During the postharvest period, citrus fruits can be affected by phytopathogens such as Penicillium digitatum, which causes green mold disease and is responsible for up to 90% of total citrus losses. Chemical fungicides are widely used to prevent green mold disease, leading to concerns about environmental and health risks.
KEYWORDS: fungi, extracellular vesicles, herbicidal activity, host-pathogen interaction, P. digitatum, tryptoquialanines, Penicillium digitatum
ABSTRACT
Penicillium digitatum is the most aggressive pathogen of citrus fruits. Tryptoquialanines are major indole alkaloids produced by P. digitatum. It is unknown if tryptoquialanines are involved in the damage of citrus fruits caused by P. digitatum. To investigate the pathogenic roles of tryptoquialanines, we initially asked if tryptoquialanines could affect the germination of Citrus sinensis seeds. Exposure of the citrus seeds to tryptoquialanine A resulted in a complete inhibition of germination and an altered metabolic response. Since this phytotoxic effect requires the extracellular export of tryptoquialanine A, we investigated the mechanisms of extracellular delivery of this alkaloid in P. digitatum. We detected extracellular vesicles (EVs) released by P. digitatum both in culture and during infection of citrus fruits. Compositional analysis of EVs produced during infection revealed the presence of a complex cargo, which included tryptoquialanines and the mycotoxin fungisporin. The EVs also presented phytotoxicity activity in vitro and caused damage to the tissues of citrus seeds. Through molecular networking, it was observed that the metabolites present in the P. digitatum EVs are produced in all of its possible hosts. Our results reveal a novel phytopathogenic role of P. digitatum EVs and tryptoquialanine A, implying that this alkaloid is exported in EVs during plant infection.
INTRODUCTION
Citriculture is a worldwide multi-billion-dollar activity (1). Brazil, China, and the United States are the major producers of citrus (2). In Brazil, 230,000 direct and indirect jobs are related to citriculture (3). The citrus industry in Brazil corresponded to US$6.5 billion in revenues in 2019 (3). Citrus fruits can be affected by different diseases, leading to up to 50% of fruit losses and causing a negative economic impact (4–6).
Diseases caused by fungal pathogens are the most adverse factors causing fresh fruit and vegetable losses during the postharvest period (7, 8). Postharvest losses due to fungal diseases can reach 30 to 55% of production (8–10). The most damaging postharvest disease in citrus is the green mold caused by Penicillium digitatum, which accounts for up to 90% of citrus losses (4–6).
Demethylation inhibitors (DMI), including prochloraz and imazalil, are fungicides used to combat P. digitatum (4). However, this practice has raised concerns about its effects on human health and the development of antifungal resistance (10, 11). Therefore, developing safer approaches to control postharvest diseases has become a global trend (7, 8, 10, 11). The development of alternative antifungal tools demands an improved knowledge of how P. digitatum causes damage to citrus fruits. Most efforts in this direction have focused on the use of biocontrol agents, antagonist microorganisms, and natural products (8, 11, 12) to neutralize virulence factors (4, 13). Nevertheless, the molecular mechanisms underlying the induction of P. digitatum-mediated damage in host cells remain poorly known (4, 5, 14).
Secondary metabolites were reported as essential for fungal pathogenicity and a consequent attenuation of the plant defense responses (4, 5, 14). Siderophores, for instance, have been associated with fungal virulence by iron sequestration (4, 15). Secondary metabolites have not yet been associated with the pathogenic process promoted by P. digitatum (4). Tryptoquialanines are major metabolites produced by P. digitatum (16). However, their role in P. digitatum phytopathogenicity is unclear, even though these compounds displayed insecticidal (17) and antifungal (18) activities.
In addition to secondary metabolites, extracellular vesicles (EVs) have been associated with the pathogenesis of several infectious diseases (19, 20). EVs are spherical structures that are released by bacteria, fungi, and plant cells (19–21). EVs are delimited by a lipid bilayer membrane in association with proteins, lipids, enzymes, pigments, polysaccharides, and RNAs (19–21). In fungi, EVs were first reported in the human-pathogenic yeast Cryptococcus neoformans and subsequently characterized in several fungal pathogens (20, 21). In host-microbe interactions, EVs are key players determining the pathogenic outcome (22, 23) and mediating the transfer of virulence traits (24). In plant infections, the roles of EVs have been superficially explored, and the knowledge of how EVs impact the plant physiology is limited (22, 23). It is known that under stress conditions, release of EVs by plants is increased in response to infection (22, 23). EVs are involved in the defense of plants against pathogens, forming physical barriers or delivering molecules that are toxic to invading microbes (22, 23). On the other hand, pathogen EVs can inhibit plant immune responses through the export of virulence factors (22). So far, no information on the production of EVs by phytopathogens and their association with secondary metabolite cargo is available.
Here, we report the phytotoxic activity of indole alkaloids and EVs produced by P. digitatum in germination assays of Citrus sinensis seeds. The export of tryptoquialanines in P. digitatum involved EVs. Untargeted metabolomics was applied to confirm the EV-mediated metabolite export in the possible hosts (citrus and stone fruits) affected by P. digitatum.
RESULTS
Phytotoxicity activity of tryptoquialanine.
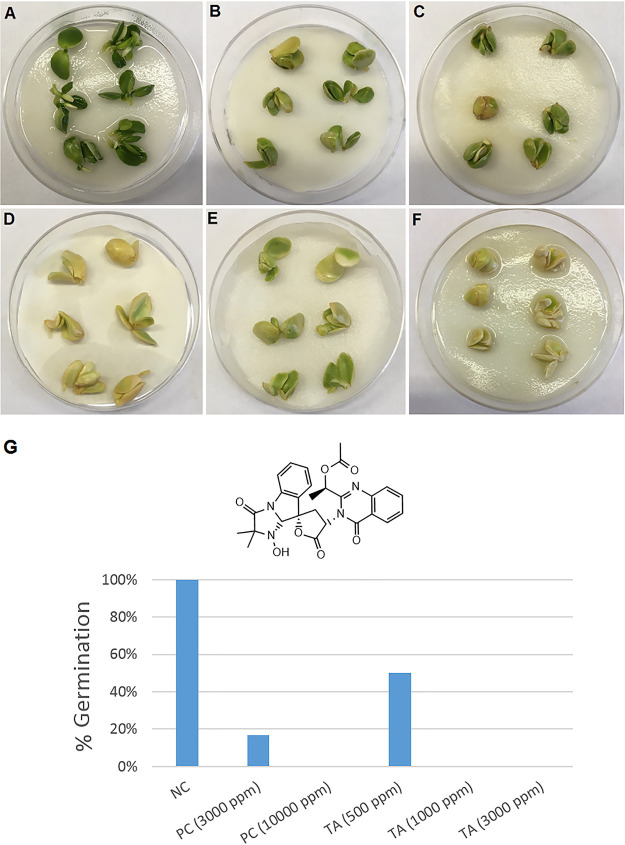
To investigate the pathogenic potential of tryptoquialanine A (TA), we first purified this alkaloid from P. digitatum’s crude extracts (Fig. S1). We then evaluated its phytotoxicity in germination assays of Citrus sinensis seeds. TA significantly inhibited seed germination at all tested concentrations. Seeds exposed to concentrations of TA lower than 1,000 ppm exhibited a delay in their germination time compared to that of the negative control (NC), as evidenced by the changes in seed color and size (Fig. 1). Seeds treated with the highest concentration of TA (3,000 ppm) showed a stronger phytotoxic effect, and no formation of radicle was observed (Fig. 1).
FIG 1.
Tryptoquialanine A (TA) is an efficient inhibitor of germination in C. sinensis seeds. (A) Untreated seeds (negative control) showed a regular pattern of germination. (B to F) Seeds exposed to TA (500, 1,000, or 3,000 ppm; panels B, C, and D, respectively) or to the commercial herbicide Roundup (3,000 or 10,000 ppm; panels E and F, respectively) manifested defective germination. Seeds exposed to TA (3,000 ppm) and the positive control (PC) (10,000 ppm) did not germinate. (G) This visual perception was confirmed by the quantitative determination of germination (%) of C. sinensis seeds under different treatments. Six seeds were used in each treatment.
(A and B) HPLC chromatograms (280 nm) for (A) P. digitatum extract and (B) purified tryptoquialanine A. (C and D) HPLC-MS analysis of purified tryptoquialanine A (m/z 519); (C) extracted ion chromatogram and (D) mass spectrum. Download FIG S1, TIF file, 0.2 MB (162.4KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
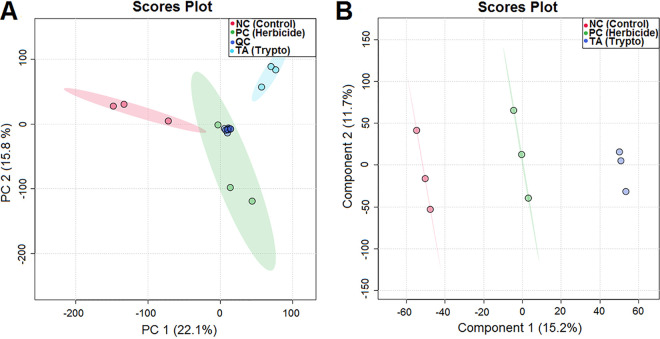
Following the observation of the phytotoxic activity of TA against the C. sinensis seeds, we compared the metabolite profiles of the seeds exposed to the different treatments by ultra-high-pressure liquid chromatography-mass spectrometry (UHPLC-MS). Principal-component analysis (PCA) of quality control (QC), negative control (NC), herbicide (PC), and tryptoquialanine A (TA) extracts was performed to observe data reproducibility and grouping tendencies (Fig. 2A). Data reproducibility was verified, as QC samples formed a distinct cluster. The two principal components, PC1 and PC2, were responsible for 37.9% of the variance of the data, revealing a separation between the seed groups related to the treatment received (water, glyphosate herbicide, or TA).
