Abstract
Background.
Exercise training is associated with functional improvements in persons with multiple sclerosis (MS), perhaps based on neuroplasticity. However, inferences regarding neuroplasticity require observations of exercise-related changes in the central nervous system that explain functional adaptations. This systematic review critically evaluated studies on exercise training, neuroimaging outcomes, and functional outcomes in MS based on consistency with a well-established conceptual model for characterizing exercise training as a possible neuroplasticity-inducing behavior in this population.
Methods.
We performed targeted and comprehensive searches of multiple databases for papers involving exercise training interventions on functional and neuroimaging outcomes in persons with MS. Acceptable study designs included randomized controlled trials, single-group pre/post designs, and quasi-experimental designs. Four independent reviewers extracted relevant data from each eligible paper on characteristics of participants, exercise interventions, neuroimaging outcomes, functional outcomes, pattern of study results, and potential risks of bias.
Results.
The literature search returned only 10 papers (involving 8 original interventions) that met eligibility criteria wherein inferences regarding neuroplasticity could be drawn, based on inclusion of neuroimaging and functional endpoints. Within those 10 papers, there is mixed evidence for exercise training as a neuroplasticity-inducing behavior in persons with MS.
Conclusions.
Such a paucity of evidence supporting exercise-induced neuroplasticity in MS is likely a product of a very small number of papers that do not sufficiently examine hypothesized mechanisms of action. Future research might consider examining specific neural changes that might result from exercise prescriptions that are specifically designed to induce certain functional changes among persons with MS.
Keywords: exercise, neuroimaging, function, rehabilitation, multiple sclerosis, neuroplasticity
Introduction
Exercise training is associated with a myriad of functional benefits among persons with multiple sclerosis (MS). For example, there is a large body of evidence that exercise training improves walking mobility, balance, and possibly cognition,1 presumably, in part, via adaptive changes in the central nervous system (CNS) in MS.2–5 This hypothesis of CNS adaptations is largely based on evidence of adaptive and regenerative exercise-related effects on brain structure, function, and connectivity that explain improvements in physical and cognitive functioning (ie, exercise as a neuroplasticity-inducing behavior) in the general population.6–9 For example, studies have reported that walking exercise training is associated with interrelated improvements in cognition (ie, executive functions/learning and memory), brain volume, and brain activation in large samples of healthy older adults.6–8 Of note, one recent international group of experts identified the importance of mechanistic examinations of the benefits of exercise training in MS as a major avenue for knowledge translation of exercise promotion through health care providers.1
To date, it has been difficult to characterize exercise training as a neuroplasticity-inducing behavior among persons with MS based on changes in functional outcomes alone, as neuroplasticity reflects behavior-related changes in function that are explained by changes in the CNS (ie, changes in neuroimaging outcomes).10 There too is the possibility that exercise-related functional adaptations are driven by peripheral mechanisms (eg, enhanced muscle oxidative capacity).11 This highlights the importance of including neuroimaging metrics of brain structure, function, and/ or connectivity in exercise research on functional outcomes in persons with MS for generating appropriate inferences regarding exercise-related neuroplasticity.2 Furthermore, there is an inconsistent focus on how exercise training, in particular, might induce functional adaptations based on CNS changes in human and animal models of MS.12–14 Collectively, this underscores the need for applying high-quality conceptual frameworks for better understanding and characterizing neuroplasticity within the context of exercise research in MS, beyond observations of exercise-related changes in function. The application of well-established conceptual frameworks for this purpose would be advantageous for providing a stronger biological foundation for prescribing exercise within the clinical setting among persons with MS.1
One particularly well-established model for characterizing neuroplasticity involves a hypothesized causal pathway connecting behavior and functioning via changes in the CNS.10 This model posits that neural plasticity is an inherent feature of the CNS that is present throughout the life span and allows the brain to continuously adapt to changes in environmental and behavioral experience via remodeling of neural networks; this can result in adaptive (or maladaptive) functional change.10 That is, changes in behavior and/or the environment induce CNS changes that, in turn, lead to concomitant behavioral/functional adaptations10 (Figure 1). This model10 is highly relevant for examining the effects of exercise training on functional adaptations in MS via neuroplasticity. Within the context of the model, if exercise is indeed a neuroplasticity-inducing behavior among persons with MS, exercise training should induce changes at the brain-systems level, which should yield consequent changes in functioning. By extension, evidence supporting exercise-induced neuroplasticity from clinical trials of MS should align with the following pattern of results: (a) exercise-related improvements in neuroimaging outcomes (as proxies of brain-systems level changes), (b) exercise-related improvements in functional outcomes, and (c) associations among changes in neuroimaging and functional outcomes. This latter component provides the connective link that is crucial for characterizing exercise as a neuroplasticity-inducing behavior (ie, exercise-related CNS adaptations resulting in functional adaptations), and such a pattern of results would be entirely consistent with the aforementioned model of neuroplasticity.10
Figure 1.

Research framework for characterizing exercise training as a neuroplasticity-inducing behavior in persons with MS based on true brain-behavior relationships.10 MS, multiple sclerosis; MRI, magnetic resonance imaging; fMRI, functional magnetic resonance imaging; TMS, transcranial magnetic stimulation; SPECT, single-photon emission computed tomography; MEG, magnetoencephalography; PET, positron emission tomography.
