Abstract
Long non-coding RNAs are a kind of endogenous ncRNAs with a length of more than 200 bp. Accumulating evidence suggests that long non-coding RNAs function as pivotal regulators in tumorigenesis and progression. However, their biological roles in breast cancer remain largely unknown. Here, we found that IGF2 antisense RNA (IGF2-AS) was significantly decreased in breast cancer tissues, cell lines, and plasma. Patients with low IGF2-AS were more likely to develop larger tumor size and later clinical stage. Overexpression of IGF2-AS evidently inhibited the proliferation and induced apoptosis of MCF-7 and T47D cells in vitro, as well as retarded tumor growth in vivo. Further investigation revealed that IGF2-AS inhibited the expression of its sense-cognate gene IGF2 in an epigenetic DNMT1-dependent manner, resulting in the inactivation of downstream oncogenic PI3K/AKT/mTOR signaling pathway. Enforced expression of IGF2 could significantly block the tumor inhibitory effect of IGF2-AS. Importantly, we found that IGF2-AS could be used as an effective biomarker for breast cancer diagnosis and prognosis. Taken together, our study indicates that IGF2-AS is a tumor suppressor in breast cancer, restoration of IGF2-AS may be a promising treatment for this fatal disease.
Keywords: Long non-coding RNAs, epigenetic modification, breast cancer, PI3K/AKT/mTOR signaling
Impact statement
The tumor suppressors play the pivotal role in repressing cancer initiation, development, and progression. In this study, we for the first time described the tumor-inhibiting role of IGF2-AS in breast cancer. IGF2-AS inhibited breast cancer cell proliferation and induced apoptosis by increasing the occupation of DNMT1 on IGF2 promoter, resulting in IGF2 hypermethylation, thereby dampening IGF2/PI3K/AKT/mTOR signaling. Importantly, we also found that IGF2-AS could be used as an effective diagnostic and prognostic biomarker of breast cancer. Thus, our data provide new evidence for the important role of lncRNA in breast cancer as well as a promising therapeutic target for breast cancer patients.
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related death worldwide. In 2018, 2.1 million new cases of breast cancer will be diagnosed in women globally, accounting for almost a quarter of all female cancer cases.1 The incidence of breast cancer has been on the rise due to the insufficient understanding of its pathogenesis and the lack of effective means of prevention and control.2 Although progress has been made in early-stage screening, detection and combined therapy strategies of breast cancer, the mortality rate continues to rise, claiming 181,004 lives and resulting in 17.7 million disability-adjusted life years,3 making it one of the most serious burdensome cancers in the world. Therefore, it is urgently needed to identify the key regulators in breast cancer, which may provide new strategies for breast cancer diagnosis, treatment, and prevention.
In the human genome, 98% of DNA sequences belong to non-coding sequences, and their transcription products are called as non-coding RNAs (ncRNAs). As a major member of ncRNAs, long non-coding RNAs (lncRNAs) are characterized by a length longer than 200 bp, most of which are transcribed by RNA polymerase II with strong tissue and cell specificity.4,5 Emerging evidence indicates that lncRNAs are frequently deregulated in various human cancers, and tightly control cancer initiation, development, and progression by regulating different genes and signaling pathways.6 Recently, a novel lncRNA, IGF2 antisense RNA (IGF2-AS), was identified as a critical player in human disease. IGF2-AS was proposed to be closely associated with high-glucose-induced apoptosis in diabetic retinopathy 7and angiogenesis in type 2 diabetes.8 Moreover, IGF2-AS was shown to be activated by STAT3, and increased hepatitis C virus replication.9 A very recent study revealed that IGF2-AS was frequently overexpressed in gastric adenocarcinoma, and promoted BGC823 and SGC7901 cell proliferation and motility by regulating the miR-503/SHOX2 axis.10 Nevertheless, an in-depth investigation of its role in breast cancer has never been undertaken.
In the current study, we explored the expression level, biological function, and clinical implication of IGF2-AS in breast cancer. Furthermore, we elucidated the underlying mechanism of its inhibition of breast cancer as a protein binding partner.
Materials and methods
Breast cancer samples and cell lines and transfection
This study was approved by the Ethics Committee of Benxi Central Hospital. A total of 95 paired breast cancer and adjacent normal tissues were collected from Benxi Central Hospital. The clinical data of these patients are shown in Table 1, and the follow-up interval began on the date of surgery and ended on the date of death or the last clinical investigation. We obtained informed consent from all patients. Four breast cancer cell lines including MCF-7, SK-BR-3, T47D, and MDA-MB-231 and one normal MCF-10A were all purchased from ATCC and maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 100 U mL−1 penicillin and streptomycin. For cell transfection, siRNAs targeting IGF2-AS (si-IGF2-AS#1: 5ʹ-GCUCCCUCUUUCAAAGUAU-3ʹ; si-IGF2-AS#2: CAAAGUAUAAGGGAGGGAA) and DNMT1 (si-DNMT1: 5ʹ-CUCAACUUGAACCGCUUCA-3ʹ) were transfected into BC cells using Lipofectamine 3000 (Invitrogen, CA, USA) based on standard protocol.
