orf72, 3 GRN) and 2 of the FTLD-tau cases were also mutation carriers. Plasma t-tau concentrations did not differ between FTLD subtypes (figure 1E). Plasma NfL concentrations were higher in the FTLD-TDP subgroup (85.6 ± 46 pg/mL) compared to the FTLD-tau subgroup (50.4 ± 26 pg/mL; p = 0.001). The effect size of this difference was small but remained significant after accounting for age, sex, and disease severity at plasma sampling (p < 0.001; partial η2 = 0.20), and also when the analysis was restricted to FTLD-causing mutation carriers without neuropathologic confirmation (p < 0.001; partial η2 = 0.19) or after excluding ALS-FTD cases (p = 0.015; partial η2 = 0.09).
Plasma t-Tau and NfL in Pathology-Confirmed AD and in FTLD with AD Copathology or Positive Amyloid PET
As shown in figure 1F, both FTLD-tau and FTLD-TDP pathologic groups had higher plasma NfL concentrations than the pathologically confirmed AD group or positive amyloid PET. Since a significant proportion of patients with FTLD-S were found to have some degree of comorbid AD (table 1), we also investigated whether plasma t-tau and NfL concentrations varied between participants with increased certainty of underlying AD (either positive amyloid PET or at least intermediate AD likelihood on autopsy, n = 43) and participants without AD (n = 103, negative amyloid PET or absent/low comorbid AD at autopsy). There were no differences in plasma t-tau or NfL concentrations regarding presence or absence of AD pathophysiology.
Diagnostic Value of Plasma t-Tau and NfL
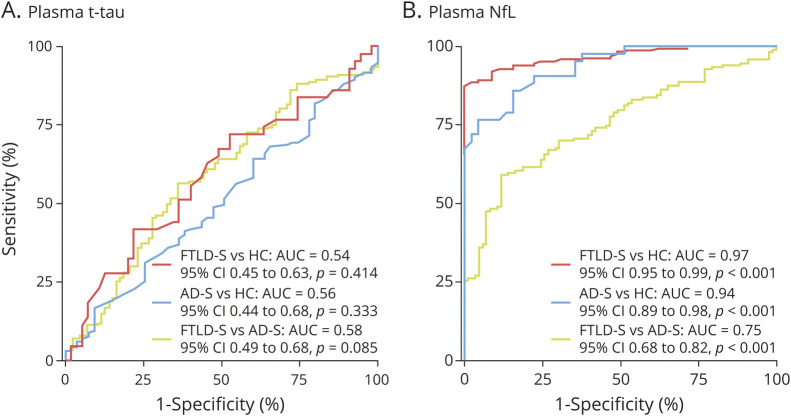
Plasma t-tau had no diagnostic utility to differentiate between FTLD-S and AD-S (AUC 0.58, 95% CI 0.49–0.68, p = 0.085), FTLD-S and healthy controls (AUC 0.54, 95% CI 0.45–0.63, p = 0.414), or AD-S and healthy controls (AUC 0.56, 95% CI 0.44–0.68, p = 0.333). In contrast, plasma NfL showed an excellent performance in the differentiation of FTLD-S from HC (AUC 0.97, 95% CI 0.95–0.99, p < 0.001) and of AD-S from controls (AUC 0.94, 95% CI 0.89–0.98, p < 0.001), but a poor performance for the discrimination between FTLD-S and AD-S (AUC 0.75, 95% CI 0.68–0.82, p < 0.001) (figure 2). Of note, we observed almost identical diagnostic performance for plasma t-tau and NfL after excluding ALS-FTD participants and participants with FTLD-related mutations (supplementary figure e-1, 10.5061/dryad.12jm63xvv). Importantly, the combination of plasma NfL and plasma t-tau in a ratio did not improve the diagnostic performance of plasma NfL alone.
Figure 2. Diagnostic Value of Plasma Total Tau (t-Tau) and Neurofilament Light (NfL) for the Differentiation of Frontotemporal Lobar Degeneration Syndromes (FTLD-S), Alzheimer Disease Syndromes (AD-S), and Healthy Controls (HC).
Diagnostic value of plasma t-tau (A) and NfL (B) for the differentiation of FTLD-S, AD-S, and HC. AUC = area under the curve.
