Abstract
Background: Although the precise pathogenesis of acute lymphoblastic leukemia (ALL) remains unclear, studying gene-regulating mechanisms during ALL pathogeneses may shed light on the underlying mechanisms driving malignant behavior. There is some evidence showing the promoter hypermethylation and silencing of RASSF1A tumor suppressor gene in ALL cells; however, there is a lack of evidence for whether the gene indeed alters during different phases of ALL or in response to therapy. Thus, the current study aimed to clarify this issue using groups of adult ALL patients who have been scarcely investigated regarding expression levels and promoter methylation status.
Materials and Methods: In this case/control study, the expression levels and methylation status of the gene promoter was evaluated using quantitative real-time PCR and methylation-specific PCR (MSP), respectively in adults with ALL. The study included peripheral blood of patients with newly diagnosed ALL (n=10), complete remission (CR) (n=10), or relapse (n=10), and 10 control samples from healthy individuals.
Results: MSP results revealed an unmethylated status for almost all patients and control samples, except a case with relapsing ALL, which showed a hemimethylated pattern. RASSF1A also showed no difference in terms of gene expression in the patients compared with the control group (p>0.05).
Conclusion: The results revealed an up-regulation of RASSF1A tumor suppressor in adult ALL patients experiencing CR, suggesting this to be a marker of therapy response. However, further investigations using more sensitive methylation detecting tools with larger sample sizes may better clarify the involvement of the promoter methylation of RASSF1A in these patients.
Key Words: Expression level, Methylation pattern, Ras association domain family member 1(RASSF1A) gene, Acute lymphoblastic leukemia (ALL)
Introduction
Acute lymphoblastic leukemia (ALL) is characterized by the excessive accumulation of lymphoblasts and lymphoid progenitors. Although ALL is the most common type of leukemia in children (25%), there is also a peak of incidence in adults 1 . The disease had been regarded incurable until 1970, which caused fatal consequences in the affected individuals 2 . A novel therapeutic development could overcome the irreversible complications of ALL, and understanding the contributing factors associated with the disease pathogenesis can further help to prevent and treat affected individuals or recognize other mechanisms driving malignant hematopoiesis or non-hematological cancers 3,4 . Recently, innovations in ALL therapy in children have led to complete remission in approximately 70-80% of children; however, adult patients suffer from a more resistant version of the disease 5 .
Several lines of evidence have revealed multiple layers of gene regulation mechanisms affecting the pathogenesis course of ALL. The role of structural genetic changes in cancer-associated genomic regions which involve signaling, cell cycle, proliferation, differentiation, apoptosis, and transcription factor genes have been well described6. However, some cases lack these structural abnormalities, suggesting the parallel role of epigenetic mechanisms which influence gene expression without altering the original DNA backbone 7 . Extensive studies have proven the roles of these factors in regulating gene expression, mostly affecting tumor suppressor genes, oncogenes, and other genes involved in tumorigenesis 8,9 . Promoter methylation of tumor suppressor genes has been the focus of many previous publications which proposed it as a key mechanism of tumor pathogenesis 10, 11 .
Despite structural changes, the epigenetic signatures result in reversible changes in gene expression, proposed as an ideal platform for the development of epigenetic-based medications modulating the expression of tumor suppressors or oncogenes 12, 13 .
The Ras-association domain family 1, isoform A (RASSF1A gene) is a member of the RASSF family and contains 10 tumor suppressor genes 14,15 . Various investigations have highlighted the aberrant expression of this gene in a wide range of human solid cancers and hematologic malignancies. An aberrant promoter methylation was previously reported in acute myeloid leukemia (AML) patients with an attempt to alter the conventional molecular model for disease prognosis 16 . Moreover, RASSF1A was also shown to be hypermethylated in a group of chronic myeloid leukemia patients 17 .
Although there are some shreds of evidence showing the promoter hypermethylation and silencing of RASSF1A in ALL cells 18,19 , there is a lack of evidence showing whether the gene indeed alters during different phases of ALL or in response to therapy. Thus, the current study aimed to clarify this issue using groups of adult ALL patients who have been scarcely investigated regarding expression levels and promoter methylation status. This study investigated the changes of promoter methylation of the RASSF1A gene and its possible association with gene expression levels in adult ALL patients in the diagnosis stage, complete remission, and relapse. Promoter methylation changes of this gene can help identify subgroups of poor prognosis patients not sufficiently treated with conventional therapies.