Table 1.
Correlations between IGF2-AS expression and clinical characteristics in breast cancer patients (n = 95).
| Clinicopathologic parameters | Total (n = 95) |
IGF2-AS expression |
P value | |
|---|---|---|---|---|
| Low (n = 48) | High (n = 47) | |||
| Age (years) | ||||
| ≤40 | 20 | 9 | 11 | 0.578 |
| >40 | 75 | 39 | 36 | |
| Menopausal status | ||||
| Premenopausal | 53 | 26 | 27 | 0.748 |
| Postmenopausal | 42 | 22 | 20 | |
| Tumor size (cm) | ||||
| ≤2 | 51 | 18 | 33 | 0.001 |
| >2 | 44 | 30 | 14 | |
| TNM stage | ||||
| I–II | 60 | 24 | 36 | 0.007 |
| III–IV | 35 | 24 | 11 | |
| ER status | ||||
| Negative | 38 | 20 | 18 | 0.738 |
| Positive | 57 | 28 | 29 | |
| PR status | ||||
| Negative | 55 | 25 | 30 | 0.246 |
| Positive | 40 | 23 | 17 | |
| HER2 status | ||||
| Negative | 63 | 30 | 33 | 0.426 |
| Positive | 32 | 18 | 14 | |
| Ki-67 status | ||||
| ≤14% | 29 | 9 | 20 | 0.012 |
| >14% | 66 | 39 | 27 | |
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2.
RNA isolation and qRT-PCR analysis
Total RNA was extracted from tissues and cell lines by using Trizol solution (Invitrogen) in accordance with manufacturer's instructions, followed by assessment of sample quality using 1.5% agarose gel and concentration testing using NanoDrop microspectrophotometer with 1 µL RNA sample and reverse transcription into cDNA. Then, cDNA was amplified and relatively quantified by using SYBR® Green qPCR SuperMix (Invitrogen). The 2−ΔΔCt method was applied to calculate gene expression level normalized to GAPDH.
Generation of stable IGF2-AS-overexpressing breast cancer cell lines
The full-length sequence of IGF2-AS (ENST00000381361.4) was inserted into the pSinGFP vector. Then, the pSinGFP-IGF2-AS plasmid was cotransfected with pMD2G and psPAX2 packaging plasmids into 293 T cells by using Lipofectamine 2000 (Invitrogen) as per manufacturer’s protocol. Subsequently, the supernatants were collected and infected into MCF-7 and T47D cells for 24 h with 8 µg/mL polybrene, followed by selection of stable IGF2-AS-overexpressing cells in the presence of 1.5 µg/mL puromycin for two weeks. The infection efficiency was evaluated by qRT-PCR analysis.
Cell phenotype assays
For the colony formation assay, control or IGF2-AS-overexpressing MCF-7 and T47D cells were seeded on the 6-well plate and routinely cultured for two weeks, then the cells were washed by PBS and stained by crystal violet dye. For the CCK-8 assay, control or IGF2-AS-overexpressing MCF-7 and T47D cells were plated on 96-well plate and cultured for 24 h, 48 h, and 72 h, followed by incubation with 10 µL CCK-8 reagent (Dojindo Laboratories, Kumamoto, Japan), and the absorbance at 450 nm was recorded an automatic microplate reader. Besides, the apoptotic analysis was performed by using the Annexin V-PE/7-AAD double staining kit purchased from BD Biosciences (San Jose, CA, USA) as per the manufacturer's protocols.
Western blot
Total protein was extracted by using RIPA lysis buffer and separated on 10% SDS-PAGE gels, followed by transfer onto PVDF membrane (Millipore, Schwalbach, Germany) and blockade with 5% skimmed milk powder. Then, the membrane was incubated with primary antibodies and corresponding secondary antibodies, followed by visualization by using enhanced chemiluminescence (ECL kit, Santa Cruz, USA). The primary antibodies used are as follows: anti-IGF2 (1: 500 dilution, #sc-515805, Santa Cruz), anti-p-PI3K (1: 2000 dilution, #17366, CST), anti-PI3K (1: 2000 dilution, #4255, CST), anti-p-AKT (1: 1000 dilution, #66444–1-Ig, Proteintech), anti-AKT (1: 3000 dilution, #10176–2-AP, Proteintech), anti-p-mTOR (1: 2000 dilution, #5536, CST), anti-mTOR (1: 2500 dilution, #20657–1-AP, Proteintech), and anti-GAPDH (1: 2000 dilution, #sc-47724, Santa Cruz).
