Abstract
Due to the common symptoms of COVID-19, patients are similar to influenza-like illness. Therefore, the detection method would be crucial to discriminate between SARS-CoV-2 and influenza virus-infected patients. In this study, CRISPR-Cas12a-based detection was applied for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza A virus, and influenza B virus which would be a practical and attractive application for screening of patients with COVID-19 and influenza in areas with limited resources. The limit of detection for SARS-CoV-2, influenza A, and influenza B detection was 10, 103, and 103 copies/reaction, respectively. Moreover, the assays yielded no cross-reactivity against other respiratory viruses. The results revealed that the detection of influenza virus and SARS-CoV-2 by using RT-RPA and CRISPR-Cas12a technology reaches 96.23% sensitivity and 100% specificity for SARS-CoV-2 detection. The sensitivity for influenza virus A and B detections was 85.07% and 94.87%, respectively. In addition, the specificity for influenza virus A and B detections was approximately 96%. In conclusion, the RT-RPA with CRISPR-Cas12a assay was an effective method for the screening of influenza viruses and SARS-CoV-2 which could be applied to detect other infectious diseases in the future.
Keywords: Detection, SARS-CoV-2, influenza A, influenza B, CRISPR-Cas, RT-RPA
Impact statement
In this study, the isothermal RT-RPA and CRISPR-Cas12a were applied for simple, rapid turn-around time, and cost-effective detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza A virus (IAV), and influenza B virus (IBV) which would be practical and attractive for the screening of patients with COVID-19 and influenza in rural areas.
Introduction
The pandemic of coronavirus disease starting in 2019 (COVID-19) is caused by the infection of SARS-CoV-2.1 This emerging disease becomes global health problem and causes major economic loss worldwide. Globally, there were increasing positive infected cases and deaths have been reported (https://www.worldometers.info/coronavirus). The symptoms of COVID-19 are varying from asymptomatic to severe respiratory failure and death. The common symptoms of COVID-19 patients are similar to influenza-like illness including high fever, cough, sore throat, headache, and difficultly breathe. In severe cases, the pneumonia might be found with acute respiratory distress syndrome (ARDS) within a week after infected.2 However, 20% of COVID-19 positive cases are asymptomatic but contagious.3 According to the similar symptoms observed between COVID-19 and influenza, the detection method would be very crucial to discriminate SARS-CoV-2 from influenza viruses-infected patients. One of the nucleotide-based methods approved by the US Food and Drug Administration (FDA) for SARS-CoV-2 detection is CRISPR-based test.4 This technique combines recombinase polymerase amplification (RPA) with the CRISPR-Cas system for amplification and detection of specific DNA target sequences. The Cas protein cleaves the specific target via recognition of the matching sequences to CRISPR RNA (crRNA). The presence of a matched DNA sequence activates the collateral effect of Cas12a; then the fluorescent reporter will be cleaved from the quencher and then illuminates the fluorescent signal. Without the need of a specialized instrument, this method can be used at point-of-need area including airports, schools, and local community hospitals.5
Materials and methods
Primers and CRISPR RNA
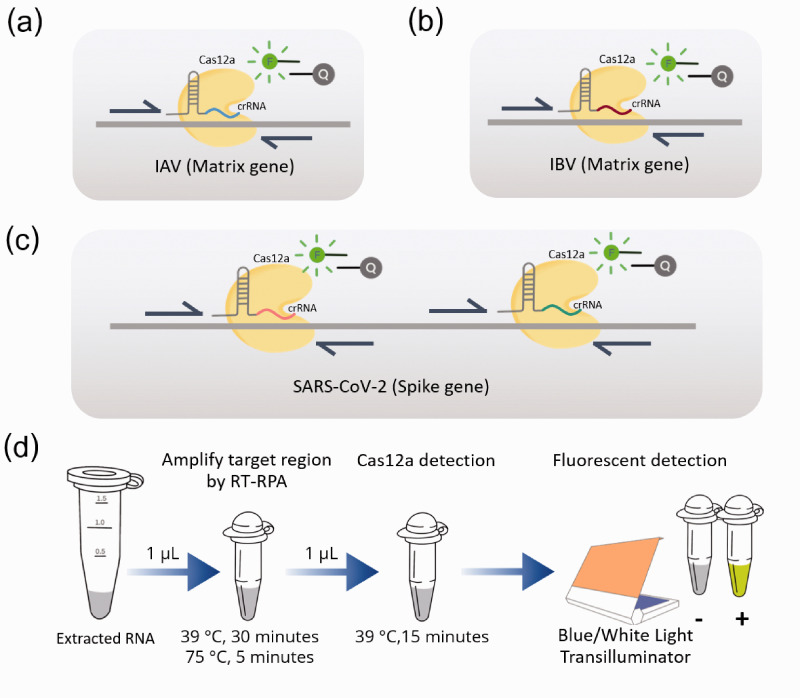
The primers and CRISPR RNA (crRNA) specific to IAV and IBV were designed to target on Matrix (M) gene (Figure 1(a) and 1(b)), whereas those for SARS-CoV-2 were specific to Spike (S) gene (Figure 1(c)). The sequences of primers and crRNA were selected from conserved regions of sequences available in NCBI (https://www.ncbi.nlm.nih.gov/nuccore) and GISAID (https://www.gisaid.org/).