FIG 2.
(A and B) PCA (A) and PLS-DA (B) for extracts of C. sinensis seeds treated with water (negative control), glyphosate (herbicide), or tryptoquialanine A (TA).
In order to verify the classification of the seeds according to the treatment received, partial least-squares discriminant analysis (PLS-DA) was performed. The PLS-DA score plot confirmed a clear separation between the seed groups (Fig. 2B). In PLS-DA, PC1 and PC2 accounted for 26.9% of the variance (15.2% for PC1 and 11.7% for PC2). As in the PCA score plot, control seeds were distributed in the opposite way of seeds treated with TA along PC1, while seeds treated with herbicide were plotted in the center.
P. digitatum produces EVs in vitro and in vivo.
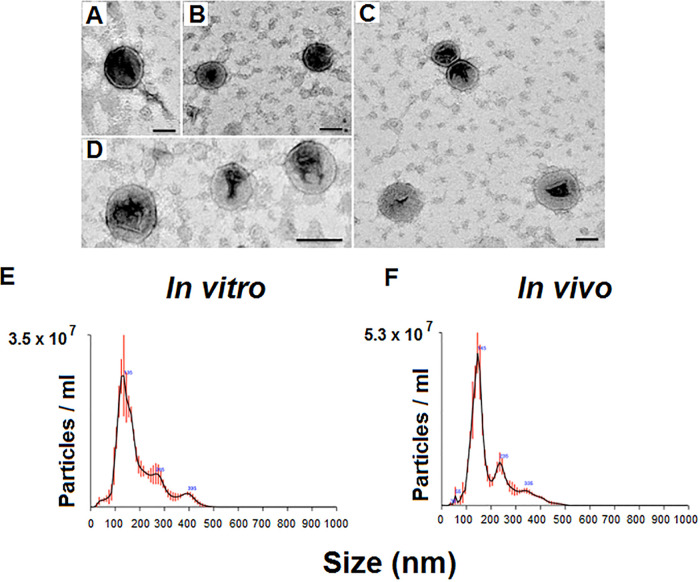
To inhibit the germination of C. sinensis seeds, TA is required to reach the extracellular environment. We then asked if the extracellular export of TA could be vesicle-mediated. However, the production of EVs by P. digitatum has not been reported so far. To address this question, we used methods for EV detection in different models of P. digitatum growth. Specifically, the production of EVs was evaluated in both solid agar medium and infected citrus fruits. Transmission electron microscopy (TEM) of P. digitatum samples grown in vitro revealed membranous structures with the typical features of vesicles, including round-shaped structures with bilayered membranes in the 100- nm size range (Fig. 3A to D). Similar results were observed for vesicles isolated from infected fruits. These results were confirmed by a second experimental approach. Nanoparticle tracking analysis (NTA) of the same samples revealed particles mostly concentrated in the 100- to 200-nm range, with subpopulations in the 200- to 300-nm and 300- to 400-nm size ranges (Fig. 3E and F). In vitro and in vivo samples had similar properties, which were consistent with those previously described for fungal EVs (19, 25, 26).
FIG 3.
Production of EVs by P. digitatum. (A to D) Analysis of ultracentrifugation pellets by TEM revealed the presence of the typical round-shaped structures presenting double membranes. Similar results were obtained with samples obtained in vivo (panels A and B) and in vitro (panels C and D). The visual observations using TEM were confirmed using NTA, which detected particles mostly concentrated in the 100 to 200 nm range, with subpopulations in the 200 to 300 and 300 to 400 nm size ranges. (E and F) Similar results were obtained with in vitro (E) and in vivo (F) samples. One representative experiment of three independent replicates producing similar results is illustrated.
Tryptoquialanine A is a component of P. digitatum EVs.
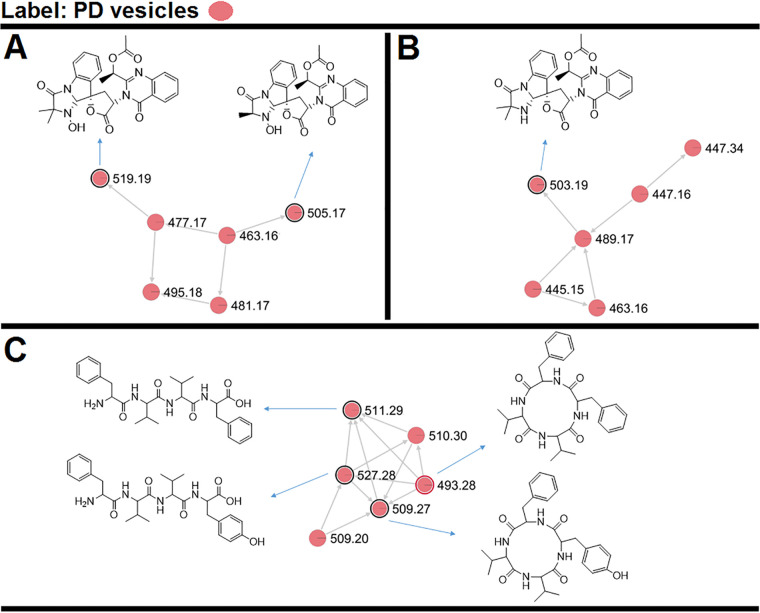
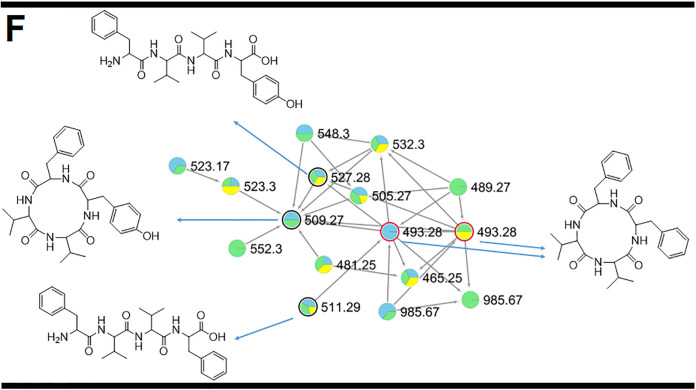
The metabolite composition of the P. digitatum EVs was investigated by UHPLC-MS/MS in EV extracts obtained in vivo (Fig. S2 and S3), followed by molecular networking in the Global Natural Products Social Molecular Networking (GNPS) platform and, when available, compared with standard metabolites. Molecular networking revealed three clusters (A, B, and C) that exhibited compounds present in the EVs (Fig. 4, pink symbols). Metabolites were manually identified by accurate mass analysis, MS/MS fragmentation profiles, or comparison with authentic standards (tryptoquialanine A and B) or as a hit in the GNPS database. The observed signals corresponded, respectively, to tryptoquialanine A (m/z 519.19), tryptoquialanine B (m/z 505.17), deoxytryptoquialanine (m/z 503.19), cyclo-(Phe-Val-Val-Tyr) (m/z 509.27), Phe-Val-Val-Phe (m/z 511.29), Phe-Val-Val-Tyr (m/z 527.28), and cyclo-(Phe-Phe-Val-Val) (m/z 493.28).
FIG 4.
Molecular networking obtained for the P. digitatum EV cargo. (A to C) Indole alkaloids were grouped in clusters A and B, and tetrapeptides were grouped in cluster C. Nodes circled in black indicate molecules identified manually through exact masses and MS/MS fragmentation pattern comparison to the literature. Nodes circled in red indicate molecules identified by comparison with the GNPS platform database.
(A to C) Extracted ion chromatograms of m/z 519.18 for (A) P. digitatum EVs extract, (B) MS analysis control, and (C) EV isolation methodology control. (D) Mass spectrum of ion [M + H]+ m/z 519.1877 obtained for tryptoquialanine A (error, 0.61 ppm) at 9.2 min. (E to G) Extracted ion chromatograms of m/z 505.17 for (E) P. digitatum EV extract, (F) MS analysis control, and (G) EV isolation methodology control. (H) Mass spectrum of ion [M + H]+ m/z 505.1719 obtained for tryptoquialanine B (error, 0.26 ppm) at 8.7 min. (I to K) Extracted ion chromatograms of m/z 503.19 for (I) P. digitatum EV extract, (J) MS analysis control, and (K) EV isolation methodology control. (L) Mass spectrum of ion [M + H]+ m/z 505.1926 obtained for deoxytryptoquialanine (error = 0.23 ppm) at 8.6 min. Download FIG S2, TIF file, 0.2 MB (222KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A to C) Extracted ion chromatograms of m/z 509.27 for (A) P. digitatum EV extract, (B) MS analysis control, and (C) EV isolation methodology control. (D) Mass spectrum of ion [M + H]+ m/z 509.2761 obtained for cyclo-(Phe-Val-Val-Tyr) (error, 0.48 ppm) at 8.6 min. (E to G) Extracted ion chromatograms of m/z 511.29 for (E) P. digitatum EV extract, (F) MS analysis control, and (G) EV isolation methodology control. (H) Mass spectrum of ion [M + H]+ m/z 511.2917 obtained for Phe-Val-Val-Phe (error, 0.43 ppm) at 7.1 min. (I to K) Extracted ion chromatograms of m/z 527.28 for (I) P. digitatum EV extract, (J) MS analysis control, and (K) EV isolation methodology control. (L) Mass spectrum of ion [M + H]+ m/z 527.2866 obtained for Phe-Val-Val-Tyr (error, 0.40 ppm) at 6.4 min. (M to O) Extracted ion chromatograms of m/z 493.28 for (M) P. digitatum EV extract, (N) MS analysis control, and (O) EV isolation methodology control. (P) Mass spectrum of ion [M + H]+ m/z 493.2809 obtained for cyclo-(Phe-Phe-Val-Val) (error,= 0.03 ppm) at 9.7 min. Download FIG S3, TIF file, 0.3 MB (301.9KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In the molecular networking analysis, each consensus MS/MS spectrum is represented by a node, and all nodes are labeled with their precursor mass. Indole alkaloids produced by P. digitatum were grouped in clusters A and B since they showed similar fragmentation patterns, with typical indole alkaloid fragments observed at [M+H]+ m/z 156.07, m/z 197.10, and m/z 213.10 (Fig. S4). Tryptoquialanines A and B and deoxytryptoquialanine are the final products of the tryptoquialanine biosynthetic pathway (27), and as already mentioned in this section, these indole alkaloids were reported as major secondary metabolites for P. digitatum (16). In cluster C, the GNPS database indicated the presence of cyclo-(Phe-Phe-Val-Val) (Fig. S5A), a mycotoxin known as fungisporin. We also observed that fungisporin analogues were grouped in this cluster. A fragmentation pattern with typical ions observed at [M+H]+ m/z 120.08, m/z 219.15, and m/z 247.14 was previously described for compounds Phe-Val-Val-Phe and Phe-Val-Val-Tyr (18, 28, 29) (Fig. S5B and C).