RNA pull-down and immunocoprecipitation (RIP) assays
IGF2-AS and its anti-sense were in vitro transcribed with the MEGAscript™ T7 Transcription Kit (Invitrogen) and biotinylated with the Pierce RNA 3′-End Desthiobiotinylation Kit (Thermo Fisher Scientific, MA, USA) as per manufacturer’s protocols. Then, the above biotin-labeled probes were incubated with MCF-7 and T47D cell lysates at 4°C overnight, followed by incubation with M-280 streptavidin magnetic beads (Invitrogen) at room temperature for 2 h. The probe-bound proteins were eluted and subjected to Western blot analysis of DNMT1 levels (#ab13537, Abcam). The RIP assay was carried out by using the Magna RIP™ RNA-binding Protein Immunoprecipitation Kit (Millipore, Massachusetts, USA) according to the manufacturer’s instructions.
Chromatin immunoprecipitation assay
The ChIP assay was conducted by using the EpiQuik Chromatin Immunoprecipitation Kit with magnetic beads (Epigentek, NY, USA) according to the manufacturer’s manual. In short, MCF-7 and T47D cell lysates were treated with 1% formaldehyde for 10 min and subsequently treated with glycine for 5 min, followed by digestion using a micrococcal nuclease for 30 min and addition with anti-DNMT1 antibody (#ab13537, Abcam). Lastly, the DNA fragments enriched by DNMT1 were washed and eluted for qPCR analysis.
Tumor xenografts in vivo and immunohistochemistry
The animal studies were authorized by the Animal Ethic Review Committees of Benxi Central Hospital; 1 × 107 control or IGF2-AS-overexpressing MCF-7 cells were subcutaneously injected into the right axillas of nude mice (n = 6 per group), which were grown under the specific-pathogen-free conditions. Tumor volume was weekly measured with a caliper and was calculated as length × width2 × 0.5. In the fourth week, all mice were euthanized and tumor tissues were collected for IHC staining by using the Mouse and Rabbit Specific HRP/DAB (ABC) Detection IHC kit (#ab64264, Abcam) based on the manufacturer’s instructions.
Statistical analysis
The comparison between the two groups was performed by Student's t test or Chi-square test. The survival curves were plotted by Kaplan–Meier method and were analyzed by log-rank test. The ROC curve was used to measure the diagnostic value of plasma IGF2-AS. Two-sided P value less than 0.05 was considered statistically significant.
Results
IGF2-AS is significantly downregulated in breast cancer
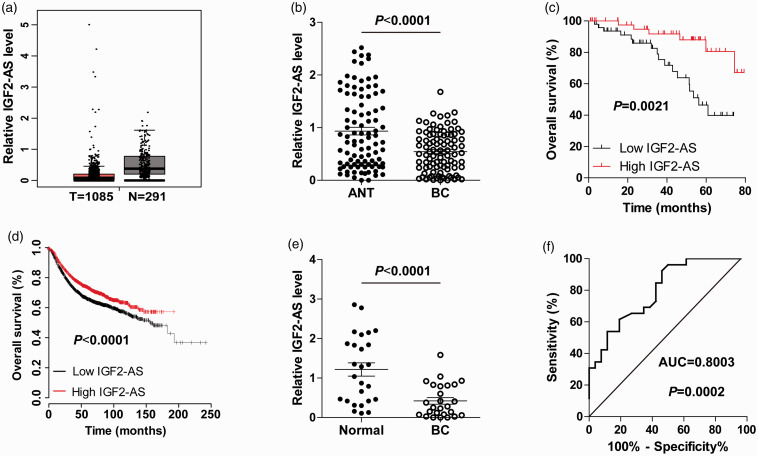
We analyzed the expression of IGF2-AS in TCGA database containing 1085 breast cancer and 291 normal tissues through using the GEPIA online tool (http://gepia.cancer-pku.cn/). As shown in Figure 1(a), IGF2-AS was dramatically decreased in breast cancer, and its downregulation was confirmed in our 95 paired breast cancer and adjacent normal tissues (Figure 1(b)). Low IGF2-AS was positively correlated with larger tumor size, advanced clinical stage, and more Ki-67-positive cells (Table 1). Importantly, patients with high IGF2-AS had longer survival time than patients with low IGF2-AS (Figure 1(c)), which was also confirmed by analyzing the KM plotter database (http://kmplot.com/analysis/) (Figure 1(d)). Further, we collected plasma samples from breast cancer patients and healthy controls and detected IGF2-AS expression. The qRT-PCR results showed that the expression of plasma IGF2-AS was evidently downregulated in breast cancer compared with that of healthy controls (Figure 1(e)), and the receiver operating characteristic (ROC) curve displayed that the area under curve (AUC) was 0.8003 (95% CI: 0.6831 to 0.9175) (Figure 1(f)). These data suggest that IGF2-AS may be a promising biomarker for the diagnosis and prognosis of breast cancer.