We are aware of 3 recent reviews of exercise and/or rehabilitation in MS that support the need for a focal examination and characterization of exercise as a neuroplasticity-inducing behavior.12–14 For example, one review paper examined the effects of cognitive and motor rehabilitation on neuroimaging outcomes in human and animal models of MS.12 Another review focused on identifying potential mechanisms of exercise training effects on biomarkers of MS disease progression.13 The third review paper primarily focused on the sensitivity and specificity of neuroimaging techniques for capturing cognitive and motor rehabilitation-related changes in the brain in MS.14 None of those review papers were registered using Prospero,12–14 and registration is important for transparency, reducing potential for bias, and avoiding unintended duplication of reviews. Of note, the present systematic review approach is different from those undertaken in recent reviews that have focused on the effects of rehabilitative interventions on various neuroimaging outcomes in MS from an effect size perspective,12–14 as those papers have not considered the overall evidence within the context of a well-established framework10 for characterizing exercise, in particular, as a neuroplasticity-inducing behavior. This is important, as evaluating research papers against such a framework permits stronger considerations for exercise as inducing neuroplasticity in MS, if such research provides supportive evidence using that framework.
The current systematic review provides a critical evaluation of studies on exercise training, neuroimaging outcomes, and functional outcomes among persons with MS based on the consistency with a well-established conceptual model10 in order to characterize exercise training as a possible neuroplasticity-inducing behavior in this population. To do so, the current systematic review categorized such research based on the number of methodological components that were present within a given study that could allow for appropriate interpretations of exercise training as a neuroplasticity-inducing behavior in MS. Those components were (a) examination of exercise training effects on functional outcomes, (b) examination of exercise training effects on functional outcomes, and (c) examination of associations among exercise-related changes in functional and neuroimaging outcomes. To permit additional evaluations of exercise training as a possible neuroplasticity-inducing behavior in MS, the present systematic review assessed the methodological rigor of research that involved those aforementioned components. As exercise-related neuroplasticity has been, to date, poorly characterized in MS, the present review and synthesis provides a streamlined, foundational step for informing the development of subsequent exercise training interventions for improving function based on specific neural changes in this population.
Methods
Search Strategy and Article Selection
This systematic review is based on a comprehensive search of different bibliographic databases (PubMed, Google Scholar, MEDLINE, CINAHL, and PsychINFO) and was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).15 The search was completed in March, 2019 and updated in February, 2020. The present review was registered on Prospero (Prospero ID: 142223). The search was restricted to English-language papers that focused on exercise training studies in humans with MS. In order to be eligible for full-review, papers had to include the following components: (a) exercise training (defined as planned, structured, and repetitive bodily movement done to improve or maintain one or more components of physical fitness16) that lasted a minimum of 2 weeks; (b) neuroimaging outcomes (operationalized as magnetic resonance imaging [MRI], functional MRI [fMRI], transcranial magnetic stimulation [TMS], single-photon emission computed tomography [SPECT], magnetoencephalography [MEG], and/or positron emission tomography [PET]); and (c) functional outcomes (operationalized as measures of physical fitness, walking mobility, balance, and/or cognition) in persons with MS. Acceptable study designs included randomized controlled trials (RCTs), single-group pre/post designs, and quasi-experimental designs. Textbook chapters, conference posters, systematic reviews, meta-analyses, animal studies, or exercise training studies that involved pediatric MS samples were not evaluated for full review. Papers were not excluded based on publication date.
The search was conducted using the following terms across electronic databases: “multiple sclerosis” OR “MS,” AND “exercise training,” OR “aerobic,” OR “resistance,” OR “cardiovascular,” OR “strength,” OR “yoga,” AND “neuroimaging,” OR “MRI,” OR “TMS,” OR “SPECT,” OR “MEG,” OR “PET,” AND “mobility,” OR “balance,” OR “walking,” OR “cognition,” OR “gait,” OR “MSFC,” OR “posturography,” OR “ambulation.” Abstracts of papers were reviewed by a single reviewer (CDJ) who applied the aforementioned inclusion/exclusion criteria for determining a paper’s eligibility for full review. Of note, reference sections of selected studies were further reviewed to identify additional eligible studies.
Data Extraction
Following the removal of duplicates, each paper meeting eligibility criteria was fully reviewed by 4 independent reviewers (CDJ, JFB, RWM, BMS). The data extracted from each eligible paper included participant characteristics, exercise intervention characteristics, neuroimaging outcome characteristics, functional outcome characteristics, study results (ie, exercise-related changes in neuroimaging outcomes, exercise-related changes in functional outcomes, associations among changes in neuroimaging and functional outcomes, study limitations, adherence/compliance, and information on statistical power). This allowed for the critical evaluation of exercise training as a possible neuroplasticity-inducing behavior among persons with MS that is consistent with a well-established framework.10 We further evaluated risks of various biases per study using a modified version of the Cochrane Risk of Bias Assessment Tool.17
Data Synthesis
The primary research question for the present systematic review involved the overall extent to which exercise training can be considered a neuroplasticity-inducing behavior among persons with MS based on the aforementioned conceptual model.10 To address that question, we initially characterized all papers that underwent full-review based on (a) reporting of analyses of exercise training effects on neuroimaging outcomes, (b) reporting of analyses of exercise training effects on functional outcomes, and (c) reporting of analyses of associations among changes in neuroimaging outcomes and changes in functional outcomes. Papers were initially categorized based on the number of those aspects that were reported on (ie, all 3 aspects or aspects b and c only). Within each category, reviewers then designated papers as either supporting or not supporting exercise training as a neuroplasticity-inducing behavior in MS. Such a designation was based on several factors, including: study parameters and results (ie, study design, participant characteristics, exercise training intervention parameters, neuroimaging outcomes, and functional outcomes), sample size/statistical power, inclusion of significance testing for the analyses above (given that many of the papers that underwent full review were pilot or feasibility studies), along with risks of bias.17
Results
The detailed summary of the literature search is depicted in Figure 2. The search of electronic databases and reference sections yielded 2405 citations. After removal of duplicates, 191 abstracts were identified for assessment of eligibility for full review. Of those abstracts, 10 met eligibility criteria and were subsequently fully reviewed for data extraction and synthesis.18–27
Figure 2.
