Abstract
As a selective estrogen receptor β agonist, the natural flavonoid liquiritigenin reportedly inhibits invasiveness of breast cancer cells, but its specific role and mechanism remain largely unclear. In this study, cells from the triple negative breast cancer lines MDA-MB-231 and BT549 were incubated with different concentrations of liquiritigenin. The results indicated that low concentrations had no significant cytotoxic effect, whereas high concentrations decreased viability of both MDA-MB-231 and BT549 cells. Liquiritigenin treatment also resulted in increased apoptosis and enhanced Caspase3 activity. After liquiritigenin treatment, we observed decreased invasive and migratory capacities of cells, as well as upregulated E-cadherin and downregulated N-cadherin, vimentin, and MMP9. Interestingly, liquiritigenin increased the mRNA and protein expression of breast cancer 1 (BRCA1). It also increased p21 and growth arrest and DNA-damage-inducible 45 alpha (GADD45A) levels, accompanied by decreased cellular DNA methyltransferase (DNMT) activity and downregulation of DNMT1, DNMT3a, and DNMT3b. These findings suggest that liquiritigenin can inhibit malignant behavior of triple negative breast cancer cells by inhibiting DNMT activity and increasing BRCA1 expression and its transcriptional activity. Liquiritigenin thus may be a promising candidate for the treatment of breast cancer.
Keywords: Breast cancer, liquiritigenin, breast cancer 1, DNA methyltransferase
Impact statement
Triple negative breast cancer (TNBC) is an aggressive cancer with a poor prognosis and higher metastatic rates and relapse frequencies than other breast cancers. Natural flavonoid liquiritigenin reportedly inhibits invasiveness of TNBC MDA-MB-231 cells, but its specific role and mechanism remain unclear. This study administered different doses of liquiritigenin into TNBC cell lines MDA-MB-231 and BT549, and found that it hindered cell proliferation, increased apoptosis, and repressed cell invasion and migration. BRCA1 exerts multiple functions and is closely related to the occurrence and development of breast cancer. Interestingly, the mRNA and protein expression of BRCA1 increased after liquiritigenin administration. Liquiritigenin also upregulated two downstream genes of BRCA1 (p21 and DNA-damage-inducible 45 alpha), decreased cellular DNA methyltransferase (DNMT) activity, and reduced BRCA1 promoter methylation. Thus, liquiritigenin may be a promising candidate for the treatment of TNBC due to its inhibition of DNMT activity and upregulation of BRCA1.
Introduction
Breast cancer is the most common malignant cancer worldwide, causing millions of deaths every year.1 Despite advances in chemotherapy, surgical resection, and endocrine therapy, prognoses remain poor among some patients, especially those with high-metastatic types.1 Approximately 10% to 20% patients of all breast cancer have triple negative breast cancer (TNBC), meaning their immunohistochemical results are estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor 2 negative.2,3 This aggressive type of breast cancer usually presents with a higher tumor grade, larger tumor size, higher metastatic rate, and more frequent recurrence than other breast cancers.3,4 TNBC thus is associated with shorter survival and increased mortality rates, and requires more effective therapeutic agents and treatment strategies.
Breast cancer 1 (BRCA1) protein is a versatile tumor suppressor that has critical roles in DNA damage repair and transcriptional regulation.5,6 It is closely related to mammary tumorigenesis and progression.4,5 BRCA1 mutation or its promoter methylation is closely associated with increased risks of familial and sporadic breast cancers,4,5,7 as well as the incidence of TNBC.8 Inactivation of BRCA1 reportedly induces malignant cell behaviors, including reduced apoptosis and accelerated proliferation, invasion, and migration.5 Therefore, inhibiting BRCA1 mutations or reducing its promoter methylation is helpful for prevention and treatment of breast cancer.
Due to their various physiological activities and low risk of side effects, many diet-derived compounds have been examined for their chemopreventive and therapeutic properties.9 Many flavonoids, such as triptolide and genistein, have been reported to affect DNA damage repair, DNA methylation, and BRCA1 expression and thus affect the progression of breast cancer.2,9,10 Moreover, epidemiological studies show a negative correlation between breast cancer risk and flavonoid intake.11 Liquiritigenin is a natural flavonoid isolated from Glycyrrhizae radix that exhibits various pharmacological effects, including estrogenic,12 anti-oxidative,13 anti-inflammatory,14 and anti-tumor activities.15 Liquiritigenin reportedly restrains proliferation, invasion, and epithelial-to-mesenchymal transition (EMT) of colorectal cancer HCT116 cells.12 Through inhibiting VEGF, liquiritigenin reduces angiogenesis and tumor growth, a mouse model of HeLa cells.16 In B16F10 melanoma cells and mice model, liquiritigenin reportedly potentiates cisplatin-mediated inhibitory effects on invasion and metastasis.17 As an ERβ agonist, liquiritigenin also reportedly represses invasiveness of TNBC cell lines HCC1806 and HCC1937.18 However, its specific role and related mechanism in breast cancer progression and whether it affects BRCA1 expression remain largely unclear.