Figure 1.
IGF2-AS is lowly expressed in breast cancer. (a, b) The expression of IGF2-AS in breast cancer and normal tissues from GEPIA database and our own cohort. (c, d) The overall survival curves of patients with low (n = 48) or high (n = 47) IGF2-AS expression from our own cohort and KM plotter database. (e) qRT-PCR analysis of plasma IGF2-AS expression in breast cancer patients (n = 26) and healthy controls (n = 26). (f) The ROC curve testing the diagnostic utility of plasma IGF2-AS. (A color version of this figure is available in the online journal.)
IGF2-AS inhibits breast cancer cell proliferation and induces apoptosis
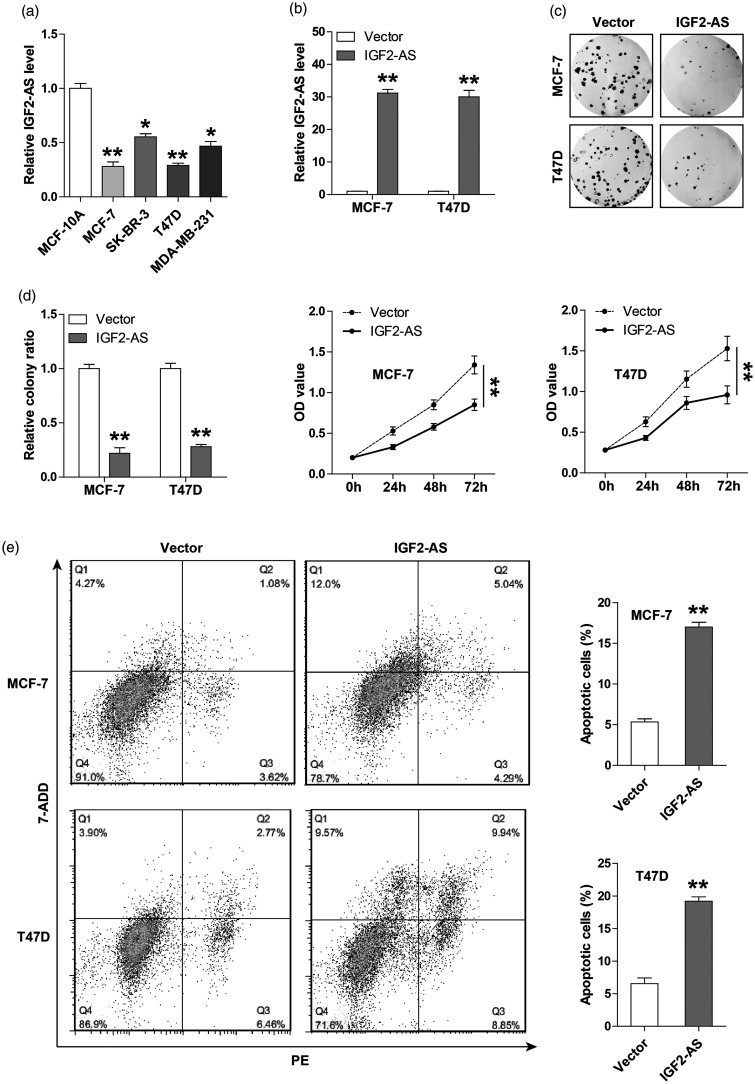
To explore the functional role of IGF2-AS, we first tested its expression in breast cancer cell lines, and the qRT-PCR results showed that IGF2-AS was lowly expressed in four breast cancer cells in comparison to normal MCF-10A cells (Figure 2(a)). Due to the relatively low level of IGF2-AS in MCF-7 and T47D cells, we thus chose them to generate stable IGF2-AS-overexpressing cell lines and performed a series of functional assays (Figure 2(b)). As shown in Figure 2(c), overexpression of IGF2-AS resulted in a significant decrease in the number of cell clones. Likewise, the CCK-8 results showed that cell viability was evidently decreased after exogenous expression of IGF2-AS (Figure 2(d)). In addition, more apoptotic cells were observed in IGF2-AS-overexpressing MCF-7 and T47D cells as compared to their respective control cells, as illustrated by flow cytometry (Figure 2(e)). Moreover, we also designed two siRNAs to silence IGF2-AS (Figure S1(a)) in SK-BR-3 and MDA-MB-231 cells, and found that IGF2-AS knockdown promoted cell proliferation (Figure S1(b)). These results indicate that IGF2-AS is negative regulator of breast cancer malignant phenotype.