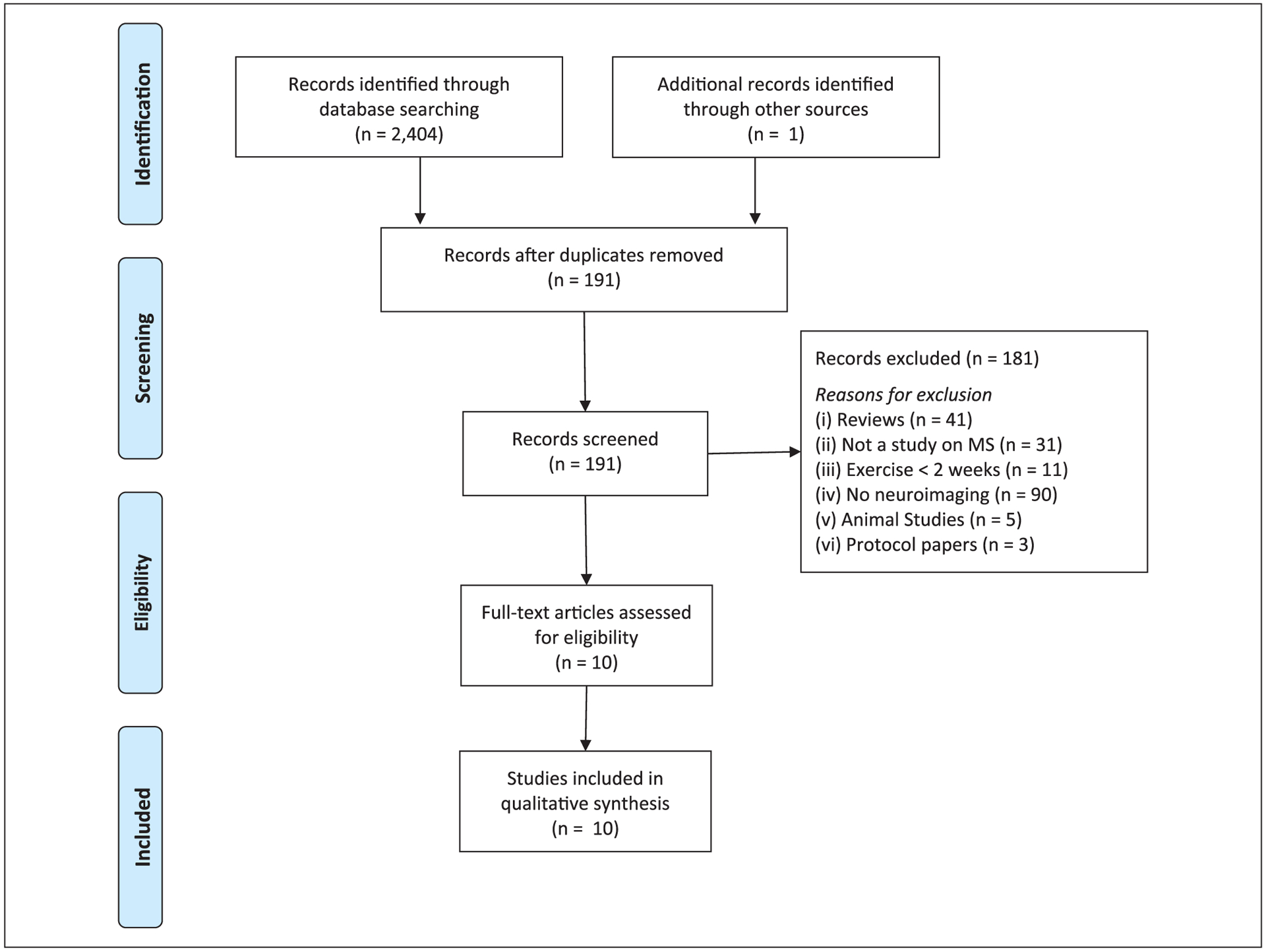
Summary of literature search results based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines.
Participant Characteristics, Interventions, Outcomes
Table 1 summarizes the participant characteristics, study characteristics, and primary study results for each of the 10 papers reviewed. Briefly, a majority of the papers involved persons with mild ambulatory disability with relapsing-remitting MS, and pilot/feasibility RCTs in relatively small samples of persons with MS (ranging from 2 to 35 persons with MS). Of note, 2 of the 10 selected papers were secondary data analyses (ie, reporting on different neuroimaging outcomes from an original intervention) of a primary paper.21,25 All 8 of the original papers included in the present systematic review involved different exercise prescriptions and all 10 papers (including secondary analyses) involved different neuroimaging outcomes.
Table 1.
Participant Characteristics, Study Characteristics, and Primary Study Results for 10 Papers That Satisfied Eligibility Criteria for the Systematic Review.
