Highlights
-
•
Tomato yield and quality was compared in soil and two hydroponic culture systems.
-
•
Environmental factors were controlled and measured across three growth systems.
-
•
Higher water use efficiency in hydroponics was associated with crop transpiration.
-
•
Deep water culture produced higher beta-carotene and lycopene contents.
-
•
Control of environmental factors allowed improved understanding of potential benefits.
Abbreviations: DI, drip irrigation; DW, dry weight; DWC, deep water culture; EC, electrical conductivity; NFT, Nutrient Film Technique; PWU, product water use; S, soil; TAA, total antioxidant activity; TSS, total soluble solids; WUE, water use efficiency; CEA, Controlled environment agriculture
Keywords: Deep Water culture, Fertilization, Hydroponic, Lycopene, Tomato, Water use efficiency
Abstract
There are many different types of systems used to grow food that are distinguished by ideology or the technology used. It is often difficult to directly compare yield and quality in different growth systems due to the complicated interactions between genotype, physiology and environment. Many published comparisons do not identify and acknowledge confounding factors. However, there is urgency to undertake controlled comparisons to identify the most efficient and effective food production systems, because the world faces considerable challenges to food supply with population rise, ongoing environmental degradation and the threat of climatic change. Here we compared soil with two hydroponic growth systems, drip irrigation and deep-water culture (DWC). It is often claimed that such systems differ in water use, yield and crop quality; however, such comparisons are often confounded by assessing plant and system parameters in different growth environments or where factors that are difficult to standardise between systems, such as nutrient status, are not controlled. We grew tomato (Solanum lycopersicum L.) in the three growth systems in two replicated experiments, in either a polytunnel or glasshouse. We controlled and monitored water use and nutrient levels across all systems as different fertilizer applications can influence the nutritional values of produce. Plants in the two hydroponic systems transpired less water and were more water-efficient with a lower product water use than plants grown in soil. Fruit yield was similar and total soluble solids and sugar levels were not significantly different between the three growing systems. However, levels of lycopene and β-carotene were either similar or significantly higher in DWC compared to growth systems using soil or drip irrigation. Our results identify hydroponic systems as more water use efficient with DWC also capable of producing higher quality produce.
1. Introduction
Food production is facing many global challenges such as adapting to climate change and the resulting extremes of weather, the increasing and increasingly urbanised human population, challenges to the supply of macronutrients such as phosphorus and the need to reduce the impact of agrochemicals on the environment. These challenges occur at a time when there is a potential slowdown in improvements in yield per unit area for some crops and large-scale degradation of land used for food production for example from irrigation and the increase in saline land (Brekke et al., 2011). There are many possible solutions to specific challenges, one is to produce more food within controlled environments (Jensen, 1999). Controlled environment agriculture (CEA) may be considered as an extension of covered crop production and horticulture but includes hydroponics and the application of novel technologies such as light-emitting diodes (LEDs) (Darko et al., 2014), robotics and imaging and some speculative high tech approaches including intensive urban vertical farming (Despommier, 2013; Touliatos et al., 2016). Such approaches do not always require hydroponics but growing plants in the absence of soil has many potential benefits. One example is that the harvested products (leaves or fruits) contain fewer soil particles (Gonnella et al., 2004) which leads to less soil-borne disease and fewer washing treatments with a consequent saving in water and energy. Furthermore, closed hydroponic systems can substantially reduce the pollution of water sources, while contributing to a reduction in water and fertilizer consumption (Carmassi et al., 2005; Bar-Yosef, 2008).
In general, hydroponic systems allow flexibility and intensification, providing high crop yield and high-quality products, even in areas with adverse growing conditions (Grillas et al., 2001). In hydroponic systems, soil preparation and weed control are avoided and land not suitable for conventional soil-based cultivation can be used. Horticulture using conventional soil systems is facing new challenges, for example soil sterilization is becoming difficult (Matthiessen and Kirkegaard, 2006) due to international efforts to reduce the use of ozone depleting substances such as Methyl Bromide (Marcotte, 1998). This compound not only increases the levels of ammonium while altering the soil microbial community (Yamamoto et al., 2008), but also is harmful to humans and banned in several countries (The Montreal Protocol on Substances that Deplete the Ozone Layer, 1987). The application of hydroponics using artificial substrates could minimise the need for chemical pest control (Polycarpou et al., 2005). Hydroponics is also ideal for holistic control of crop development, quality and uniformity (Ho, 2004; Gruda, 2009) and yield has been reported to be up to 20 times higher (per area) than equivalent open field systems (Jensen, 1999).
Crop quality has attracted increasing interest recently and for some consumer’s the choice of food includes consideration of health values along with taste (Ho, 2004). Some plant compounds have been linked to potential health benefits, for example, anthocyanin showed anti-carcinogenic activity in cell culture models and in animal model tumour systems (Wang and Stoner, 2008). Levels of other pigments have been suggested to be important, leading to the general advice to eat a more varied and colourful diet (WHO, 2005). More specifically, some pigments can protect lipoproteins and vascular cells from oxidation which is the widely accepted theory for the genesis of atherosclerosis (Willcox et al., 2003).
The tomato is a staple product in many different countries and is the second most important vegetable crop next to potato in terms of production quantity (FAOSTAT, 2018). In fact, current world production is about 100 million tons fresh fruit from 3.7 million ha (FAOSTAT, 2001) with an annual value estimated to be around $65.4 billion (FAOSTAT 2016). Tomatoes contain high levels of important pigments such as lycopene and the inclusion of tomatoes in the diet can be linked to a lower risk of cancer (Giovannucci, 1999). Tomatoes are routinely grown in both soil and hydroponic systems and produce has been compared from these different production systems. For example, Kunsch et al. (1994) found that tomatoes grown hydroponically had a higher sugar/acid ratio compared to tomatoes grown in soil and tomatoes grown hydroponically by Nutrient Film Technique (NFT) were firmer and higher in vitamin C (Benoit and Ceustermans, 1987). Tomatoes grown using NFT had higher levels of titratable acids and potassium levels compared to tomatoes grown in peat bags (Cronin and Walsh, 1983). In some studies tomatoes grown in soil were reported to have improved quality characteristics, for example, Granges (1980) found that soil-grown tomatoes had a higher dry matter. Levels of ascorbic acid were increased by growing tomatoes on organic substrates when compared with peat-perlite (Premuzic et al., 1998) and during post-harvest storage, hydroponic-grown tomatoes synthesised lycopene at a lower rate compared to soil-grown tomatoes (Ajlouni et al., 2001).