Longitudinal Changes in Plasma t-Tau and NfL
We explored the longitudinal changes in tau and NfL plasma concentrations in the subgroup of participants with a second longitudinal sample available. After controlling for age, sex, baseline CDR+NACC/FTLD-SB, and time between samples, we observed a significant increase in plasma NfL concentrations in FTLD-S and AD-S compared to baseline but not in healthy controls (supplementary figure e-2, 10.5061/dryad.12jm63xvv). Conversely, we did not observe longitudinal changes in t-tau concentrations in any diagnostic group (supplementary figure e-2).
Relationship Between Baseline Plasma Biomarkers and Clinical Progression
Figure 3 shows the association between baseline plasma t-tau and NfL concentrations and longitudinal disease severity, measured by the CDR+NACC/FTLD-SB. In FTLD-S, baseline plasma t-tau concentrations were associated with worse decline (3.7 points change in CDR+NACC/FTLD-SB score per log t-tau pg/mL increase per year, 95% CI 1.4 to 6.0, p = 0.006). However, this effect was evident only in the bvFTD and PSP FTLD-S subgroups (3.6 points change in CDR+NACC/FTLD-SB, 95% CI 0.1–7.1, p = 0.015 and 11.8 points change in CDR+NACC/FTLD-SB, 95% CI 0.3–23.3, p = 0.023) and was not observed in the AD-S group. In contrast, baseline plasma NfL concentrations related to faster annual worsening in both FTLD-S (2.4 point change per log NfL ng/mL increase per year, 95% CI 0.78–2.4, p < 0.001) and AD-S (4.6 points change per log NfL ng/mL increase per year, 95% CI 1.2–5.5, p = 0.002). The relationship between baseline NfL and worse disease severity was significant in all FTLD-S subgroups (except for nfvPPA and CBS) and both typical amnestic and nonamnestic AD presentations (supplementary table e-4, 10.5061/dryad.12jm63xvv). The combination of plasma NfL and tau levels did not improve the ability of plasma NfL alone to predict longitudinal CDR+NACC/FTLD-SB score changes.
Figure 3. Estimates of Annualized Clinical Deterioration as a Function of Baseline Plasma Biomarkers in Frontotemporal Lobar Degeneration Syndromes (FTLD-S) and Alzheimer Disease Syndromes (AD-S).
Clinical Dementia Rating plus National Alzheimer's Coordinating Center FTLD sum of boxes (CDR+NACC/FTLD-SB) estimates were obtained from linear mixed-effects models adjusted for age, sex, and basal CDR+NACC/FTLD-SB. For illustrative purposes, we show the groups with high levels of plasma biomarker (higher than the median) and low levels of plasma biomarker (lower than the median). Error bars represent 95% confidence intervals. NfL = neurofilament light.
Relationship Between Plasma Biomarkers and Cortical Thickness
When compared to the HC group, the FTLD-S group showed expected decreases in cortical thickness in dorsolateral prefrontal, superior frontal, inferior frontal, temporal poles, and medial and lateral temporal regions. The AD-S group also showed the expected pattern of atrophy in temporal and parietal regions (figure 4, A and B). Plasma t-tau concentrations did not correlate with cortical thickness in either the FTLD-S or AD-S groups (figure 4, C and D). In contrast, plasma NfL showed strong correlations with cortical thickness in frontal regions in FTLD-S and the right lateral temporal lobe, right inferior parietal, and left superior frontal in the AD-S group (figure 4, E and F).
Figure 4. Relationship Between Plasma Biomarkers and Cortical Thickness in Frontotemporal Lobar Degeneration Syndromes (FTLD-S) and Alzheimer Disease Syndromes (AD-S) Groups.

Group comparison of cortical thickness between healthy controls (HC) and FTLD-S (A) and AD-S (B). Correlation between basal plasma levels of tau and cortical thickness in FTLD-S group (C); correlation between basal plasma levels of total tau (t-tau) and cortical thickness in AD-S group (D); correlation between basal plasma levels of NfL and cortical thickness in FTLD-S group (E); correlation between basal plasma levels of NfL and cortical thickness in AD-S group (F). For group comparisons, only clusters that survived false discovery rate correction (p < 0.05) are shown. For correlation analyses (C–F), the threshold for statistically significant correlation was set at p < 0.001.
