Abstract
Human papillomavirus (HPV) is a principal driver for most oropharyngeal squamous cell carcinomas (OPSCCs), where it is strongly associated with improved survival. HPV is much less frequently detected in squamous cell carcinomas arising in nonoropharyngeal sites (non-OPSCCs), and its pathogenic role and prognostic value in these tumors is unclear. We evaluated the clinicopathologic features of 52 non-OPSCCs considered HPV-positive based upon p16 immunohistochemistry and direct HPV detection using RNA in situ hybridization (ISH), DNA ISH, or real-time DNA polymerase chain reaction. The HPV-positive non-OPSCCs were from the larynx (n = 27), oral cavity (n = 21), and hypopharynx (n = 4). While most cases (n = 34, 65%) showed classic histologic features of HPV-positive OPSCC, including endophytic growth, minimal keratinization, and hyperchromatic nuclei without koilocytic changes, a subset (n = 13, 25%) were characterized by exophytic growth, exuberant surface hyperkeratosis and parakeratosis, marked nuclear pleomorphism, and prominent koilocytic atypia. These antithetical features were highly reminiscent of the warty variant of HPV-positive squamous cell carcinoma described in anogenital sites. Compared with tumors without warty features, the warty tumors presented at lower stage and were not associated with lymph node metastasis, local recurrence, or distant spread (4 y disease-free survival of 100% vs. 66%, P = 0.069). The presence of transcriptionally active HPV as detected by RNA ISH suggests a pathogenic role for HPV in these nonoropharyngeal sites. While most HPV-positive non-OPSCCs are morphologically similar to their tonsillar counterparts, this study highlights a previously unrecognized warty variant that may be associated with a highly favorable clinical outcome.
Keywords: head and neck squamous cell carcinoma, human papillomavirus, oropharyngeal squamous cell carcinoma, oral cavity squamous cell carcinoma, laryngeal squamous cell carcinoma, p16
In the head and neck, the role of high-risk human papillomavirus (HPV) in cancer development is largely dictated by anatomic site. HPV is well-established as a principal driver for squamous cell carcinomas arising in the oropharynx (OPSCCs), where it is detected in 70% to 90% of tumors arising from tonsillar tissues in United States populations.1 Its importance as a pathologic agent is far less clear for squamous cell carcinomas (SCCs) arising in nonoropharyngeal sites (non-OPSCCs) including the oral cavity, larynx, and hypopharynx. While historical literature that relied on DNA polymerase chain reaction (PCR)-based methods reported widely disparate HPV prevalence of 10% to 50% in nonoropharyngeal tumors, more recent studies that strictly excluded OPSCCs and employed highly specific in situ hybridization (ISH) and RNA PCR-based approaches have recognized high-risk HPV in ~5% of oral and laryngeal SCCs.2–11 In the oropharynx, the presence of HPV is associated with a consistent phenotype including localization to the tonsillar crypts, a lack of surface squamous dysplasia, scant cytoplasm without significant keratinization, and the absence of viral cytopathic changes such as koilocytosis.12 Although similar features have been reported in a subset of HPV-positive non-OPSCCs,3,6,13 it is still not entirely clear whether this signature morphologic profile reflects intrinsic properties related to HPV or largely mirrors the unique tonsillar crypt microenvironment from which HPV-positive OPSCCs arise.
HPV-positive OPSCCs also have a characteristic clinical profile that includes a propensity to metastasize to regional lymph nodes, sensitivity to radiation and chemotherapy, and improved survival.14–18 Accordingly, HPV status has emerged as a major biomarker for predicting patient outcomes, randomizing patients in clinical trials, and triaging patients to HPV-specific treatment protocols. There is a predictable inclination to generalize the clinical experience for patients with HPV-positive OPSCCs to patients with HPV-positive SCCs arising outside of the oropharynx; however, the assumption that HPV positivity confers unique biological and clinical properties irrespective of the anatomic site is not well validated. Indeed, previous studies have produced conflicting results regarding the prognostic implications of HPV-positive SCCs in nonoropharyngeal sites.7,9,10,13,19,20 Like the quandary surrounding its morphologic profile, it remains unclear whether the prototypic clinical properties of HPV-positive OPSCCs are intrinsic to HPV or related to the unique histologic, ultrastructural, and immunologic terrain of the tonsillar crypts.
Variable detection methods including highly sensitive assays that are unable to distinguish biologically active from inactive infections have consistently overestimated the incidence of HPV-positive non-OPSCCs.21 This together with the paucity of true HPV-positive SCCs of the oral cavity, larynx, and hypopharynx has impeded complete assessment of pathologic and clinical features. This study aims to provide a comprehensive clinicopathologic characterization of a large multi-institutional cohort of well-documented HPV-positive SCCs from these sites. In doing so, we hope to (1) define the full morphologic spectrum of HPV-positive non-OPSCCs; (2) establish HPV as a true pathogen for SCCs arising in the oral cavity, larynx, and hypopharynx; and (3) determine the clinical implications of various HPV-positive tumor phenotypes in these sites.
MATERIALS AND METHODS
Case Selection
We identified 52 primary HPV-positive oral cavity, laryngeal, and hypopharyngeal SCCs from the surgical pathology archives of the Johns Hopkins Hospital (JHH), University of California-San Francisco Medical Center (UCSF), and Mount Sinai Hospital (MSH) between 1995 and 2018. Of these, 26 tumors were tested systematically for HPV as part of the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) epidemiological study, which assessed HPV prevalence in SCCs across a randomly sampled group of tumors representing all head and neck anatomic subsites.22 Another 26 HPV-positive cases were identified through a search of the surgical pathology archives at JHH and MSH, where HPV testing was performed selectively as part of pathologic evaluation based on clinical or pathologic suspicion for HPV involvement. To maximize specificity for HPV status, tumors were only included only if they demonstrated both p16 overexpression by immunohistochemistry and direct positivity for HPV using ISH or real-time polymerase chain reaction (RT-PCR). The platforms used for direct HPV detection varied across the study site and time as indicated in Table 1. If not performed originally, HPV RNA ISH was added to recent cases (<5 y old) when the tissue was available. Tumors were strictly excluded if they demonstrated any clinical evidence of involvement of the oropharynx (eg, supraglottic laryngeal SCCs involving the vallecula or posterior oral tongue SCCs extending into the base of tongue) via detailed review of all available imaging studies and procedure/operative notes; for this reason, all large tumors that spanned the oropharynx, as well as oral cavity and/or larynx, were excluded by definition. Tumors were also excluded if there was any histologic evidence of involvement of tonsillar tissue on the review of slides.
TABLE 1.
Details of Direct HPV Testing
| Probe | Type | Source | HPV Genotypes | Cohorts and Dates Tested |
|---|---|---|---|---|
| GenPoint HPV16 Probe | DNA ISH | DAKO. Carpintcria. CA | 16 | PROVE cases 1995–2014 |
| Vcntana Inform HPV III Family 16 Probe | DNA ISH | Vcntana Mcdical Systems. Tucson, AZ | 16. 18. 31, 33, 35, 39. 45. 51. 52. 56. 58. 66 | JHH cases 2010–2014 |
| RNAScopc HPV 16 Probe | RNA ISH | Advanced Cell Diagnostics. Hayward. CA | 16 | PROVE cases 2015–2018 |
| RNAScopc HPV High Risk Cocktail Probe | RNA ISH | Advanced Cell Diagnostics. Hayward. CA | 16. 18. 26. 31. 33, 35, 39. 45. 51. 52. 53, 56. 58. 59. 66. 68. 73. 82 | HPV 16-ncgativc PROVE cases from any date; JHH cases 2015–2018; available JHH/PROVE cases for retesting |
| LightCyclcr 480 | DNA RT-PCR | Roche Diagnostics. Indianapolis. IN | Any | MSH cases 2012–2018; JHH/PROVE cases for gcnotyping |
JHH indicates The Johns Hopkins Hospital; MSH, Mount Sinai Hospital; UCSF, University of California-San Francisco Medical Center.