MATERIALS AND METHODS
Study population and sample collection
This case/control study was performed on adult patients with ALL, including newly diagnosed individuals (n=10), patients with complete remission (CR) (n=10), and patients with relapsed ALL (n=10) who were referred to the Hematology, Oncology and Stem Cell Transplantation Research Center at Shariati Hospital, Tehran, Iran. The mean age of patients was 32.62 ± 15.55 years. Twenty (67.3%) patients were male, and 10 (32.7%) were female. The inclusion criteria were having a complete set of traditional hematological and molecular tests, and being diagnosed and confirmed to have ALL by specialists. Of note, those who refused to participate in the study were excluded, and all participants provided written consent. The current study also incorporated 10 control samples from healthy individuals with normal complete blood count (CBC) profiles. The age range, geographical areas, sex distribution, career, etc. were considered to be matched with the selected patients. All subjects were asked to sign a written consent form describing the study goals and procedures. This study was approved by the Ethics Committee of Tehran University of Medical Sciences. Peripheral blood samples (10 ml each) were collected under sterile conditions using vacuum tubes containing K2-EDTA as the anticoagulant.
Extraction of DNA and RNA from peripheral blood
DNA and RNA samples were extracted using the salting out method and the RNXTM kit (Plus), respectively. The sample quality was measured using the Nanodrop system and optical absorption comparisons at wavelengths of 260 and 280 nm. The extracted DNA and RNA samples were stored at -20 °C.
Sodium bisulfite treatment and methylation specific PCR (MSP)
The DNA was treated with sodium bisulfite using the Fast EpiTect Qiagen Kit. Sodium bisulfite converts non-methylated cytosines to uracil, while methylated cytosines remain unchanged. To investigate the promoter methylation pattern of the RASSF1A gene, the treated DNA was prepared for methylation specific PCR (MSP). To perform the MSP reactions, two pairs of methylated and non-methylated primers were designed using the meth-primer tool. The sequence of primers is shown in Table 1.
Table 1.
Sequence of primers of RASSF1A gene used in the MSP experiments
| Sequence type | Primer's sequence | GC content | TM (°C) | Length (bp) | PCR product size (bp) |
|---|---|---|---|---|---|
| MF | 5'- GGGTTTTGCGAGAGCGCG-3' | 66.7 | 60.7 | 18 | 168 |
| MR | 5'- GCTAACAAACGCGAACCG-3' | 55.6 | 56.1 | 18 | |
| UF | 5'- GGTTTTGTGAGAGTGTGTTTAG-3' | 40.9 | 58.4 | 22 | 168 |
| UR | 5'- CACTAACAAACACAAACCAAAC-3' | 36.4 | 56.6 | 22 |
MF: Methylated Forward, MR: Methylated Reverse, UF: Unmethylated Forward; UR: Unmethylated Reverse; TM: temperature of melting
The MSP reactions were performed using HotStarTaq® PCR (QIAGEN) Kit in a PEQLAB Thermo cycler device. Briefly, 0.25 μL of methylated/unmethylated forward primer, 0.25 μl of reverse methylated/unmethylated primer, 2 μl of buffer (2x), 0.4 μl dNTP, 0.1 μl Hot Start Taq polymerase, 8.5 μl of d.d.H2O, and 1 μl of DNA were mixed in a microtube. The thermal program proceeded with 5 min at 95 °C as the primary denaturation, followed by 6 cycles of each 95 °C for 30 seconds, 62 °C for 30 seconds, and 72 °C for 30 seconds. The extension phase was continued by a further 35 cycles as follows: 95 °C for 30 seconds, 59 °C (for unmethylated primer)/64 °C (for methylated primer) for 30 seconds, 72 °C for 30 seconds, and a final extension at 72 °C for 10 min. Finally, the products were electrophoresed on a 2.5% agarose gel stained with cyber safe dye.