The comparisons of quantity, quality or efficiency of production between culture systems is complicated by inappropriate control of factors that differ between systems. For example, the quality of tomatoes is influenced by the environment in which the crop is grown, this includes light levels and the nutrient content in the feed. High proportions of potassium in the nutrient solution can increase the production of lycopene and total soluble solids (TSS) (Fanasca et al., 2006) and a higher presence of sulphur can increase the production of lycopene (Zelená et al., 2009) while low N results in a positive effect on vitamin C and some phenolics (Erba et al., 2013). Thus, a number of comparative studies may have been confounded by the complex environmental interactions that impact tomato crop quality and some studies simply made comparisons of marketed produce between different production systems (Hernández Suárez et al., 2007, 2008). Decisions to adopt approaches in agriculture should include controlled comparisons between production systems in which factors that may impact on crop quantity or quality are as far as possible monitored and standardised between growing systems. One example of the potential for confounding factors to obscure accurate conclusions is the recent interest in the relative benefits of organic and conventional growth systems. Some of the differences reported for example in TSS attributed recently to growing systems may actually be attributed to varieties, disparities in agronomic practices and differences in harvest stages (Pieper and Barrett, 2008).
The aim of our experiments was to provide a controlled comparison between three different growth systems to reduce the impact of confounding factors. We compared the production of tomatoes growing at the same location in either soil, deep water culture or drip irrigation. Similar levels of key nutrients N, P, K, Ca, Mg and S were supplied across all three growing systems and we examined the uptake of nutrients by the crop and water use efficiency between systems. We replicated experiments in two different growth environments, a polytunnel and glasshouse. This ensured that our conclusions were generalisable across the most popular growth environments for tomatoes. We compared total yield and growth rates and recorded environmental variables including temperature and relative humidity (RH) throughout the experiments. The nutritional quality of the harvested tomatoes was compared in terms of levels of lycopene, the total antioxidant activity (TAA) and TSS in harvested fruit.
2. Materials and methods
The study took place at the Institute of Biological, Environmental and Rural Sciences (IBERS), Aberystwyth University Gogerddan Campus (Wales, United Kingdom) during late summer 2018 and spring 2019 using seeds of round tomato cv. Forticia F1 (Rijk Zwaan seeds, Holland). Seeds were sown in low nutrient soil plugs (S) (Bulrush Horticulture Ltd), in 2 cm3 cubes rockwool (Grodan Rockwool B.V., Holland) with drip irrigation (DI) and in 2 cm3 cubes inert growing sponge (Changzhou Dengyue Sponge Co., Ltd.) for deep-water culture (DWC). However, the inert sponge was found to perform poorly in the first experiment in the polytunnel and subsequently rockwool was used in both soilless systems. Plants were grown to seedling stage in the two environments and watered with the standard nutrient solution used in the rest of the experiment. After 3 weeks the established seedlings were transferred to the larger growing units, either into a larger soil volume or inserted into the polystyrene float or rockwool slab as described below for the two hydroponic systems. The seedlings were transferred on 06/08/2018 in the polytunnel experiment and on 04/03/2019 in the glasshouse experiment to allow comparisons from contrasting temperature profiles in the two environments. The experimental design within each environment was fully randomized with 7 replicates per treatment. Plants were grown until the first truss and the vegetative growth was stopped on the 8th week and only 3 leaves were maintained after the first truss (Jiang et al., 2017). Once per week side shoots were removed. Fruit were pruned to four per truss (Fig S1), following the commercial practice as discussed in Wu and Kubota (2008) and fruits were harvested on the same day from all the three treatments. During the whole growing period, temperature (minimum and maximum) and relative humidity were recorded using data loggers placed in the growing area (Tinytag Ultra 2, Gemini Data Loggers, UK). At the end of the experiments the numbers of fruit harvested was recorded; in the polytunnel experiment low night temperatures during the ripening stage resulted in the loss of some fruit, but losses were never more than one fruit per truss.
Soil treatment (S).
In the polytunnel experiment, the growing unit was a 35 L pot and in the glasshouse experiment, the growing unit was a 14 L pot. The soil media used was Bulrush compost (Bulrush Horticulture Ltd, Magherafelt, UK) which has a negligible nutrient content as measured by the manufacturer (Table S1). During the experiment each plant was irrigated by a dripper that supplied nutrient solution. The soil treatment was provided with the same nutrient solution as the other treatments.
Drip irrigation (DI) treatment.
Seedlings were established in 2 cm3 rockwool (Grodan Rockwool B.V., Holland) which were inserted into larger rockwool slabs. Rockwool is inert and lacks nutrients providing only mechanical support. The Rockwool slabs were placed on top of a rounded container in order to collect the excess flow of the nutrient solution after each irrigation event. The excess solution was collected along with previous irrigations to further irrigate the plants, making a complete closed-loop from which total nutrient supplied and water use could be determined.
Deep water culture (DWC) treatment.
Seedlings were sown in the polytunnel into 2 cm3 growing sponge cubes (Changzhou Dengyue Sponge Co., Ltd.) and placed in a polystyrene disc floating on the liquid surface in a 35 L pot. In the glasshouse experiment the same size rockwool cube was used and the seedlings were placed in a polystyrene disc floating on the liquid surface in a 14 L pot. In both cases the liquid was the nutrient solution of the same composition as other treatments. The polystyrene float in both cases covered most of the liquid surface. The pot was covered internally with a plastic film to prevent leakage and at the bottom was placed an air stone to keep the solution oxygenated and avoid root anoxia.