Survival Analyses
As shown in table 2, in FTLD-S, only the clinical phenotype was independently associated with shorter survival, whereas in AD-S, only the CDR+NACC/FTLD-SB score was independently associated with shorter survival. When we introduced plasma biomarkers in the Cox regression models, only in FTLD-S, plasma NfL, but not plasma t-tau, predicted survival after accounting for age at plasma sampling, CDR+NACC/FTLD-SB, sex, and clinical phenotype. Figure 5 shows example survival curves in the FTLD-S group after a median split of baseline plasma t-tau and NfL concentrations. FTLD-S participants with high NfL concentrations (≥42 pg/mL) showed increased mortality compared to those with low concentrations (<42 pg/mL, log-rank 14.4, p < 0.001). Neither plasma t-tau nor plasma NfL predicted survival in the AD-S group (table 2).
Table 2.
Cox Proportional Hazard Models With Plasma Biomarkers Associated With Survival
Figure 5. Kaplan-Meier Survival Curves for Total Tau (t-Tau) and Neurofilament Light (NfL) in Frontotemporal Lobar Degeneration Syndromes (FTLD-S).
Kaplan-Meier survival curves in the FTLD-S group for t-tau (A) and NfL (B). High t-tau and high NfL represent levels superior to 2.2 ng/mL and 42 ng/mL, respectively (median split).
Discussion
The goal of this multimodal biomarker study was to compare the diagnostic and prognostic value of plasma t-tau and NfL in FTLD-S and AD-S participants with deep clinical, neuropsychological, and neuroimaging phenotyping. We observed a striking contrast between the clinical performances of plasma t-tau and NfL. The main findings of this study are that (1) only plasma NfL provided between-group clinical discrimination, predicted disease progression and survival, and correlated with neuroimage measures of neurodegeneration; and (2) the combination of plasma NfL and plasma t-tau did not improve the performance of plasma NfL alone. Plasma NfL was higher in both FTLD-S and AD-S than HC, and it was higher in FTLD-S compared to AD-S. Within FTLD-S, the highest plasma NfL levels were observed in the ALS-FTD subgroup. In both FTLD-S and AD-S, plasma NfL correlated with faster disease progression, and in FTLD-S, it was associated with shorter survival. Also, plasma NfL correlated with reduced frontal cortical thickness in FTLD-S and with reduced cortical thickness in parietotemporal regions in AD-S. In pathology-confirmed cases, plasma NfL was higher in FTLD than AD, and in FTLD-TDP, compared to FTLD-tau, independently of the inclusion of FTLD-related mutations. In marked contrast, plasma t-tau showed none of these associations, except for being low in ALS-FTD, compared to other FTLD phenotypes, and an association with more aggressive disease course in FTLD-S. Most pathology-confirmed FTLD cases had at least some degree of AD co-pathology, but this did not influence the performance of plasma t-tau or NfL. This adds to accumulating evidence supporting that t-tau and NfL reflect different aspects of neurodegeneration and provide different information compared to other neurodegeneration biomarkers, such as FDG-PET, or structural neuroimaging biomarkers, and that their longitudinal trajectories may be differently affected by demographic variables or disease stage.34 These results are particularly relevant for the application of biomarker-based classification systems.34
Tau is a microtubule-stabilizing protein encoded by MAPT and has been implicated in the pathophysiology of AD and FTLD. Tau hyperphosphorylation leads to the formation of paired helical filaments that aggregate in neurofibrillary tangles, a defining pathologic hallmark of AD.3 Elevated CSF levels of t-tau and p-tau are considered markers of neurodegeneration and tau pathology in AD and are used in the clinical setting to increase the diagnostic certainty of AD,1 and together with CSF Aβ42 recommended for diagnostic use in the Alzheimer's Association Appropriate Use Criteria for CSF testing in the diagnosis of AD.35 High CSF t-tau relates to clinical progression in AD36 and FTLD.37 Only a single previous study, however, investigated plasma t-tau levels in FTLD-S.38 In that study, plasma t-tau was elevated in bvFTD, PPA, and symptomatic MAPT mutation carriers, but the effect sizes were small, pathologic data were not available, and analyses for prediction of disease progression with clinical scales, neuropsychological testing, and survival were not conducted. In agreement with our results, no associations were found between plasma t-tau and baseline measures of disease severity or brain volume and AD pathophysiology (as measured by the CSF tau/Aβ1–42 ratio).38 Also in agreement with the present results are those from 2 large AD cohorts, in which plasma t-tau was associated with faster clinical decline.13 Plasma t-tau was weakly elevated and correlated with more severe longitudinal hypometabolism in patients with AD compared to controls.13