For each MSP reaction, positive and negative control (methylated and unmethylated DNA samples of EpiTect PCR control DNA (Qiagen Inc., cat. 59695) commercial kits) were used, respectively. Distilled water was used as a negative control in each set of PCR reactions.
cDNA synthesis and real-time PCR
Real-time PCR was used to check expression levels. To perform this, cDNA was constructed using the cDNA synthesis kit (TAKARA). The RASSF1A and ABL primers were used as previously described 20 . Then they were aligned with the BLAST tool from NCBI. The sequence of primers is shown in Table 2.
Table 2.
Primer's sequences of target (RASSF1A) and reference (ABL) genes
|
Primer's
name |
Primer's type | Primer's sequence | GC content | TM (°C) | Length (bp) | PCR product size (bp) |
|---|---|---|---|---|---|---|
| RASSF1A | F | 5'- CCCTGCTGCGAAAGTTCTTG-3' | 55 | 60.5 | 20 | 60 |
| RASSF1A | R | 5'- CGCGCTCAAAGAGTGCAAA-3' | 52.6 | 57.3 | 19 | |
| ABL | F | 5'- TGGAGATAACACTCTAAGCATAACTAAAGG-3' | 36.67 | 60.8 | 30 | 124 |
| ABL | R | 5'- GATGTAGTTGCTTGGGACCCA-3' | 52.38 | 60 | 21 |
F: forward, R: reverse, TM: temperature of melting
The expression level was measured using real-time PCR with the Syber®premix Ex taqTm ll (Tli RNase H Plus) (TAKARA) in an ABI thermal cycler system. The reaction tubes contained a mix of 10 μl master mix (2X), 0.5 μL of each forward and reverse primer, 7 μl of d.d.H2O, and 2 μl of the cDNA as template. The thermal program started with a primary denaturation at 95 °C for 30 sec, followed by 40 cycles of each 95 °C for 5 sec, 60 °C for 30 sec, and 72 °C for 30 sec, and a melting program, including 95 °C for 15 sec, 60 °C for 1 min, and 95 °C for15 sec. The results were ultimately analyzed using the ΔΔCT method.
Statistical analysis
Statistical analysis was performed using SPSS, version 21 software. To determine the statistically significant differences among the different patients and control groups, ANOVA was performed. A level of p<0.05 was considered statistically significant.
Results
Comparison of Real-Time PCR results on cDNA of RASSF1A gene in both ALL and control groups
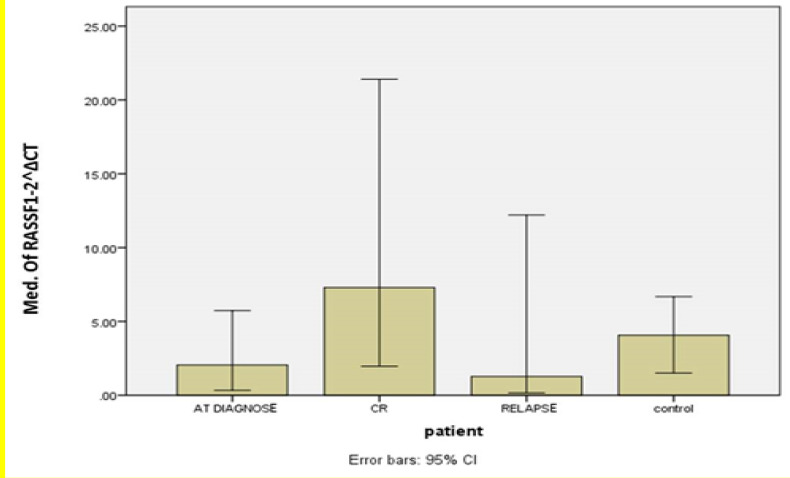
To measure the mRNA levels of the RASSF1A gene and compare it among ALL patients in different stages, the qRT-PCR technique was applied with the ABL gene as the internal control. The data calculation was performed using the ΔΔCT method, and the differences were analyzed by statistical tests. According to the findings, no significant correlation was observed between ALL groups individually and the control group using ANOVA tests (p>0.05) (Figure 1).
Figure 1.