| Study | Sample size | Design | Exercise prescription | Neuroimaging outcomes | Functional outcomes | Exercise training effects on neuroimaging outcomes | Exercise training effects on functional outcomes | Associations among changes in neuroimaging and functional outcomes |
|---|---|---|---|---|---|---|---|---|
| Prosperini et al, (2014)18 | n = 27 | Randomized controlled crossover design | Modality: Home-based, high-intensity, task-oriented feedback balance exercise (WBBS) Duration: 12 weeks Frequency: 5 times/week Time: 30 min/session Intensity: WBBS games automatically progressed in difficulty based on achievement of criterion scores |
Modality: MRI (DTI) Outcome: White matter integrity (FA, MD, RD) of cerebellar peduncles |
Domain: Balance Outcome: Eyes-open postural sway (static posturography) |
↑ FA of bilateral superior cerebellar peduncles relative to waitlist control condition ↓ RD of left superior cerebellar peduncle relative to waitlist control condition No Δ in FA, MD, AD, and RD of middle or inferior cerebellar peduncles relative to waitlist control condition |
↓ in eyes-open postural sway relative to waitlist control condition | ↓ in eyes-open postural sway associated with ↑ FA of bilateral superior cerebellar peduncles ↓ in eyes-open postural sway associated with ↓ RD of bilateral superior cerebellar peduncles |
| Leavitt et al (2014)19 | n = 2 | Quasi-experimental, pre/post | Modality: Supervised aerobic stationary cycling Duration: 12 weeks Frequency: 3 times/week Time: 30 min/session Intensity: Not specified; used a program of graduated resistance developed by an exercise physiologist |
Modality: MRI Outcomes: Hippocampal volume Cortical and subcortical gray matter volume Modality: fMRI Outcome: Hippocampal RSFC |
Domain: Physical fitness Outcome: CRF (VO2peak) Domain: Cognition Outcomes: LM (CVLT-II, BVMT-R) CPS (SDMT, PASAT) EF (Stroop) Working memory (Digit Span) |
Exercise participant: ↑ in hippocampal volume No Δ in cortical or subcortical gray matter volume ↑ in left hippocampal RSFC Stretching participant: No Δ in hippocampal, cortical, or subcortical gray matter volume No Δ in hippocampal RSFC |
Exercise participant: ↑ in VO2peak ↑ in CVLT-II and BVMT-R No Δ in CPS, EF, working memory Stretching participant: No Δ in CRF or any cognitive outcome |
NR |
| Sandroff et al (2017)20 | n = 8 | RCT | Modality: Supervised aerobic treadmill walking exercise Duration: 12 weeks Frequency: 3 times/week Time: Progressive (15–40 min/session) Intensity: Progressive (40%-80% HRR) |
Modality: MRI (MRE) Outcome: Hippocampal viscoelasticity (shear stiffness, damping ratio) |
Domain: Physical fitness Outcome: CRF (VO2peak) Domain: Cognition Outcome: LM (CVLT-II) |
↑ hippocampal shear stiffness relative to waitlist control condition ↓ hippocampal damping ratio relative to waitlist control condition |
↑ VO2peak relative to waitlist control ↑ CVLT-II scores relative to waitlist control |
↑ hippocampal shear stiffness associated with ↑ CVLT-II scores ↓ hippocampal damping ratio associated with ↑ CVLT-II scores |
| Sandroff et al (2018)21 | n = 8 | RCT | Same as Sandroff et al.20 | Modality: fMRI Outcome: Thalamocortical RSFC |
Domain: Physical fitness Outcome: CRF (VO2peak) Domain: Cognition Outcome: CPS (SDMT) |
↑ RSFC between thalamus and right SFG relative to waitlist control condition ↑ RSFC between thalamus and left MFG relative to waitlist control condition |
↑VO2peak relative to waitlist control condition ↑SDMT scores relative to waitlist control condition |
↑ RSFC between thalamus and right SFG/left MFG associated with ↑VO2peak ↑ RSFC between thalamus and right SFG/left MFG associated with ↑SDMT scores ↑VO2peak associated with ↑SDMT scores |
| Kjølhede et al (2018)22 | n = 35 | RCT | Modality: Supervised PRT of LE and UE Duration: 24 weeks Frequency: 2 times/week Time: NR Intensity: Progressive (3–4 sets of 6–12 repetitions between 6 and 15 repetition maximum) |
Modality: MRI Outcome: Whole-brain volume, PBVC, cortical thickness Cortical thickness of 74 exploratory ROI |
Domain: Physical fitness Outcome: LE muscle strength (MVC of KE/KF, 5-STS) Domain: Walking mobility Outcome: Walking endurance (2MWT) Walking speed (T25FW) Domain: Cognition Outcome: CPS (PASAT) Domain: Manual dexterity Outcome: 9HPT scores |
No Δ in whole-brain PBVC, white/grey matter volume, volumes of subcortical structures relative to waitlist control condition ↑ in cortical thickness in 19/74 exploratory ROI relative to waitlist control |
↑ in MVC of KE/KF relative to waitlist control condition ↑ in T25FW relative to waitlist control condition ↑ in 9HPT scores relative to waitlist control condition No Δ in 5-STS, 2MWT, PASAT relative to waitlist control condition |
↑ in cortical thickness of anterior cingular sulcus and gyrus associated with ↑ in MVC of KE/KF and ↑ in 5-STS |
| Tavazzi et al (2018)23 | n = 26 | RCT | Modality: Supervised treadmill endurance training; Supervised PRT of LE Duration: 4 weeks Frequency: 10 times/week Time: 30–45 min/session Intensity: Endurance training: Moderate (below 16–17 on Borg RPE) PRT: Progressive (2–3 sets of 10–15 repetitions) |
Modality: MRI (DTI) Outcome: White matter integrity (FA, MD, RD) Modality: fMRI Outcome: Activation during plantar dorsiflexion Sensorimotor RSFC |
Domain: Balance Outcome: BBS Domain: Mobility Outcome: Walking endurance (2MWT) Walking speed (T25FW) Gait (DGI) |
No Δ in any DTI measures ↓ activation in left precentral gyrus for both groups ↑ RSFC in bilateral precentral and postcentral gyri for both groups Between-groups comparisons NR |
↑ 2MWT for both groups ↑ BBS for both groups No Δ in T25FW, DGI for both groups Between-groups comparisons NR |
No association among ↓ precentral gyrus activation, ↑ 2MWT, and ↑ BBS Associations among Δ in RSFC and Δ in functional outcomes NR |
| Feys et al (2019)24 | n = 29 | RCT | Modality: Community-based aerobic running training Duration: 12 weeks Frequency: 3 times/week Time: NR, distance-based Intensity: Progressive (1-minute running bouts through running 5 km without interruption) |