Studies of plasma t-tau in AD and FTLD, including the present one, have found no relationship between plasma and CSF t-tau.13 This may be related to differential expression of tau species in each compartment or different sensitivities of the techniques used for detection. For example, the CSF t-tau immunoassay used here relies on a combination of monoclonal antibodies with epitopes in the mid-protein region. In contrast, the plasma t-tau assay detects epitopes going from the N-terminal region to the more distal amino acid 224.39 This contrasts with a strong correlation of plasma and CSF t-tau concentration in Creutzfeldt-Jakob disease, in which the range of t-tau concentrations and species in plasma and CSF is higher than in AD or FTLD.40 In acute conditions, like traumatic brain injury and hypoxic brain injury, plasma t-tau concentrations measured using the same technology as the one employed here increase rapidly and show an apparent half-life of around 10 hours,41 which contrasts with the half-life of CSF t-tau, which is about 20 days.42 This may also explain the weak correlation of plasma and CSF t-tau and the poor diagnostic performance of plasma t-tau in chronic neurodegeneration. The clinical performance of plasma t-tau is in marked contrast with that of other AD biomarkers in plasma. Specifically, plasma β-amyloid 42/40 measured with mass spectrometry is strongly associated with amyloid status determined with amyloid PET scan.43 We also recently observed that phosphorylated plasma tau at threonine 181 differentiates autopsy-diagnosed AD from FTLD with better accuracy than clinically diagnosed or amyloid PET-defined cases.22 Together, the data support that plasma t-tau has a poor performance and will likely be of little value at a single subject level. These results may be related to limitations of the methodology to measure plasma t-tau, and the measurement of phosphorylated species of tau may have better clinical performance, as suggested by recent studies.44
NfL has emerged as a nonspecific CSF and plasma biomarker of neuronal injury in degenerative and nondegenerative disorders.6 Our results add to a large body of evidence showing that plasma or serum NfL concentrations are elevated in both FTLD and AD, compared to healthy individuals,6 but it is nonspecific and has weak discriminatory power between FTLD and AD or between FTLD clinical subtypes. Our results, however, support that plasma NfL has high prognostic value in FTLD, including bvFTD,45 svPPA,46 FTD-ALS,47 and PSP5 clinical subtypes. This study also replicated the findings of previous investigations supporting that, in AD, high plasma NfL correlates with faster worsening in global cognition and faster atrophy rates.4,15 Our results are also consistent with earlier reports showing a high correlation between plasma and CSF NfL.5 This further supports that plasma NfL reflects brain pathophysiology,14 and that plasma and CSF NfL may provide equivalent prognostic information, with the added value of plasma being more convenient for clinical use. In sharp contrast, plasma levels of t-tau only predicted clinical decline in some clinical subgroups of FTLD-S (bvFTD and PSP) but not in the whole FTLD-S and AD-S groups. This finding does not support the role of t-tau as a general marker of neurodegeneration. However, our results in the AD-S group should be interpreted cautiously due to its relatively small size and the lack of AD participants at the preclinical stage. Of note, 2 recent studies suggested that plasma t-tau could be an early surrogate marker of neurodegeneration in preclinical AD.48,49 Future studies should precise the role of t-tau and other tau species in the AD continuum and other tauopathies. The main contributions of this study are the analyses of plasma NfL in relation to specific pathology-confirmed FTLD subtypes and of its prognostic value for survival estimates in FTLD-S. We studied a sizeable FTLD-S cohort with available pathologic data (i.e., 70 cases or 42% of the FTLD-S sample). Plasma NfL was higher in FTLD-TDP than FTLD-tau or AD, a result driven by high plasma NfL concentrations in patients with ALS-FTD. Nevertheless, the variability of plasma NfL is high, especially in FTLD-TDP, which makes it a poor discriminator of FTLD pathology subtypes.
This study has some limitations. The study is not representative of genetic FTLD forms, because it included only a small number of participants with genetic FTLD and did not include prodromal forms of disease. Our results, however, are in line with a recent European multicenter study of plasma NfL in genetic FTLD.50 The results were not confirmed in a replication cohort.