p16 Immunohistochemistry
For p16 immunohistochemistry, 5-μm whole-slide tumor sections were deparaffinized and antigen retrieval was performed using 10 mM citrate butter at 92°C for 30 minutes. A mouse monoclonal antibody for p16 (clone E6H4; Ventana Medical Systems, Tucson, AZ; prediluted) was applied using a BenchMark XT autostainer (Ventana Medical Systems) with incubation for 8 minutes at 36°C, and signals were visualized using the ultraView polymer detection kit (Ventana Medical Systems). Staining was performed according to the manufacturer’s instructions in the presence of appropriate controls. p16 overexpression was defined as strong nuclear and cytoplasmic staining in > 70% of tumor cells.23,24
HPV In Situ Hybridization
HPV ISH was performed on PROVE and JHH cases using various probes as indicated in Table 1. Full staining procedures for the HPV ISH platforms used are described in detail elsewhere.2,22,25 Briefly, 5-μm whole-slide sections were deparaffinized and pretreated with heat and protease. DNA ISH was performed using the type-specific GenPoint HPV16 probe (DAKO, Carpinteria, CA) or the Ventana Inform HPV III Family 16 cocktail probe (Ventana Medical Systems) recognizing HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 66. RNA ISH was performed using the RNAScope HPV16 type-specific probe (Advanced Cell Diagnostics, Hayward, CA), or the RNAScope high-risk cocktail probe (Advanced Cell Diagnostics) recognizing HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82. All steps were performed according to the manufacturers’ instructions in the presence of appropriate controls. Positivity for DNA ISH was defined as single punctate signals in tumor cell nuclei. For RNA ISH, positivity was defined as multiple punctate signals in the tumor cell cytoplasm and/or nuclei.
HPV DNA Genotyping
The Maxwell 16 formalin-fixed paraffin-embedded Tissue LEV DNA Kit (Promega, Madison, WI) was used for DNA extraction from formalin-fixed paraffin-embedded tissue sections, in accordance with the manufacturer’s instructions. The concentrations and quality of extracted DNAs were measured by a NanoDrop ND-2000c spectrophotometer (Thermo Fisher Scientific, Wilmington, DE).
HPV genotyping was performed in a stepwise fashion. An initial HPV screening was performed by consensus PCR by amplifying the HPV L1 region using consensus primers GP5+ and GP6+ and the LightCycler 480 High-Resolution Melting Master kit (Roche Diagnostics, Indianapolis, IN). The L1 region is conserved in over 31 different genotypes including low-risk and high-risk HPV types found in cervical and head and neck cancer (HPV6, 11, 13, 16, 18, 26, 30, 31, 32, 33, 34, 35, 39, 40, 42, 45, 51, 52, 53, 56, 58, 61, 62, 66, 67, 68, 69, 74, 81, 90, 91).26,27 If HPV DNA was amplified in the sample, a high resolution melting curve analysis was performed on the LightCycler 480 instrument (Roche) to confirm the presence of HPV. Positive samples were reflexed for HPV16/18 genotyping using HPV16 and HPV18 specific PCR. Beta-actin was amplified simultaneously as a housekeeper gene to evaluate DNA integrity and quantity.
For type-specific detection of HPV, RT-PCR by LightCycler 480 instrument was utilized to amplify E6 regions of HPV16 and 18. Amplicons were detected by 2 specific pairs of fluorescence hybridization probes, consisting of 4 different short oligonucleotides that hybridized to an internal sequence of the amplified fragments of HPV16 and 18 during the annealing phase of the amplification cycle. Two probes were labeled at the 5′-end with LightCycler-Red 640 for HPV16 or LightCycler-Red 670 for HPV18, and to avoid extension, modified at the 3′-end by phosphorylation. The other probes were labeled at the 3′-end with fluorescein. After specific hybridization to the template DNA, 2 probes of each target came in close proximity, resulting in fluorescence resonance energy transfer between the 2 fluorophores. The emitted fluorescence of LC-Red 640 or LC-Red 670 was then measured by the LC480 Instrument.
In the event HPV16/18-specific PCR was negative, Sanger Sequencing was performed to detect other HPV genotypes after amplification with GP51 and GP61 primer pairs. HPV subtypes other than HPV16/18 were determined by aligning sequences in GenBank.
Case Review and Data Analysis
All available hematoxylin and eosin sections, immunohistochemical stains, and ISH studies for each non-OPSCC were reviewed by 2 head and neck pathologists (W.H.W. and L.M.R.). The histologic features of each tumor were tabulated, and tumors were classified according to histologic subtypes previously described in the head and neck as well as the gynecologic and external anogenital tracts. Clinical, demographic, and follow-up information was collected from the electronic medical records and the PROVE study database. Sex, smoking status, tumor site, and TNM stages based on the American Joint Committee on Cancer (AJCC) Seventh Edition Cancer Staging Manual28 were compared across histologic variants using χ2 analysis with a significance level set at 0.05. Disease-free survival was compared using the Kaplan-Meier method. All data analysis was performed using the statistical programming language R (R Foundation, Vienna, Austria).
RESULTS
Clinical Background
The clinical characteristics of the study population are summarized in Table 2. The 52 HPV-positive tumors occurred in 51 individual patients, including 35 men and 16 women, with a median age of 59 years (range: 29 to 85). Smoking history was available for 49 patients, of which 38 (78%) were either current (n = 18) or former (n = 20) smokers; these individuals had a mean 36 pack years tobacco exposure (range: 1 to 100). There were 27 laryngeal tumors, 21 oral cavity tumors, and 4 hypopharyngeal tumors, with the most common subsites overall being the glottic larynx (n = 15), supraglottic larynx (n = 10), and floor of mouth (n = 9). There were 15 patients (33%) treated with surgery alone, 4 patients (9%) with radiation alone, 4 patients (9%) with definitive chemoradiation, 6 patients (13%) with surgery and adjuvant radiation, and 12 patients (26%) with surgery and adjuvant chemoradiation therapy.
TABLE 2.
Clinical and Staging Information
| n (%) | ||||
|---|---|---|---|---|
| Nonkeratinizing | Warty | Keratinizing | Total | |
| Histology | ||||
| SCC | 34 (65) | 13 (25) | 5 (10) | 52 (100) |
| Sex | ||||
| Female | 11 (33) | 2 (15) | 3 (60) | 16 (31) |
| Male | 22 (67) | 11 (85) | 2 (40) | 35 (69) |
| Smoking status | ||||
| Current smoker | 11 (33) | 5 (45) | 2 (40) | 18 (38) |
| Former smoker | 15 (45) | 4 (36) | 1 (20) | 20 (41) |
| Never smoker | 7 (21) | 2 (18) | 2 (40) | 11 (22) |
| Tumor site | ||||
| Hypopharynx | ||||
| Postcricoid | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| Pyriform sinus | 3 (9) | 0 (0) | 0 (0) | 3 (6) |
| Larynx | ||||
| Glottis | 10 (29) | 4 (31) | 1 (20) | 15 (29) |
| Larynx NOS | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| Subglottis | 0 (0) | 0 (0) | 1 (20) | 1 (2) |
| Supraglottis | 8 (24) | 1 (8) | 1 (20) | 10 (19) |
| Oral cavity | ||||
| Buccal | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| Floor of mouth | 6 (18) | 3 (23) | 0 (0) | 9 (17) |
| Lateral tongue | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| Mandibular alveolus | 0 (0) | 1 (8) | 2 (40) | 3 (6) |
| Maxillary alveolus | 1 (3) | 1 (8) | 0 (0) | 2 (4) |
| Tongue NOS | 2(6) | 1 (8) | 0 (0) | 3 (6) |
| Ventral tongue | 0 (0) | 2 (15) | 0 (0) | 2 (4) |
| TNM staging (AJCC Seventh Edition) | ||||
| T stage | ||||
| T1 | 5 (15) | 8 (62) | 1 (20) | 14 (27) |
| T2 | 7 (21) | 2 (15) | 2 (40) | 11 (22) |
| T3 | 11 (33) | 0 (0) | 0 (0) | 11 (22) |
| T4 | 10 (30) | 3 (23) | 2 (40) | 15 (29) |
| N stage | ||||
| NO | 13 (39) | 13 (100) | 4 (80) | 30 (59) |
| N1 | 7 (21) | 0 (0) | 0 (0) | 7 (14) |
| N2 | 10 (30) | 0 (0) | 1 (20) | 11 (22) |
| N3 | 3 (9) | 0 (0) | 0 (0) | 3 (6) |
| M stage | ||||
| MO | 32 (97) | 13 (100) | 5 (100) | 50 (98) |
| Ml | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
AJCC indicates American Joint Committee on Cancer; NOS, not otherwise specified.