Modality: MRI Outcome: Whole-brain gray/white matter volume, volumes of subcortical ROI Modality: MRI (DTI) Outcome: Nonspecific structural connectivity outcomes |
Domain: Physical fitness Outcomes: CRF (VO2peak), LE muscular strength (5-STS) Domain: Walking mobility Outcomes: Walking endurance (6MWT) Walking speed (T25FW) Domain: Cognition CPS (PASAT, DSST) EF (WLG) LM (SRT, 10/36 SPART) |
No Δ in whole-brain gray/white volume relative to waitlist control condition ↑ in left pallidum volume relative to waitlist control condition No Δ in other subcortical volumes or structural connectivity relative to waitlist control condition |
↑ in VO2peak relative to waitlist control condition ↑ in 5-STS relative to waitlist control condition ↑ in 10/36 SPART scores relative to waitlist control condition No Δ in walking mobility or other cognitive outcomes relative to waitlist control condition |
NR |
| Huiskamp et al (2019)25 | n = 29 | RCT | Same as Feys et al24 | Modality: fMRI Outcome: RSFC of the bilateral hippocampus with cortical regions related to the DMN |
Domain: Physical fitness Outcome: CRF (VO2peak) Domain: Cognition Outcome: LM (SRT, 10/36 SPART) |
No Δ in hippocampal/DMN RSFC relative to waitlist control condition | ↑ in VO2peak relative to waitlist control condition ↑ in 10/36 SPART scores relative to waitlist control condition |
↑ in 10/36 SPART was associated with ↑ hippocampal-DMN RSFC in the exercise group only |
| Orban et al (2019)26 | n = 17 | Quasi-experimental, known-groups, pre/post | Modality: Supervised aerobic treadmill walking and stationary cycling exercise training Duration: 8 weeks Frequency: 4 times/week Time: 30 min/session Intensity: 70% of peak HR |
Modality: MRI (MRS) Outcome: Whole-brain 31P MRS (PCr/ Pi, ATP/ Pi, ATP/PCr ratios) |
Domain: Physical fitness Outcome: CRF (VO2peak) Body composition (lean muscle mass; BIA) Domain: Walking mobility Outcome: Functional mobility (TUG) Walking endurance (6MWT) Domain: Cognition Outcome: CPS (SDMT) |
No Δ in any 31P MRS outcomes relative to stretching control condition | ↑ in VO2peak relative to stretching control condition ↑ in SDMT score relative to stretching control condition No Δ in walking mobility or body composition relative to stretching control condition |
NR |
| Akbar et al (2020)27 | n = 10 | Quasi-experimental, known-groups, pre/post | Modality: Partially supervised PRT of LE and UE Duration: 16 weeks Frequency: 3 times/week Time: NR Intensity: Progressive (1–3 sets of 10–15 repetitions; increasing resistance of resistance bands, complexity of exercises, as determined by exercise leader) |
Modality: fMRI Outcome: Left caudate RSFC |
Domain: Physical fitness Outcome: Grip strength (hand dynamometry) |
↑ RSFC between left caudate and left inferior parietal region, bilateral inferior frontal regions, left middle frontal region, right insula relative to waitlist control condition | ↑ in grip strength relative to waitlist control condition | ↑ RSFC between left caudate and left inferior parietal lobule associated with ↑ grip strength |
Abbreviation: Δ, change; ↑, improvement/increase; ↓, reduction/decrease; 2MWT, 2-minute walk test; 5-STS, 5 times sit-to-stand; 6MWT, 6-minute walk test; 9HPT, 9-hole peg test; 31P, phosphorus; AD, axial diffusivity; ATP, adenosine triphosphate; BBS, Berg Balance Scale; BIA, bioelectrical impendence analysis; BVMT-R, Brief Visuospatial Memory Test-Revised; CPS, cognitive processing speed; CRF, cardiorespiratory fitness; CVLT-II, California Verbal Learning Test-II; DGI, Dynamic Gait Index; DMN, default-mode network; DSST, Digit-Symbol Substitution Test; DTI, diffusion tensor imaging; EF, executive function; FA, fractional anisotropy; HRR, heart rate reserve; KE, knee extensors; KF, knee flexors; LE, lower extremity; LM, learning and memory; MD, mean diffusivity; MFG, medial frontal gyrus; MRE, magnetic resonance elastography; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; MVC, maximal voluntary contraction; NR, not reported; PASAT, Paced Auditory Serial Addition Test; PBVC, percent brain volume change; PCr, phosphocreatine; Pi, inorganic phosphate; PRT, progressive resistance training; RCT, randomized controlled trial; RD, radial diffusivity; ROI, regions of interest; RSFC, resting-state functional connectivity; SDMT, Symbol Digit Modalities Test; SFG, superior frontal gyrus; SPART, Spatial Recall Test; SRT, selective reminding task; TUG, timed up-and-go; T25FW, timed 25-foot walk; UE, upper extremity; WBBS, Wii Balance Board System; WLG, Word List Generation.
There were 8 different exercise training interventions that were examined across the 10 papers. Those interventions involved various modalities of aerobic exercise training,19–21,23–26 progressive resistance training (PRT),22,23,27 and balance training.18 Duration of exercise training ranged from 4 to 24 weeks in length. Across the 8 identified exercise training interventions, 1 intervention involved 4 weeks of exercise training,23 1 involved 8 weeks of exercise training,26 4 involved 12 weeks of exercise training,18–21,24,25 1 involved 16 weeks of exercise training,27 and 1 intervention involved 24 weeks of exercise training.22 Regarding frequency, exercise training took place between 2 and 10 times per week across the 8 interventions. One intervention took place 2 times per week,22 4 took place 3 times per week,19–21,24,25,27 1 took place 4 times per week,26 1 took place 5 times per week,18 and another intervention took place 10 times per week (ie, twice per day on 5 days per week).23 Some studies reported exercise intensity and progression in great detail (eg, Sandroff et al20), whereas others did not (eg, Leavitt et al19). Regarding exercise setting, 1 intervention was community based,24,25 1 was home based,18 1 intervention was partially supervised and partially home based,27 and 5 interventions were fully supervised.19–23,26 One intervention that was partially supervised27 and 1 community-based intervention24,25 involved several group-based, in-person sessions whereas the other 6 interventions involved exercise training on an individual basis.