Figure 2.
Overexpression of IGF2-AS inhibits breast cancer cell proliferation and induces apoptosis. (a) qRT-PCR analysis of IGF2-AS expression in breast cancer cell lines. (b) qRT-PCR analysis confirming the infection efficiency of IGF2-AS-overexpressing lentiviral vector. (c, d) The colony formation and CCK-8 assays testing the proliferation of MCF-7 and T47D cells after IGF2-AS overexpression. (e) The annexin V-PE/7-AAD double staining testing the number of apoptotic MCF-7 and T47D cells after IGF2-AS overexpression by flow cytometry. **P < 0.01.
IGF2-AS dampens IGF2/PI3K/AKT/mTOR signaling in breast cancer cells
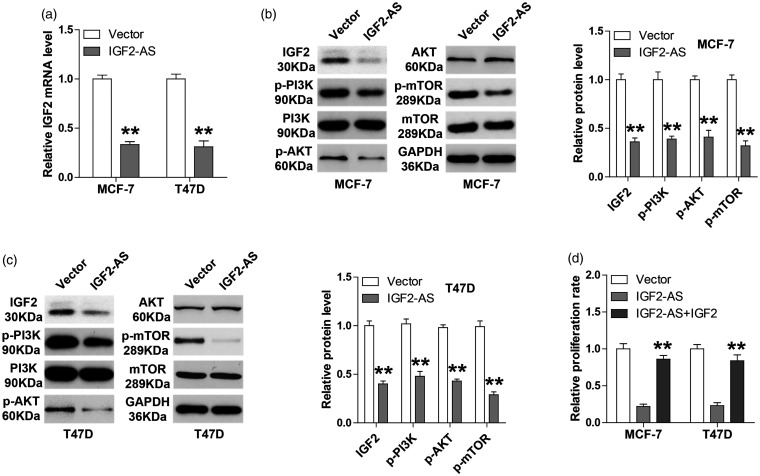
Emerging evidence shows that lncRNA is involved in cancer cell biology through regulation of its natural anti-sense linear transcript.11 We then tested whether IGF2-AS affected IGF2 expression. As shown in Figure 3(a), overexpression of IGF2-AS significantly decreased IGF2 mRNA expression. Consistently, less protein levels of IGF2, p-PI3K, p-AKT, and p-mTOR were observed in IGF2-AS-overexpressing MCF-7 and T47D cells as compared with their respective control cells, while total protein levels of PI3K, AKT and mTOR remained unchanged (Figure 3(b) and (c)). Functionally, ectopic expression of IGF2 could evidently rescued the decreased cell proliferation caused by IGF2-AS overexpression (Figure 3(d)). These results suggest that IGF2-AS is a negative regulator of its sense-cognate gene IGF2.
Figure 3.
IGF2-AS decreases IGF2 expression. (a) qRT-PCR analysis of IGF2 expression in IGF2-AS-overexpressing MCF-7 and T47D cells. (b, c) Western blot analysis of IGF2, p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR protein expression in IGF2-AS-overexpressing MCF-7 and T47D cells. GAPDH was used as loading control. (d) CCK-8 assay detecting the proliferation of IGF2-AS-overexpressing MCF-7 and T47D cells transfected with IGF2 expressing vector. **P < 0.01.