In this study, cells from the TNBC cell lines MDA-MB-231 and BT549 were incubated with liquiritigenin, and the results indicated that liquiritigenin hindered cell proliferation, increased apoptosis, and repressed cell invasion and migration in both. The possible mechanisms in this process are related to the liquiritigenin-mediated decrease in DNA methyltransferase (DNMT) activity and upregulation of BRCA1.
Materials and methods
Cell culture and experimental treatments
Cells from the human TNBC cell lines MDA-MB-231 and BT549 were purchased from the cell bank of the Chinese Academy of Medical Science (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (ATTC, Manassas, VA, USA) with 10% fetal bovine serum (Gibco Life Technologies Inc., Grand Island, NY, USA) and maintained at 37°C in 5% CO2. Liquiritigenin was obtained from Sigma-Aldrich (Shanghai, China) and diluted to final concentrations of 0, 10, 20, 50, and 100 µM in culture broth. After 24 h of incubation with or without liquiritigenin, follow-up detection of cell viability, apoptosis, and invasion was carried out.
Cell viability detection
Following the manufacturer's instructions, cell viability of MDA-MB-231 and BT549 cells was detected using a Cell Counting Kit-8 (CCK-8; Sigma). Cells were seeded into 96-well plates and allowed to attach for 24 h, followed by 24 h of exposure to the different doses of liquiritigenin. Then, 10 µL of CCK-8 solution was added to each well and incubated at 37°C for an additional 3 h. Absorbance was detected at 450 nm using a microplate reader (Thermo, San Jose, CA, USA).
Colony formation assay
Cells were inoculated on 6-well plates at 1 × 103 cells per well and cultured with different doses of liquiritigenin for 12 days until visible colonies were formed. After fixing and staining, the numbers of colonies were counted under a microscope.
Apoptosis analysis
As recommended by the manufacturer, a Cell Death Detection ELISA kit (Roche, Nutley, NJ, USA) was used to assess apoptosis. Absorbance at 405 nm was read, and apoptosis was measured as a percentage of control. In addition, a Caspase3 colorimetric assay kit (Promega, Madison, WI, USA) was applied to evaluate Caspase3 activity.
Cell invasion and migration determination
After different administration, cells were collected separately and suspended in serum-free medium. Transwell chambers with 8-µM pores (Corning, Inc., Corning, NY, USA) were employed to assess cell invasion and migration. To detect cell invasion, 200 µL of cell suspension containing 2 × 104 cells was added to the upper chamber, which was precoated with Matrigel (BD Biosciences, Bedford, MA, USA). Concomitantly, 500 µL of Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum was added to the lower chamber. After 24 h of incubation, the invaded cells were fixed with methanol and stained with 0.5% crystal violet (Sigma), then counted at ×200 magnification in five random fields. The experimental procedures for migration detection were similar to the invasion assay, except that the upper chambers were not pre-coated with Matrigel.
Cell cycle analysis
Following the manufacturer's instructions, cell cycle was analyzed by a cell cycle Detection Kit (KeyGen Biotech, Nanjing, China). Cells were seeded into 6-well plates and exposed to different doses of liquiritigenin for 24 h, then were harvested and fixed with ice-cold 70% ethanol at 4°C overnight. After washed with phosphate-buffered saline and stained with propidium iodide/RNase A for 30 min in the dark, cells were detected by FACS Calibur Flow Cytometer (BD Biosciences).
Quantitative real-time polymerase chain reaction
Following the manufacturer's recommended procedure, total RNA from cells in each group was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). After obtaining template cDNA by reverse transcription, a quantitative real-time polymerase chain reaction (PCR) was conducted on an ABI Prism 7900HT Sequence Detector (Applied Biosystems, Foster City, CA, USA). The relative mRNA levels were calculated using the 2−ΔΔCT method and normalized to β-actin.
Western blot assay
After extracting with RIPA buffer (Beyotime, Shanghai, China) and quantitating by a BCA assay, 20 µg of protein sample was separated via SDS-polyacrylamide gel electrophoresis and electro-transferred onto polyvinylidene difluoride membranes (Millipore, Watford, UK). The membranes were blocked with 5% skim milk for 1 h, followed by overnight incubation with primary antibodies against E-cadherin, N-cadherin, vimentin, matrix metallopeptidase (MMP) 9, BRCA1, p21, GADD45A, DNMT1, DNMT3a, DNMT3b, and β-actin at 4°C. After that, the horseradish peroxidase-linked secondary antibodies were incubated with these membranes at room temperature for 1 h. Finally, the bands were visualized and imaged using an ECL detection system (Pierce, Rockford, IL, USA). Densitometry was performed using Image J software. The relative protein levels were normalized to β-actin.