Figure 1.
Detection of influenza A virus, influenza B virus, and SARS-CoV-2 based on RT-RPA and CRISPR-Cas12a assay. Schematic representation of specific primers and crRNAs for (a) M gene of influenza A virus, (b) M gene of influenza B virus, and (c) spike gene of SARS-CoV-2. (d) The workflow of the overall process for rapid detection based on RT-RPA and CRISPR-Cas12a assay within 1 h. (A color version of this figure is available in the online journal.)
Oligonucleotides for crRNA template (4 μM) were annealed to T7 promoter primer (4 μM) in 1× T4 DNA Ligase Buffer (Thermo Scientific, USA) and incubated in the following condition; 95°C for 3 min, 65°C for 3 min, 42°C for 5 min, and 37°C for 45 min. Then crRNA was transcribed by using Riboprobe® In Vitro Transcription Systems (Promega, USA) according to the manufacturer protocol. Transcribed crRNA (approximately 40 nucleotides) was purified by miRNA isolation kit (Geneaid, Taiwan) and quantified by Qubit™ microRNA Assay Kit (Thermo Scientific™, USA).
Reverse transcription recombinase polymerase amplification
To amplified RNA target, isothermal reverse transcription and amplification were conducted by RT-RPA technique. Briefly, each detection assay consisting of 0.48 μM forward primer, 0.48 μM reverse primer (Table 1), 200 U RevertAid Reverse Transcriptase (Thermo Scientific™, USA), and rehydration buffer was mixed with lyophilized reaction of the TwistAmp® Basic Kit (TwistAmp®, UK). Then 1 µL of template and 14 mM MgOAC were added before incubating at 39°C for 30 min followed by heat inactivation at 75°C for 5 min.
Table 1.
Oligonucleotides used for qRT-PCR, RT-RPA, and crRNA production.
| Assay | Primer name | Sequence (5′ ⟶ 3′) | Product size (bp) | Ref. |
|---|---|---|---|---|
| RT-RPA | SARS2_spike1-F | CCACTGAGAAGTCTAACATAATAAGAGGCTG | 201 | This study |
| SARS2_spike1-R | AATAAACTCTGAACTCACTTTCCATCCAACT | |||
| SARS2_spike2-F | AATCTATCAGGCCGGTAGCACACCTTGTAAT | 166 | ||
| SARS2_spike2-R | TCCACAAACAGTTGCTGGTGCATGTAGAAGTT | |||
| FluA-M-RPA-F | ACCGAGGTCGAAACGTATGTTCTCTCTATC | 219 | ||
| FluA-M-RPA-R | TCTACGCTGCAGTCCTCGCTCACTGGGCAC | |||
| FluB_M-RPA-F | CACAATTGCCTACYTGCTTTCATTRACAGAAGA | 168 | ||
| FluB_M-RPA-R | GCACCAATTAGTGCTTTCTGTATATCAGTTAAG | |||
| GAPDH_RPA-F | ACCAGGTGGTCTCCTCTGACTTCAACAGCG | 178 | ||
| GAPDH_RPA-R | ATGGCCCACATGGCCTCCAAGGAGTAAGAC | |||
| qRT-PCR | SARS-CoV2-N-F | CACATTGGCACCCGCAATC | 128 | 7 |
| SARS-CoV2-N-R | GAGGAACGAGAAGAGGCTTG | |||
| SARS-CoV2-N-P | FAM-ACTTCCTCAAGGAACAACATTGCCA-BBQ | |||
| SARS-CoV-RdRp-F | GTGARATGGTCATGTGTGGCGG | 100 | 7 | |
| SARS-CoV-RdRp-R | CARATGTTAAASACACTATTAGCATA | |||
| SARS-CoV-RdRp-P-Pan | FAM-CCAGGTGGWACRTCATCMGGTGATGC-BBQ | |||
| SARS-CoV2-RdRp-P | FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ | |||
| SARS-CoV2-E-F | ACAGGTACGTTAATAGTTAATAGCGT | 113 | 7 | |
| SARS-CoV2-E-R | ATATTGCAGCAGTACGCACACA | |||
| SARS-CoV2-E-P | FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ | |||
| FluA_M_F | CATGGARTGGCTAAAGACAAGACC | 126 | 6 | |
| FluA_M_R | AGGGCATTTTGGACAAAKCGTCTA | |||
| FluA_M_P | FAM-ACGC T CACCG T GCCC A GT-BHQ1 | |||
| FluB_MP_F | CTCTGTGCTTTRTGCGARAAAC | 233 | ||