The nutrients supplied were the same across all three treatments and both experiments. The fertilization formula was based on Kaya and Higgs, 2002 (Table S2) and was adjusted to account for nutrients in the compost (Table S1). The nutrient solution was periodically checked, and pH and electrical conductivity (EC) were recorded and adjusted. The pH was maintained between 5.5 and 6.0. EC was kept low during the initial 4 weeks to aid acclimation and subsequently, to reach the desired levels of nutrients, EC was increased to 2.4 mS cm−1. The nutrient levels were measured using ion exchange chromatography on a Metrohm IC 700 series system fitted with Metrosep A sup 5 anion column and a Metrosep C4 250/C4 cation column (Metrohm AG, Switzerland). These samples were collected on a weekly basis for DI and DWC in the polytunnel experiment (2018) and on a bi-weekly basis in the glasshouse experiment (2019).
Crop parameters were estimated as detailed in Hussain Shah et al., 2011 and briefly as follows. Plant height was measured from the media surface to the uppermost leaf node weekly until vegetative growth stopped. Biomass was measured as the fresh weight of aerial biomass including all pruned parts. Dry weight was estimated after drying the aerial biomass at 80 °C until constant weight was achieved. Yield (fresh weight of fruit per plant in grams) was measured as the weight of tomato fruit without the vine which was considered part of the aerial biomass. Fruit were harvested only when at least three out of four fruits per truss reached the “Red” stage of the U.S. Tomato Grades and Standards (United States Standards for Grades of Fresh Tomatoes, 1991) (Fig. 1).
Fig. 1.
Example of the different ripening stages of tomatoes. At 9 weeks after transplant stage 2 “BREAKERS” (A) and at the harvest stage 6 “RED” (B) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Water consumed was recorded on a weekly basis in both soilless systems as the water required to refill each independent growing unit to a pre-set maximum level. The water consumed in the soil system was estimated from the flow rate of the drippers (L h−1) and number of irrigation events per day. In both experiments were placed three growing units with soil without plants and three hydroponic units without plants. These growth units were treated the same as those containing plants and used to estimate evaporative loss. By subtracting the averaged evaporative loss from the total water used in the soil and hydroponic systems in growing each plant, total transpired water was estimated which was used to calculate water use efficiency (WUE). The WUE was calculated as the fresh weight of fruit produced per litre of transpired water. Product Water Use (PWU) was estimated as the total volume of evapotranspired water used to produce one kilogram of fresh produce (Nederhoff and Stanghellini, 2010).
Total soluble solids (TSS) were determined from the refractive index using a refractometer (HI-96,801 Hanna Instruments Ltd. USA) according to the methodology of Patanè et al. (2017). All four fruits from each plant were homogenized to produce a representative sample purée. A drop of the tomato purée was placed on the prism of the refractometer and the reading was recorded in °Brix (AOAC, 1990).
To estimate total antioxidant activity (TAA) a modified colorimetric assay using DPPH (2, 2-diphenyl-1-picrylhydrazyl) was used (Barbagallo et al., 2013). Each analysis comprised 3 technical replicates. To 2 mL of freshly prepared DPPH stock solution (25 mg in 100 mL methanol) was added 100 μL of tomato extract (1 g mL−1 in methanol) or 100 μL of methanol (control) and mixed thoroughly. After incubation at room temperature for 30 min in the dark the absorbance was measured at 517 nm using a UV–vis spectrophotometer compared with the control assay lacking tomato extract. When a solution of DPPH is mixed with a substance that can donate a hydrogen atom it gives rise to the reduced form with the loss of violet colour (Molyneux, 2004), therefore total antioxidant activity was calculated from the absorbance using the following equation:
To estimate lycopene and β-carotene contents of the fruit a 4:6 mixture of acetone:hexane (10 mL) was added to one gram of tomato sample in a 15 mL test tube and the mixture was homogenized. The optical density of the polar extract supernatant was determined at 663, 645, 505 and 453 nm in a glass cuvette (1400 μL). The values of lycopene and β-carotene (mg 100 mL−1) were calculated using equations 2 and 3 respectively (Nagata & Yamashita, 1992):
Where A453, A505, A645 and A663 are the absorbance values at 453, 505, 645 and 663 nm respectively.
All results were analysed using R version 3.0.3 (The R Development Core Team, 2010). Equality of variance was tested using Levene’s test and a one-way ANOVA was used to test for significant differences between the treatments.
3. Results
The polytunnel and glasshouse environments combined with the later sowing time in the polytunnel provided quite different environments in which to test the three growing systems (Figure S2). In the polytunnel experiment the minimum night temperatures were low especially in October and in November (Table 1). In October the minimum monthly average was 6.5 °C ± 0.8, but for several days in the last week minimum temperature values of below 0 °C were recorded (Fig. S2B). Although the daily averages in the first month of growth (vegetative stage) were quite similar in both the experiments (21.1 °C ± 0.5 in 2018 and 21.9 °C ± 0.2 in 2019), the daily temperatures differed by around 5 °C during the first stage of fruit set (18.0 °C ± 0.5 in 2018 and 23.0 °C ± 0.3 in 2019). These differences were more than 8 °C in the 3rd month (15.0 °C ± 0.6 in 2018 and 23.7 °C ± 0.2 in 2019), and more than 11 °C during the last part of the ripening stage in the 4th month (12.2 °C ± 0.6 in 2018 and 23.6 °C ± 0.3 in 2019).
Table 1.