Histologic Findings
The majority of HPV-positive tumors in the oral cavity, larynx, and hypopharynx, including 34 tumors (65%) overall and 20 tumors (77%) drawn from the PROVE cohort, exhibited the prototypic nonkeratinizing morphology of HPV-positive OPSCC (Fig. 1). These tumors were composed of invasive sheets, nests and/or lobules of basaloid cells characterized by syncytial cytoplasm, an absence of intracellular bridges, a high nuclear-cytoplasmic ratio, and relatively uniform oval nuclei with hyperchromasia. Multinucleation, nuclear halos, raisinoid nuclear membranes, and other koilocytic changes were not observed. Cytoplasmic keratinization either was absent or only focally developed. While a subset of these nonkeratinizing SCC had areas of papillary architecture, they did not exhibit prominent exophytic growth with hyperkeratosis. Likewise, despite somewhat basaloid cytology, these tumors lacked specific features of the basaloid variant of SCC, including peripheral palisading, adenoidal architecture with pseudoglandular spaces, or prominent basement membrane material.
FIGURE 1.
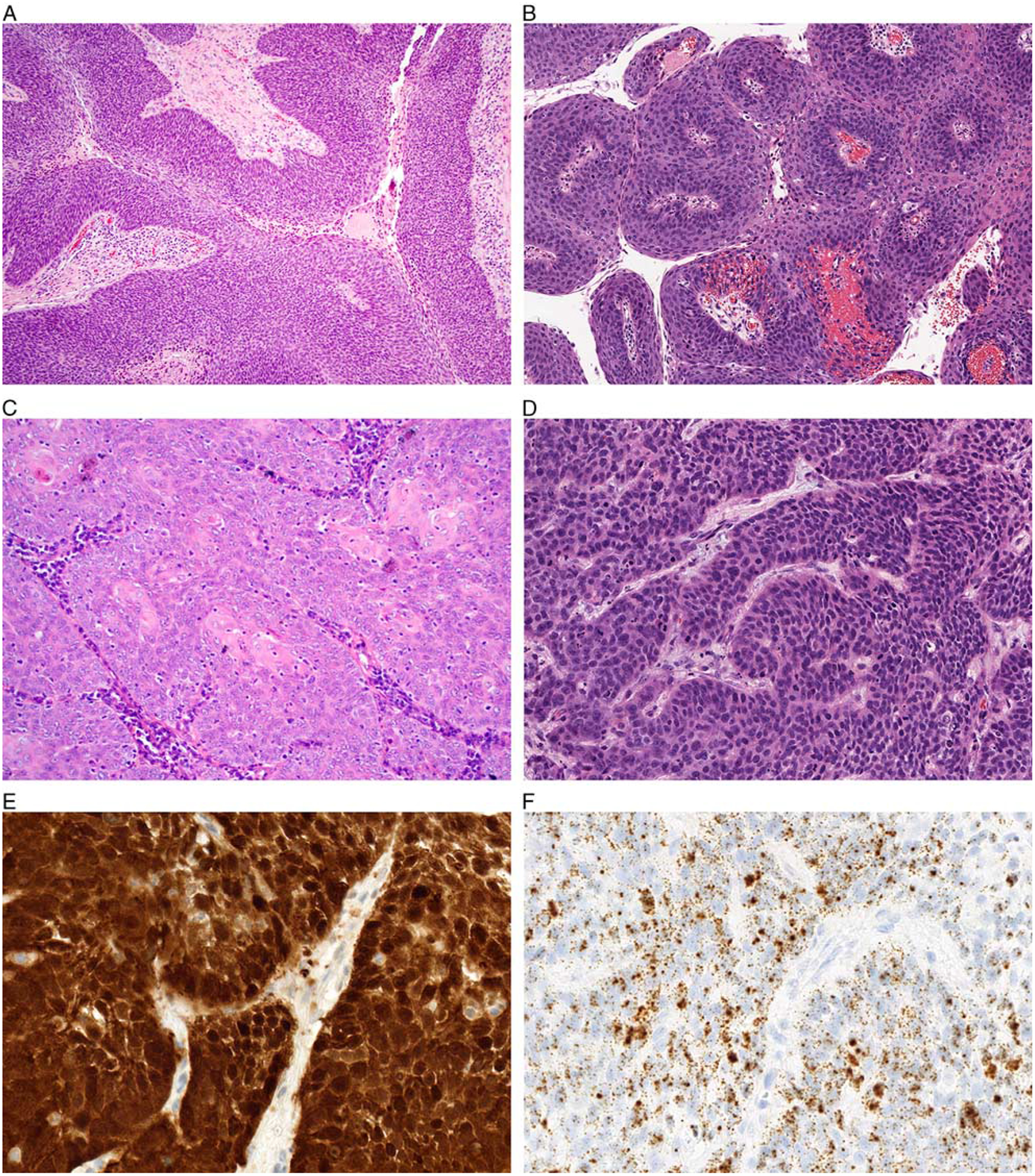
The majority of nonoropharyngeal HPV-positive SCCs demonstrated a nonkeratinizing morphology with nests and lobules of somewhat syncytial basaloid cells with minimal desmoplastic stroma (A, oral cavity). Although no tumors were entirely exophytic, a subset demonstrated papillary architecture (B, larynx). The tumors showed minimal keratinization in a central or peripheral distribution (C, larynx). While cells had a high nuclear-cytoplasmic ratio, nuclei were oval and relatively uniform with only focal pleomorphism (D, larynx). These nonkeratinizing tumors were diffusely positive for p16 (E, larynx) and HPV RNA ISH (F, larynx).
The second subset of tumors, including 13 tumors (25%) overall and 3 tumors (12%) from the PROVE cohort, were designated as warty based on their striking resemblance to the warty variant of HPV-positive SCCs described in the anogenital tract (Fig. 2).29–31 These tumors demonstrated predominantly exophytic growth with abundant bright orange surface parakeratosis and hyperkeratosis with orthokeratinization. The invasive tumor front tended to be broad and pushing. The tumor cells were large with voluminous and glassy eosinophilic cytoplasm, prominent koilocytic change including perinuclear halos and multinucleation, and striking nuclear pleomorphism. In addition to scattered atypical mitotic figures, a subset of tumor cells demonstrated notable chromatin fragmentation similar to the mitosoid bodies seen in focal epithelial hyperplasia. Five (10%) of the 52 non-OPSCCs demonstrated at least 30% of both warty and nonkeratinizing components, present either as discrete zones or closely intermixed (Fig. 3). These tumors were classified as mixed warty/nonkeratinizing.
FIGURE 2.