| FluB_MP_R | CCTTCYCCATTCTTTTGACTTGC | |||
| FluB_M_P | Cy5-TCAG C A A T GA A C ACAGCAA-BHQ3 | |||
| crRNA | SARS2-S1 | UAAUUUCUACUAAGUGUAGAUGAUUCGAAGACCCAGUCCCU | This study | |
| SARS2-S2 | UAAUUUCUACUAAGUGUAGAUCAAUCAUAUGGUUUCCAACC | |||
| FluA-M | UAAUUUCUACUAAGUGUAGAUGCCAUUCCAUGAGAGCCUCA | |||
| FluB-M | UAAUUUCUACUAAGUGUAGAUACCUAGACUCAGCCUUGGAA | |||
| GAPDH | UAAUUUCUACUAAGUGUAGAUCUGGUAUGACAACGAAUUUG |
Note: Bold nucleotide represents LNA residue; Underline sequence represents spacer sequence.
Limit of detection and cross-reactivity testing
Standard RNA was prepared by using T7 Riboprobe® in vitro transcription systems (Promega, USA) as previously described.6 The limit of detection was performed in triplicate by using 10-fold serial dilution of each standard in vitro transcribed RNA (ranging from 107 to 10 copies/reaction) as templates for RT-RPA with CRISPR-Cas12a. The limit of detection was observed from reaction tubes containing the lowest concentration of RNA template that yielded fluorescent signal. The assays were tested against several human respiratory viruses including respiratory syncytial virus, coronaviruses (OC43 and NL63 strains), bocavirus, rhinovirus, parainfluenza virus, adenovirus, metapneumovirus, IAV, IBV, and SARS-CoV-2
Sample collection and processing
Nasopharyngeal and/or throat swab samples were collected from patients with ILI. The viral RNA was extracted from 200 µL of swab samples by using GenUP™ Virus RNA kit (BiotechRabbit, German) following manufacturing instruction. The samples were stored and tested at Center of Excellence in Clinical Virology, Chulalongkorn University. All clinical samples in this study were tested with blind.
CRISPR-Cas12a detection
The reaction of CRISPR-Cas12a-based-nucleic acid detection was consisted of 30 nM crRNA, 330 nM of EnGen® Lba Cas12a (Cpf1) (New England Biolabs, USA), 200 nM fluorescent reporter/quencher probe, 1× NEBuffer 2.0 reaction buffer (New England Biolabs, USA), and 1 µL of RPA product in a final volume of 15 µL. For COVID-19 detection, there are two crRNAs to bind to two specific regions within the spike gene of the SARS-CoV-2 (Figure 1(c)). The Cas reaction was incubated at 39°C for 15 min and then the fluorescent signal was visualized by BluPAD Dual LED Blue/White Light Transilluminator (BIO-HELIX, Taiwan) (Figure 1(d)). The interpretation of the CRISPR-Cas assay was recorded from three independent persons and then the concordant results obtained from at least two of three interpreters were used as the end results.
The results of RT-RPA with CRISPR-Cas assay were compared to the qRT-PCR (RdRp, E, and N genes) which served as the gold standard for diagnosis. The qRT-PCR for IAV, IBV, and SARS-CoV-2 was performed by using primers/probes (Table 1), reaction mixtures, and thermal profiles as previously described.6,7 The performances of the assay including sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and accuracy were calculated by web-based diagnostic test evaluation calculator implemented in MEDCALC® easy-to-use statistical software (https://www.medcalc.org/calc/diagnostic_test.php).