Quantitative real-time PCR results of RASSF1A mRNA levels in different
groups of ALL patients and control group
MSP analysis revealed the unmethylated status of the RASSF1A gene in adult ALL patients
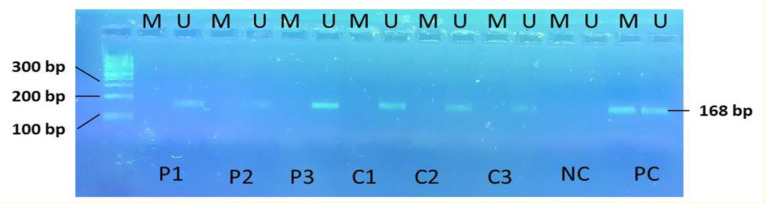
To analyze the promoter methylation pattern of the RASSF1A gene, the methylation-specific PCR technique was applied using two primer pairs specific for the methylated and un-methylated status of the gene. After being resolved on a 2.5% agarose gel, the MSP products showed an unmethylated status for the ALL patients at diagnosis and those who experienced a complete remission. A single case of disease relapse was observed to be hemi-methylated for the RASSF1A promoter region. However, this cannot indicate a significant difference in promoter methylation level, nor can it be significantly correlated with the gene expression levels. The promoter methylation pattern was also shown to be in an unmethylated status in the control group, suggesting an intact methylation level in ALL patients when compared with healthy individuals, at least in the region where the primers had been designed. Figure 2 indicates the MSP products for samples of ALL patients and the control group showing an unmethylation pattern in both groups.
Figure 2.
Electrophoresis results on 2.5% agarose gel for MSP products of the RASSF1A gene.
P1, P2, P3: samples from ALL patients. C1, C2, C3: Control's samples. NC-: Negative control. PC: Positive control. M: Methylated. U: Un-methylated. PCR product size: 168 bp
Discussion
ALL is the most common acute leukemia in children. Understanding the factors associated with the onset of this disease can help prevent, treat, and recognize other mechanisms that trigger hematologic and even non-hematological cancers21.
Recent efforts have been focused on discerning the underlying mechanisms regulating the pathogenesis of leukemia with the purpose of designing novel therapeutics with minimal adverse effects. Among the gene regulatory mechanisms, epigenetic changes, as reversible biochemical reactions in chromatin organization without structural alterations in the DNA sequence, have been increasingly studied 7 . Promoter methylation analysis in the minimal residual disease (MRD)-related genes can be used as a new biomarker to assess the risk of leukemia recurrence. Epigenetic changes can be reversed, and thus, re-activating these pathways, including the return of aberrant promoter methylation of genes effective in disease development, can potentially offer therapeutic effects for these diseases 13 . In this regard, RASSF1A gene promoter methylation in ALL patients in different stages of disease progression was assessed as a scarcely addressed issue. Promoter methylation changes of this gene can help in the diagnosis of adults in ALL subgroups as well as the different stages of response to treatment in this disease. The findings of this study also lead to a better understanding of the mechanisms developing cancer, including adult ALL. The current findings suggest that despite having CpG islands in its promoter region, the RASSF1A gene seems to rely on gene-regulating mechanisms other than promoter methylation, which is, at least, not detected using the MSP technique.
Analysis of the MSP results of the RASSF1A gene promoter methylation status in ALL patients showed that only one case of relapse had a promoter hemi-methylation pattern (3.3%). However, the remaining patients in all phases of ALL disease had a promoter non-methylation status for the promotor of this gene (96.6%). Furthermore, the healthy individuals considered as control groups were revealed to have the unmethylated pattern in the RASSF1A gene promoter, suggesting an unchanged promoter methylation status of the gene during the disease pathogenesis, at least in the region where the MSP primers were designed. Furthermore, real-time PCR results showed no statistically significant difference in the RASSF1A gene in ALL patients compared with the control group.