This study supports the superiority of plasma NfL compared with plasma t-tau as a diagnostic and prognostic biomarker for both FTLD-S and AD-S. Plasma t-tau may not be equivalent to other blood-based neurodegeneration biomarkers, which should be considered for the refinement of biomarker-based classification schemes.
Acknowledgment
The authors thank the patients and their relatives for their support for this study and the laboratory technicians at the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden, for performing the biomarker analyses.
Glossary
- AD
Alzheimer disease
- AD-S
Alzheimer disease syndromes
- ALS-FTD
amyotrophic lateral sclerosis with frontotemporal dementia
- AUC
area under the receiver operator characteristic curve
- bvFTD
behavioral variant frontotemporal dementia
- CBS
corticobasal syndrome
- CDR+NACC/FTLD-SB
Clinical Dementia Rating plus National Alzheimer's Coordinating Center FTLD sum of boxes
- FTLD
frontotemporal lobar degeneration
- FTLD-S
frontotemporal lobar degeneration syndromes
- HC
healthy control
- MMSE
Mini-Mental State Examination
- NfL
neurofilament light
- nfvPPA
nonfluent/agrammatic variant of primary progressive aphasia
- PSP
progressive supranuclear palsy
- t-tau
total tau
- svPPA
semantic variant of primary progressive aphasia
- UCSF
University of California, San Francisco
Appendix. Authors



Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was supported by the Fondo de Investigaciones Sanitario/Instituto de Salud Carlos III PI14/1561 and PI17/01896 (A.L.); Alzheimer's Association AARF-16-443577 (R.L.J.); Departament de Salut de la Generalitat de Catalunya SLT002/16/00408 (A.L.); NIH K23AG059888 (J.C.R.); NIH K23AG061253 (A.M.S.); NIH K08 AG052648 (S.S.); NIH K24AG053435 (L.T.G.). I. Illán-Gala is supported by the Rio Hortega grant (CM17/00074) from Acción Estratégica en Salud 2013-2016 and the Global Brain Health Institute (Atlantic Fellow for Equity in Brain Health). H. Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (2018-02532), the European Research Council (681712), Swedish State Support for Clinical Research (ALFGBG-720931) and the UK Dementia Research Institute at UCL. K. Blennow is supported by the Swedish Research Council (2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (RDAPB-201809-2016615), the Swedish Alzheimer Foundation (AF-742881), Hjärnfonden, Sweden (FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (ALFGBG-715986), and European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236). A.M. Staffaroni is also supported by the Larry L. Hillblom Foundation (2018-A-025-FEL).
Disclosure
I. Illán-Gala reports no disclosures relevant to the manuscript. A. Lleó has served on scientific advisory boards from Fujirebio-Europe, Nutricia, and Biogen, and has filed a patent application of synaptic markers in neurodegenerative diseases. A. Karydas and A.M. Staffaroni report no disclosures relevant to the manuscript. H. Zetterberg has served on scientific advisory boards for Roche Diagnostics, Wave, Samumed, and CogRx, has given lectures in symposia sponsored by Alzecure and Biogen, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. R. Sivasankaran, L.T. Grinberg, S. Spina, and R. La Joie report no disclosures relevant to the manuscript. G.D. Rabinovici reports receiving grants from the NIH and Tau Consortium during the conduct of the study; grants from Avid Radiopharmaceuticals, Eli Lilly and Company, GE Healthcare, and Life Molecular Imaging outside the submitted work; and personal fees from GE Healthcare, Axon Neurosciences, Merck, Eisai, Roche, and Genentech outside the submitted work. D.C. Perry, M.L. Gorno-Tempini, W.W. Seeley, B.L. Miller, and H.J. Rosen report no disclosures relevant to the manuscript. K. Blennow has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis and Roche Diagnostics, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. A.L. Boxer receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimers Drug Discovery Foundation, and the Alzheimer's Association. He has served as a consultant for Aeton, AbbVie, Alector, Amgen, Arkuda, Arvinas, Asceneuron, Ionis, Lundbeck, Novartis, Passage BIO, Samumed, Third Rock, Toyama and UCB, and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche, and TauRx. J.C. Rojas is a principal investigator for clinical trials sponsored by Eli Lilly. Go to Neurology.org/N for full disclosures.