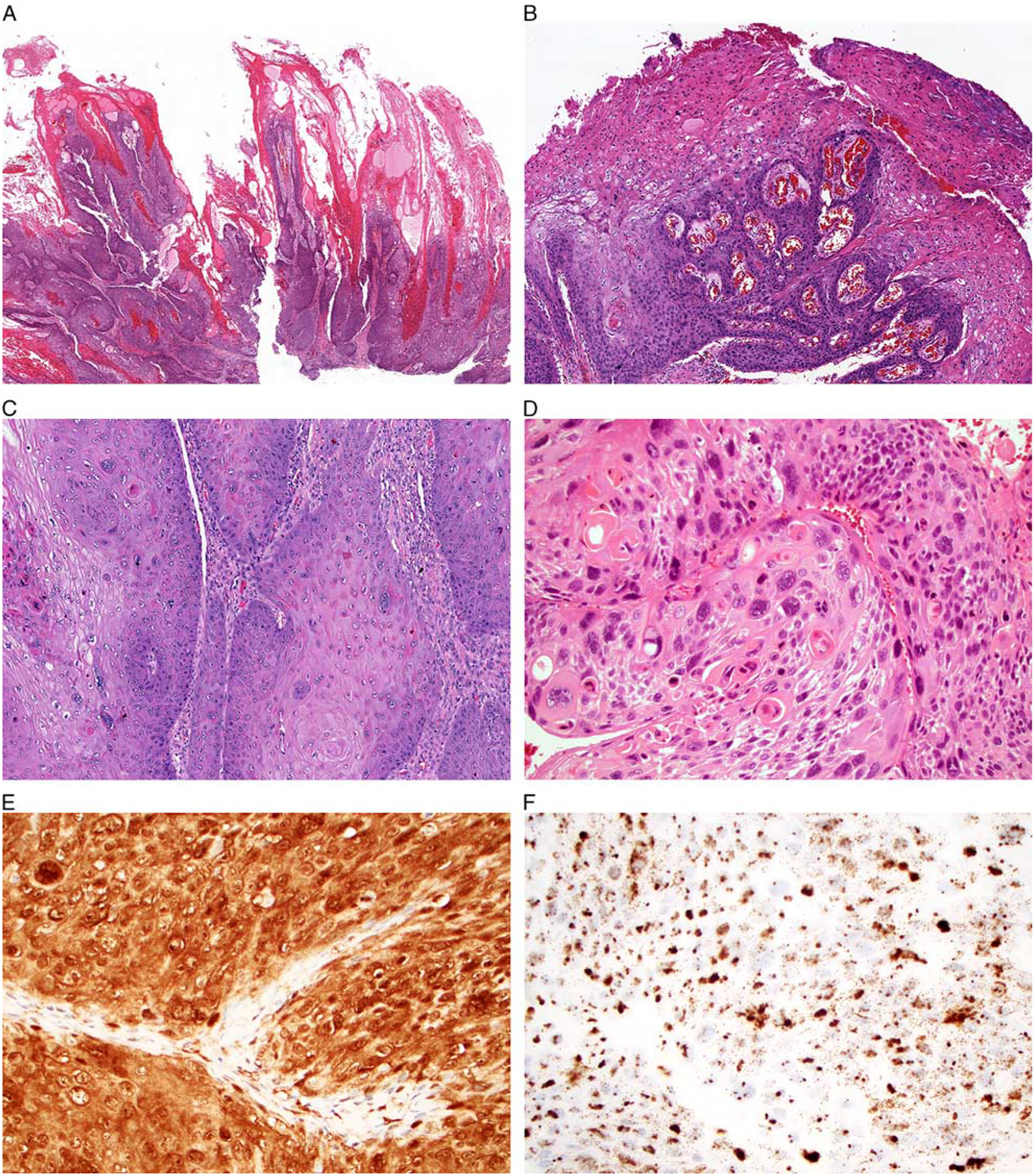
A subset of nonoropharyngeal HPV-positive SCCs had a unique warty morphology with prominent exophytic growth (A, larynx). The surface of the tumors showed abundant production of hard orange parakeratin (B, oral cavity). Tumor cells demonstrated an abundant eosinophilic cytoplasm with marked nuclear pleomorphism and multinucleation (C, oral cavity). They were also notable for areas of koilocytic change and chromatin fragmentation (D, larynx). These warty tumors were diffusely positive for p16 (E, larynx) and HPV RNA ISH (F, larynx).
FIGURE 3.
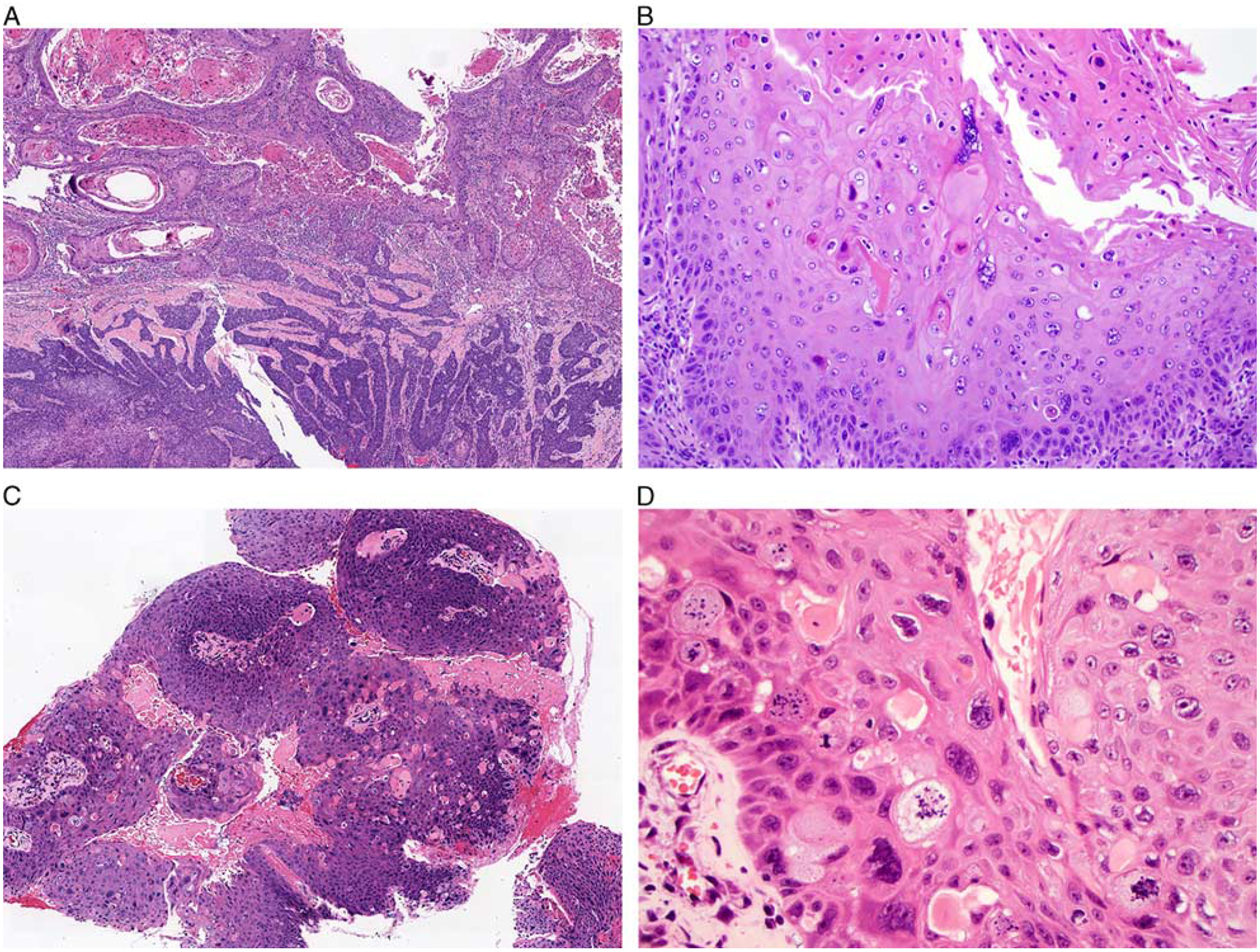
A few tumors demonstrated discrete areas with both exophytic warty and lobulated nonkeratinizing components (A, oral cavity). The warty regions of these tumors demonstrated the same exophytic growth, surface parakeratin production, and marked nuclear pleomorphism as pure warty tumors (B, oral cavity). Other tumors demonstrated warty and nonkeratinizing components that were closely intermixed (C, larynx). The warty areas had prominent nuclear pleomorphism, multinucleation, koilocytosis, and chromatin fragmentation with the appearance of mitosoid bodies (D, larynx).