Monthly averages of relative humidity (RH), minimum (Tmin) and maximum (Tmax) temperature recorded during both experiments in a polytunnel (2018) and glasshouse (2019).
| Month | Polytunnel (2018) |
Month | Glasshouse (2019) |
||||
|---|---|---|---|---|---|---|---|
| Tmin | Tmax | RH | Tmin | Tmax | RH | ||
| August | 12.8 ± 0.6 | 29.1 ± 1.0 | 51.5 ± 2.9 | March | 18.1 ± 0.1 | 28.8 ± 0.7 | 59.1 ± 1.4 |
| September | 9.9 ± 0.6 | 27.1 ± 0.9 | 57.9 ± 2.7 | April | 18.5 ± 0.2 | 30.5 ± 0.7 | 62.1 ± 1.6 |
| October | 6.5 ± 0.8 | 23.9 ± 0.9 | 66.8 ± 2.9 | May | 19.3 ± 0.2 | 30.0 ± 0.5 | 64.7 ± 1.3 |
| November | 5.7 ± 0.6 | 17.9 ± 0.9 | 71.6 ± 2.8 | June | 19.8 ± 0.1 | 30.5 ± 0.9 | 67.8 ± 1.5 |
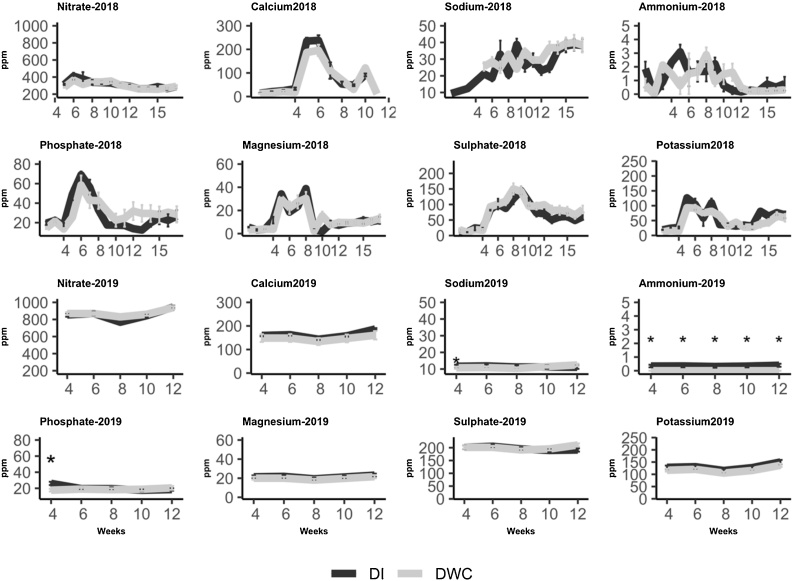
In the polytunnel experiment no significant differences in any nutrient contents of the fertigation solution were detected; however, in the glasshouse experiment and more specifically during the 4th week of growth, there were differences in phosphate levels (F2,18 = 12.01, p = 0.00467). Across the whole experiment in the glasshouse, higher levels of ammonium were measured in solution from the DI system (F2,18 = 12.91, p = 0.0037)) (Fig.2). However, these levels of ammonium were very low (<1 ppm) and are likely to be of negligible significance.
Fig. 2.
Nutrient levels (ppm) in the hydroponic solutions of tomatoes growing in either deep water culture (DWC) (black line) or using drip irrigation (DI) (light grey line); the sampling was on a weekly basis in polytunnel grown plants (2018) and bi-weekly in glasshouse grown plants (2019).
There was a significant effect of cultivation system on total water consumed in both the polytunnel (F2,18 = 46.79, p = 7.39 × 10−8) and the glasshouse experiments (F2,18 = 65.41, p = 5.54 × 10-9) and the ranked order was similar in both experiments. In the polytunnel, plants grown in soil consumed the highest amount of water (87.44 ± 3.97 L) and DWC the lowest (41.70 ± 8.14 L) whilst plants in the DI system consumed intermediate amounts of water (70.34 ± 12.55 L). Similarly, in the glasshouse, plants grown in soil consumed the highest amount of water (102.89 ± 4.14 L) and DWC the lowest (48.07 ± 8.3 L) and again plants growing in the DI system were intermediate in their water consumption (92.53 ± 13.64 L). When comparing the two experimental settings, polytunnel and glasshouse, plants in the DWC system consumed a similar amount of water (F1,12 = 2.09, p = 0.173), this was despite there being very different climatic conditions especially in the latter part of the growth cycle (Figure S2, Table 1).
In the polytunnel experiment WUE was not significantly different between the three growth systems (F2,18 = 2.007, p = 0.163) but the ranked order in WUE was DWC > DI > S; however, in the glasshouse experiment there was a significant effect of cultivation system on WUE (F2,18 = 10.61, p = 0.0009) (Table 2). The trend in WUE in the glasshouse was the same as in the polytunnel, plants grown using DWC (0.0129 ± 0.005 Kg L −1) and DI (0.0099 ± 0.0039 Kg L −1) were significantly more water efficient than those grown in soil (0.0044 ± 0.0008 Kg L −1).
Table 2.
Total water consumed, water use efficiency (WUE), product water use (PWU) and transpired water in tomatoes growing in soil (S), deep water culture (DWC) and drip irrigation (DI) in a polytunnel (2018) and glasshouse (2019).
| Parameter | Growth environment |
Growth environment |
||||
|---|---|---|---|---|---|---|
| Polytunnel experiment (2018) |
Glasshouse experiment (2019) |
|||||
| DWC | DI | S | DWC | DI | S | |
| Water Consumption (L plant−1) | 41.7 ± 8.14c | 70.34 ± 12.55b | 87.44 ± 3.97a | 48.07 ± 8.3b | 92.53 ± 13.64a | 102.89 ± 4.14a |
| WUE (Kg L−1) | 0.0059 ± 0.0050 | 0.0072 ± 0.0029 | 0.0035 ± 0.0022 | 0.0124 ± 0.0046a | 0.0099 ± 0.0033a | 0.0044 ± 0.0008b |
| PWU (L Kg−1) | 325.1 ± 89.9ab | 192.5 ± 68.5b | 497.7 ± 298.4a | 120.2 ± 23.7c | 224.98 ± 30.7b | 275.9 ± 41.1a |
| Water Transpired (L plant−1) | 31.8 ± 15.3b | 57.16 ± 9.9a | 70.83 ± 3.24a | 35.83 ± 11.38b | 49.77 ± 30.28b | 87.64 ± 5.65a |
Lower case letters indicate significant differences after Tukey’s post hoc test (significance level p < 0.05). Means with standard errors were calculated from 7 replicates.