Across the 10 selected papers, all the neuroimaging outcomes involved some variation of MRI; some paradigms involved structural MRI whereas others involved fMRI. No selected papers involved other neuroimaging modalities (eg, PET, functional near-infrared spectroscopy [NIRS], SPECT). Structural MRI outcomes included lesion burden, volumetric outcomes, cortical thickness, diffusion tensor imaging (DTI), magnetic resonance spectroscopy (MRS), and magnetic resonance elastography (MRE) outcomes at the whole-brain level and specific brain structures or regions of interest (ROI). Specific ROI for structural neuroimaging that were identified a priori included the hippocampus,19,20,24 cerebellar peduncles,18 and basal ganglia.19,24 One paper involved a priori hypotheses pertaining to exercise effects on whole-brain structural outcomes that further included exploratory cortical ROI.22 By comparison, 5 papers involved fMRI outcomes.19,21,23,25,27 All 5 papers used resting-state functional connectivity (RSFC) paradigms and no papers included task-based fMRI measures or effective connectivity measures. Specific network hubs of interest for RSFC outcomes included the hippocampus,19,25 thalamus,21 caudate nucleus,27 and default-mode network.25 Of note, 1 paper involved task-based fMRI for measuring sensorimotor network activation during a plantar dorsiflexion task.23
Across the 10 selected papers, functional outcomes included measures of physical fitness, walking mobility, balance, manual dexterity, and cognition. Physical fitness outcomes included measures of cardiorespiratory fitness,19–21,24,26 body composition,26 grip strength,27 and lower extremity muscular strength.22,24 Measures of walking mobility included walking speed and walking endurance,22–24,26 gait,23 and functional mobility.26 One paper measured balance using static posturography with eyes open18 and another paper measured balance using the Berg Balance Scale.23 One paper measured manual dexterity using the 9-hole peg test.22 Measures of cognition included neuropsychological tests of cognitive processing speed,19,21,22,24,26 learning and memory,19,20,24,25 and executive function.19,24
Risks of Bias
Information on potential risks of biases per paper is presented in Figure 3. Briefly, across the 10 selected papers, the most common risks of bias included lack of statistical power (ie, 9 of 10 papers) and lack of masked participants or personnel (9 of 10 papers). Risks associated with concealed allocation and selective reporting were largely not reported in a majority of papers. Nine of 10 papers demonstrated low rates of attrition (ie, <20%), consistent with a previous systematic review of exercise training RCTs in MS.28 Six of the 10 papers included blinded outcome assessors, but there was no reporting on the fidelity of maintaining the blinding.
Figure 3.

Modified Cochrane Risk of Bias Assessment Tool17 for the 10 papers that satisfied eligibility criteria for the systematic review. +, low risk of bias; ?, unclear risk of bias; −, high risk of bias.
Exercise Training as a Neuroplasticity-Inducing Behavior
Papers Reporting on 3 Aspects of Conceptual Model for Neuroplasticity.
Seven of the 10 selected papers (70%) reported on exercise-related changes in both neuroimaging and functional outcomes along with associations among exercise-related changes in those outcomes (ie, fully consistent with the aforementioned conceptual model10). Six of those papers involved an RCT design.18,20–23,25 and 1 involved a quasi-experimental, known-groups, pre/post design.27 Four of those papers supported exercise training as a neuroplasticity-inducing behavior based on improvements in neuroimaging and functional outcomes, as well as correlations among neuroimaging and functional improvements.18,20,21,27 Of note, 2 of those papers involved the reporting of separate outcomes from the same intervention.20,21
Papers Supporting Exercise as Neuroplasticity-Inducing Behavior in MS.
One paper reported on the effects of a 12-week, high-intensity, video game-based balance training intervention compared with a waitlist control condition on static posturography and MRI outcomes in 27 persons with MS.18 That study reported participants who underwent the 12-week balance training intervention demonstrated statistically significant increases in fractional anisotropy (FA) (Cohen’s f2 > 0.20) and decreases in radial diffusivity (RD) (Cohen’s f2 > 0.16) in the superior cerebellar peduncles relative to the waitlist control condition. That study also reported statistically significant, balance training related improvements (ie, reductions) in postural sway (F = 4.61, P = .016).29 The reductions in postural sway were significantly associated with improved white matter integrity in the superior cerebellar peduncles (P < .037).18 Two other papers that involved 3 aspects of the conceptual framework10 provided support for exercise as a neuroplasticity-inducing behavior. Both of those papers examined the effects of 12 weeks of supervised, progressive treadmill walking exercise training compared with a waitlist control condition on neuroimaging, cardiorespiratory fitness, and cognitive outcomes in 8 fully ambulatory persons with MS.20,21 One paper reported exercise-related improvements in hippocampal viscoelastic properties (based on MRE) (|d| = 0.94–1.20) and verbal learning and memory (d = 0.34); such improvements were strongly correlated with one another (|r| > 0.93, P < .01).20 The other paper using that intervention reported that treadmill walking exercise training improved RSFC between the thalamus and pre-frontal cortical regions (d > 1.70), cardiorespiratory fitness (d = 0.88), and cognitive processing speed (d = 0.72) relative to the waitlist control, and that the improvements in thalamocortical RSFC, cardiorespiratory fitness, and cognitive processing speed were intercorrelated (ρ > 0.43).21 Of note, the results reported in that second paper were largely based on effect sizes as opposed to significance testing. One final paper reported partially supervised, PRT-related improvements in grip strength (d = 1.11) and RSFC between the caudate and left inferior parietal lobule, bilateral inferior frontal regions, left middle frontal region, and right insula (all Ps < .01) relative to a stretching control condition among 10 persons with MS.27 That paper further reported on large correlations between improvements in grip strength and increased RSFC between the caudate and left inferior parietal lobule (r = 0.80).27
Papers Not Supporting Exercise as Neuroplasticity-Inducing Behavior in MS.