The final group of tumors, including 5 tumors (10%) overall and 3 tumors (12%) from the PROVE cohort, exhibited conventional keratinizing morphology and were designated as keratinizing type (Fig. 4). These tumors were characterized by infiltrative nests and cords of squamous cells eliciting a prominent desmoplastic stromal reaction. Tumor cells exhibited well-defined intracellular bridges and keratin pearl formation. Although the keratinizing tumors demonstrated eosinophilic cytoplasm and more diffuse nuclear atypia than nonkeratinizing tumors, they lacked the bizarre nuclear pleomorphism, koilocytosis, multinucleation, mitosoid bodies, and bright orange parakeratin production seen in warty SCCs.
FIGURE 4.
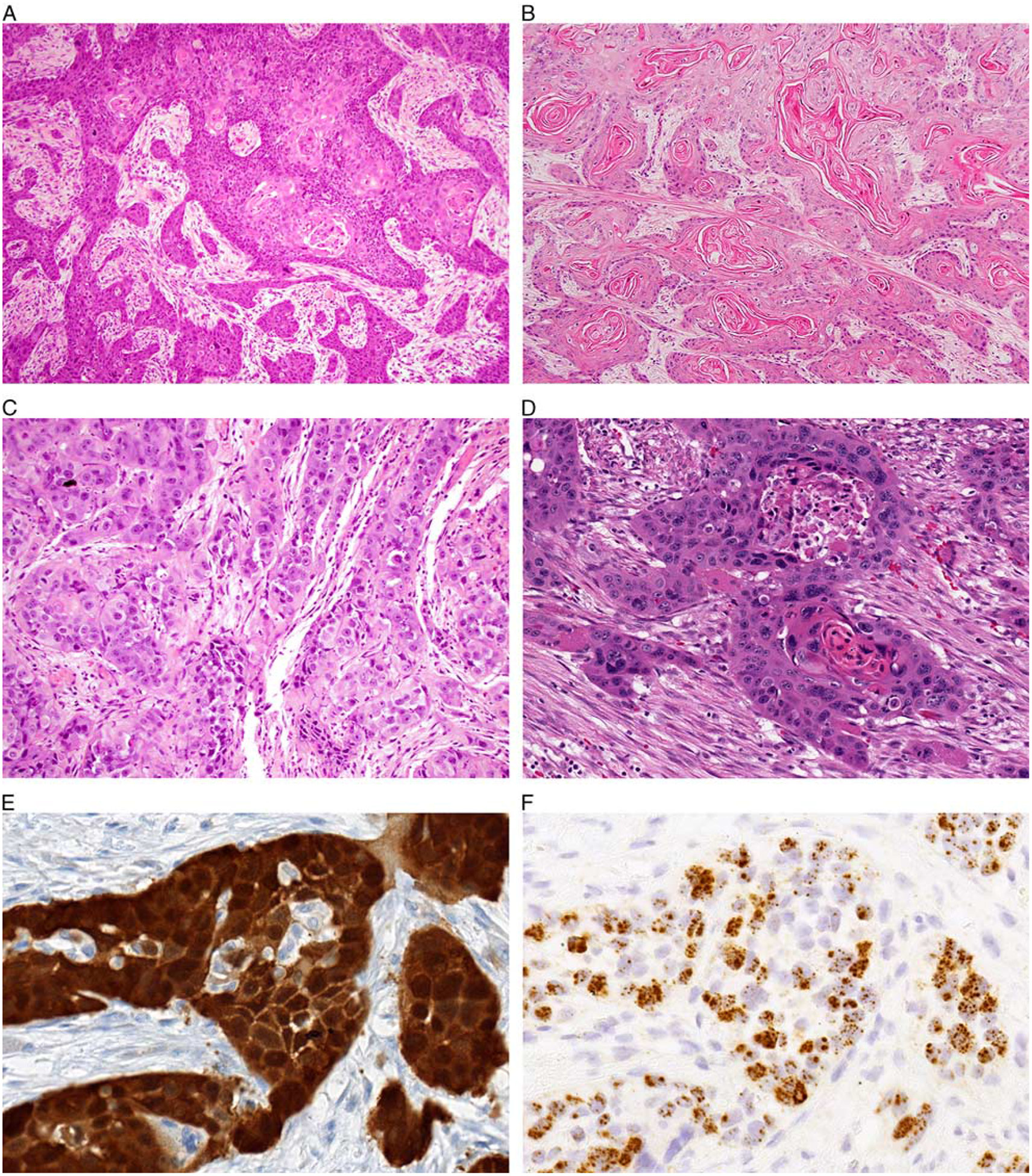
A group of nonoropharyngeal HPV-positive SCCs showed keratinizing morphology characterized by extensively infiltrative growth with marked stromal desmoplasia (A, oral cavity). Prominent keratin production was identified throughout the tumor (B, oral cavity). These tumors demonstrated discrete cell borders with intracellular bridge formation (C, larynx). Tumor cells had abundant eosinophilic cytoplasm with moderate nuclear atypia but lacked the degree of pleomorphism, multinucleation, or chromatin fragmentation seen in warty tumors (D, oral cavity). These keratinizing tumors were diffusely positive for p16 (E, oral cavity) and HPV RNA ISH (F, oral cavity).
Of the 25 biopsies and 27 resection specimens included in this cohort, 38 also had normal mucosa available for evaluation. HPV-related squamous dysplasia was identified adjacent to 12 tumors (32%) including 6 nonkeratinizing (26%), 4 warty (40%), and 2 keratinizing (40%) variants. This group comprised 9 oral cavity (75%) and 3 laryngeal (25%) tumors, including synchronous contralateral nonkeratinizing and warty oral cavity SCCs that arose in a single patient in a background of extensive dysplasia. Regardless of the histologic subtype of the associated SCCs, all precursor lesions demonstrated distinctive features of HPV-related dysplasia characterized by a full-thickness proliferation of atypical basaloid cells with high nuclear-cytoplasmic ratio, prominent apoptotic bodies and karyorrhectic debris, and a thick corrugated layer of surface parakeratin (Fig. 5).
FIGURE 5.