In the polytunnel experiment the product water use (PWU) was significantly lower in the DI than S cultivation systems (F2,18 = 4.831, p = 0.0209) but in DWC the PWU was not significantly different from either system. In the glasshouse experiment there was also a significant effect of cultivation system on PWU (F2,18 = 41.36, p = 1.86 × 10−7) and each system was significantly different with the trend of decreasing PWU being DWC < DI < S (Table 2), meaning that soil cultivation required more water in both experiments to produce the same amount of fruit.
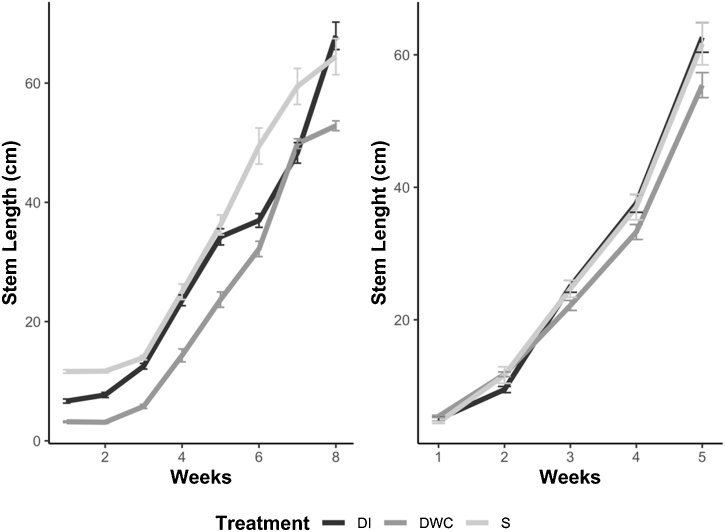
Vegetative growth in the two experiments stopped at different times as seen from stem growth because plants reached maturity and developed the truss at different rates (Fig.3). Although the different flowering times affected the length of the vegetative stage, the final heights were not significantly different either when comparing the two experiments or the three growth systems (Fig. 3), except plants growing in DWC in the polytunnel experiment due to relatively poor establishment of the seedlings in this system as described previously.
Fig. 3.
Stem growth of tomato plants growing in soil (light grey line), and two hydroponic systems, drip irrigation (DI) (black line) and deep-water culture (DWC) (dark grey line) in either a polytunnel (A) or glasshouse (B).
Total fresh weights and dry weights of vegetative biomass were not significantly affected by cultivation system in the glasshouse experiment (F2,18 = 0.66, p = 0.53; F2,18 = 1.946, p = 0.172 F W and DW respectively). In the polytunnel experiment, even though plants growing in the DWC system established slowly, a similar fresh weight was generated in all three cultivation systems (F2,18 = 1.458, p = 0.259). However, dry weight (DW) was significantly different (F2,18 = 4.133, p = 0.033) (Table 3), and soil grown tomatoes produced significantly more DW biomass (57.6 ± 12.5) than tomatoes grown in DWC (41.7 ± 9.9) and DW values from tomatoes grown with DI were intermediate (49.8 ± 7.9) and not significantly different from the other two growth systems. The variance in biomass values, especially FW, from all three cultivation systems was considerably higher in the polytunnel experiment than the glasshouse experiment (Table 3).
Table 3.
Fresh weight of biomass (FW), total dry weight (DW) and total fruit per plant and fruit weight (Yield) per plant of tomatoes growing in soil (S), and two hydroponic systems; deep water culture (DWC) and drip irrigation (DI).
| Parameter | Growth environment |
Growth environment |
||||
|---|---|---|---|---|---|---|
| Polytunnel experiment (2018) |
Glasshouse experiment (2019) |
|||||
| DWC | DI | S | DWC | DI | S | |
| FW (g) | 594.69 ± 172.5 | 649.8 ± 128.4 | 739.4 ± 174.9 | 405 ± 67.6 | 351 ± 48.4 | 389.9 ± 132.4 |
| DW (g) | 41.7 ± 9.9b | 49.9 ± 7.9ab | 57.6±12.5a | 24.9±8.6 | 17.1±4.7 | 23.5±9.4 |
| Yield (g) | 138.1 ± 52.5b | 399.5 ± 127.3a | 247.1 ± 159.2ab | 401.3 ± 14.8 | 411.1 ± 15.7 | 379.4 ± 51.8 |
| Fruit per plant | 3.0 ± 0.0b | 3.7 ± 0.5a | 3.3 ± 0.5ab | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 |
Means with standard errors were calculated from 7 replicates. Each replicate comprised a maximum of 4 but sometimes 3 fruits.
Lycopene content, TSS and TAA content were significantly affected by cultivation system in the polytunnel experiment but these differences were largely derived from low values associated with the poor initial growth in the DWC system due to use of growth sponge. The composition of fruits from plants grown in DI and S were similar. In the glasshouse experiment, cultivation system did not significantly affect TSS and TAA although values were always lowest in tomatoes cultured in soil and more similar in the two hydroponic systems. There was a significant effect of growth system on lycopene content (F2,18 = 32.59, p = 1.04 × 10−6) in the glasshouse experiment and fruit of soil grown tomatoes were significantly lower in lycopene content than fruit grown in both hydroponic systems (Table 4). An additional quality parameter was assessed in the glasshouse experiment and there was a significant effect of growth system on β-carotene content (F2,18 = 10.8, p = 8.29 × 10-4) and tomatoes grown in DWC had significantly higher levels of β-carotene and the lowest levels were produced by plants grown in soil.
Table 4.