IGF2-AS reduces IGF2 expression in a DNMT1-dependent manner
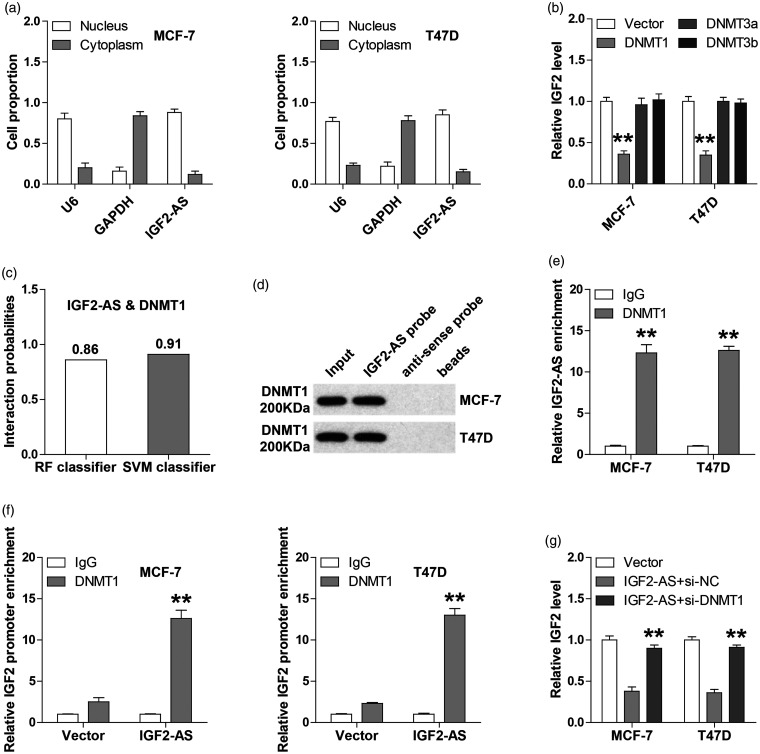
To determine how IGF2-AS affected IGF2 expression, we first tested the subcellular localization of IGF2-AS, and the qRT-PCR results showed that IGF2-AS was mainly located in the nucleus of both MCF-7 and T47D cells (Figure 4(a)). Given that IGF2 is frequently overexpressed due to the hypomethylation of its promoter,12 we then tested which DNA methyltransferases were involved in this process in breast cell lines. As shown in Figure 4(b), overexpression of DNMT1, not DNMT3a and DNMT3b, could evidently decrease IGF2 expression. Further, we found that IGF2-AS1 are likely to directly interact with DNMT1 by analyzing the RPISeq online tool (http://pridb.gdcb.iastate.edu/RPISeq)13(RF classifier = 0.86, SVM classifier = 0.91) (Figure 4(c)). As expected, the RNA pull-down assays showed that DNMT1 protein was abundantly enriched by IGF2-AS1 probe in both MCF-7 and T47D cells (Figure 4(d)). Likewise, IGF2-AS1 was evidently immunoprecipitated by DNMT1 antibody as compared with IgG antibody (Figure 4(e)). Importantly, more DNMT1 was occupied on IGF2 promoter which was observed in IGF2-AS1-overexpressing cells in comparison to control cells (Figure 4(f)), as demonstrated by ChIP assay. And the downregulation of IGF2 by IGF2-AS was blocked in the absence of DNMT1 (Figure 4(g)). These indicate that DNMT1 is responsible for the regulation of IGF2-AS on IGF2.
Figure 4.
IGF2-AS directly bins to DNMT1 and enhances the occupation of DNMT1 on IGF2 promoter. (a) qRT-PCR analysis of the location of IGF2-AS1 in MCF-7 and T47D cells. U6 and GAPDH were used as nucleus and cytoplasmic reference fractions, respectively. (b) qRT-PCR analysis of IGF2 expression in MCF-7 and T47D cells after DNMT1, DNMT3a, and DNMT3b overexpression. (c) The interaction possibility between IGF2-AS and DNMT1 predicted by RPISeq online tool. (d, e) RNA pull-down and RIP assays confirming the interaction between IGF2-AS and DNMT1 using biotinylated IGF2-AS probe and DNMT1 antibody, respectively. (f) ChIP assay analyzing the occupation of DNMT1 on IGF2 promoter in MCF-7 and T47D cells after IGF2-AS overexpression. (g) qRT-PCR analysis of IGF2 expression in IGF2-AS-overexpressing cells after DNMT1 knockdown. **P < 0.01.
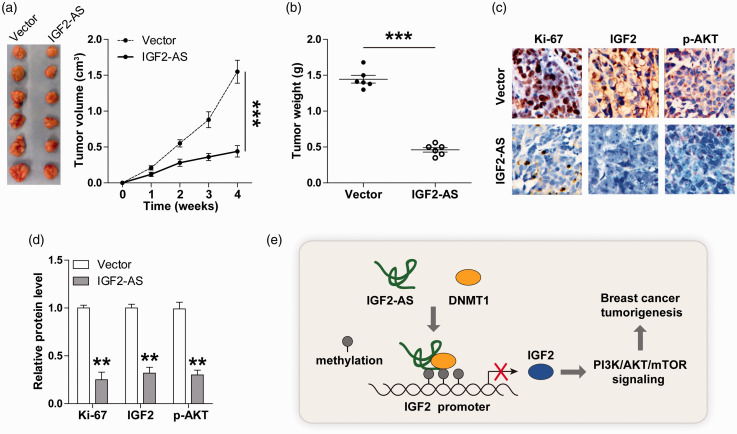
Restoration of IGF2-AS delays tumor growth in vivo
To test whether IGF2-AS also functioned in vivo, we established a xenograft model by inoculating control or IGF2-AS-overexpressing MCF-7 cells into nude mice. The results showed that the tumor volume and weight of IGF2-AS-overexpressing group were significantly smaller than those of control group (Figure 5(a) and (b)). Moreover, less Ki-67-, IGF2-, and p-AKT-positive cells were observed in IGF2-AS-overexpressing tumor tissues in comparison with control tumor tissues (Figure 5(c) and (d)). These in vivo data suggest that IGF2-AS is a suppressor of breast cancer growth, which is consistent with the findings in vitro.