(A to F) Comparison of MS/MS spectra between (A) P. digitatum EV extract and (B) purified tryptoquialanine A, (C) P. digitatum EV extract and (D) purified tryptoquialanine B, and (E) P. digitatum EV extract and (F) purified deoxytryptoquialanine. Download FIG S4, TIF file, 0.1 MB (152.7KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) MS/MS match between GNPS database (green) and compound cyclo-(Phe-Phe-Val-Val) (black). (B and C) MS/MS spectrum of (B) m/z 511.29 and (C) m/z 527.28 obtained from P. digitatum EV extract. The fragmentation pattern was compared with MS/MS data of compounds Phe-Val-Val-Phe and Phe-Val-Val-Tyr reported in the literature. Download FIG S5, TIF file, 0.08 MB (86.7KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Quantification of tryptoquialanine A in P. digitatum EVs.
The quantitative composition of alkaloids in P. digitatum EVs was evaluated using UHPLC-MS/MS analyses. First, a calibration curve was prepared using standard TA (tR = 7.2 min) (Fig. S1) purified from P. digitatum’s crude extracts (17). The coefficient of determination (r2) obtained was greater than 0.998, indicating an excellent linearity (Fig. S6). Extracts of P. digitatum EVs isolated from in vivo assays were again analyzed for the presence of TA. Each 1.0 × 1010 P. digitatum EV contained 0.0184 ± 0.0002 μg of TA.
UHPLC-MS/MS calibration curve obtained for tryptoquialanine A. Download FIG S6, TIF file, 0.01 MB (13.7KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
P. digitatum EVs are phytotoxic to seeds.
We asked whether the phytotoxic effects of TA alone would be comparable to its vesicle-exported form. To address this question, we isolated EVs produced during infection and performed the seed germination tests in the presence of the vesicles. EVs were adjusted to a final concentration of 2.1 × 1010 EVs ml−1 to allow comparisons between the effects of purified TA and the vesicle preparations.
After 10 days of incubation, seeds exposed to EVs had germination rates similar to those observed in untreated systems. Positive controls of inhibition of germination revealed seeds with different colors and patterns and absence of radicle formation, as expected. However, the seeds that were exposed to the P. digitatum EVs showed altered tissues. Tissular alteration included injured areas with differences in pigmentation (Fig. 5). No color alteration or tissue damage was observed in the negative controls.
FIG 5.

P. digitatum EVs affect C. sinensis seeds. (A) Negative control (NC), consisting of C. sinensis seeds incubated in PBS. (B) Positive control (PC), consisting of the citrus seeds incubated in glyphosate (10,000 ppm). (C) Incubation of C. sinensis seeds with P. digitatum EVs produced in vivo (2.1 × 1010 EVs ml−1). Seeds exposed to fungal vesicles presented injured tissues (orange spots on their surface; red arrows).
Comparison of secondary metabolite production of P. digitatum in different hosts.
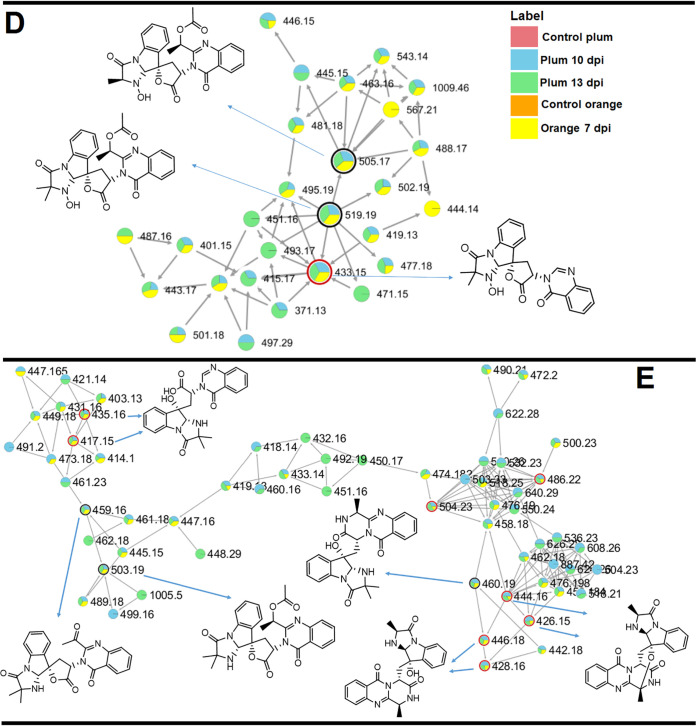
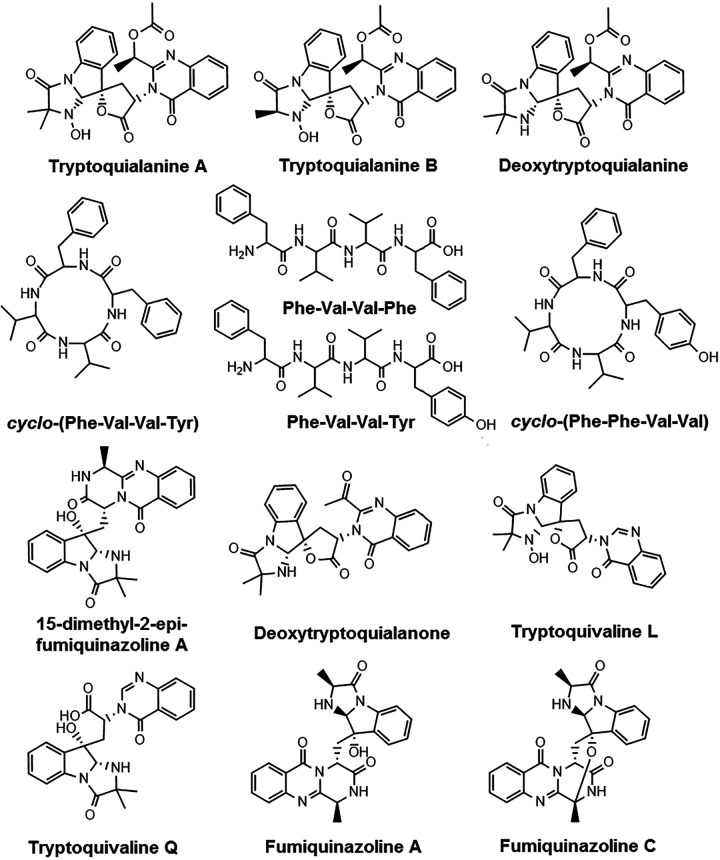
After evaluating the citrus response to TA and EVs, we next analyzed the metabolite response of different hosts to the infection caused by P. digitatum. Similar to what we observed for the P. digitatum EV extracts, molecular networking of extracts from plums and oranges infected with P. digitatum showed three clusters (D, E, and F) with compounds present only in the infected fruits (blue, green, and yellow nodes) and absent in control fruits (orange and pink nodes) (Fig. 6 and 7). Metabolites were manually identified by their accurate masses and fragmentation profiles or identified as hits in the GNPS database. Fragmentation patterns obtained by MS/MS analyses are represented in Fig. S7. Accurate mass measurements showed mass errors below 5 ppm (Table 1). The structures of the detected metabolites are shown in Fig. 8.
FIG 6.
MS/MS molecular network of different extracts of P. digitatum. Nodes circled in black indicate molecules identified manually through accurate mass and fragmentation pattern analysis. Nodes circled in red indicate molecules identified by comparison with the GNPS database. Indole alkaloids produced by P. digitatum were grouped in clusters D and E.
FIG 7.
MS/MS molecular network of different extracts of P. digitatum. Nodes circled in black indicate molecules identified manually through accurate mass and fragmentation pattern analysis. Nodes circled in red indicate molecules identified by comparison with the GNPS database. Fungisporin and analogues were grouped in cluster F.
TABLE 1.