Although q-PCR analysis showed an overall significant difference among different ALL groups (p=0.044), no statistically significant correlation was observed between ALL groups individually and the control group (p=0.278, p=0.104, p=0.156 for the new case, CR, and relapse groups, respectively). Interestingly, intergroup analysis revealed a significant up-regulation of the RASSF1A tumor suppressor gene in patients experiencing complete remission compared with the newly diagnosed (p=0.041) and relapsing patients (p=0.016). This seems to offer a rationale for the fact that patients reactivate the tumor suppressor gene RASSF1A as a mechanism to reverse the malignant phenotype of tumor cells while undergoing remission, highlighting the importance of the gene as a biomarker of ALL therapy response in adult patients.
The RASSF1A gene modulates some of the RAS-mediated inhibitory responses and serves as a tumor suppressor gene. Therefore, this gene counteracts with RAS inhibitors such as RAF and PI3K, which are regarded as oncogenes. Nevertheless, according to the current findings, this gene indicated no alteration in ALL patients. This may propose that this gene, unlike solid tumors, plays no key role in the pathogenesis of ALL.
Several lines of studies have supported the role of the RASSF family in the pathogenesis of solid tumors. Hill et al. revealed that the RASSF1 gene is linked to the protected pathway between Drosophila and mammals, the tumor suppressor network SWH (Salvador/Warts/Hippo), which was connected to MST1,2 22 . Danielsen et al. also verified the prognostic role of promoter hypermethylation of the RASSF1A gene in peripheral neuropathic malignant tumors (MPNSTs) limited to type 1 neurofibromatosis (NF1) 23 . Moreover, Colombara et al. noted the prognostic value of the RASSF1 gene in non-small cell lung cancer (NSCLC) 24 . Based on the above-mentioned reports, the RASSF1A gene showed a hypermethylated pattern in most solid tumors, which could be due to the different role of this gene in various human tissues, underscoring its tumor suppressor role in solid tumors.
Some other investigations studied the role of the RASSF family in the pathogenesis of leukemia. In this context, RASSF1-10 genes were shown to inactivate as a result of DNA promoter hypermethylation. Hesson et al. reported this as a frequent phenomenon during ALL development. It was also demonstrated that the inactivation of tumor suppressors RASSF6 and 10 is a common event in the pathogenesis of leukemia. This study showed that the promoter hypermethylation profile of RASSF genes in leukemia is different from that in solid tumors and presented the first report of the inactivation of RASSF6 or RASSF10 in cancer. This data showed that the epigenetic inactivation of tumor suppressor genes RASSF6 and RASSF10 is a frequent occurrence in the pathogenesis of childhood leukemia. It also revealed the recently recognized RASSF, RASSF10, and its potential role in leukemia. It was found that the promoter methylation of RASSF1A, RASSF5A, and RASSF6 may be responsible for the inactivation of the RAS route in most T-ALL cases. It was argued that RASSF1A, RASSF5A, and RASSF6 were hypermethylated in leukemia cell lines, but RASSF1A and RASSF5A were rarely methylated in childhood T-ALL patients 19 . The current findings, however, revealed no promoter methylation changes for the RASSF1A gene in adult ALL patients.
Shinawi et al. found that the hypermethylation of the promotor's CPG islands inactivated the expression of the RASSF1A gene in many conditions such as lung, breast, prostate, glioma, neuroblastoma, kidney, and epithelial cancers, and in chronic lymphoblastic leukemia (CLL), it was unmethylated or had less than 10% methylation 25 . In line with these results, the current study showed that the promoter of the RASSF1A gene remained un-methylated or unchanged in ALL disease in which the same cell lineage was affected as CLL.
Avramoulia et al. proposed that the RASSF1A gene played a key role in the cell cycle and was expressed in all hematopoietic cells. The results of this study on chronic myeloblastic leukemia (CML) showed that although the promoter of this gene was methylated in the K562 BCR-ABL + cell line, it was not methylated in CML patients, and as a result, the promoter methylation of this gene did not play a significant role in the pathogenesis or progression of CML 18 .