Figure 5.
IGF2-AS inhibits tumor growth in vivo. (a, b) The volume and weight of tumor of nude mice in control and IGF2-AS-overexpressing groups (n = 6 in each group). (c, d) IHC staining of Ki-67, IGF2, and p-AKT in control and IGF2-AS-overexpressing tumor tissues. Scale bar = 20 µM. (e) The proposed model showing the tumor-inhibiting role of IGF2-AS in breast cancer by acting as DNMT1 protein binding partner and dampening oncogenic IGF2/PI3K/AKT/mTOR signaling. **P < 0.01, ***P < 0.001. (A color version of this figure is available in the online journal.)
Discussion
Recent studies suggest that lncRNAs are emerging as important regulators of cancer initiation, maintenance, and progression, attracting great attention.14 Many dysregulated lncRNAs have been identified in human cancers, such as the well-characterized MALAT1,15 H1916, HOTAIR,17 and ANRIL18 and so on. In the present study, we found a breast cancer-related lncRNA, IGF2-AS, which was significantly decreased in human breast cancer tissues, cells, as well as plasma. Ectopic expression of IGF2-AS evidently inhibited breast cancer cell aggressive phenotype in vitro and tumor growth in vivo. Stepwise investigation revealed that IGF2-AS could directly bind to methyltransferase DNMT1 and facilitate the occupation of DNMT1 on the promoter of oncogenic IGF2, resulting in decreased IGF2 expression and subsequently inactivation of PI3K/AKT/mTOR signaling pathway, thereby repressing breast cancer malignancy and progression (Figure 5(e)). Importantly, we also confirmed this regulatory axis in vivo. Thus, our results highlight the crucial regulatory role of IGF2-AS in breast cancer, meanwhile advance our understanding of epigenetic regulation of IGF2.
Accumulated evidence demonstrates that lncRNAs are closely linked with cancer development and progression through regulation of its sense-cognate genes.11 For example, lncRNA ZNF667-AS1 was reported to be frequently downregulated in esophageal squamous cell carcinoma, and inhibited cancer cell viability, migration, and invasion by epigenetic activation of ZNF667.19 LIN28B-AS1 was shown as an oncogene in lung adenocarcinoma, and it activated LIN28B by directly interacting with IGF2BP1.20 Similarly, in this study, we found that overexpression of IGF2-AS could significantly reduce the expression of natural anti-sense linear transcript IGF2. IGF2 is a well-documented tumor driver that can bind to IGF1R and activate downstream PI3K/AKT/mTOR signaling pathway via a series of phosphorylation cascades.21 And overexpression of IGF2 evidently rescued the impaired malignant phenotype of breast cancer cells caused by IGF2-AS overexpression, suggesting that IGF2-AS functions as a negative regulator in breast cancer through its sense-cognate gene.
Extensive research suggests that lncRNAs participate in various cell biological processes including transcriptional activation/interference, chromatin remodeling, RNA processing, and mRNA translation, through acting as a protein binding partner.22 The most well-known is HOTAIR, its 5ʹ domain could bind PRC2, while its 3ʹ domain bound the LSD1/CoREST/REST complex, thus regulating the pattern of histone modifications on target genes.23 Herein, we found that IGF2-AS was able to directly interact with DNMT1 (a methyltransferase that induces both de novo and maintenance of methylation24) and increase the binding of DNMT1 to IGF2 promoter. Importantly, the reduction of IGF2 induced by IGF2-AS almost disappeared in the absence of DNMT1, implying that DNMT1 is required for the silencing effect of IGF2-AS on IGF2. Further studies are needed to explore which regions are necessary for the interaction between IGF2-AS and DNMT1.
Collectively, our findings clearly indicate that IGF2-AS inhibits breast cancer tumorigenesis by negatively regulating IGF2/PI3K/AKT/mTOR signaling axis via binding to DNMT1, which provides a potential diagnostic and prognostic indicator as well as therapeutic target for breast cancer patients.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220966253 for Long non-coding RNA IGF2-AS represses breast cancer tumorigenesis by epigenetically regulating IGF2 by Yanan Zhang, Hanbing Yan, Yan Jiang, Tao Chen, Zhijin Ma, Fei Li, Min Lin, Yanzhi Xu, Xuemei Zhang, Jianming Zhang and Hui He in Experimental Biology and Medicine
Authors’ contributions
YZ, HBY, YJ, and TC performed the assays. ZM, FL, ML, YZX collected data and provided the support of experimental technology. XMZ, JMZ, and HH designed this study. YZ drafted this manuscript and HH revised it. All authors read and approved the final manuscript.