MS data obtained for P. digitatum secondary metabolites observed on GNPS molecular network
| Compound | Ion formula ([M + H]+) | Calculated m/z | Exptl m/z | Error (ppm) |
|---|---|---|---|---|
| Tryptoquialanine A | C27H27N4O7 | 519.1874 | 519.1876 | –0.4 |
| Tryptoquialanine B | C26H25N4O7 | 505.1723 | 505.1735 | 2.4 |
| Deoxytryptoquialanine | C27H27N4O6 | 503.1925 | 503.1948 | 4.6 |
| 15-dimethyl-2-epi-fumiquinazoline A | C25H26N5O4 | 460.1979 | 460.1983 | 0.9 |
| Deoxytryptoquialanone | C25H23N4O5 | 459.1663 | 459.1678 | 3.3 |
| Tryptoquivaline L | C23H21N4O5 | 433.1512 | 433.1523 | 2.5 |
| Tryptoquivaline Q | C23H23N4O5 | 435.1668 | 435.1673 | 1.1 |
| Fumiquinazoline A | C24H24N5O4 | 446.1828 | 446.1838 | 2.2 |
| Fumiquinazoline C | C24H22N5O4 | 444.1672 | 444.1687 | 3.4 |
| cyclo-(Phe-Phe-Val-Val) | C28H37N4O4 | 493.2814 | 493.2822 | 1.6 |
| cyclo-(Phe-Val-Val-Tyr) | C28H37N4O5 | 509.2763 | 509.2770 | 1.4 |
| Phe-Val-Val-Phe | C28H39N4O5 | 511.2920 | 511.2920 | 0.0 |
| Phe-Val-Val-Tyr | C28H39N4O6 | 527.2869 | 527.2859 | –1.9 |
FIG 8.
Structures of secondary metabolites identified manually or through the GNPS MS/MS database.
(A to M) Mass spectrum and MS/MS spectrum for (A) tryptoquialanine A ion [M + H]+ m/z 519.1876, (B) tryptoquialanine B ion [M + H]+ m/z 505.1735, (C) deoxytryptoquialanine ion [M + H]+ m/z 503.1948, (D) cyclo-(Phe-Val-Val-Tyr) ion [M + H]+ m/z 509.2770, (E) Phe-Val-Val-Phe ion [M + H]+ m/z 511.2920, (F) Phe-Val-Val-Tyr ion [M + H]+ m/z 527.2859, (G) cyclo-(Phe-Phe-Val-Val) ion [M + H]+ m/z 493.2822, (H) 15-dimethyl-2-epi-fumiquinazoline A ion [M + H]+ m/z 460.1983, (I) deoxytryptoquialanone ion [M + H]+ m/z 459.1678, (J) tryptoquivaline L ion [M + H]+ m/z 433.1523, (K) tryptoquivaline Q ion [M + H]+ m/z 435.1673, (L) fumiquinazoline A ion [M + H]+ m/z 446.1838, and (M) fumiquinazoline C ion [M + H]+ m/z 444.1687. Download FIG S7, TIF file, 1.1 MB (1.1MB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The compounds identified in our analysis included 15-dimethyl-2-epi-fumiquinazoline (m/z 460.19) A, deoxytryptoquialanone (m/z 459.16), tryptoquivaline L (m/z 433.15), tryptoquivaline Q (m/z 435.16 and 417.15), fumiquinazoline A (m/z 446.18 and 428.16), and fumiquinazoline C (m/z 444.16 and 426.15). Compounds 15-dimethyl-2-epi-fumiquinazoline A and deoxytryptoquialanone are intermediates of the tryptoquialanine biosynthetic pathway (27), while the tryptoquivalines and fumiquinazolines were previously identified as P. digitatum metabolites (17). Fungisporin and analogues were also identified in EVs (cluster C, Fig. 4). A few differences were observed in clusters D, E, and F (Fig. 7 and 8) considering the production of secondary metabolites by P. digitatum in different fruits. All identified compounds were detected in infected plums (at 10 and 13 days postinoculation[dpi]) and oranges (at 7 dpi) (Fig. S8).
Fruits infected with P. digitatum. (A to C) (A) plum at 10 dpi, (B) plum at 13 dpi, and (C) orange at 7 dpi. Download FIG S8, TIF file, 0.3 MB (329.5KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The complete molecular networking obtained for P. digitatum is represented in the supplemental information (Fig. S9). P. digitatum molecular networking was composed of 235 clusters, 83 of which (36%) were composed of unknown metabolites that were only present in the infected fruits and absent in the control fruits. Molecular networking also showed clusters containing unknown metabolites present only in infected oranges or only in infected plums (Fig. S10).
Complete molecular networking obtained for extracts of plums and citrus fruits infected by P. digitatum. Download FIG S9, JPG file, 2.7 MB (2.7MB, jpg) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Examples of clusters obtained by molecular networking for extracts of P. digitatum infection in different fruits. The unknown compounds are present in just one type of the fruits and absent in the control fruits Download FIG S10, TIF file, 0.03 MB (30.8KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
Tryptoquialanines are the major secondary metabolites produced by P. digitatum (16). The involvement of tryptoquialanines during the infection of citrus fruits by P. digitatum was evaluated after deletion of the tqaA gene (nonribosomal peptide synthetase) responsible for the biosynthesis of tryptoquialanines. P. digitatum mutants deficient in tryptoquialanine A production did not have their virulence affected compared to wild-type P. digitatum cells (30). Thus, tryptoquialanines were initially thought to be dispensable for the pathogenesis in fruits. Other damaging roles could not be ruled out, since they were not investigated in detail. It has been recently reported that TA is accumulated in the citrus surface during the P. digitatum pathogenic process (17), suggesting extracellular export. TA also exhibited insecticidal activity against Aedes aegypti larvae (17). These results suggested that tryptoquialanines are involved in the fruit protection against insects that could compete with the fungus for the rotten fruit (17). In coculture models, it has been observed that P. digitatum tryptoquialanines were present in the confrontation zone with citrus pathogens, suggesting that tryptoquialanines participate in antifungal defense mechanisms that could provide competitive advantages during infection of the citrus host (18).
The reports described above and the fact that tryptoquialanines are the major metabolites produced by P. digitatum led us to ask if these metabolites could be involved in P. digitatum phytotoxic activity. To address this question, we investigated the phytotoxic effects of tryptoquialanines in a seed germination model, as previously established for the evaluation of the phytotoxicity of chemicals (31, 32). This method is simple, sensitive, and of low cost (31). Seed germination is a vulnerable stage in the plant life cycle, during which seedlings are weak, sensitive, and more affected by unfavorable conditions (32). Our results indicated that TA was comparable to the herbicide Roundup in its ability to inhibit germination. Such an inhibitory effect requires the extracellular export of TA, as suggested by its accumulation on the surface of citrus infected with P. digitatum (17). We then hypothesized that the transport of the indole alkaloids from P. digitatum cells to the extracellular environment would involve EVs, as previously described for fungal proteins, glycans, and RNA (25, 33). In our model, EVs were detected in culture and infected citrus fruits. The possibility of coisolation of plant EVs in the in vivo samples cannot be ruled out, since it is well known that plant cells also produce EVs during interaction with fungi (34). However, the similar features of EVs obtained in vivo and in vitro and our vesicle compositional analysis reinforce the notion that P. digitatum produces EVs in vitro and during plant infection. The observation of P. digitatum EVs gains additional significance considering that most of the studies characterizing fungal EVs used human pathogens as models, which implies that the importance of EV production by phytopathogens has been underscored so far. In this context, it has been only recently demonstrated that EVs from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a phytotoxic response in plants (25). In the EV cargo of F. oxysporum f. sp. vasinfectum, 482 enzymes were identified, including two polyketide synthases, yet the isolated EVs presented a deep purple color, indicating that a naphthoquinone pigment is packaged into the EVs (25). The authors suggested that EVs could be a site of biosynthesis and transport of pigments and other secondary metabolites (25), an idea that is quite complementary to what is presented in our study.
Secondary metabolites participate in the virulence mechanisms of some phytopathogenic fungi, implying that knowledge of metabolite exportation could improve the understanding of the molecular basis of plant infection and fruit protection (4, 15). However, the association of fungal metabolites and EVs has not been established so far in plant infection models. Based on the observation of P. digitatum EVs in vitro (potato dextrose agar) and in vivo (citrus fruits), we identified indole alkaloids and mycotoxins in EV samples. Penicillium species are known to produce mycotoxins such as fungisporin (35, 36). Fungisporin and analogues were reported in the cultures of P. canescens (28), P. roqueforti (29), P. citrinum (18) and P. chrysogenum (37). Therefore, the production of fungisporin compounds by P. digitatum was expected. To the best of our knowledge, the presence of secondary metabolites and mycotoxins in EVs produced by a phytopathogen in vivo is reported here for the first time.
An estimate of the tryptoquialanine levels in EVs could provide insights into the biosynthesis and metabolic flow of these molecules in P. digitatum. Previous studies with oranges infected by P. digitatum showed that, at 5 days postinfection, TA was detected in the orange epicarp, mesocarp, and endocarp, with concentrations of 24.810, 388, and 24 μg kg−1, respectively (38). TA concentration in the EVs was considerably lower. We then speculated that the biosynthesis of tryptoquialanines may occur in the fungal cells with further export in EVs. This mechanism would differ from that described by Bleackley et al. in the F. oxysporum f. sp. vasinfectum EVs (25).