Composition of lycopene, β-carotene, total soluble solids (TSS) and total antioxidant activity (TAA) content of tomatoes growing in soil, and two hydroponic systems; deep water culture and drip irrigation.
| Parameter | Growth environment |
Growth environment |
||||
|---|---|---|---|---|---|---|
| Polytunnel experiment (2018) |
Glasshouse experiment (2019) |
|||||
| DWC | DI | S | DWC | DI | S | |
| Lycopene (mg/100 m L−1) | 0.008 ± 0.007b | 0.02 ± 0.006a | 0.016 ± 0.01ab | 0.16 ± 0.03a | 0.14 ± 0.01a | 0.06 ± 0.02b |
| β-carotene (mg/100 m L−1) | n.d. | n.d. | n.d. | 0.098 ± 0.03a | 0.039 ± 0.03b | 0.028 ± 0.01b |
| TSS (Brix°) | 2.9 ± 0.9b | 4.2 ± 0.3a | 3.6 ± 0.6ab | 3.4 ± 0.6 | 3.7 ± 0.24 | 3.0 ± 0.8 |
| TAA (%) | 20.2 ± 10.1b | 53.2 ± 26.1a | 67.3 ± 9.0a | 79.1 ± 9.1 | 77.3 ± 16.7 | 65.9 ± 25.1 |
n.d. = β-carotene levels in tomatoes were not assessed in the polytunnel experiment. Means with standard errors were calculated from 7 replicates.
4. Discussion
The benefits of hydroponic culture systems have often been stated but rarely are cultivation systems compared directly while trying to control as far as possible environmental variables that may confound comparisons, particularly when comparing crop quality. Different growth environments may impact variables more or less significantly for example warm and cool environments impact vapour pressure deficit and therefore evaporative stress (Lu et al., 2015) and may reduce or exaggerate differences in water use (Leonardi et al., 2000). Therefore, it is useful to compare different environments to allow more generalisable conclusions. Other environmental parameters may influence nutritional comparisons. High electrical conductivity (EC) levels can increase TSS in the tomato fruits (Rodríguez et al., 2019), and nutrients can affect total antioxidant capacity (Fanasca et al., 2006). To overcome these limitations and potential confounding or obfuscating factors we tested the three growth systems in both a polytunnel and a glasshouse, two different growth environments commonly used for tomato cultivation.
The sponge used as support medium in the DWC system in the polytunnel experiment in 2018 resulted in relatively poor establishment of the seedlings although once established plants displayed similar growth rates across all three systems (Fig.3), biomass accumulation was not significantly different and fruit production was completed in all three growth systems. The two experiments exposed tomatoes to quite different average temperatures, 8.7 °C ± 0.4 in the polytunnel and 18.9 ± 1.0 in the glasshouse (Fig. S2). High and low temperatures are abiotic stresses that can damage horticultural crops and tomato is a warm season crop, air temperatures lower than 10 °C will delay seed germination, inhibit vegetative development, reduce fruit set, and impair fruit ripening (Haghighi et al., 2014). Therefore, we may expect that some results from the polytunnel experiment in 2018 might be influenced by low temperature. For example, water use was generally lower in the polytunnel perhaps due to higher humidity in cooler air and thus lower vapour pressure gradient. The variance in data was higher and lycopene levels lower (Table 4) and different to some published values (Barbagallo et al., 2013) in the cooler polytunnel grown tomatoes perhaps reflecting the slower and less complete ripening of the fruit. Light and temperature have no significant effect on final sugar content of tomatoes (Gautier et al., 2008), this was reflected in similar TSS values (Table 4) despite the environmental differences between the two experiments and in the similarity in TSS levels to those published in other studies (Nasrin et al., 2008).
Despite the contrasting environments used there were several consistencies between experiments, the main one being the difference in water use between cultivation systems. Soil-grown tomatoes used more water than either hydroponic system and DWC used less than DI (Table 2). DWC was the only growing system to use a similar amount of water in both experiments, despite the different climatic conditions, suggesting that water use in this growing system is less susceptible to variation in climate. Product water use (PWU) was always higher for soil-grown plants in both experiments, meaning that it required more water to produce the same yield. Although we anticipated the design of the two hydroponic systems would limit evaporation the difference in WUE (based on transpired water) and PWU (based on total water use) was consistent and water use efficiency was not due to difference in evaporation only. The significant reduction to transpired water, particularly in DWC, may be caused by the partial induction of responses that are common in flooded plants which include stomatal closure (Kozlowski, 1984) and in tomatoes flooding induces stomatal closure due to leaf dehydration linked to a low root hydraulic permeability (Dell’Amico et al., 2001).
Using less water may reflect a lower yield or lower biomass accumulation and therefore less leaf transpiration but this was not the case and yields and biomass accumulations were not significantly different except for DWC in the first experiment (Table 3). Most nutrient use was similar between cultivation systems, especially in the polytunnel; however, some differences in nutrient levels in the solution were noted such as levels of phosphate, potassium and ammonium (Fig.2); however, these differences were either not consistent, occurred only at a specific date of sampling or were negligible due to a very low amounts e.g. ammonium concentration was below 1 ppm. Therefore, we anticipate that any differences in nutritional content (Table 4) were not caused by variation in nutrient content (Simone Fanasca et al., 2006; Zelená et al., 2009; Erba et al., 2013).
There is a common perception that soil grown tomatoes have better taste or are healthier. The taste of tomato fruit is determined by ripening conditions, and when tomatoes are ripened off-the-vine the palatability decreases significantly (Sorrequieta et al., 2013). Together with breeding that has focused on yield rather than quality (Klee and Tieman, 2013), this may contribute to the perception of poor taste in tomatoes. Thus the ‘lack of taste’ may be more about early harvesting and the ripening conditions during shipping and less about growth conditions. We demonstrated that the soilless cultivation systems tested produced at least as good tomatoes as the soil system with similar sugar levels particularly in the glasshouse experiment. Fruit quality represents the complex interactions of many chemicals and the analysed parameters in this study might not be fully representative; however, lycopene and β-carotene levels were significantly lower in tomatoes growing in soil in the glasshouse experiment representing a lower nutritional value from this system. The higher levels of these pigments in hydroponically grown plants, particularly those plants growing in DWC, may be induced by increased levels of reactive oxygen species often experienced by flooded plants (Rasheed et al., 2018). Whatever the stomatal and stress response mechanisms responsible for improved WUE and nutritional contents, these did not limit biomass accumulation in hydroponically grown plants. We demonstrate that with a similar fertilization regime provided to quite different cultivation systems, it is possible to produce similar or better crop quality in hydroponic systems compared to soil and to produce the crop using significantly less water.