DNA methyltransferase activity analysis
After the different treatments, the nuclear extract of cells was obtained via a nuclear extraction reagent (Pierce). The overall DNMT activity was then quantified using the EpiQuik™ DNA Methyltransferase Activity/Inhibition Assay Ultra Kit (EpiGentek, Brooklyn, NY, USA) in line with the manufacturers’ instructions.
BRCA1 promoter methylation determination
The genomic DNA of cells in each group was extracted using the phenol-chloroform-isoamyl alcohol method. After bisulfite modification, methylation-specific PCR was performed, as described previously.19 The unmethylated (U)- and methylated (M)-specific primers for BRCA1 were synthesized by Shanghai Sangon Biotech (Shanghai, China) as follows: BRCA1, U-sense: 5′-TTGAGAGGTTGTTGTTTAGTGG-3′ and U-antisense: 5′-AACAAACTCACACCACACAA-3′; M-sense: 5′-TCGTGGTAACGGAAAAGCGC-3′ and M-antisense 5′-AACGAACTCACGCCGCGCAA-3′.
Statistical analysis
All of the final data were determined from at least three repeated experiments and are presented as the mean ± standard deviation (SD). Differences between two groups or more groups were analyzed via t test or one-way analysis of variance using GraphPad Prism 7.0 software. P < 0.05 was considered statistically significant.
Results
Liquiritigenin inhibited cell survival and colony formation of TNBC cells
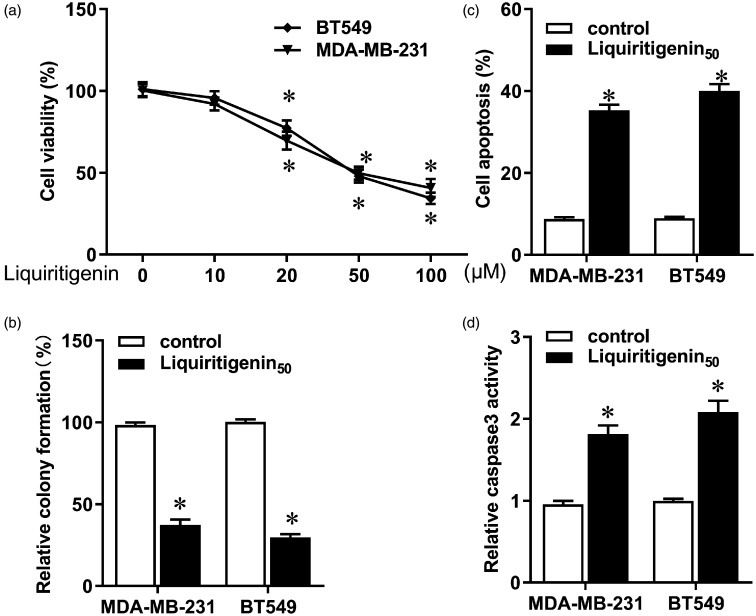
Different doses of liquiritigenin were added to the medium containing TNBC cell lines MDA-MB-231 and BT549. The CCK-8 assay showed that low doses of liquiritigenin had no significant cytotoxic effect on these two cell lines, whereas high concentrations of liquiritigenin notably reduced viability of both cells (Figure 1(a), P < 0.05). Liquiritigenin also significantly decreased colony formation in MDA-MB-231 and BT549 cells (Figure 1(b), P < 0.05). Moreover, apoptosis increased in response to liquiritigenin treatment (Figure 1(c), P < 0.05), accompanied by enhanced Caspase3 activity (Figure 1(d), P < 0.05). These results indicate that liquiritigenin impeded the occurrence and progression of TNBC by inhibiting tumorigenesis and inducing apoptosis of breast cancer cells.
Figure 1.
Effect of liquiritigenin on proliferation and apoptosis of triple negative breast cancer cells. MDA-MB-231 and BT549 cells were incubated with different concentrations of liquiritigenin (0, 10, 20, 50, and 100 µM) for 24 h. (a) Cell viability was analyzed using a CCK-8 assay. (b) Colony formation was assessed to evaluate cellular growth and tumorigenesis. (c) Apoptosis was detected using a Cell Death Detection ELISA kit. (d) Activity of Caspase3 was determined using a Caspase3 colorimetric assay kit. Data are expressed as the mean ± SD from three independent experiments. *P < 0.05 versus the control group.