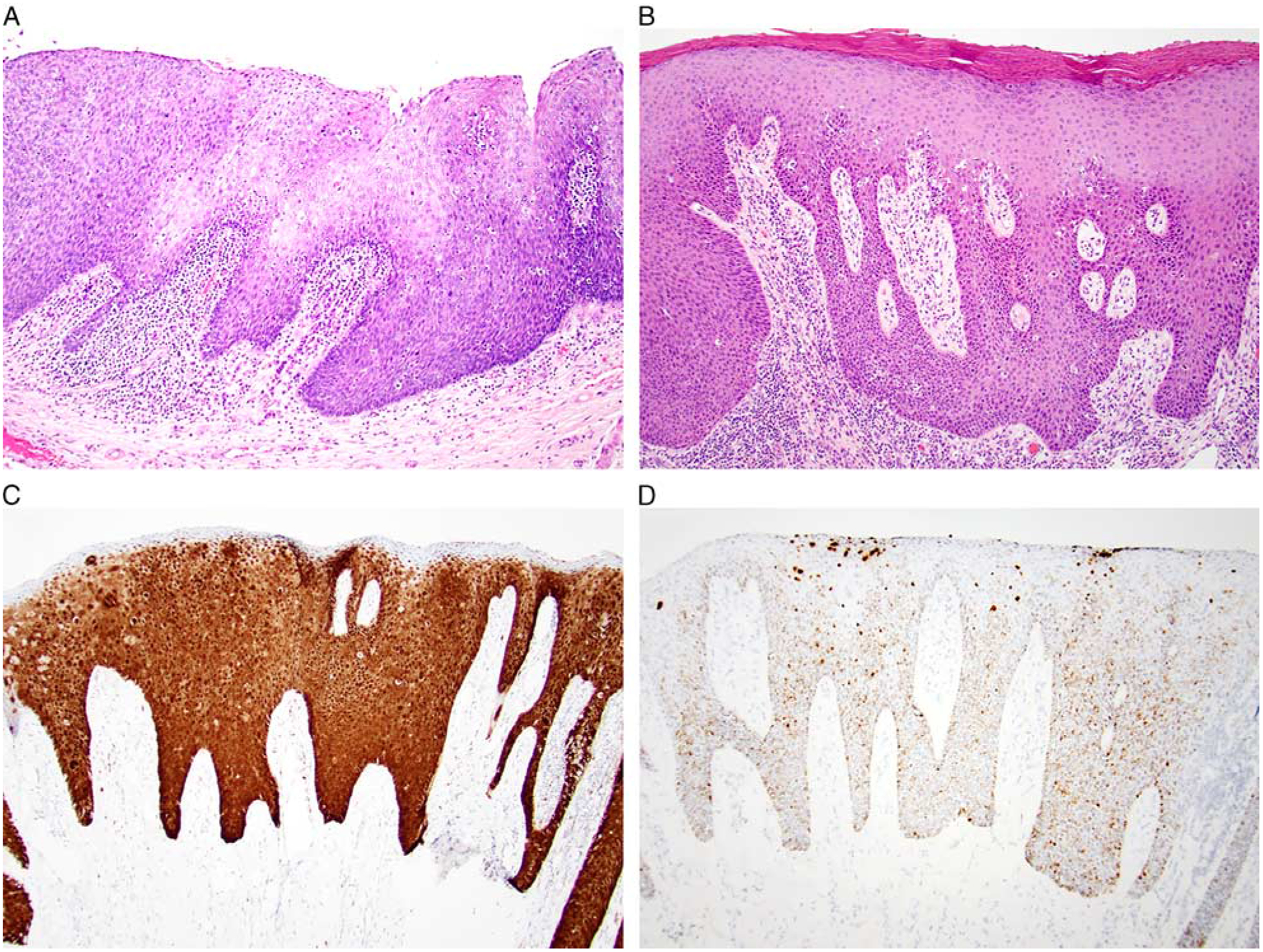
HPV-positive squamous dysplasia was identified in association with a subset of nonoropharyngeal HPV-positive SCCs and was characterized by acanthoticss epithelium with a full-thickness proliferation of atypical basaloid cells with prominent apoptotic and karyorrhectic debris (A, larynx); a dense layer of surface parakeratin was also frequently seen (B, oral cavity). HPV-positive squamous dysplasia was diffusely positive for p16 (C, oral cavity) and HPV RNA ISH (D, oral cavity).
HPV Testing
Per the study inclusion criteria, all 52 tumors in this cohort were p16 positive by immunohistochemistry, and high-risk HPV positive by direct testing (Figs. 1, 2, 4). HPV testing was performed in 30 tumors (58%) using RNA ISH, 16 tumors (31%) using DNA ISH, and 17 tumors (33%) using DNA RT-PCR. The 11 tumors that underwent dual testing by both ISH and RT-PCR demonstrated concordant HPV positivity with both platforms. There were 42 cases (80%) that underwent specific testing to delineate the involvement of HPV16 versus non-HPV16 types. Of these, 29 (69%) were positive for HPV16 including 15 of the nonkeratinizing type (65%), 9 of the warty type (69%) and 5 of the keratinizing type (100%). Of the remaining 13 tumors (31%) that were HPV16 negative, 5 underwent HPV genotyping. The non-HPV16 types identified from this group were 33 (n = 1), 45 (n = 2), 46 (n = 1), and 69 (n = 1). All associated foci of HPV-related squamous dysplasia were also positive for p16 immunohistochemistry and high-risk HPV ISH (Fig. 5). Eight (73%) of the 11 cases with HPV-related dysplasia that underwent HPV16-specific testing were HPV16 positive in both the tumor and associated dysplasia.
Clinical-Histologic Correlation
Comparisons of clinicopathologic features between warty and nonwarty tumors are summarized in Table 3. Warty tumors (including pure and mixed warty/nonkeratinizing) and nonwarty tumors had a similar male:female ratio (11:2 vs. 24:14, odds ratio [OR] = 3.14; 95% confidence interval [CI]: 0.56–33.26, P = 0.18) and similar rates of current or former tobacco use (81% vs. 76%; OR=1.39; 95% CI: 0.22–15.52, P = 1). However, while HPV-positive non-OPSCCs more commonly arose in the larynx in general, encompassing 52% of all cases, warty tumors were somewhat more likely to be seen in the oral cavity (62% vs. 33%; OR=3.12, 95% CI: 0.19–3.78, P = 0.10). Indeed, 38% of HPV-positive oral cavity SCCs were warty compared with 19% of HPV-positive laryngeal and 0% of HPV-positive hypopharyngeal SCCs. In addition, although HPV-positive non-OPSCCs were overall evenly distributed between AJCC Seventh Edition stages at presentation, including 25 low-stage (T1/T2) tumors (49%) and 26 high-stage (T3/T4) tumors (51%) as well as 30 node-negative (N0) tumors (59%) and 21 node-positive (N1/N2/N3) tumors (41%), tumors with warty histology were significantly more likely than nonwarty tumors to present at low tumor stage (77% vs. 39%; OR = 4.95; 95% CI: 1.05–32.59; P = 0.03) and without lymph node metastasis (100% vs. 45%, OR = 0; 95% CI: 0–0.32; P < 0.001).
TABLE 3.
Clinical-Histologic Correlation
| n (%) | |||
|---|---|---|---|
| Non warty | Warty | P; OR (95% Cl) | |
| Sex | |||
| Female | 14 (37) | 2 (15) | 0.18; 3.14 (0.56–33.26) |
| Male | 24 (63) | 11 (85) | |
| Smoking status | |||
| Current/former smoker | 29 (76) | 9 (81) | 1; 1.39 (0.22–15.52) |
| Never smoker | 9 (24) | 2 (19) | |
| Tumor site | |||
| Larynx/hypopharynx | 26 (67) | 5 (38) | 0.1; 3.12 (0.19–3.78) |
| Oral cavity | 13 (33) | 8 (62) | |
| Tumor stage (AJCC Seventh Edition) | |||
| Low T stage (T1/T2) | 15 (39) | 10 (77) | 0.03; 4.95 (1.05–32.59) |
| High T stage (T3/T4) | 23 (61) | 3 (23) | |
| Nodal stage (AJCC Seventh Edition) | |||
| Node negative (NO) | 17 (45) | 13 (100) | <0.001; 0 (0–0.32) |
| Node positive (N1/N2/N3) | 21 (55) | 0 (0) | |
| Metastasis stage (AJCC Seventh Edition) | |||
| MO | 37 (97) | 13 (100) | 1; 0 (0–113.80) |
| Ml | 1 (3) | 0 (0) | |
AJCC indicates American Joint Committee on Cancer; NOS, not otherwise specified.
Survival
Clinical follow-up information was available for 46 patients with a median duration of 24 months (range: 0 to 81 mo); the follow-up interval was somewhat longer in the patients with nonwarty tumors (median: 28 mo) compared with warty tumors (median: 16 mo). The median survival was not reached in this cohort in the available follow-up. Overall, 9 patients (20%) developed progressive disease, including 4 with locoregional recurrence, 1 with distant metastases, and 4 with both. Of these, 8 patients (89%) had nonkeratinizing histology and 1 (11%) had keratinizing histology; none of the 12 patients with warty or mixed warty/nonkeratinizing tumors who had follow-up information available went on to develop the progressive disease after definitive therapy in this limited follow-up interval. In Kaplan-Meier analysis based on histology alone, warty tumors had better oncologic outcomes with a 100% 4-year disease-free survival compared with 66% for nonwarty histologies, although this did not reach statistical significance (P=0.069; Fig. 6). At last follow-up, 4 patients (9%) had died of disease, 8 (17%) had died of other or unknown causes, 2 (4%) were alive with disease, and 33 (72%) had no evidence of disease.
FIGURE 6.