CRediT authorship contribution statement
Salvatore Gaetano Verdoliva: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft. Dylan Gwyn-Jones: Funding acquisition, Writing - review & editing. Andrew Detheridge: Resources, Writing - review & editing. Paul Robson: Funding acquisition, Conceptualization, Resources, Writing - original draft, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Knowledge Economy Skills Scholarships (KESS 2) led by Bangor University on behalf of the HE sector in Wales, and partly funded by the Welsh Government’s European Social Fund (ESF) convergence programme for West Wales and the Valleys and the UK Biotechnology and Biological Sciences Research Council (grant number BBS/E/W/0012843A).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.scienta.2021.109896.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ajlouni S., Kremer S., Masih L. Lycopene content in hydroponic and non-hydroponic tomatoes during postharvest storage. Food Australia. 2001;53(5):1–14. [Google Scholar]
- AOAC . Vol 1. Association of Official Agricultural Chemists; Washington, D.C: 1990. pp. 136–138. (AOAC Official Methods of Analysis). 15th. [Google Scholar]
- Barbagallo R.N., Di Silvestro I., Patanè C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in Mediterranean climate. J. Sci. Food Agric. 2013;93(6):1449–1457. doi: 10.1002/jsfa.5913. [DOI] [PubMed] [Google Scholar]
- Bar-Yosef B. Vol. 2008. Academic Press; San Diego: 2008. Fertigation management and crops response to solution recycling in semi-closed greenhouses; pp. 341–424. (Soilless Culture: Theory and Practice). [Google Scholar]
- Benoit F., Ceustermans N. Some qualitative aspects of tomatoes grown on NFT. Soilless Cult. 1987;3(3):3–7. [Google Scholar]
- Brekke B., Edwards J., Knapp A. Selection and adaptation to high plant density in the Iowa stiff stalk synthetic maize (L.) population. Crop Sci. 2011;51(5):1965. [Google Scholar]
- Carmassi G., Incrocci L., Maggini R., Malorgio F., Tognoni F., Pardossi A. Modelling salinity build-up in recirculating nutrient solution culture. J. Plant Nutr. 2005;28(3):431–445. [Google Scholar]
- Cronin D.A., Walsh P.C. A comparison of chemical composition, firmness and flavour in tomatoes from peat and nutrient film growing systems. Irish J. Food Sci. Technol. 1983;Vol. 7 [Google Scholar]
- Darko E., Heydarizadeh P., Schoefs B., Sabzalian M.R. Photosynthesis under artificial light: the shift in primary and secondary metabolism. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2014;369(1640) doi: 10.1098/rstb.2013.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Amico J., Torrecillas A., Rodríguez P., Morales D., Sánchez-Blanco M.J. Differences in the effects of flooding the soil early and late in the photoperiod on the water relations of pot-grown tomato plants. Plant Sci. 2001;160(3):481–487. doi: 10.1016/s0168-9452(00)00409-x. [DOI] [PubMed] [Google Scholar]
- Despommier D. Farming up the city: the rise of urban vertical farms. Trends in Biotechnology. Trends Biotechnol. 2013;31(7):388–389. doi: 10.1016/j.tibtech.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Erba D., Casiraghi M.C., Ribas-Agustí A., Cáceres R., Marfà O., Castellari M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013;31(2):245–251. [Google Scholar]
- Fanasca S., Colla G., Maiani G., Venneria E., Rouphael Y., Azzini E., Saccardo F. Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J. Agric. Food Chem. 2006;54(12):4319–4325. doi: 10.1021/jf0602572. [DOI] [PubMed] [Google Scholar]
- Gautier H., Diakou-Verdin V., Bénard C., Reich M., Buret M., Bourgaud F. How Does Tomato Quality (Sugar, Acid, and Nutritional Quality) Vary with Ripening Stage, Temperature, and Irradiance? J. Agric. Food Chem. 2008;27(4):1241–1250. doi: 10.1021/jf072196t. 56. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J. Natl. Cancer Inst. 1999;91(4):317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- Gonnella M., Serio F., Conversa G., Santamaria P. Production and nitrate content in lamb’s lettuce grown in floating system. Acta Hortic. 2004;644:61–68. [Google Scholar]
- Granges A. Tomates en culture hydroponique sur film nutritif (NFT). Influence du mode de culture sur la composition chimique des fruits. Revue Suisse Vitic. Arboric. Hortic. 1980;12:59–63. [Google Scholar]
- Grillas S., Lucas M., Bardopoulou E., Sarafopoulos S., Voulgari M. Perlite based soilless culture systems: current commercial applications and prospects. Acta Hortic. 2001;548:105–114. [Google Scholar]
- Gruda N. Do soilless culture systems have an influence on product quality of vegetables? J. Appl. Bot. Food Qual. 2009;82:141–147. [Google Scholar]
- Haghighi M., Abolghasemi R., Teixeira da Silva J.A. Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Hortic. 2014;178:231–240. [Google Scholar]
- Hernández Suárez M., Rodríguez Rodríguez E.M., Díaz Romero C. Mineral and trace element concentrations in cultivars of tomatoes. Food Chem. 2007;104(2):489–499. [Google Scholar]
- Hernández Suárez M., Rodríguez Rodríguez E.M., Díaz Romero C. Chemical composition of tomato (Lycopersicon esculentum) from Tenerife, the Canary Islands. Food Chem. 2008;106(3):1046–1056. [Google Scholar]
- Ho L.C. The contribution of plant physiology in glasshouse tomato soilless culture. Acta. Hortic. 2004;648:19–25. [Google Scholar]