Liquiritigenin repressed invasion, migration, and EMT of TNBC cells
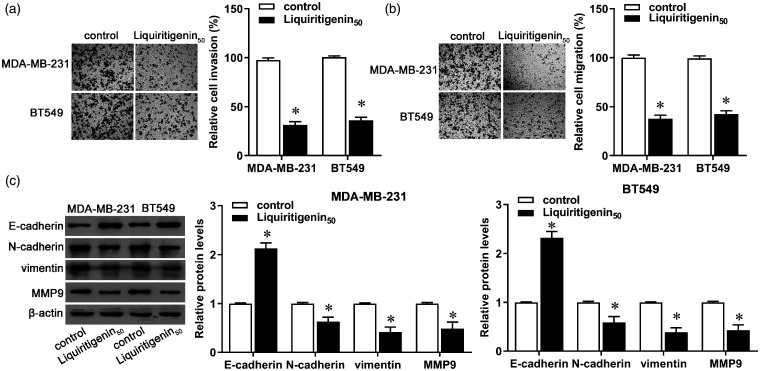
Compared with the control group, the invasive and migratory capacities of MDA-MB-231 and BT549 cells decreased more in cells treated with liquiritigenin (Figure 2(a) and (b), P < 0.05). Synchronously, the protein expression of E-cadherin increased, whereas protein expression of N-cadherin, vimentin, and MMP9 decreased in both cell lines (Figure 2(c), P < 0.05). These results indicate that liquiritigenin prevented progression of TNBC by inhibiting invasion, migration, and EMT in breast cancer cells.
Figure 2.
Effect of liquiritigenin on invasion and migration of triple negative breast cancer cells. MDA-MB-231 and BT549 cells were incubated with 50 µM of liquiritigenin for 24 h. (a) Cell invasion was measured via Transwell invasion assay. (b) Cell migration was measured via Transwell migration assay. (c) Protein expression of E-cadherin, N-cadherin, vimentin, and MMP9 was evaluated by Western blot. β-actin was used as the loading control. The bar graph shows the relative protein expression level. Data are expressed as the mean ± SD from three independent experiments. *P < 0.05 versus the control group. (A color version of this figure is available in the online journal.)
Liquiritigenin enhanced BRCA1 expression in TNBC cells
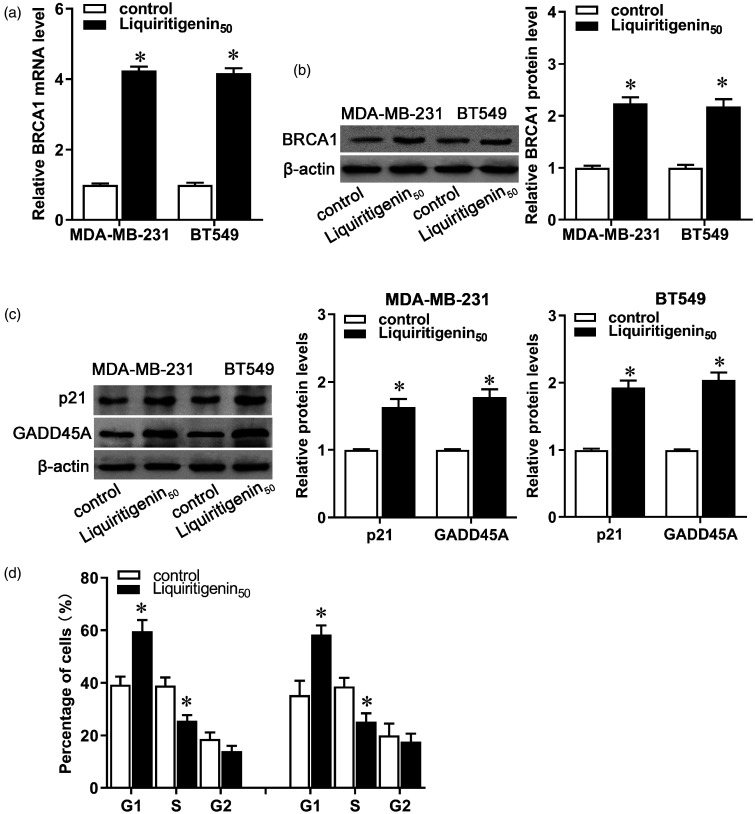
Given the functional diversity of BRCA1 and its importance in breast cancer, we measured the expression of BRCA1 and some of its downstream and interacting genes in liquiritigenin-treated breast cancer cells. After administration with liquiritigenin, both mRNA and protein expression of BRCA1 increased in MDA-MB-231 and BT549 cells (Figure 3(a) and (b), P < 0.05). Moreover, protein expression of p21 and GADD45A increased after liquiritigenin treatment in both MDA-MB-231 and BT549 cells (Figure 3(c), P < 0.05), along with increase in the G1 fraction accompanied by a significant decrease in the S phase (Figure 3(d), P < 0.05). These results indicate that liquiritigenin may inhibit carcinogenesis and progression of breast cancer by affecting BRCA1 expression and its mediated transcriptional activity.