P. digitatum EVs induced alterations in the C. sinensis seeds, as concluded from the observation of color alteration resulting from tissue lesions. Similar results were reported in recent studies with cotton cotyledons infiltrated with F. oxysporum f. sp. vasinfectum. In this model, EVs induced discoloration around the sites of infiltration (25). Therefore, the phytotoxic effect observed for the isolated TA was different from that caused by the EVs. These differences were, in fact, expected, considering that vesicular TA is accompanied by hundreds of other molecules. Those molecules could, for instance, physically interact with TA, altering its relative concentration. In addition, if those additional vesicular molecules have biological effects that differ from those observed for TA alone, it would be very hard to predict what kind of effect would prevail, since the relative concentration of vesicular molecules in the P. digitatum model is still unknown. In any case, our results provide a proof-of-concept model showing that P. digitatum exports bioactive molecules in EVs that can directly impact the pathogenic process.
P. digitatum pathogenesis was believed to be restricted to citrus fruits (13). However, this fungus is also an aggressive pathogen of stone fruits, including nectarines and plums (39, 40). Few studies have investigated the infection of stone fruits by P. digitatum. Even though P. digitatum disease was characterized at the physical (incidence, lesion diameter, pH) and molecular (gene expression) levels (40, 41), no information on secondary metabolite production has been presented in the literature for this host-pathogen interaction. Since tryptoquialanines and mycotoxins were found in EVs produced during infection, the metabolic profile of P. digitatum in different fruits was evaluated in order to verify if the same metabolites were found in the different hosts. Molecular networking analyses indicate that intermediates of the tryptoquialanine biosynthetic pathway are present in fruits and absent in EVs. These data are in agreement with the quantification level of TA in EVs, reinforcing the idea that tryptoquialanines are only transported by the EVs. Also, our results are the first to identify the production of tryptoquialanines and other indole alkaloids in the P. digitatum-stone fruit interaction. Likewise, the similarity between the metabolic profile in the fruits suggests that the production of EVs by P. digitatum is not restricted to the citrus fruits, since the same metabolites found in EV cargo obtained from infection in citrus were detected in plums. The clusters containing unknown metabolites present only in infected oranges or only in infected plums (Fig. S9) suggest that the metabolite production of P. digitatum can vary depending on the infected fruit.
Conclusions.
This work is the first to report that P. digitatum is able to release EVs and to report secondary metabolites in EVs produced by a phytopathogen in vivo. Furthermore, we suggested that TA is synthesized intracellularly and exported in EVs. Molecular networking confirmed our hypothesis that tryptoquialanines and mycotoxins are delivered through EVs during the infection process, since the intermediates of the tryptoquialanine biosynthetic pathway are absent in the EVs. This delivery system is not restricted to citrus and occurs in different types of fruits, such as plums.
A novel phytotoxic function for P. digitatum EVs and for tryptoquialanines was observed. EVs caused alterations in the physiology of C. sinensis seed tissues, while TA inhibited 100% of seed germination. The presence of alkaloids and mycotoxins in phytotoxic EVs opens new venues for the investigation of fungal secretion and its relationship with plant pathogenesis. Also, our results provided new insights into the biological role of the indole alkaloids and the infection strategies used by the phytopathogen P. digitatum.
MATERIALS AND METHODS
Fungal strain and culture conditions.
The P. digitatum strain is deposited in the Spanish Type Culture Collection (CECT) (accession code CECT20796). The fungus was cultured in commercial potato dextrose agar (PDA) (darkness, 7 days at 25°C). Conidial suspensions were prepared in sterile distilled water and adjusted to a final concentration of 1.0 × 106 conidia ml−1.
Purification of tryptoquialanine A by high-performance liquid chromatography (HPLC).
P. digitatum was cultivated in 12 liters of PDA distributed in petri dishes. After cultivation, the content of the petri dishes was sliced and transferred to Erlenmeyer flasks. The content of the Erlenmeyer flasks was extracted twice with ethyl acetate (EtOAc) under sonication in an ultrasonic bath for 1 h. The mixture of agar, mycelia, and EtOAc was filtered, and the solvent was removed under reduced pressure.
The P. digitatum EtOAc extract was suspended in methanol (MeOH), filtered, and subjected to separation by high-performance liquid chromatography (HPLC) in order to obtain pure tryptoquialanine A. HPLC separation was performed with a Phenomenex column Luna 5-μm phenyl-hexyl (250 × 4.6 mm) using a Shimadzu prominence HPLC LC-20AT instrument connected to a CBM-20A communication bus module, to an SPD-M20A photodiode array detector, and to a SIL-20A auto sampler. The mobile phases were 0.1% (vol/vol) formic acid in water (A) and acetonitrile (B). The flow rate was 1.0 ml min−1. Elution was performed as follows (A:B): gradient from 95:5 up to 55:45 for 30 min, then up to 35:65 from 30 to 52 min, then up to 5:95 from 52 to 55 min, remaining under this condition for 5 min. Column reconditioning between each injection was a gradient to 95:5 from 60 to 61 min, remaining under this condition for 9 min. Semipreparative HPLC separations were performed with a Phenomenex column Luna 5 μm phenyl-hexyl (250 × 10 mm) using a Waters 1525 binary HPLC pump equipped with a Waters 2998 photodiode array detector and a Waters fraction collector III. The eluent was the same as indicated above with a flow rate of 4.7 ml min−1.
Seed germination test (phytotoxicity assay).
The phytotoxicity of tryptoquialanine A on seed germination was evaluated as previously described with a few modifications (42–45). Briefly, C. sinensis seeds were manually collected from oranges purchased at a local grocery store (Campinas, São Paulo, Brazil). Seeds coats were removed, and seeds were immersed in a 50% (vol/vol) commercial bleach solution for 15 min for surface sterilization. Six sterilized seeds were placed in each petri dish (6 cm) lined with two filter papers. A volume of 2.5 ml of treatment solution was added to the plate. As the negative control (NC), seeds were treated with sterile distilled H2O containing dimethyl sulfoxide (DMSO) 3% (vol/vol). Tryptoquialanine A (TA) was solubilized in DMSO and diluted in sterile distilled water to a final concentration of 500, 1,000, and 3,000 ppm. The commercial herbicide Roundup was utilized as a positive control (PC) diluted to the concentrations of 10,000 and 3,000 ppm in sterile distilled water containing DMSO 3% (vol/vol). Treatment solutions were filtered through 0.22-μm membranes. Petri dishes were sealed with tape and incubated in a biochemical oxygen demand (BOD) chamber at 25°C with photoperiods of 12 h for 10 days. After incubation, the percentage of seed germination was calculated as described in Equation 1, considering complete, proportionate, and healthy development.
| (1) |
To evaluate the phytotoxic activity of EVs, uncoated and sterilized C. sinensis seeds were placed in a 24-well cell culture plate lined with filter papers (1 seed per well). The seeds were treated with 100 μl of a phosphate-buffered saline (PBS) solution of P. digitatum EVs (2.1 × 1010 EVs ml−1). Negative controls (NC) were performed using 100 μl of PBS, and for positive controls (PC), 100 μl of the herbicide Roundup diluted in PBS (10,000 ppm) was used. The plate was sealed and incubated as described above.
Infection of fruits by P. digitatum (in vivo assays) and metabolite extraction.
For in vivo assays, mature oranges (C. sinensis) and plums (Prunus salicina) obtained from a local grocery store (Campinas, São Paulo, Brazil) were surfaced sterilized and wounded (17). Four fruits (2 oranges and 2 plums) were infected with 15 μl of a P. digitatum 1.0 × 106 conidia ml−1 solution. Control fruits (2 oranges and 2 plums) were also included. Infected and control fruits were stored in sterile 500-ml beakers in darkness at 25°C. The fruits were incubated for different numbers of days postinoculation (dpi) in triplicates.
After the infection period (7 dpi for oranges, 10 and 13 dpi for plums), extraction of infected fruits was performed as previously described, with few modifications (46). Fruits were cut around the infected area (4 cm by 4 cm), and collected fruit pieces were extracted with 5 ml of MeOH for 1 h in ultrasonic bath. The same procedure was performed for control fruits. MeOH extracts were filtered, dried with a N2 flux, and stored at −20°C.
Isolation of P. digitatum EVs and metabolite extraction.
Isolation of P. digitatum EVs produced in vitro was performed as previously described, with a few modifications (26). Fungal cells were cultivated and softly scraped from PDA plates (triplicates, 20 ml of PDA per plate) using a sterile spatula. Fungal mycelia were transferred to a Falcon tube filled with 30 ml of sterile phosphate-buffered saline (PBS). For the analysis of EVs in vivo, nine oranges (C. sinensis) were infected with P. digitatum (as described above). Infected fruits were incubated for 7 days (darkness, 25°C). Then, fungal cells in the infected areas of fruits were softly scraped using a sterile spatula and transferred to a Falcon tube filled with 30 ml of PBS. Then, 30-ml cell suspensions obtained in vivo or in vitro were sequentially centrifuged to remove fungal cells (5,000 × g for 15 min at 4°C) and possible debris (15,000 × g for 15 min at 4°C). The remaining supernatants were filtered through 0.45-μm-pore syringe filters and ultracentrifuged to collect EVs (100,000 × g for 1 h at 4°C). Ultracentrifugation pellets were negatively stained and analyzed by transmission electron microscopy (TEM) as previously described (26). Briefly, EV samples were transferred to carbon- and Formvar-coated grids and negatively stained with 1 % (vol/vol) uranyl acetate for 10 min. The grids were then blotted dry before immediately being observed in a JEOL 1400Plus transmission electron microscope at 90 kV. The same samples were subjected to nanoparticle tracking analysis (NTA) on an LM10 nanoparticle analysis system, coupled with a 488-nm laser and equipped with an SCMOS camera and a syringe pump (Malvern Panalytical, Malvern, United Kingdom). Recorded data were acquired and analyzed using the NTA v.3.0 software (Malvern Panalytical).