Footnotes
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from Liaoning million talents engineering training project (SY-2018–065).
ORCID iD: Hui He https://orcid.org/0000-0001-6160-6557
Supplemental material: Supplemental material for this article is available online.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Goding SA, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017; 67:439–48 [DOI] [PubMed] [Google Scholar]
- 3. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392:1789–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 2016; 17:47–62 [DOI] [PubMed] [Google Scholar]
- 5.Umlauf D, Fraser P, Nagano T. The role of long non-coding RNAs in chromatin structure and gene regulation: variations on a theme. Biol Chem 2008; 389:323–31 [DOI] [PubMed] [Google Scholar]
- 6.Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol 2018; 28:287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Luo Y, Chen G, Liu H, Tian N, Zen X, Liu Q. Long noncoding RNA IGF2AS regulates high-glucose induced apoptosis in human retinal pigment epithelial cells. Iubmb Life 2019; 71:1611–8 [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Liu B, Li B, Song C, Diao H, Guo Z, Li Z, Zhang J. Inhibition of long noncoding RNA IGF2AS promotes angiogenesis in type 2 diabetes. Biomed Pharmacother 2017; 92:445–50 [DOI] [PubMed] [Google Scholar]
- 9.Xiong Y, Jia M, Yuan J, Zhang C, Zhu Y, Kuang X, Lan L, Wang X. STAT3regulated long noncoding RNAs lnc7SK and lncIGF2AS promote hepatitis C virus replication. Mol Med Rep 2015; 12:6738–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Chen YX, Zhang B. IGF2-AS affects the prognosis and metastasis of gastric adenocarcinoma via acting as a ceRNA of miR-503 to regulate SHOX2. Gastric Cancer 2020; 23:23–38 [DOI] [PubMed] [Google Scholar]
- 11.Deniz E, Erman B. Long noncoding RNA (lincRNA), a new paradigm in gene expression control. Funct Integr Genomics 2017; 17:135–43 [DOI] [PubMed] [Google Scholar]
- 12.Murphy SK, Erginer E, Huang Z, Visco Z, Hoyo C. Genotype-epigenotype interaction at the IGF2 DMR. Genes 2015; 6:777–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muppirala UK, Honavar VG, Dobbs D. Predicting RNA-protein interactions using only sequence information. BMC Bioinform 2011; 12:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017; 36:5661–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Zhu C, Jin Y. The oncogenic and tumor suppressive functions of the long noncoding RNA MALAT1: an emerging controversy. Front Genet 2020; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Y, Shen A, Liu A. Long non-coding RNA H19 and cancer: a competing endogenous RNA. Bull Cancer 2019; 106:1152–9 [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Hann SS. HOTAIR: an oncogenic long non-coding RNA in human cancer. Cell Physiol Biochem 2018; 47:893–913 [DOI] [PubMed] [Google Scholar]
- 18.Deng W, Zhang Y, Cai J, Zhang J, Liu X, Yin J, Bai Z, Yao H, Zhang Z. LncRNA-ANRIL promotes gastric cancer progression by enhancing NF-kB signaling. Exp Biol Med 2019; 244:953–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Z, Li S, Wu X, Niu Y, Liang X, Yang L, Guo Y, Shen S, Liang J, Guo W. Aberrant hypermethylation-mediated downregulation of antisense lncRNA ZNF667-AS1 and its sense gene ZNF667 correlate with progression and prognosis of esophageal squamous cell carcinoma. Cell Death Dis 2019; 10:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Gu Y, Zhang E, Zhang K, Qin N, Dai J, Zhu M, Liu J, Xie K, Jiang Y, Guo X, Liu M, Jin G, Ma H, Jiang T, Yin R, Xia Y, Liu L, Wang S, Shen B, Huo R, Xu L, Sha J, Qu B, Shen H, Hu Z. A cancer-testis non-coding RNA LIN28B-AS1 activates driver gene LIN28B by interacting with IGF2BP1 in lung adenocarcinoma. Oncogene 2019; 38:1611–24 [DOI] [PubMed] [Google Scholar]
- 21.Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–39 [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Wen L, Zhu H. Unveiling the hidden function of long non-coding RNA by identifying its major partner-protein. Cell Biosci 2015; 5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010; 329:689–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gowher H, Jeltsch A. Mammalian DNA methyltransferases: new discoveries and open questions. Biochem Soc Trans 2018; 46:1191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220966253 for Long non-coding RNA IGF2-AS represses breast cancer tumorigenesis by epigenetically regulating IGF2 by Yanan Zhang, Hanbing Yan, Yan Jiang, Tao Chen, Zhijin Ma, Fei Li, Min Lin, Yanzhi Xu, Xuemei Zhang, Jianming Zhang and Hui He in Experimental Biology and Medicine