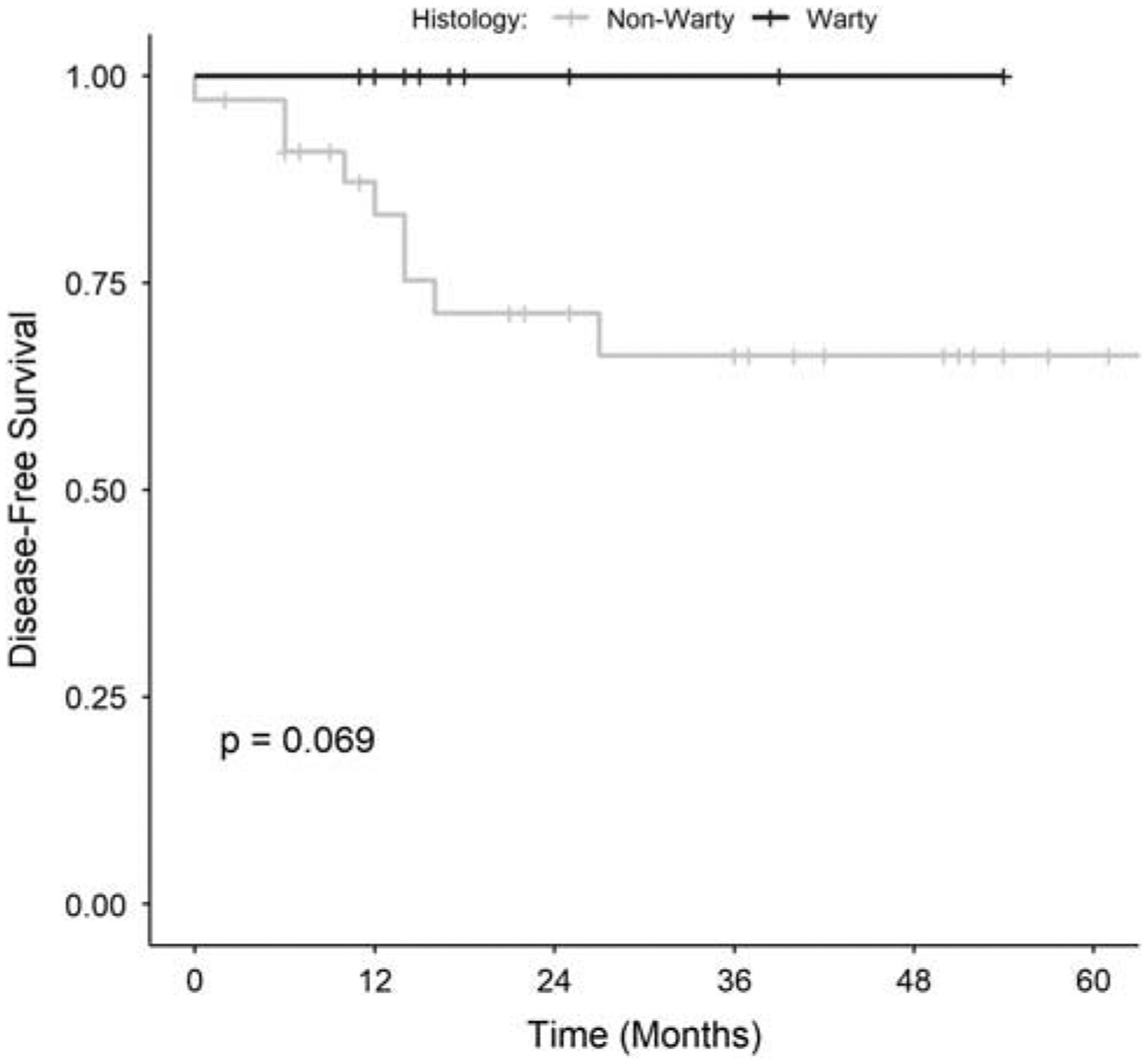
Kaplan-Meier analysis comparing disease-free survival in nonoropharyngeal HPV-positive SCCs with warty (including pure warty and mixed warty/nonkeratinizing) and nonwarty histologies.
DISCUSSION
For SCCs arising from the oropharynx where HPV prevalence is high, detection of high-risk HPV defines a specific tumor entity that is pathologically and clinically distinct from its HPV-negative counterpart. For SCCs arising outside the oropharynx where the prevalence of HPV is low, meaningful pathologic and clinical correlations have been difficult to establish due to an inability to assemble sufficiently powered studies of HPV-positive cases. Although previous studies have observed that a small number of HPV-positive SCCs of the oral cavity, larynx, and hypopharynx share nonkeratinizing morphologic features common to those of the oropharynx,3,6,13 these findings have never been validated in a large group of nonoropharyngeal tumors. Taking advantage of a large multi-institutional cohort of rigorously tested non-OPSCCs, we identified and characterized 52 HPV-positive SCCs of the oral cavity, larynx, and hypopharynx. In doing so, we hoped to determine whether the distinctive features of HPV-positive SCCs are exclusive to the oropharynx and better understand the pathogenic role and clinical implications of HPV involvement in nonoropharyngeal sites.
HPV-positive OPSCCs consistently exhibit a nonkeratinizing morphology with rounded nests of basaloid tumor cells with hyperchromatic nuclei and a high nuclear to cytoplasmic ratio. Although this somewhat immature appearance has long led to HPV-related OPSCCs being regarded as poorly differentiated, there is growing recognition that these tumors may actually be highly differentiated carcinomas that consistently recapitulate the specialized lymphoepithelium of the tonsillar crypts where they preferentially occur.16,32,33 By evaluating the morphologic appearance of HPV-related SCCs arising from sites entirely unrelated to the crypt epithelium, we were able to verify that nonkeratinizing morphology (1) correlates with the presence of HPV and (2) is not restricted to the tonsillar regions of the oropharynx. The majority of HPV-positive oral, laryngeal, and hypopharyngeal SCCs in this cohort, regardless of how cases were selected for testing, demonstrate equivalent histologic features with the prototypic nonkeratinizing HPV-related SCCs of the oropharynx. These findings confirm those of Chakravarthy et al,13 who likewise noted in a smaller sample of 15 cases that the HPV-positive non-OPSCCs were typically nonkeratinizing even when arising in sites usually dominated by the classic keratinizing morphology. Thus, it is likely that the prototypic morphology of HPV-positive head and neck SCCs is a viral-related property that cannot be entirely attributed to origin from the tonsillar crypts.
Our review of a large number of HPV-positive non-OPSCCs further facilitates the recognition of phenotypes that appear to diverge from the classic HPV-associated morphology. In OPSCCs, surface keratosis, cytoplasmic keratinization, and koilocytic changes are conspicuously absent.12 However, a subset of our laryngeal, oral, and hypopharyngeal cases demonstrate a dramatic and complete departure from this phenotype with a unique warty morphology defined by exophytic growth, prominent surface keratosis and parakeratosis, koilocytosis, multinucleation and marked nuclear pleomorphism. The overall incidence of the warty morphology and its specificity for HPV are not known and were not addressed in this study, although it was somewhat less common in the randomly-sampled PROVE cohort than the selectively-tested clinical cases. Even though this warty phenotype has only rarely been noted the head and neck,4,34 it is a well-recognized form of HPV-positive SCC in the anogenital tract.18–20,26 In the anogenital tract, the warty variant does not seem to behave any differently from the nonkeratinizing type.35 While caution is merited because these data are limited by small numbers and a shorter median follow-up of just 16 months in warty tumors, this study raises the possibility that tumors with pure or mixed warty elements in nonoropharyngeal head and neck sites not only differ histologically, but clinically in terms of stage presentation and clinical outcome. In our experience with HPV-positive non-OPSCCs, the presence of warty morphology was associated with a trend toward better outcomes in limited follow-up and sample size. Patients with warty tumors presented with lower stage disease, no regional or distant metastases, and no tumor recurrences.