To study the vesicular cargo, EVs obtained in vivo were extracted with 1 ml of MeOH HPLC grade for 1 h in an ultrasonic bath.
Mass spectrometry (MS) analyses.
In vivo extracts. in vivo extracts were resuspended in 1 ml of MeOH HPLC grade. An aliquot of 100 μl was diluted in 900 μl of MeOH HPLC grade, filtered through 0.22-μm membranes, and collected in glass vials. UHPLC-MS analyses were performed in a Waters Acquity UPLC H-class chromatograph coupled to a Waters Xevo G2-XS QToF mass spectrometer using electrospray ionization. The conditions were as follows: positive mode, capillary voltage at 1.2 kV; source temperature at 100°C; cone gas (N2) flow of 50 liters h−1; desolvation gas (N2) flow of 750 liters h−1, and m/z range of 100 to 1,500. MS/MS analyses were performed using a collision energy ramp of 6 to 9 V (low mass) and 60 to 80 V (high mass). A BEH C18 column (2.1 mm by 100 mm by 1.7 μm) was used. Mobile phases were 0.1% (vol/vol) formic acid in water (A) and acetonitrile (B). Eluent profile (A:B) 0 to 6 min, gradient from 90:10 up to 50:50; 6 to 9 min, gradient up to 2:98; 9 to 10 min, gradient up to 90:10. The flow rate was 0.2 ml min−1. The injection volume was 2 μl. Operation and spectrum analyses were conducted using Waters MassLynx v.4.1. software.
P. digitatum EV extracts. First, 1 ml of EV extracts was filtered through 0.22-μm membranes into glass vials. UHPLC-MS analyses were performed using a Thermo Scientific QExactive hybrid Quadrupole-Orbitrap mass spectrometer with the following parameters: electrospray ionization in positive mode, capillary voltage at +3.5 kV; capillary temperature at 250°C; S-lens of 50 V, and m/z range of 133.40 to 2,000.00. MS/MS was performed using normalized collision energy (NCE) of 30 eV, and 5 precursors per cycle were selected. Stationary phase: Thermo Scientific Accucore C18 2.6 μm (2.1 mm x 100 mm) column. Mobile phases were 0.1% (vol/vol) formic acid in water (A) and acetonitrile (B). Eluent profile (A:B) 0 to 10 min, gradient from 95:5 up to 2:98; held for 5 min; 15 to 16.2 min gradient up to 95:5; held for 8.8 min. The flow rate was 0.2 ml min−1. The injection volume was 3 μl. Operation and spectrum analyses were conducted using Xcalibur software (v.3.0.63) developed by Thermo Fisher Scientific.
Seed extracts.
Two seeds of each condition, TA (3,000 ppm), PC (10,000 ppm), and NC, were macerated with liquid nitrogen in triplicate. Aliquots of 100 mg of macerated seeds were extracted in plastic tubes with 2 ml of MeOH containing 0.1% (vol/vol) formic acid during 1 h in an ultrasonic bath. The extracts were filtered (0.22 μm), dried with a N2 flux, and stored at −20°C.
Seed extracts were resuspended in 1 ml of MeOH and aliquots of 100 μl and diluted with 900 μl and filtered through a 0.22-μm membrane. UHPLC-MS analyses were performed using a Thermo Scientific QExactive hybrid Quadrupole-Orbitrap mass spectrometer with the following parameters: electrospray ionization in positive mode, capillary voltage at 3.5 kV; capillary temperature at 300°C; S-lens of 50 V, and m/z range of 100.00 to 1,500.00. MS/MS was performed using normalized collision energy (NCE) of 20, 30, and 40 eV, and a maximum of 5 precursors per cycle were selected. A Waters Acquity UPLC BEH C18 1.7-μm (2.1 mm by 50 mm) column was used. Mobile phases were 0.1% (vol/vol) formic acid in water (A) and acetonitrile (B). Eluent profile (A:B) 0 to 10 min, gradient from 95:5 up to 2:98; held for 5 min; 15 to 16.2 min gradient up to 95:5; held for 3.8 min. The flow rate was 0.2 ml min−1. UHPLC-MS operation and spectrum analyses were performed using Xcalibur software (v.3.0.63). Samples were injected in random order. A quality control (QC) sample was prepared with 50 μl of each sample and was injected three times at the beginning of the batch and after three sample injections (47–49).
Quantification of tryptoquialanine A.
Standard TA isolated from P. digitatum and EV extract were analyzed using a Waters Acquity UPLC system coupled to a Waters Micromass Quattro Micro TM API with electrospray ionization source and a triple quadrupole mass analyzer. Analyses were performed in the positive mode with an m/z range of 100 to 1,200, capillary voltage of 3 kV, cone voltage of 25 V, inlet capillary temperature of 150°C, and nebulizing gas temperature of 200°C. Stationary phase: Thermo Scientific column Accucore C18 2.6 μm (2.1 mm by 100 mm). Mobile phase: 0.1% formic acid (A) and acetonitrile (B). Eluent profile (A/B): 95/5 up to 2/98 within 10 min, held for 5 min, up to 95/5 within 1.2 min, and held for 3.8 min. The total run time was 20 min for each run, and the flow rate was 0.2 ml min−1. Injection volume: 10 μl. All the operation and spectrum analyses were conducted using Waters MassLynx v.4.1.
For the construction of the calibration curve, standard TA was diluted in the range concentration of 6.25 to 0.006 μg ml−1, and selected reaction monitoring (SRM) analyses were performed following conditions as previously described: m/z 519 → 197 (quantification) and m/z 519 → 213 (monitoring), collision energy of 22 eV (38).
For quantification of tryptoquialanine A in EVs, 40 μl of a 2.1 × 1010 EV ml−1 solution was dried and extracted with 100 μl of MeOH HPLC grade as previously described. Then, 100 μl of EV extract solution was transferred to glass vials and analyses were performed in duplicate.
Statistical and metabolomic analyses.
Feature detection was performed on XCMS online (v.3.5.1) using the following parameters: method: centWave, prefilter peaks and intensity: 3 and 5,000, ppm: 2.5, Signal/noise threshold: 10, peak width: 5 to 20, mzdiff: 0.01, and noise filter: 1,000. Preprocessing included median fold change normalization on XCMS Online. Multivariate and univariate analyses of the feature list were performed with the MetaboAnalyst tool (v.4.0). Pareto scaling was applied. One-way analysis of variance (ANOVA) was performed, and all the results were analyzed using a confidence level of 95% and a significance level corresponding to P < 0.05. Principal-component analysis (PCA) was performed for an exploratory analysis, followed by partial least-squares discriminant analysis (PLS-DA). A permutation test (cross validation) was performed to determine the reliability of the created PLS-DA model.
Molecular networking analyses.
MS data were converted to mzXML format using MSConvert GUI, a tool of the ProteoWizard package. Molecular networks for in vivo assays and EV extracts were created using the mzXML files on the online workflow at the Global Natural Products Social Molecular Networking (GNPS) platform (http://gnps.ucsd.edu). Data were filtered by removing all MS/MS peaks within ±17 Da of the precursor ion. MS/MS spectra were window filtered by choosing only the top 6 peaks in the ±50-Da window throughout the spectrum. The data were then clustered with MS-Cluster with a parent mass tolerance of 0.02 Da and an MS/MS fragment ion tolerance of 0.02 Da to create consensus spectra. Consensus spectra that contained fewer than 2 spectra were discarded. A network was then created where edges were filtered to have a cosine score above 0.6 and more than 5 matched peaks. The spectra in the network were then searched against GNPS’s spectral libraries. The library spectra were filtered in the same manner as the input data. All matches between network spectra and library spectra were required to have a score above 0.6 and at least 5 matched peaks (50).
ACKNOWLEDGMENTS
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, Fundação de Amparo a Pesquisa no Estado de São Paulo (grant numbers 2019/11563-2, 2019/06359-7, 2017/24462-4, 2013/50228-8, and 2015/01017-0) L’Oréal Brazil, together with ABC and UNESCO in Brazil. M.L.R. was supported by grants from the Brazilian Ministry of Health (grant 440015/2018-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grants 405520/2018-2 and 301304/2017-3), and Fiocruz (grants PROEP-ICC 442186/2019-3, VPPCB-007-FIO-18, and VPPIS-001-FIO18). We also acknowledge support from the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças de Populações Negligenciadas (INCT-IDPN).
Conflict of interest: M.L.R. is currently on leave from the position of associate professor at the Microbiology Institute of the Federal University of Rio de Janeiro, Brazil.
Footnotes
This article is a direct contribution from Marcio L. Rodrigues, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Aaron Mitchell, University of Georgia, and Maysa Furlan, Universidade Estadual Paulista.
Citation Costa JH, Bazioli JM, Barbosa LD, dos Santos Júnior PLT, Reis FCG, Klimeck T, Crnkovic CM, Berlinck RGS, Sussulini A, Rodrigues ML, Fill TP. 2021. Phytotoxic tryptoquialanines produced in vivo by Penicillium digitatum are exported in extracellular vesicles. mBio 12:e03393-20. https://doi.org/10.1128/mBio.03393-20.
REFERENCES
- 1.Jr-Mattos D, Carlos EF. 2018. The role of the International Society of Citriculture on the world citrus industry. Citrus Res Technol 38:228–232. doi: 10.4322/crt.ICC171. [DOI] [Google Scholar]
- 2.Citrus: World Markets and Trade. 2020. United States Department of Agriculture. Foreign Agricultural Service. https://downloads.usda.library.cornell.edu/usda-esmis/files/w66343603/00000g55g/kp78h0193/citrus.pdf. Accessed 14 April 2020.