- Hussain Shah A., Munir S.U., Hussain Shah S. Evaluation of two nutrient solutions for growing tomatoes in a non-circulating hydroponics system. Sarhad J. Agric. 2011;27(274):557–567. [Google Scholar]
- Jensen M.H. Hydroponics worldwide. Acta. Hortic. 1999;481:719–729. [Google Scholar]
- Jiang C., Johkan M., Hohjo M., Tsukagoshi S., Ebihara M., Nakaminami A., Maruo T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci. Hortic. 2017;222:221–229. [Google Scholar]
- Kaya C., Higgs D. Response of tomato (Lycopersiconesculentum L.) cultivars to foliar application of zinc when grown in sand culture at low zinc. Sci. Hortic. 2002;93(1):53–64. [Google Scholar]
- Klee H.J., Tieman D.M. Genetic challenges of flavor improvement in tomato. Trends in Genetics. Elsevier Curr. Trends. 2013;29(4):257–262. doi: 10.1016/j.tig.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Kozlowski T.T. Plant responses to flooding of soil. BioScience. 1984;34(3):162–167. [Google Scholar]
- Kunsch U., Scharer H., Durr P., Hurter J., Martinoni A., J., & G, Sulser H., Seeger B. Do differences exist in the quality between soilless and conventionally produced tomatoes and head lettuce. Mitt. Geb. Lebensmittelunters. Hyg. 1994;85:18–21. [Google Scholar]
- Lu N., Nukaya T., Kamimura T., Zhang D., Kurimoto I., Takagaki M. Control of vapor pressure deficit (VPD) in greenhouse enhanced tomato growth and productivity during the winter season. Sci. Hortic. 2015;197:17–23. [Google Scholar]
- Marcotte M. Irradiation as a disinfestation method — update on methyl bromide phase out, regulatory action and emerging opportunities. Radiat. Phys. Chem. 1998;52(6):85–90. [Google Scholar]
- Matthiessen J.N., Kirkegaard J.A. Biofumigation and enhanced biodegradation: opportunity and challenge in Soilborne Pest and disease management. Crit. Rev. Plant Sci. 2006;25(3):235–265. [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004;26(2):211–219. [Google Scholar]
- Nasrin T., Molla M.M., Alamgir Hossaen M., Alam M.S., Yasmin L. Effect pf postharvest treatments on shelf life and quality of tomato. Bangladesh J. Agril. Res. 2008;33(3):579–585. [Google Scholar]
- Nederhoff E.M., Stanghellini C. Vol. 2010. 2010. pp. 52–59. (Water Use Efficiency of Tomatoes - in Greenhouses and Hydroponics). 115. [Google Scholar]
- Patanè C., Pellegrino A., Saita A., Siracusa L., Ruberto G., Barbagallo R. Mediterranean long storage tomato as a source of novel products for the agrifood industry: nutritional and technological traits. Lwt Food Sci. Technol. 2017;85:445–448. [Google Scholar]
- Pieper J.R., Barrett D.M. 2008. Effects of Organic and Conventional Production Systems on Quality and Nutritional Parameters of Processing Tomatoes. [Google Scholar]
- Polycarpou P., Neokleous D., Dora Chimonidou I.P. A closed system for soil less culture adapted to the Cyprus conditions. Proceedings of the ICID Conference 7-11 Decemeber 2004; Cairo Egypt. Options Mediterrameemes, Series B, No 53; 2005. pp. 237–241. 241. [Google Scholar]
- Premuzic Z., Bargiela M., Garvia A., Rendina A., Iorio A. Calcium, Iron, potassium, phosporus and vitamin C content of organic and hydroponic tomatoes. Hortic. Sci. 1998;33(2) [Google Scholar]
- Rasheed R., Iqbal M., Ashraf M.A., Hussain I., Shafiq F., Yousaf A., Zaheer A. Glycine betaine counteracts the inhibitory effects of waterlogging on growth, photosynthetic pigments, oxidative defence system, nutrient composition, and fruit quality in tomato. J. Hortic. Sci. Biotechnol. 2018;93(4):385–391. [Google Scholar]
- Sorrequieta A., Abriata L., Boggio S., Valle E. Off-the-Vine ripening of tomato fruit causes alteration in the primary metabolite composition. Metabolites. 2013;3(4):967–978. doi: 10.3390/metabo3040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Montreal Protocol on Substances that Deplete the Ozone Layer . 1987. The Montreal Protocol on Substances That Deplete the Ozone Layer. [Google Scholar]
- The R Development Core Team . 2010. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Touliatos D., Dodd I.C., McAinsh M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016;5(3):184–191. doi: 10.1002/fes3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Standards for Grades of Fresh Tomatoes . 1991. United States Standards for Grades of Fresh Tomatoes. [Google Scholar]
- Wang L.-S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO; 2005. Promoting Fruit and Vegetable Consumption Around the World. [Google Scholar]
- Willcox J.K., Catignani G.L., Lazarus S. Tomatoes and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2003;43(1):1–18. doi: 10.1080/10408690390826437. [DOI] [PubMed] [Google Scholar]
- Wu M., Kubota C. Effects of high electrical conductivity of nutrient solution and its application timing on lycopene, chlorophyll and sugar concentrations of hydroponic tomatoes during ripening. Sci. Hortic. 2008;116(2):122–129. [Google Scholar]
- Yamamoto T., Ultra V.U., Tanaka S., Sakurai K., Iwasaki K. Effects of methyl bromide fumigation, chloropicrin fumigation and steam sterilization on soil nitrogen dynamics and microbial properties in a pot culture experiment. Soil Sci. Plant Nutr. 2008;54(6):886–894. [Google Scholar]
- Zelená E., HolaSoVá M., ZElEný F., FIEdlEroVá V., NoVotná P., Houška M. Effect of sulphur fertilisation on lycopene content and colour of tomato fruits. Special Issue Czech J. Food Sci. 2009:27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.