Unlike with HPV-positive OPSCCs where the premalignant changes in the tonsillar crypts have yet to be well defined, we were also able to confirm the presence of squamous precursor lesions in association with HPV-positive non-OPSCCs. A distinctive HPV-related intraepithelial proliferation has recently been recognized in the oral cavity and larynx.19,36–38 This HPV-related squamous dysplasia is defined not only by positivity for p16 and high-risk HPV but also by unique histologic features, including a full-thickness proliferation of basaloid cells with prominent apoptosis and karyorrhexis and corrugated surface parakeratosis. Between 15% and 70% of cases of HPV-related squamous dysplasia have been shown to be associated with invasive SCC.19,36 Our results conversely demonstrate that as many as 30% of nonoropharyngeal HPV-positive SCCs arise in the setting of identifiable HPV-related squamous dysplasia. The concomitant presence of HPV in these precursor lesions and their invasive components suggests that the presence of transcriptionally active high-risk HPV plays an important role in the initiation of tumor development at these nonoropharyngeal sites.
Because tumor HPV status is being considered as a risk stratification biomarker for patients with head and neck SCCs, there is a pressing need to establish the prognostic impact of HPV status for head and neck SCCs arising at nonoropharyngeal sites. But while some studies have demonstrated a survival advantage for HPV-positive tumors in the oral cavity and larynx,20,39 others have not.9,10,13 Conceivably these inconsistencies could reflect, in part, differences in the distribution of nonkeratinizing and warty subtypes among study populations. The College of American Pathologists and the American Society for Clinical Oncology currently do not endorse routine screening of non-OPSCCs for HPV.23,24 Our findings are not intended to challenge these recommendations. Nevertheless, the presence of well-developed warty features could guide selective HPV testing in the context of clinical trials in a way that will be necessary to identify and evaluate a larger cohort of HPV-positive tumors to confirm the prognostic advantage of the warty variant. The histologic features characterizing warty carcinomas likely fall along a spectrum with conventional keratinizing SCCs and may not represent an HPV-specific alteration, but when present and overtly developed, these changes may help guide this selective HPV testing.
In summary, a more complete understanding of HPV-positive SCCs in nonoropharyngeal sites including the oral cavity, larynx, and hypopharynx has been impeded by low prevalence and highly variable methods of detection resulting in small and suspect study collections. Using p16 staining combined with highly specific methods of direct HPV detection to screen head and neck SCCs from a large multi-institutional study and clinical experience, we were able to evaluate a large number of HPV-positive non-OPSCCs relative to other studies. On the basis of our observations, the nonkeratinizing phenotype remains the dominant HPV phenotype even for those SCCs arising from nontonsillar squamous epithelia, indicating that these phenotypic changes are likely an HPV effect. But we also demonstrated that there is a second, less common warty phenotype characterized by exophytic growth, abundant surface hyperkeratosis and parakeratosis, prominent koilocytosis, multinucleation, and marked nuclear pleomorphism—features in direct contrast to the prototypic nonkeratinized morphology. This warty variant showed a high tendency to present at an early stage and thus a trend toward improved outcomes in limited experience and may contribute to conflicting data about the prognostic significance of HPV in non-OPSCCs.
Conflicts of Interest and Source of Funding:
Funding was provided by the National Institute of Dental and Craniofacial Research (grant P50 DE019032). W.R.R. is on the scientific advisory boards of Olympus and reports consulting fees from Ziteo and Medtronic outside the submitted work. P.K.H. reports consultant fees from Bristol-Myers Squibb Loxo Oncology, Stryker, and Ethicon outside the submitted work. W.H.W. reports consultation fees from Merck and from Cancer Panels outside the submitted work. The remaining authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Gelwan E, Malm IJ, Khararjian A, et al. Nonuniform distribution of high-risk human papillomavirus in squamous cell carcinomas of the oropharynx: rethinking the anatomic boundaries of oral and oropharyngeal carcinoma from an oncologic HPV perspective. Am J Surg Pathol. 2017;41:1722–1728. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JS Jr, Ukpo OC, Ma XJ, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas—a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60:982–991. [DOI] [PubMed] [Google Scholar]
- 4.Poling JS, Ma XJ, Bui S, et al. Human papillomavirus (HPV) status of non-tobacco related squamous cell carcinomas of the lateral tongue. Oral Oncol. 2014;50:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8. [DOI] [PubMed] [Google Scholar]
- 6.Chernock RD, Wang X, Gao G, et al. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combes JD, Franceschi S. Role of human papillomavirus in nonoropharyngeal head and neck cancers. Oral Oncol. 2014;50:370–379. [DOI] [PubMed] [Google Scholar]
- 9.Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RJ, Urban D, Angel C, et al. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;112:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zammit AP, Sinha R, Cooper CL, et al. Examining the contribution of smoking and HPV towards the etiology of oral cavity squamous cell carcinoma using high-throughput sequencing: a prospective observational study. PLoS One. 2018;13:e0205406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34:4132–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010; 363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 16.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. [DOI] [PubMed] [Google Scholar]
- 17.Marur S, D’Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Lewis JS Jr, El-Mofty SK, et al. Nonkeratinizing squamous cell carcinoma in situ of the upper aerodigestive tract: an HPV-related entity. Head Neck Pathol. 2017;11:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao D, Xu QG, Chen XM, et al. Human papillomavirus as an independent predictor in oral squamous cell cancer. Int J Oral Sci. 2009;1:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isayeva T, Li Y, Maswahu D, et al. Human papillomavirus in nonoropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(suppl 1):S104–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV Detection method. JAMA Oncol. 2017;3:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakhry C, Lacchetti C, Rooper LM, et al. Human papillomavirus testing in head and neck carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol. 2018;36:3152–3161. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142:559–597. [DOI] [PubMed] [Google Scholar]
- 25.Rooper LM, Gandhi M, Bishop JA, et al. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:11–16. [DOI] [PubMed] [Google Scholar]
- 26.de Roda Husman AM, Walboomers JM, van den Brule AJ, et al. The use of general primers GP5 and GP6 elongated at their 3’ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76(pt 4):1057–1062. [DOI] [PubMed] [Google Scholar]
- 27.Cai YP, Yang Y, Zhu BL, et al. Comparison of human papillomavirus detection and genotyping with four different prime sets by PCR-sequencing. Biomed Environ Sci. 2013;26:40–47. [DOI] [PubMed] [Google Scholar]
- 28.Edge SB. American Joint Committee on Cancer AJCC Cancer Staging Manual. New York, NY: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 29.Gregoire L, Cubilla AL, Reuter VE, et al. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87: 1705–1709. [DOI] [PubMed] [Google Scholar]
- 30.Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993;17: 133–145. [DOI] [PubMed] [Google Scholar]
- 31.Matoso A, Ross HM, Chen S, et al. Squamous neoplasia of the scrotum: a series of 29 cases. Am J Surg Pathol. 2014;38:973–981. [DOI] [PubMed] [Google Scholar]
- 32.Westra WH. The pathology of HPV-related head and neck cancer: implications for the diagnostic pathologist. Semin Diagn Pathol. 2015;32:42–53. [DOI] [PubMed] [Google Scholar]
- 33.Westra WH, Lewis JS Jr. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Oropharynx. Head Neck Pathol. 2017;11:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piattelli A, Rubini C, Fioroni M, et al. Warty carcinoma of the oral mucosa in an HIV+ patient. Oral Oncol. 2001;37:665–667. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez DF, Canete S, Fernandez-Nestosa MJ, et al. HPV- and non-HPV-related subtypes of penile squamous cell carcinoma (SCC): Morphological features and differential diagnosis according to the new WHO classification (2015). Semin Diagn Pathol. 2015;32:198–221. [DOI] [PubMed] [Google Scholar]
- 36.Lerman MA, Almazrooa S, Lindeman N, et al. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod Pathol. 2017;30:1646–1654. [DOI] [PubMed] [Google Scholar]
- 37.McCord C, Xu J, Xu W, et al. Association of high-risk human papillomavirus infection with oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:541–549. [DOI] [PubMed] [Google Scholar]
- 38.Woo SB, Cashman EC, Lerman MA. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013;26:1288–1297. [DOI] [PubMed] [Google Scholar]
- 39.Jiang H, Lin PF. Human papillomavirus infection a favorable prognostic factor in laryngeal squamous cell carcinoma is associated with the expression of proliferating cell nuclear antigen. Pak J Med Sci. 2013;29:1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]