By comparison, 3 papers22,23,25 did not fully support exercise training as a neuroplasticity-inducing behavior based on the presence of all 3 components of the aforementioned conceptual model.10 One paper examined the effects of 24 weeks of PRT (involving upper and lower body exercises) compared with a waitlist control condition on structural MRI outcomes, muscular strength, mobility, manual dexterity, and cognitive processing speed in 35 persons with MS.22 Although 24 weeks of PRT did significantly improve lower extremity muscular strength (P < .01), walking speed (P < .01), and manual dexterity (P < .05) relative to the waitlist control condition, there were no significant improvements in lesion load, global brain volume, percentage brain volume, or cortical thickness (P > .13). However, despite nonsignificant improvements in structural neuroimaging outcomes, exploratory, post hoc analyses revealed that increases in cortical thickness of the anterior cingular sulcus and anterior cingular gyrus were associated with improvements in lower extremity muscular strength (r = 0.35).22 Another paper examined the effects of 4 weeks of treadmill-based endurance training compared with PRT of the lower extremities on structural and functional MRI outcomes and measures of mobility, balance, and gait in 26 persons with MS.23 The 4-week interventions resulted in significantly reduced activation of the left precentral gyrus during a plantar dorsiflexion task (P value not reported), enhanced RSFC in bilateral pre- and postcentral gyri (P < .01), as well as improved walking endurance (P = .03), balance (P < .01), and gait (P = .03). However, exercise-related improvements in brain function were not associated with improvements in mobility and balance (effect sizes not reported).23 The other paper involved a secondary analysis of neuroimaging and cognitive data from a 12-week, community-based running training intervention among persons with mild MS.25 That study reported that running training was associated with statistically significant improvements in visuospatial memory compared with a waitlist control condition (). However, running training was not associated with statistically significant improvements in RSFC between the hippocampus and default-mode network hubs (effect size not reported). Despite such nonsignificant effects of the intervention on brain connectivity, improvements in visuospatial memory were significantly associated with increased hippocampal/default-mode network RSFC (r = 0.62).25
Papers Reporting on 2 Aspects of Conceptual Model of Neuroplasticity.
Three papers that reported on exercise training intervention effects on neuroimaging and functional outcomes in persons with MS did not report on the associations among exercise-related changes in neuroimaging and functional outcomes.19,24,26 One such paper provided limited support for exercise as a neuroplasticity-inducing behavior based on improvements in neuroimaging and functional outcomes in a quasi-experimental study in 2 persons with MS,19 whereas 2 papers did not support exercise training as a neuroplasticity-inducing behavior based on the lack of improvement in neuroimaging outcomes.24,26
Papers Supporting Exercise as a Neuroplasticity-Inducing Behavior in MS.
The lone paper that reported on 2 aspects of the conceptual model that provided limited support for exercise-induced neuroplasticity was a pre/post, quasi-experimental study involving only 2 memory-impaired persons with MS.19 That paper reported that a participant who was randomly assigned to 12 weeks of aerobic cycle ergometry training demonstrated increased hippocampal volume (+16.5%), RSFC (magnitude not reported), verbal learning and memory (+55.9%), and visuospatial learning and memory (+51.3%) compared with a participant who was randomly assigned to 12 weeks of supervised stretching.19 Of note, the small sample size precluded the ability to perform correlations among changes in neuroimaging and cognitive outcomes.
Papers Not Supporting Exercise as a Neuroplasticity-Inducing Behavior in MS.
Another paper involved a RCT of 12 weeks of remotely supervised, community-based running training compared with a waitlist control condition (ie, the primary paper to Huiskamp et al25) on structural MRI outcomes, physical fitness, mobility, and cognition in persons with mild MS disability.24 That paper reported that running training was associated with significantly increased left pallidum volume (P < .05) and improvements in various functional domains (ie, cardiorespiratory fitness, lower extremity muscular strength, visuospatial memory; all Ps < .05). However, there were no intervention effects on whole-brain volume, volumes of other subcortical structures, or measures of structural connectivity (P > .05), and it is unknown if the exercise-related change in left pallidum volume was associated with any functional change.24 The final paper on exercise effects on neuroimaging and functional outcomes among persons with MS involved a quasi-experimental, known-groups, pre/post design.26 That study compared the effects of 8 weeks of vigorous intensity aerobic exercise training compared with static stretching on MRS outcomes and measures of physical fitness (ie, cardiorespiratory fitness, body composition), cognitive processing speed, and mobility (walking speed, walking endurance, functional mobility) in 17 persons with MS.26 That study reported exercise-related improvements in physical fitness (+12.7%) and cognitive processing speed (+15.1%), but there were minimal exercise-related improvements in 31P levels in the brain (based on MRS; <+3.8%).26
Discussion
There is a large body of literature on the beneficial effects of exercise training on functional outcomes in persons with MS,1 and it has been suggested that such effects might be driven by neuroplasticity.12–14 Such a possibility would be important for the clinical translation of evidence into practice of MS care.1 The current systematic review characterized neuroplasticity using a well-established conceptual model,10 and sought to evaluate the overall extent to which exercise training induces neuroplasticity among persons with MS based on brain-behavior relationships. Despite well over a hundred published papers reporting on exercise-related benefits on functioning in MS,1 the literature search returned only 10 papers (involving 8 original interventions) wherein inferences regarding neuroplasticity could be drawn, based on the inclusion of both neuroimaging and functional endpoints in the same paper. Within those 10 papers, there was mixed evidence for exercise training as a neuroplasticity-inducing behavior in persons with MS. Overall, most of the studies involved pilot/feasibility trials (ie, high risk of demonstrating lack of statistical power; Figure 3), there was an inconsistent pattern of results pertaining to exercise training effects on neuroimaging outcomes as proxies of CNS adaptations, as well as insufficient reporting of mechanistic analyses (ie, inclusion of correlations between changes in neuroimaging and functional outcomes). Collectively, such a paucity of consistent data renders it difficult to draw definitive conclusions on the extent to which exercise training can be characterized as a neuroplasticity-inducing behavior in MS. The existing evidence would only support a tentative statement that exercise training has the potential to induce neuroplasticity in persons with MS based on preliminary evidence from a few select papers.