In 2012, Weyden et al. found that the lack of RASSF1A and RUNX2 was associated with the multi-stage model of tumorigenesis, and reductions in the expression of RASSF1A and RUNX2 were observed in both T-ALL and colorectal cancer 26 . Furthermore, San et al. reported that RASSF1 and other genes involved in apoptosis were hypermethylated in ALL 27 . The results of this study contradicted the current study results, possibly because of different gene isoforms. Although the current data clarified the methylation status of part of the RASSF1A promoter region, a full analysis of the region using higher resolution methodologies such as bisulfite sequencing is recommended to give better insight into the total methylation pattern of the region. Therefore, using a relatively low resolution as well as the limited sample size and, to some extent, patients’ refusal to participate were among the most important limitations of the current study.
CONCLUSION
Overall, in the current study, the promoter methylation pattern of the RASSF1A gene and its expression level showed no significant correlation. However, the findings highlighted the importance of RASSF1A mRNA measurement as a valuable tool to determine the therapy response because the gene levels were shown to be significantly up-regulated in patients experiencing a complete remission compared with newly-diagnosed ALL patients and those experience a relapse of the disease. It is highly recommended to study the gene family using precise and newer techniques such as advanced and quantitative methylation techniques, protein level analysis, sequence analysis, other epigenetic mechanism studies, and investigate different isoforms of the RASSF1 gene in the disease to gain a deeper understanding of this gene in the pathogenesis of adult ALL.
Funding
This work was supported by Tehran University of Medical Sciences under grant number: 94-03-31-29963.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Mousa Vatanmakanian for his technical and critical revision of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Rahmani T, Azad M, Chahardouli B, et al. Patterns of DNMT1 Promoter Methylation in Patients with Acute Lymphoblastic Leukemia. Int J Hematol Oncol Stem Cell Res . 2017;11(3):172–177. [PMC free article] [PubMed] [Google Scholar]
- 2.Yaniv I, Betzer H, Naumov I, et al. Hypermethylation and Expression of Rassf1a in Childhood Acute Lymphoblastic Leukemia (ALL). Blood. 2007;110(11):4141. [Google Scholar]
- 3.Azad M, Biniaz RB, Goudarzi M, et al. Short view of leukemia diagnosis and treatment in Iran. Int J Hematol Oncol Stem Cell Res. 2015;9(2):88–94. [PMC free article] [PubMed] [Google Scholar]
- 4.Wong IH, Chan J, Wong J, et al. Ubiquitous aberrant RASSF1A promoter methylation in childhood Neoplasia1. Clin Cancer Res. 2004;10(3):994–1002. doi: 10.1158/1078-0432.ccr-0378-3. [DOI] [PubMed] [Google Scholar]
- 5.Dehghanifard A, Kaviani S, Abroun S et al. Various signaling pathways in multiple myeloma cells and effects of treatment on these pathways. Clin Lymphoma Myeloma Leuk. 2018;18(5):311–320. doi: 10.1016/j.clml.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Naderi M, Reykande SE, Tabibian S, et al. Childhood acute lymphoblastic leukemia: refusal and abandonmentof treatment in the southeast of Iran. Turk J Med Sci. 2016;46(3):706–11. doi: 10.3906/sag-1412-42. [DOI] [PubMed] [Google Scholar]
- 7.Hoffbrand AV, 2016 , editors. Postgraduate haematology. 7th edn. United States: John Wiley & Sons; [Google Scholar]
- 8.Einav Nili G-Y, Saito Y, Egger G, et al. Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med. 2008;59:267–80. doi: 10.1146/annurev.med.59.061606.095816. [DOI] [PubMed] [Google Scholar]
- 9.Gallegos-Arreola M, Borjas-Gutiérrez C, Zúñiga-González G, et al. Pathophysiology of Acute Lymphoblastic Leukemia. In: Mejía-Aranguré, JM, editor. Clinical Epidemiology of Acute Lymphoblastic Leukemia-From the Molecules to the Clinic. Mexico: InTech; 2013. pp. 43–73. [Google Scholar]