Results
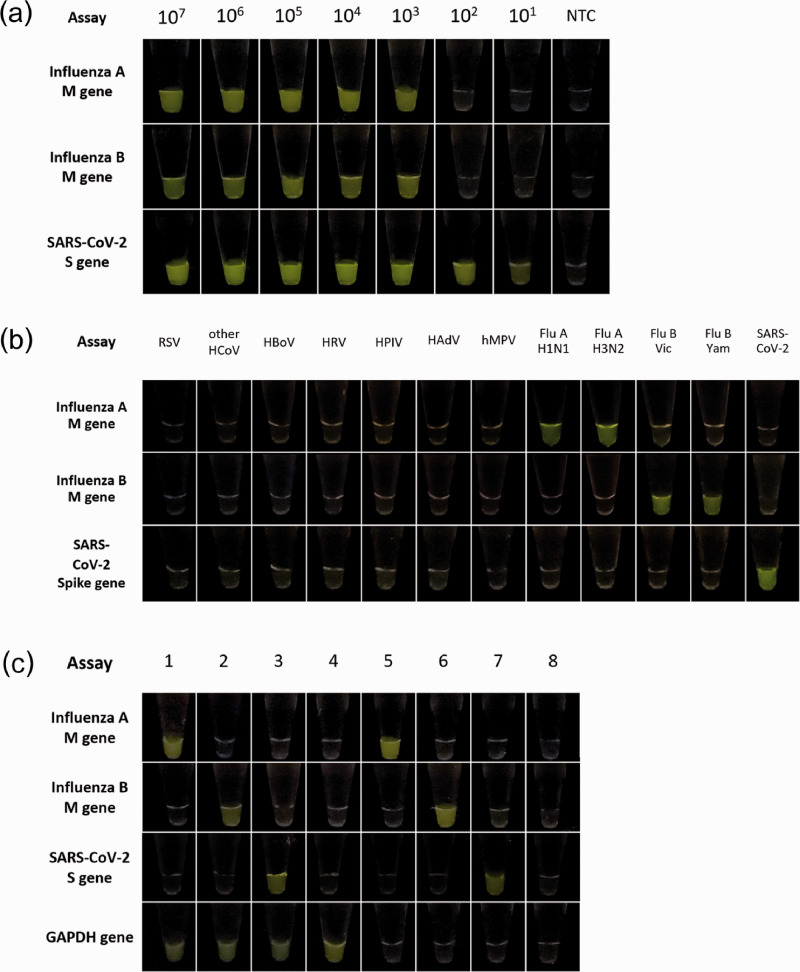
The results revealed that the limit of detection for IAV and IBV was approximately 103 copies/reaction, whereas the SARS-CoV-2 can be detected as low as 10 copies/reaction (Figure 2(a)). For testing of cross-reactivity, the RNA sample for other seven respiratory viruses was used. The results demonstrated that the primers of RT-RPA and crRNA of CRISPR-Cas12a yielded very high specificity without any cross-reactivity with other respiratory viruses (Figure 2(b)).
Figure 2.
Detection of SARS-CoV-2, influenza A virus, and influenza B virus based on CRISPR-Cas12a. (a) Limit of detection for influenza A virus, influenza B virus, and SARS-CoV-2 based on RT-RPA and CRISPR-Cas12a assay. NTC: no template control. (b) Cross-reactivities testing of RT-RPA and CRISPR-Cas12a assay against several respiratory viruses. RSV: respiratory syncytial virus; HCoV: human coronavirus strains OC43 and NL63; HBoV: human bocavirus; HRV: human rhinovirus; HPIV: human parainfluenza virus; HAdV: human adenovirus; hMPV: human metapneumovirus; Flu A-H1N1: influenza A virus subtype pH1N1; Flu A-H3N2: influenza A virus subtype H3N2; Flu B-Vic: influenza B virus Victoria lineage; Flu B-Yam: influenza B virus Yamagata lineage and SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. (c) Representative results of RT-RPA and CRISPR-Cas12a assay for IAV, IBV, SARS-CoV-2, and GAPDH by testing with RNA extracted from clinical samples. Samples 1 and 5 were as IAV-positive samples; samples 2 and 6 were IBV-positive samples; samples 3 and 7 were SARS-CoV-2-positive samples; sample 4 was a negative sample; sample 8 was interpreted as inadequate specimen collection. (A color version of this figure is available in the online journal.)