- 3.Blauth de Lima F, Félix C, Osório N, Alves A, Vitorino R, Domingues P, Correia A, Ribeiro RTS, Esteves AC. 2016. Secretome analysis of Trichoderma atroviride T17 biocontrol of Guignardia citricarpa. Biol Control 99:38–86. doi: 10.1016/j.biocontrol.2016.04.009. [DOI] [Google Scholar]
- 4.Costa JH, Bazioli JM, Pontes JGM, Fill TP. 2019. Penicillium digitatum infection mechanisms in citrus: what do we know so far? Fungal Biol 123:584–593. doi: 10.1016/j.funbio.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Qian X, Yang Q, Zhang Q, Abdelhai MH, Dhanasekaran S, Serwah BNA, Gu N, Zhang H. 2019. Elucidation of the initial growth process and the infection mechanism of Penicillium digitatum on postharvest citrus (Citrus reticulata blanco). Microorganisms 7:485. doi: 10.3390/microorganisms7110485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Shen Y, Chen C, Wan C. 2019. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: a review. Plants 8:26. doi: 10.3390/plants8020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Zheng Q, Xu B, Liu J. 2019. Identification of the fungal pathogens of postharvest disease on peach fruits and the control mechanisms of Bacillus subtilis JK-14. Toxins 11:322. doi: 10.3390/toxins11060322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spadaro D, Droby S. 2016. Development of biocontrol products for postharvest diseases of fruit: the importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci Technol 47:39–49. doi: 10.1016/j.tifs.2015.11.003. [DOI] [Google Scholar]
- 9.Sanzani SM, Reverberi M, Geisen R. 2016. Mycotoxins in harvested fruits and vegetables: insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol Tec 122:95–105. doi: 10.1016/j.postharvbio.2016.07.003. [DOI] [Google Scholar]
- 10.Nicosia MGLD, Pangallo S, Raphael G, Romeo FV, Strano MC, Rapisarda P, Droby S, Schena L. 2016. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol Tec 114:54–61. doi: 10.1016/j.postharvbio.2015.11.012. [DOI] [Google Scholar]
- 11.Dukare AS, Paul S, Nambi VE, Gupta RK, Singh R, Sharma K, Vishwakarma RK. 2019. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: a review. Crit Rev Food Sci Nutr 59:1498–1513. doi: 10.1080/10408398.2017.1417235. [DOI] [PubMed] [Google Scholar]
- 12.Bazioli JM, Belinato JR, Costa JH, Akiyama DY, Pontes JGM, Kupper KC, Augusto F, Carvalho JE, Fill TP. 2019. Biological control of citrus postharvest phytopathogens. Toxins 11:460. doi: 10.3390/toxins11080460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramón-Carbonell M, Sánchez-Torres P. 2017. The transcription factor PdSte12 contributes to Penicillium digitatum virulence during citrus fruit infection. Postharvest Biol Tec 125:129–139. doi: 10.1016/j.postharvbio.2016.11.012. [DOI] [Google Scholar]
- 14.Vu TX, Ngo TT, Mai LTD, Bui T, Le DH, Bui HTV, Nguyen HQ, Ngo BX, Tran V. 2018. A highly efficient Agrobacterium tumefaciens-mediated transformation system for the postharvest pathogen Penicillium digitatum using DsRed and GFP to visualize citrus host colonization. J Microbiol Methods 144:134–144. doi: 10.1016/j.mimet.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Scharf DH, Heinekamp T, Brakhage AA. 2014. Human and plant fungal pathogens: the role of secondary metabolites. PLoS Pathog 10:e1003859. doi: 10.1371/journal.ppat.1003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariza MR, Larsen TO, Peterson BO, Duus JO, Barrero AF. 2002. Penicillium digitatum metabolites on synthetic media and citrus fruits. J Agric Food Chem 50:6361–6365. doi: 10.1021/jf020398d. [DOI] [PubMed] [Google Scholar]
- 17.Costa JH, Bazioli JM, Araújo EV, Vendramini PH, Porto MCF, Eberlin MN, Souza-Neto JA, Fill TP. 2019. Monitoring indole alkaloid production by Penicillium digitatum during infection process in citrus by mass spectrometry imaging and molecular networking. Fungal Biol 123:594–600. doi: 10.1016/j.funbio.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Costa JH, Wassano CI, Angolini CFF, Scherlach K, Hertweck C, Fill TP. 2019. Antifungal potential of secondary metabolites involved in the interaction between citrus pathogens. Sci Rep 9:18647. doi: 10.1038/s41598-019-55204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza JAM, Baltazar LM, Carregal VM, Gouveia-Eufrasio L, Oliveira AG, Dias WG, Rocha MC, Miranda KR, Malavazi I, Santos DA, Frézard FJG, Souza DG, Teixeira MM, Soriani FM. 2019. Characterization of Aspergillus fumigatus extracellular vesicles and their effects on macrophages and neutrophils functions. Front Microbiol 10:2008. doi: 10.3389/fmicb.2019.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herkert PF, Amatuzzi RF, Alves LR, Rodrigues ML. 2019. Extracellular vesicles as vehicles for the delivery of biologically active fungal molecules. Curr Protein Pept Sci 20:1027–1036. doi: 10.2174/1389203720666190529124055. [DOI] [PubMed] [Google Scholar]
- 21.Silva VKA, Rodrigues ML, May RC. 2019. Deciphering fungal extracellular vesicles: from cell biology to pathogenesis. Curr Clin Micro Rep 6:89–97. doi: 10.1007/s40588-019-00128-1. [DOI] [Google Scholar]
- 22.Rybak K, Robatzek S. 2019. Functions of extracellular vesicles in immunity and virulence. Plant Physiol 179:1236–1247. doi: 10.1104/pp.18.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regente M, Pinedo M, Clemente HS, Balliau T, Jamet E, Canal L. 2017. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot 68:5485–5496. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 24.Bielska E, Sisquella MA, Aldeieg M, Birch C, O’Donoghue EJ, May RC. 2018. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat Commun 9:1556. doi: 10.1038/s41467-018-03991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleackley MR, Samuel M, Garcia-Ceron D, McKenna JA, Lowe RGT, Pathan M, Zhao K, Ang C-S, Mathivanan S, Anderson MA. 2019. Extracellular vesicles from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a phytotoxic response in plants. Front Plant Sci 10:1610. doi: 10.3389/fpls.2019.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reis FCG, Borges BS, Jozefowicz LJ, Sena BAG, Garcia AWA, Medeiros LC, Martins ST, Honorato L, Schrank A, Vainstein MH, Kmetzsch L, Nimrichter L, Alves LR, Staats CC, Rodrigues ML. 2019. A novel protocol for the isolation of fungal extracellular vesicles reveals the participation of a putative scramblase in polysaccharide export and capsule construction in Cryptococcus gattii. mSphere 4:e00080-19. doi: 10.1128/mSphere.00080-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X, Chooi YH, Ames BD, Wang P, Walsh CT, Tang Y. 2011. Fungal indole alkaloid biosynthesis: genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J Am Chem Soc 133:2729–2741. doi: 10.1021/ja1101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertinetti BV, Peña NI, Cabrera GM. 2009. An antifungal tetrapeptide from the culture of Penicillium canescens. Chem Biodivers 6:1178–1184. doi: 10.1002/cbdv.200800336. [DOI] [PubMed] [Google Scholar]
- 29.Hammerl R, Frank O, Schmittnägel T, Ehrmann MA, Hofmann T. 2019. Functional metabolome analysis of Penicillium roqueforti by means of differential off-line LC-NMR. J Agric Food Chem 67:5135–5146. doi: 10.1021/acs.jafc.9b00388. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Sheng D, Wu X, Wang M, Hu X, Li H, Yu D. 2017. Identification of secondary metabolite biosynthetic gene clusters associated with the infection of citrus fruit by Penicillium digitatum. Postharvest Biol Tec 134:17–21. doi: 10.1016/j.postharvbio.2017.07.011. [DOI] [Google Scholar]
- 31.Wang XD, Sun C, Gao S, Wang L, Shuokui H. 2001. Validation of germination rate and root elongation as indicator to assess phytotoxicity with Cucumis sativus. Chemosphere 44:1711–1721. doi: 10.1016/s0045-6535(00)00520-8. [DOI] [PubMed] [Google Scholar]
- 32.Ashagre H, Almaw D, Feyisa T. 2013. Effect of copper and zinc on seed germination, phytotoxicity, tolerance and seedling vigor of tomato (Lycopersicon esculentum L. cultivar ROMA VF). Int J Agric Sci Res 2:312–317. [Google Scholar]
- 33.Zamith-Miranda D, Nimrichter L, Rodrigues ML, Nosanchuk JD. 2018. Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes Infect 20:501–504. doi: 10.1016/j.micinf.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Q, He B, Weiberg A, Buck AH, Jin H. 2019. Small RNAs and extracellular vesicles: new mechanisms of cross-species communication and innovate tools for disease control. PLoS Pathog 15:e1008090. doi: 10.1371/journal.ppat.1008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisvad JC, Smedsgaard J, Larsen TO, Samson RA. 2004. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol 49:201–241. [Google Scholar]