Figure 3.
Effect of liquiritigenin on BRCA1 expression and transcriptional activity. MDA-MB-231 and BT549 cells were exposed to 50 µM of liquiritigenin for 24 h. (a) The mRNA expression of BRCA1 was detected by real-time PCR. (b) Protein expression of BRCA1 was detected by Western blot. (c) Protein expression of p21 and GADD45A was evaluated by Western blot. (d) Cell cycle was analyzed by a cell cycle Detection Kit. Data are expressed as the mean ± SD from three independent experiments. *P < 0.05 versus the control group.
Liquiritigenin antagonized DNMTs activity and BRCA1 promoter methylation in TNBC cells
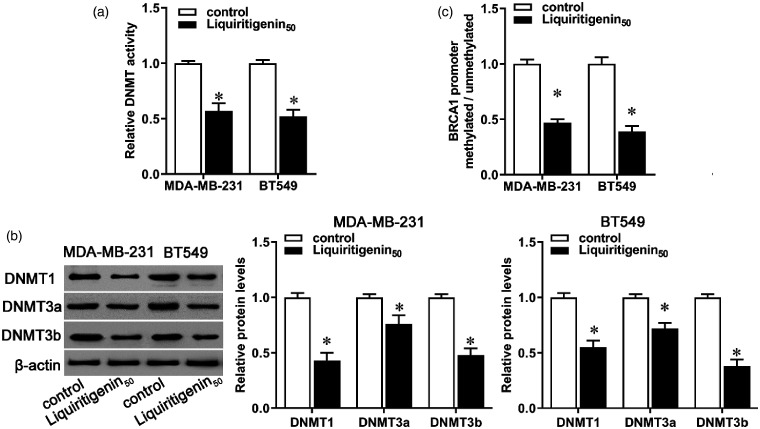
Previous studies have shown a close link between low expression of BRCA1 and its promoter methylation.20 Previous studies showed that BRCA1 promoter methylation was observed in about 20%–60% of TNBC patients.8,21,22 The methylation status of BRCA1 is significantly correlated with the co-expression of DNMTs.23 In our study, administration with liquiritigenin markedly decreased cellular DNMT activity in both MDA-MB-231 and BT549 cells (Figure 4(a), P < 0.05), accompanied by decreased expression of DNMT1, DNMT3a, and DNMT3b (Figure 4(b), P < 0.05). Synchronously, BRCA1 methylation was lower in liquiritigenin-treated cells than in the control (Figure 4(c), P < 0.05). These results indicate that liquiritigenin may regulate BRCA1 expression via regulating DNMT activity and BRCA1 promoter methylation.
Figure 4.
Effect of liquiritigenin on decreased cellular DNA methyltransferase activity and BRCA1 promoter methylation. MDA-MB-231 and BT549 cells were incubated with 50 µM of liquiritigenin for 24 h. (a) Decreased cellular DNA methyltransferase (DNMT) activity was evaluated using a commercial kit. (b) The protein expression of DNMT1, DNMT3a, and DNMT3b was evaluated by Western blot. (c) BRCA1 promoter methylation was evaluated by methylation-specific PCR. Data are expressed as the mean ± SD from three independent experiments. *P < 0.05 versus the control group. #P < 0.05 versus the liquiritigenin group.
Discussion
Despite great advances in medical technology, treatment and prognosis for breast cancer, especially TNBC is still poor at so far. Flavonoids exhibit cytotoxic, anti-proliferative, and pro-apoptotic properties and thus have been used as anti-cancer agents.24 Epidemiological studies show that high intake of flavonoids is negatively correlated with breast cancer risk.11 Because flavonoids have a chemical structure that is similar to that of estrogen, they offer potential advantages in preventing and treating breast cancer.24,25 Liquiritigenin is a natural flavonoid that inhibits invasiveness of TNBC cell lines HCC1806 and HCC1937.18 However, its specific role and related mechanism in breast cancer progression remain largely unclear. In this study, we demonstrated that liquiritigenin can impede progression of breast cancer by reducing proliferation, inducing apoptosis, and inhibiting invasion, migration, and EMT of breast cancer cells. The possible mechanisms in this process may relate to a liquiritigenin-mediated decrease in DNMT activity and upregulation of BRCA1.