To evaluate the sensitivity, specificity, and diagnostic accuracy of the assay, 164 and 130 RNA samples were used for testing with SARS-CoV-2 and influenza viruses, respectively. Detection of the fluorescent signals obtained from the assays for IAV, IBV, SARS-CoV-2, and GAPDH was used for interpretation of the results (Figure 2(c)). Briefly, samples positive for the SARS-CoV-2 yielded positive fluorescent signal in the SARS-CoV-2 tube with or without GAPDH. In the same way, interpretations of influenza A and influenza B would be determined as positive samples when fluorescent signal was observed in the influenza A or influenza B tube, respectively. When the sample yielded no fluorescent signal from any tube as well as the GAPDH internal control, the samples should be determined as inadequate sample collection.
The results of the assay were compared with the gold standard detection results from qRT-PCR. The assay for IAV was tested with 67 IAV-positive samples (Ct 14.0–39.6; average Ct 29.7) and 63 IAV-negative samples, while IBV was evaluated with 39 IBV-positive samples (Ct 16.2–37.1; average Ct 26.8) and 91 IBV-negative samples. Finally, the detection of SARS-CoV-2 was validated against 53 SARS-CoV-2-positive samples (Ct 10.5–34.7, average Ct for RdRp, E, and N genes was 24.7, 22.8, 26.1, respectively) and 111 SARS-CoV-2-negative samples. The performance of the assay yielded very high specificity (100%), sensitivity (96.23%), diagnostic accuracy (98.78%) as summarized in Table 2.
Table 2.
Numbers of samples tested and the performance of each CRISPR-Cas12a-based assay.
| Parameters | Influenza AM gene | Influenza BM gene | SARS-CoV-2S gene |
|---|---|---|---|
| Total samples | 130 | 130 | 164 |
| True positive | 57 | 37 | 51 |
| True negative | 61 | 88 | 111 |
| False positive | 2 | 3 | 0 |
| False negative | 10 | 2 | 2 |
| Sensitivity | 85.07% | 94.87% | 96.23% |
| Specificity | 96.83% | 96.70% | 100.00% |
| Diagnostic accuracy | 90.77% | 96.15% | 98.78% |
Discussion
The procedure of isothermal amplification (RPA or LAMP) followed by fluorescent detection based on the CRISPR-Cas system was recently developed for pathogen detection.8 This study is the first report for RT-RPA and CRISPR-based assay for influenza virus detection. The turn-around time of the assay from extracted RNA to fluorescent detection was approximately 50–60 min. The cost for instruments was approximately 2000 USD including pipettes, heat blocks, microcentrifuges, and the LED Blue/White Light Transilluminator. The cost of reagents and consumables for RNA extraction and detection assay was approximately 15–20 USD/sample which is comparatively cheaper than the Sherlock™ CRISPR SARS-CoV-2 kit (30 USD/sample). However, the cost might vary depending on the brands of reagents and instruments in different countries.
Previously, several assays have been designed for specific detection of N, E, and RdRp genes of SARS-CoV-2.9 Our study focused on the detection of conserved regions within the S gene of SARS-CoV-2. Although the nucleotide substitutions might occur within the S gene in the future, which raises the concerns for the detection assay. Therefore, two regions of primers and crRNAs within the S gene were used in the same reaction to avoid the false negative results due to the mismatch of primers or crRNA. Currently, there has been no obvious mutation occurring within the primers and crRNA binding sites for both SAR-CoV-2 and influenza viruses based on thousands of sequences alignment, implying that the assay would be effective for detections of those viruses worldwide.
The detection of SARS-CoV-2 using RT-LAMP and CRISPR-Cas12a on N gene yielded the limit of detection approximately 10 copies/µL and 95% sensitivity based on the evaluation with 83 RNA from clinical samples.9 Besides, RT-RAA couple with CRISPR-Cas12a detection on RdRp and E gene was reported with the limit of detection as 10 copies/µL.10,11 Our assay for SARS-CoV-2 detection reached the limit of detection as 10 copies/reaction. The performance of the assay yielded very high specificity (100%), sensitivity (96.23%), and diagnostic accuracy (98.78%) as summarized in Table 2. Recently, the RT-LAMP with colorimetric assay was developed for direct on-the-spot detection of SARS-CoV-2 from crude saliva samples which is very simple and yielded 93% true positive rate (Ct ≤ 28.8 of E gene) and 96.8% true negative rate when tested with the crude sample,12 whereas our assay provided 96.23% true positive rate (Ct ≤ 34.7 of E gene) and 100% true negative rate when tested with extracted RNA.