References
References
- 1.Lleo A, Irwin DJ, Illan-Gala I, et al. A 2-step cerebrospinal algorithm for the selection of frontotemporal lobar degeneration subtypes. JAMA Neurol 2018;75:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randall J, Mörtberg E, Provuncher GK, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation 2013;84:351–356. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattsson N, Andreasson U, Zetterberg H, Blennow K; for the Alzheimer's Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol 2016;3:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019;76:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol 2016;3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeter LHH, Steketee RME, Salkovic D, et al. Clinical value of cerebrospinal fluid neurofilament light chain in semantic dementia. J Neurol Neurosurg Psychiatry 2019;90:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjogren M, Rosengren L, Minthon L, Davidsson P, Blennow K, Wallin A. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology 2000;54:1960–1964. [DOI] [PubMed] [Google Scholar]
- 10.Irwin DJ, Lleo A, Xie SX, et al. Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Ann Neurol 2017;82:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman M, Elman L, McCluskey L, et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol 2014;71:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pijnenburg YAL, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement Amst Neth 2015;1:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashton NJ, Leuzy A, Lim YM, et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun 2019;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2019;76:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong MJ, Armstrong MJ, Litvan I, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 21.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–629. [DOI] [PubMed] [Google Scholar]
- 22.Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators, Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 2020;26:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa T, Brushaber D, Syrjanen J, et al. Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: data from the ARTFL/LEFFTDS Consortium. Alzheimers Dement 2020;16:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas JC, Bang J, Lobach IV, et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018;90:e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Joie R, Ayakta N, Seeley WW, et al. Multisite study of the relationships between antemortem [ 11 C]PIB-PET Centiloid values and postmortem measures of Alzheimer's disease neuropathology. Alzheimers Dement 2019;15:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaser C, Robert D. Computational Anatomy Toolbox [online]. Available at neuro.uni-jena.de/cat/. Accessed August 16, 2020. [Google Scholar]
- 29.Friston K, Ashburner J. Statistical Parametric Mapping [online]. Available at fil.ion.ucl.ac.uk/spm/software/spm12/. Accessed August 16, 2020. [Google Scholar]
- 30.Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain J Neurol 2017;140:3329–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010;119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol (Berl) 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illan-Gala I, Pegueroles J, Montal V, et al. Challenges associated with biomarker-based classification systems for Alzheimer's disease. Alzheimers Dement Amst Neth 2018;10:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw LM, Arias J, Blennow K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement 2018;14:1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wattmo C, Blennow K, Hansson O. Cerebro-spinal fluid biomarker levels: phosphorylated tau (T) and total tau (N) as markers for rate of progression in Alzheimer's disease. BMC Neurol 2020;20:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ljubenkov PA, Staffaroni AM, Rojas JC, et al. Cerebrospinal fluid biomarkers predict frontotemporal dementia trajectory. Ann Clin Transl Neurol 2018;5:1250–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foiani MS, Woollacott IO, Heller C, et al. Plasma tau is increased in frontotemporal dementia. J Neurol Neurosurg Psychiatry 2018;89:804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cicognola C, Brinkmalm G, Wahlgren J, et al. Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer's disease. Acta Neuropathol 2019;137:279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staffaroni AM, Kramer AO, Casey M, et al. Association of blood and cerebrospinal fluid tau level and other biomarkers with survival time in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2019;76:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zetterberg H. Review: tau in biofluids: relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 2017;43:194–199. [DOI] [PubMed] [Google Scholar]
- 42.Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau kinetics in neurons and the human central nervous system. Neuron 2018;98:861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019;93:e1647–e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blennow K, Chen C, Cicognola C, et al. Cerebrospinal fluid tau fragment correlates with tau PET: a candidate biomarker for tangle pathology. Brain 2020;143:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinacker P, Anderl-Straub S, Diehl-Schmid J, et al. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 2018;91:e1390–e1401. [DOI] [PubMed] [Google Scholar]
- 46.Steinacker P, Semler E, Anderl-Straub S, et al. Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology 2017;88:961–969. [DOI] [PubMed] [Google Scholar]
- 47.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavedo E, Lista S, Houot M, et al. Plasma tau correlates with basal forebrain atrophy rates in people at risk for Alzheimer disease. Neurology 2020;94:e30–e41. [DOI] [PubMed] [Google Scholar]
- 49.Pase MP, Beiser AS, Himali JJ, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol 2019;76:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol 2019;18:1103–1111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.