- 10.Florean C, Schnekenburger M, Grandjenette C, et al. Epigenomics of leukemia: from mechanisms to therapeutic applications. Epigenomics. 2011;3(5):581–609. doi: 10.2217/epi.11.73. [DOI] [PubMed] [Google Scholar]
- 11.Ghorban K, Shanaki M, Mobarra N, et al. Apolipoproteins A1, B, and other prognostic biochemical cardiovascular risk factors in patients with beta-thalassemia major. Hematology. 2016;21(2):113–120. doi: 10.1179/1607845415Y.0000000016. [DOI] [PubMed] [Google Scholar]
- 12.Azad M, Kaviani S, Noruzinia M, et al. Gene expression status and methylation pattern in promoter of P15INK4b and P16INK4a in cord blood CD34+ stem cells. Iran J Basic Med Sci. 2013;16(7):822–828. [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa ME, Reimers M, Thompson RF, et al. An integrative genomic and epigenomic approach for the study of transcriptional regulation. PLoS One. 2008;3(3):e1882. doi: 10.1371/journal.pone.0001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derakhshanfar E, Alizadeh S, Rafiemehr H, et al. Determination of SFRP1 and SFRP2 Genes Promoter Methylation Status in Patients with Chronic Myelogenous Leukemia. Payavard Salamat. 2017;10(6):514–22. [Google Scholar]
- 15.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 16.Bootwala Y, Bandyopadhyay D. Epigenetic therapies of cancer. Curr Canc Ther Rev. 2006;2(2):127–35. [Google Scholar]
- 17.van der Weyden L, Adams DJ. The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta. 2007;1776(1):58–85. doi: 10.1016/j.bbcan.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avramouli A, Tsochas S, Mandala E, et al. Methylation status of RASSF1A in patients with chronic myeloid leukemia. Leuk Res . 2009;33(8):1130–2. doi: 10.1016/j.leukres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Hesson LB, Dunwell TL, Cooper WN, et al. The novel RASSF6 and RASSF10 candidate tumour suppressor genes are frequently epigenetically inactivated in childhood leukaemias. Mol Cancer . 2009;8:42. doi: 10.1186/1476-4598-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang V, Davis DA, Haque M, et al. Differential gene up-regulation by hypoxia-inducible factor-1α and hypoxia-inducible factor-2α in HEK293T cells. Cancer Res. 2005;65(8):3299–306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 21.Harada K, Toyooka S, Maitra A, et al. Aberrant promoter methylation and silencing of the RASSF1A gene in pediatric tumors and cell lines. Oncogene. 2002;21(27):4345–9. doi: 10.1038/sj.onc.1205446. [DOI] [PubMed] [Google Scholar]
- 22.Hill VK, Dunwell TL, Catchpoole D, et al. Frequent epigenetic inactivation of KIBRA, an upstream member of the Salvador/Warts/Hippo (SWH) tumor suppressor network, is associated with specific genetic event in B-cell acute lymphocytic leukemia. Epigenetics. 2011;6(3):326–32. doi: 10.4161/epi.6.3.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danielsen SA, Lind GE, Kolberg M, et al. Methylated RASSF1A in malignant peripheral nerve sheath tumors identifies neurofibromatosis type 1 patients with inferior prognosis. Neuro Oncol. 2015;17(1):63–9. doi: 10.1093/neuonc/nou140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombara DV, Eidsmoe D, Stern JE, et al. No Association between p53 Immunohistochemical Staining and RASSF1 or DAPK1 Hypermethylation in Non-Small Cell Lung Cancer. J Cancer Res Ther. 2015;6(8):631–637. [Google Scholar]
- 25.Shinawi T, Hill V, Dagklis A, et al. KIBRA gene methylation is associated with unfavorable biological prognostic parameters in chronic lymphocytic leukemia. Epigenetics. 2012;7(3):211–5. doi: 10.4161/epi.7.3.19222. [DOI] [PubMed] [Google Scholar]
- 26.van der Weyden L, Papaspyropoulos A, Poulogiannis G, et al. Loss of RASSF1A synergizes with deregulated RUNX2 signaling in tumorigenesis. Cancer Res. 2012;72(15):3817–27. doi: 10.1158/0008-5472.CAN-11-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.San Jose-Eneriz E, Agirre X, Rodríguez-Otero P, et al. Epigenetic regulation of cell signaling pathways in acute lymphoblastic leukemia. Epigenomics. 2013;5(5):525–38. doi: 10.2217/epi.13.56. [DOI] [PubMed] [Google Scholar]