In conclusion, the benefits of the assays include simple, rapid turn-around time (<1 h), cost-effectiveness (reagents and instruments), and can be applied in areas with limited resources. The assays would be practical and attractive for large-scale screening in several populations and systematic retesting of individuals in order to quarantine infected patients and control the spread of COVID-19 and influenza.13
ACKNOWLEDGEMENTS
We would like to thank the staffs of the Bangpakok 1 Hospital, Bangpakok 9 International Hospital, Chum Phae Hospital and Institute for Urban Disease Control and Prevention (IUDC), Thailand for assistant with specimen collections.
Authors’ contributions: OM performed the experiments and drafted the manuscript. PN, SR, and NC assisted in sample processing and CRISPR-Cas detection. KK assisted in drafting the manuscript. JC, JP, NS, and PV assisted in qRT-PCR. YP provided the clinical samples. SP designed the study, revised the manuscript, and coordinated the project.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study protocols were approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University (IRB No. 337/57 and IRB No. 302/63).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided in part by Ratchada Pisek Sompoch Fund, Faculty of Medicine, Chulalongkorn University [RA(P0)002/63]; National Research Council of Thailand (NRCT); Thailand Research Fund (TRF) [RSA6180035]; National Science and Technology Development Agency (NSTDA) [P-17–51377]; and Chulalongkorn University Graduate Scholarship To Commemorate The 72nd Anniversary of His Majesty King Bhumibol Adulyadej.
ORCID iD: Sunchai Payungporn https://orcid.org/0000-0003-2668-110X
References
- 1.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med 2020; 382:692–4 [DOI] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim G-U, Kim M-J, Ra SH, Lee J, Bae S, Jung J, Kim S-H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect 2020. doi: 10.1016/j.cmi.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guglielmi G. First CRISPR test for the coronavirus approved in the United States. Nature 2020. doi: 10.1038/d41586-020-01402-9 [DOI] [PubMed]
- 5.Li C, Ren L. Recent progress on the diagnosis of 2019 novel coronavirus. Transbound Emerg Dis 2020;67:1485–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suwannakarn K, Payungporn S, Chieochansin T, Samransamruajkit R, Amonsin A, Songserm T, Chaisingh A, Chamnanpood P, Chutinimitkul S, Theamboonlers A, Poovorawan Y. Typing (a/B) and subtyping (H1/H3/H5) of influenza a viruses by multiplex real-time RT-PCR assays. J Virol Methods 2008; 152:25–31 [DOI] [PubMed] [Google Scholar]
- 7.Corman V, Bleicker T, Brünink S, Drosten C, Zambon M, Organization WH. Diagnostic detection of Wuhan coronavirus 2019 by real-time RT-PCR. Geneva: World Health Organization, 2020
- 8.Xiang X, Qian K, Zhang Z, Lin F, Xie Y, Liu Y, Yang Z. CRISPR-cas systems based molecular diagnostic tool for infectious diseases and emerging 2019 novel coronavirus (COVID-19) pneumonia. J Drug Target 2020;28:727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, Zorn K, Gopez A, Hsu E, Gu W, Miller S, Pan CY, Guevara H, Wadford DA, Chen JS, Chiu CY. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol 2020;38:870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Zhong M, Liu Y, Ma P, Dang L, Meng Q, Wan W, Ma X, Liu J, Yang G, Yang Z, Huang X, Liu M. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci Bull 2020;65:1436–1439 [DOI] [PMC free article] [PubMed]
- 11.Guo L, Sun XH, Wang XG, Liang C, Jiang HP, Gao QQ, Dai MY, Qu B, Fang S, Mao YH, Chen YC, Feng GH, Gu Q, Wang RR, Zhou Q, Li W. SARS-CoV-2 detection with CRISPR diagnostics. Cell Discov 2020. doi: 10.1038/s41421-020-0174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Assa N, Naddaf R, Gefen T, Capucha T, Hajjo H, Mandelbaum N, Elbaum L, Rogov P, King DA, Kaplan S, Rotem A, Chowers M, Szwarcwort-Cohen M, Paul M, Geva-Zatorsky N. Direct on-the-spot detection of SARS-CoV-2 in patients. Exp Biol Med 2020; 245:1187–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koks S, Williams RW, Quinn J, Farzaneh F, Conran N, Tsai SJ, Awandare G, Goodman SR. COVID-19: time for precision epidemiology. Exp Biol Med 2020; 245:677–9 [DOI] [PMC free article] [PubMed] [Google Scholar]