Flavonoids reportedly inhibit the occurrence and development of breast cancer.24 For example, the flavonoids apigenin, genistein, hesperidin, naringin, and quercetin exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer MDA-MB-231 and MCF-7 cells.24 By downregulating the PTHrP pathway, total flavonoids from Scutellaria barbata D. Don inhibit human breast to bone metastasis.26 As a natural flavonoid, liquiritigenin also has anti-cancer properties in breast cancer.18 In this study, high concentrations of liquiritigenin inhibited proliferation, induced apoptosis, and repressed invasion and migration in both MDA-MB-231 and BT549 cells. These results align with those of a previous study suggesting that liquiritigenin inhibits proliferation, invasion, and EMT of colorectal cancer cells.12 Moreover, similar to its pro-apoptotic and inhibition effects on EMT in HCT116 cells,12 as well as its inhibitory effects on invasion and metastasis of B16F10 melanoma cells,17 liquiritigenin treatment also enhanced Caspase3 activity, upregulated E-cadherin, and downregulated N-cadherin, vimentin, and MMP9 expression in both MDA-MB-231 and BT549 cells. These findings suggest that liquiritigenin can inhibit the occurrence and development of breast cancer by reducing cellular proliferation, inducing apoptosis, and inhibiting cell invasion and migration.
BRCA1, which is poorly expressed in breast cancer cells, is a multifunctional tumor suppressor involved in many biological processes, including cell cycle regulation, apoptosis, DNA damage repair, gene transcriptional regulation, and ubiquitination.5,27 BRCA1 deregulation or mutation is closely associated with increased risk of familial and sporadic breast cancers.4,5,7,28 Previous studies showed that BRCA1 promoter methylation was observed in about 20%–60% of TNBC patients.8,21,22 Evaluation of BRCA1 methylation was significantly related to poor overall survival of breast cancer.29 Inactivation of BRCA1 can exacerbate malignant cell behaviors, including reduced apoptosis and accelerated proliferation, invasion, and migration.5 Astragalus flavone shows anti-proliferation, pro-apoptotic, and anti-metastasis effects in breast cancer with increased expression of BRCA1.10 A combination treatment of quercetin and curcumin has been shown to induce anti-cancer activity against TNBC cells by enhancing BRCA1 expression.28 Similarly, our data showed that liquiritigenin treatment significantly increased mRNA and protein expression of BRCA1 in both MDA-MB-231 and BT549 cells. Moreover, the expression of p21 and GADD45A, two downstream genes of BRCA1, was upregulated by liquiritigenin. Studies have shown that inhibiting p21 transcription can lead to poor clinical outcomes of TNBC by promoting tumor cell growth and drug resistance,30 and upregulating p21 by PVT1 knockdown can repress cell proliferation, migration, and EMT of TNBC cells.31 GADD45A is a cell growth arrest and DNA damage repair gene that can interact with BRCA1, thereby maintaining genomic stability, regulating cell cycle arrest and apoptosis, removing carcinogenic adducts, and preventing tumorigenesis.32 Liquiritigenin thus may affect TNBC progression by upregulating BRCA1 and affecting its transcriptional activity.
Low expression of BRCA1 is closely related to its promoter methylation.20 Flavonoids can affect DNA methylation and genome stability.33,34 Moreover, DNMT can affect the expression of BRCA1.35 In this study, liquiritigenin led to a significant decrease in cellular DNMT activity and BRCA1 methylation, accompanied by decreased expression of DNMT1, DNMT3a, and DNMT3b. DNMT3b expression is reportedly responsible for the epigenetic inactivation of BRCA1.36 Reduced DNMT1 expression can lead to hypomethylation of the BRCA1 promoter and increase its expression in MDA-MB-231 cells.37 Thus, liquiritigenin may affect BRCA1 expression via regulating DNMT activity and BRCA1 promoter methylation.
Conclusions
Liquiritigenin exerts anti-cancer effects in TNBC by inhibiting proliferation, promoting apoptosis, and suppressing invasion and migration of TNBC cells. The possible mechanisms may be partially related to a liquiritigenin-mediated decrease in DNMT activity and upregulation of BRCA1. However, the mechanisms and specific molecular regulatory networks need further research.
Authors' contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; FL, HZ, HG, DC, NZ, JD, JMY, and P D conducted the experiments; HG, BBZ, and LY analyzed the data and interpreted experimental results; FL and HZ wrote the manuscript All authors contributed to and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Key Research Projects of Henan Higher Education (grant number 15A320085).