- 36.Oppong-Danquah E, Passaretti C, Chianese O, Blümel M, Tasdemir D. 2020. Mining the metabolome and the agricultural and pharmaceutical potential of sea foam-derived fungi. Mar Drugs 18:128. doi: 10.3390/md18020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali H, Ries MI, Lankhorst PP, Hoeven RAM, Schouten OL, Noga M, Hankemeier T, Peij NNME, Bovenberg RAL, Vreeken RJ, Driessen AJM. 2014. A non-canonical NRPS is involved in the synthesis of fungisporin and related hydrophobic cyclic tetrapeptides in Penicillium chrysogenum. PLoS One 9:e98212. doi: 10.1371/journal.pone.0098212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araújo EV, Vendramini PH, Costa JH, Eberlin MN, Montagner CC, Fill TP. 2019. Determination of tryptoquialanines A and C produced by Penicillium digitatum in oranges: are we safe? Food Chem 301:125285. doi: 10.1016/j.foodchem.2019.125285. [DOI] [PubMed] [Google Scholar]
- 39.Navarro D, Diáz-Mula HM, Guillén F, Zapata PJ, Castillo S, Serrano M, Valero D, Martínez-Romero D. 2011. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int J Food Microbiol 151:241–246. doi: 10.1016/j.ijfoodmicro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Louw JP, Korsten L. 2016. Postharvest decay of nectarine and plum caused by Penicillium spp. Eur J Plant Pathol 146:779–791. doi: 10.1007/s10658-016-0956-0. [DOI] [Google Scholar]
- 41.Louw JP, Korsten L. 2019. Impact of ripeness on the infection and colonisation of Penicillium digitatum and P expansum on plum. Postharvest Biol Technol 149:148–158. doi: 10.1016/j.postharvbio.2018.11.024. [DOI] [Google Scholar]
- 42.Niedz RP 2008. In vitro germination of citrus seed. Proc Fla State Hort Soc 121:148–151. [Google Scholar]
- 43.Habermann E, Pereira VDC, Imatomi M, Pontes FC, Gualtieri CJ. 2017. In vitro herbicide activity of crude and fractionated leaf extracts of Blepharocalyx salicifolius (Myrtaceae). Braz J Bot 40:33–40. doi: 10.1007/s40415-016-0317-4. [DOI] [Google Scholar]
- 44.Abdelgaleil SAM, Saad MMG, Ariefta NR, Shiono Y. 2020. Antimicrobial and phytotoxic activities of secondary metabolites from Haplophyllum tuberculatum and Chrysanthemum coronarium. S Afr J Bot 128:35–41. doi: 10.1016/j.sajb.2019.10.005. [DOI] [Google Scholar]
- 45.Gris D, Boaretto AG, Marques MR, Damasceno-Junior G, Carollo CA. 2019. Secondary metabolites that could contribute to the monodominance of Erythrina fusca in the Brazilian Pantanal. Ecotoxicology 28:1232–1240. doi: 10.1007/s10646-019-02133-y. [DOI] [PubMed] [Google Scholar]
- 46.Smedsgaard J 1997. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J Chromatogr A 760:264–270. doi: 10.1016/S0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Zhang X, Ye L, Kang Z, Jia D, Yang L, Zhang B. 2019. LC-MS-based metabolomic approach revealed the significantly different metabolic profiles of five commercial truffle species. Front Microbiol 10:2227. doi: 10.3389/fmicb.2019.02227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albóniga OE, González O, Alonso RM, Xu Y, Goodacre R. 2020. Optimization of XCMS parameters for LC-MS metabolomics: an assessment of automated versus manual tuning and its effect on the final results. Metabolomics 16:14. doi: 10.1007/s11306-020-1636-9. [DOI] [PubMed] [Google Scholar]
- 49.Le TN, da Silva D, Colas C, Darrouzet E, Baril P, Leseurre L, Maunit B. 2020. Development of an LC-MS multivariate nontargeted methodology for differential analysis of the peptide profile of Asian hornet venom (Vespa velutina nigrithorax): application to the investigation of the impact of collection period variation. Anal Bioanal Chem 412:1419–1430. doi: 10.1007/s00216-019-02372-2. [DOI] [PubMed] [Google Scholar]
- 50.Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu WT, Crüsemann M, Boudreau PD, Esquenazi E, Sandoval-Calderón M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu CC, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw CC, Yang YL, Humpf HU, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, et al. 2016. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol 34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A and B) HPLC chromatograms (280 nm) for (A) P. digitatum extract and (B) purified tryptoquialanine A. (C and D) HPLC-MS analysis of purified tryptoquialanine A (m/z 519); (C) extracted ion chromatogram and (D) mass spectrum. Download FIG S1, TIF file, 0.2 MB (162.4KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A to C) Extracted ion chromatograms of m/z 519.18 for (A) P. digitatum EVs extract, (B) MS analysis control, and (C) EV isolation methodology control. (D) Mass spectrum of ion [M + H]+ m/z 519.1877 obtained for tryptoquialanine A (error, 0.61 ppm) at 9.2 min. (E to G) Extracted ion chromatograms of m/z 505.17 for (E) P. digitatum EV extract, (F) MS analysis control, and (G) EV isolation methodology control. (H) Mass spectrum of ion [M + H]+ m/z 505.1719 obtained for tryptoquialanine B (error, 0.26 ppm) at 8.7 min. (I to K) Extracted ion chromatograms of m/z 503.19 for (I) P. digitatum EV extract, (J) MS analysis control, and (K) EV isolation methodology control. (L) Mass spectrum of ion [M + H]+ m/z 505.1926 obtained for deoxytryptoquialanine (error = 0.23 ppm) at 8.6 min. Download FIG S2, TIF file, 0.2 MB (222KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A to C) Extracted ion chromatograms of m/z 509.27 for (A) P. digitatum EV extract, (B) MS analysis control, and (C) EV isolation methodology control. (D) Mass spectrum of ion [M + H]+ m/z 509.2761 obtained for cyclo-(Phe-Val-Val-Tyr) (error, 0.48 ppm) at 8.6 min. (E to G) Extracted ion chromatograms of m/z 511.29 for (E) P. digitatum EV extract, (F) MS analysis control, and (G) EV isolation methodology control. (H) Mass spectrum of ion [M + H]+ m/z 511.2917 obtained for Phe-Val-Val-Phe (error, 0.43 ppm) at 7.1 min. (I to K) Extracted ion chromatograms of m/z 527.28 for (I) P. digitatum EV extract, (J) MS analysis control, and (K) EV isolation methodology control. (L) Mass spectrum of ion [M + H]+ m/z 527.2866 obtained for Phe-Val-Val-Tyr (error, 0.40 ppm) at 6.4 min. (M to O) Extracted ion chromatograms of m/z 493.28 for (M) P. digitatum EV extract, (N) MS analysis control, and (O) EV isolation methodology control. (P) Mass spectrum of ion [M + H]+ m/z 493.2809 obtained for cyclo-(Phe-Phe-Val-Val) (error,= 0.03 ppm) at 9.7 min. Download FIG S3, TIF file, 0.3 MB (301.9KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A to F) Comparison of MS/MS spectra between (A) P. digitatum EV extract and (B) purified tryptoquialanine A, (C) P. digitatum EV extract and (D) purified tryptoquialanine B, and (E) P. digitatum EV extract and (F) purified deoxytryptoquialanine. Download FIG S4, TIF file, 0.1 MB (152.7KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) MS/MS match between GNPS database (green) and compound cyclo-(Phe-Phe-Val-Val) (black). (B and C) MS/MS spectrum of (B) m/z 511.29 and (C) m/z 527.28 obtained from P. digitatum EV extract. The fragmentation pattern was compared with MS/MS data of compounds Phe-Val-Val-Phe and Phe-Val-Val-Tyr reported in the literature. Download FIG S5, TIF file, 0.08 MB (86.7KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
UHPLC-MS/MS calibration curve obtained for tryptoquialanine A. Download FIG S6, TIF file, 0.01 MB (13.7KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A to M) Mass spectrum and MS/MS spectrum for (A) tryptoquialanine A ion [M + H]+ m/z 519.1876, (B) tryptoquialanine B ion [M + H]+ m/z 505.1735, (C) deoxytryptoquialanine ion [M + H]+ m/z 503.1948, (D) cyclo-(Phe-Val-Val-Tyr) ion [M + H]+ m/z 509.2770, (E) Phe-Val-Val-Phe ion [M + H]+ m/z 511.2920, (F) Phe-Val-Val-Tyr ion [M + H]+ m/z 527.2859, (G) cyclo-(Phe-Phe-Val-Val) ion [M + H]+ m/z 493.2822, (H) 15-dimethyl-2-epi-fumiquinazoline A ion [M + H]+ m/z 460.1983, (I) deoxytryptoquialanone ion [M + H]+ m/z 459.1678, (J) tryptoquivaline L ion [M + H]+ m/z 433.1523, (K) tryptoquivaline Q ion [M + H]+ m/z 435.1673, (L) fumiquinazoline A ion [M + H]+ m/z 446.1838, and (M) fumiquinazoline C ion [M + H]+ m/z 444.1687. Download FIG S7, TIF file, 1.1 MB (1.1MB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Fruits infected with P. digitatum. (A to C) (A) plum at 10 dpi, (B) plum at 13 dpi, and (C) orange at 7 dpi. Download FIG S8, TIF file, 0.3 MB (329.5KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complete molecular networking obtained for extracts of plums and citrus fruits infected by P. digitatum. Download FIG S9, JPG file, 2.7 MB (2.7MB, jpg) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Examples of clusters obtained by molecular networking for extracts of P. digitatum infection in different fruits. The unknown compounds are present in just one type of the fruits and absent in the control fruits Download FIG S10, TIF file, 0.03 MB (30.8KB, tif) .
Copyright © 2021 Costa et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.