ORCID iD
Fang Liang https://orcid.org/0000-0001-6312-5925
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet 2017; 389:1134–50 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Sun C, Zhang L, Chi X, Ji J, Gao X, Wang Y, Zhao Z, Liu L, Cao X, Yang Y, Mao W. Triptolide interferes with XRCC1/PARP1-mediated DNA repair and confers sensitization of triple-negative breast cancer cells to cisplatin. Biomed Pharmacother 2019; 109:1541–6 [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Gou Q, Wang Q, Zhong X, Zheng H. The role of BRCA status on prognosis in patients with triple-negative breast cancer. Oncotarget 2017; 8:87151–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo PK, Zhang Y, Wolfson B, Gernapudi R, Yao Y, Duru N, Zhou Q. Dysregulation of the BRCA1/long non-coding RNA NEAT1 signaling axis contributes to breast tumorigenesis. Oncotarget 2016; 7:65067–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnolo AP, Romagnolo DF, Selmin OI. BRCA1 as target for breast cancer prevention and therapy. Anticancer Agents Med Chem 2015; 15:4–14 [DOI] [PubMed] [Google Scholar]
- 6.Ali R, Rakha EA, Madhusudan S, Bryant HE. DNA damage repair in breast cancer and its therapeutic implications. Pathology 2017; 49:156–65 [DOI] [PubMed] [Google Scholar]
- 7.Su L, Zhang J, Meng H, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y. Prevalence of BRCA1/2 large genomic rearrangements in Chinese women with sporadic triple-negative or familial breast cancer. Clin Genet 2018; 94:165–9 [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Shan L, Wang F, Wang J, Wang F, Shen G, Liu X, Wang B, Yuan Y, Ying J, Yang H. Hypermethylation of BRCA1 gene: implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Breast Cancer Res Treat 2015; 150:479–86 [DOI] [PubMed] [Google Scholar]
- 9.Mirza S, Sharma G, Parshad R, Gupta SD, Pandya P, Ralhan R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J Breast Cancer 2013; 16:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Zhang H, Zhu Z, Zhang Q, Ma X, Cui Z, Yao T. Effects and mechanism of flavonoids from astragalus complanatus on breast cancer growth. Naunyn-Schmiedeberg's Arch Pharmacol 2015; 388:965–72 [DOI] [PubMed] [Google Scholar]
- 11.Takemura H, Sakakibara H, Yamazaki S, Shimoi K. Breast cancer and flavonoids – a role in prevention. Curr Pharm Des 2013; 19:6125–32 [DOI] [PubMed] [Google Scholar]
- 12.Meng FC, Lin JK. Liquiritigenin inhibits colorectal cancer proliferation, invasion, and epithelial-to-mesenchymal transition by decreasing expression of Runt-related transcription factor 2. Oncol Res 2019; 27:139–46 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Huang Z, Sheng Y, Chen M, Hao Z, Hu F, Ji L. Liquiritigenin and liquiritin alleviated MCT-induced HSOS by activating Nrf2 antioxidative defense system. Toxicol Appl Pharmacol 2018; 355:18–27 [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Shi J, Li H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-kappaB and NLRP3 inflammasome pathways. Biomed Pharmacother 2018; 106:976–82 [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Wong HK, Feng YB, Zhang ZJ. Liquiritigenin exhibits antitumour action in pituitary adenoma cells via ras/ERKs and ROS-dependent mitochondrial signalling pathways. J Pharm Pharmacol 2014; 66:408–17 [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Wang Y, Xie S, Zhou Y, Ren X, Li X, Cai Y. Liquiritigenin induces mitochondria-mediated apoptosis via cytochrome c release and caspases activation in HeLa cells. Phytother Res 2011; 25:277–83 [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Wu Y, Wang Y, Zhou M, Yan S, Chen Z, Gu D, Cai Y. Liquiritigenin potentiates the inhibitory effects of cisplatin on invasion and metastasis via downregulation MMP-2/9 and PI3 K/AKT signaling pathway in B16F10 melanoma cells and mice model. Nutr Cancer 2015; 67:761–70 [DOI] [PubMed] [Google Scholar]
- 18.Hinsche O, Girgert R, Emons G, Grundker C. Estrogen receptor beta selective agonists reduce invasiveness of triple-negative breast cancer cells. Int J Oncol 2015; 46:878–84 [DOI] [PubMed] [Google Scholar]
- 19.Papoutsis AJ, Borg JL, Selmin OI, Romagnolo DF. BRCA-1 promoter hypermethylation and silencing induced by the aromatic hydrocarbon receptor-ligand TCDD are prevented by resveratrol in MCF-7 cells. J Nutr Biochem 2012; 23:1324–32 [DOI] [PubMed] [Google Scholar]
- 20.Poh W, Dilley RL, Moliterno AR, Maciejewski JP, Pratz KW, McDevitt MA, Herman JG. BRCA1 promoter methylation is linked to defective homologous recombination repair and elevated miR-155 to disrupt myeloid differentiation in myeloid malignancies. Clin Cancer Res 2019; 25:2513–22 [DOI] [PubMed] [Google Scholar]
- 21.Ignatov T, Poehlmann A, Ignatov A, Schinlauer A, Costa SD, Roessner A, Kalinski T, Bischoff J. BRCA1 promoter methylation is a marker of better response to anthracycline-based therapy in sporadic TNBC. Breast Cancer Res Treat 2013; 141:205–12 [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Stecklein SR, Kimler BF, Sethi G, Petroff BK, Phillips TA, Tawfik OW, Godwin AK, Jensen RA. The prognostic value of BRCA1 promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res 2014; 3:–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai X, Fu Y, Xue H, Guo K, Song Z, Yu Z, Jia T, Yan Y, Zhao L, Mi X, Wang E, Zheng Z, Zhao H, Yao W, Wei M. BRCA1 promoter hypermethylation in sporadic epithelial ovarian carcinoma: association with low expression of BRCA1, improved survival and co-expression of DNA methyltransferases. Oncol Lett 2014; 7:1088–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabala-Dzik A, Rzepecka-Stojko A, Kubina R, Iriti M, Wojtyczka RD, Buszman E, Stojko J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7 - a comparative study. Cell Mol Biol 2018; 64:1–10 [PubMed] [Google Scholar]
- 25.Pang BB, Chu YK, Yang H. Anti-breast cancer mechanism of flavonoids. Zhongguo Zhong Yao Za Zhi 2018; 43:913–20 [DOI] [PubMed] [Google Scholar]
- 26.Zheng X, Kang W, Liu H, Guo S. Inhibition effects of total flavonoids from Sculellaria barbata D. Don on human breast carcinoma bone metastasis via downregulating PTHrP pathway. Int J Mol Med 2018; 41:3137–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahusen TJ, Kim SJ, Miao K, Huang Z, Xu X, Deng CX. BRCA1 function in the intra-S checkpoint is activated by acetylation via a pCAF/SIRT1 axis. Oncogene 2018; 37:2343–50 [DOI] [PubMed] [Google Scholar]
- 28.Kundur S, Prayag A, Selvakumar P, Nguyen H, McKee L, Cruz C, Srinivasan A, Shoyele S, Lakshmikuttyamma A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J Cell Physiol 2018; 234:11103–18 [DOI] [PubMed] [Google Scholar]
- 29.Nindrea RD, Harahap WA, Aryandono T, Lazuardi L. Association of BRCA1 promoter methylation with breast cancer in Asia: a meta-analysis. Asian Pac J Cancer Prev 2018; 19:885–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu RZ, Vo TM, Jain S, Choi WS, Garcia E, Monckton EA, Mackey JR, Godbout R. NFIB promotes cell survival by directly suppressing p21 transcription in TP53-mutated triple-negative breast cancer. J Pathol 2019; 247:186–98 [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Wang R, Ye Z, Wang Y, Li X, Chen W, Zhang M, Cai C. PVT1 affects EMT and cell proliferation and migration via regulating p21 in triple-negative breast cancer cells cultured with mature adipogenic medium. Acta Biochim Biophys Sin 2018; 50:1211–8 [DOI] [PubMed] [Google Scholar]
- 32.Pietrasik S, Zajac G, Morawiec J, Soszynski M, Fila M, Blasiak J. Interplay between BRCA1 and GADD45A and its potential for nucleotide excision repair in breast cancer pathogenesis. Int J Mol Sci 2020; 21:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoud AM, Al-Alem U, Ali MM, Bosland MC. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J Steroid Biochem Mol Biol 2015; 152:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee N, Kumar AP, Ghosh R. DNA methylation and flavonoids in genitourinary cancers. Curr Pharmacol Rep 2015; 1:112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z, Xiao Q, Zhao L, Ren J, Bai X, Sun M, Wu H, Liu X, Song Z, Yan Y, Mi X, Wang E, Jin F, Wei M. DNA methyltransferase 1/3a overexpression in sporadic breast cancer is associated with reduced expression of estrogen receptor-alpha/breast cancer susceptibility gene 1 and poor prognosis. Mol Carcinog 2015; 54:707–19 [DOI] [PubMed] [Google Scholar]
- 36.Butcher DT, Rodenhiser DI. Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer 2007; 43:210–9 [DOI] [PubMed] [Google Scholar]
- 37.Tang H, Liu P, Yang L, Xie X, Ye F, Wu M, Liu X, Chen B, Zhang L, Xie X. miR-185 suppresses tumor proliferation by directly targeting E2F6 and DNMT1 and indirectly upregulating BRCA1 in triple-negative breast cancer. Mol Cancer Ther 2014; 13:3185–97 [DOI] [PubMed] [Google Scholar]