Across the 10 selected papers, 6 (involving 5 original interventions) provided some support for exercise training as a neuroplasticity-inducing behavior in persons with MS based on improvements in neuroimaging and functional outcomes18–21,23,27; 4 of those 6 papers18,20,21,27 reported on positive associations between exercise-related improvements in neuroimaging and functional outcomes (ie, all 3 aspects of the conceptual model). One paper reported a lack of association among exercise-related changes in cortical activation (based on fMRI) and functional outcomes.23 Importantly, there was substantial heterogeneity in parameters of the exercise training interventions (ie, home-based balance training, supervised treadmill walking exercise, cycle ergometry, PRT), improvements in neuroimaging outcomes (ie, white matter integrity of cerebellar peduncles, hippocampal viscoelasticity, thalamocortical RSFC, hippocampal volume/RSFC, task-based activation in the sensorimotor network, caudate-related RSFC), and improvements in functional outcomes (ie, balance, cardiorespiratory fitness, learning and memory, cognitive processing speed, grip strength). Despite such overall heterogeneity, 2 papers did report on a similar mechanism (ie, aerobic exercise-related improvements in hippocampal neuroimaging outcomes and concomitant improvements in learning and memory).19,20 However, 2 others papers reported that aerobic running training did not improve hippocampal neuroimaging outcomes among persons with MS, despite improvements in visuospatial learning and memory (see below24,25). In this group of 6 papers, the majority were likely statistically underpowered and were at high risk for participant and experimenter expectancy effects, given the lack of masking of participants and personnel to the intent of the exercise and/or control conditions.
By comparison, 4 of the 10 papers did not support exercise as a neuroplasticity-inducing behavior in this population based on the presence of exercise-related functional improvements without substantial improvements in neuroimaging outcomes.22,24–26 Of note, there were no papers that reported on exercise-related improvements in neuroimaging outcomes without concomitant functional improvements. There too was substantial heterogeneity in this group of papers based on different exercise training stimuli (ie, PRT, community-based running training, and aerobic exercise training based on treadmill walking and cycle ergometry), neuroimaging outcomes and approaches (ie, whole-brain, cortical, and subcortical volumetric outcomes, cortical thickness, hippocampal default-mode network RSFC, whole-brain MRS), and functional improvements (cardiorespiratory fitness, muscular strength, manual dexterity, walking speed, visuospatial learning and memory, cognitive processing speed). Indeed, there were no clear patterns of results across papers that demonstrated consistency of specific exercise training modalities leading to minimal changes in specific neuroimaging outcomes. In addition, all 4 of those papers involved high or unclear risks of bias associated with selective reporting, lack of statistical power, and lack of masked participants and personnel (Figure 3).
To that end, the overall conflicting results of the systematic review are likely a product of (a) a very limited number of studies that (b) consisted of heterogeneous interventions and outcomes and (c) a sample size that was not defined by an a priori power analyses and/or previous data. Those issues further highlight the importance of characterizing neuroplasticity based on specific brain-behavior relationships in exercise research in MS. On closer examination of the 10 selected papers, only 5 hypothesized a specific neural mechanism of exercise-related change in function, based on inclusion of selective neuroimaging and functional outcomes (as opposed to examinations of generalized outcomes that are typically included in MS rehabilitation research).18–21,25 Importantly, of those papers, 4 included all 3 aspects of the well-established conceptual model10 for characterizing neuroplasticity based on brain-behavior relationships (ie, exercise-related changes in neuroimaging, exercise-related changes in function, associations among changes in neuroimaging and functional outcomes).18,20,21,25 Of note, 2 of those 4 papers were secondary data analyses.21,25 This boils down to the identification of only 2 papers that involved original interventions18,20 that further included a priori hypotheses and rationale for the inclusion of specific exercise prescriptions for improving specific functional outcomes based on specific brain mechanisms among persons with MS. Those studies are the most consistent with the previously described conceptual model for appropriately characterizing exercise training as a neuroplasticity-inducing behavior.10 However, the results of those studies should be interpreted with caution given that both studies demonstrated a high risk for bias based on the potential of being underpowered and involving passive control conditions. The identification of only 2 papers that fully align with such a framework is alarming given that many review papers allude to the possibility that exercise training results in functional changes in MS based on changes in the CNS.1–3,12–14 Such a hypothesis might be an extension of data that suggest certain prescriptions of cognitive and motor rehabilitation (not including exercise training) exert specific effects on the CNS, leading to specific functional improvements among persons with MS.12–14
However, there exists the possibility that exercise training might affect the brain and downstream effects on functioning in a more generalized fashion among persons with MS.4 Indeed, 5 selected papers included more generalized hypotheses pertaining to exercise effects on neuroimaging and functional outcomes.22–24,26,27 Three of the 5 papers included all 3 aspects of the model for appropriately characterizing exercise-related neuroplasticity,10 but did not provide an a priori hypothesis on a specific neural mechanism of exercise-related change in function.22,23,27 Across this group of 5 selected papers, only 1 supported exercise training as a neuroplasticity-inducing behavior,27 though that intervention was not specifically designed for inducing functional improvements based on CNS changes.27 Rather, the intervention involved PRT for reducing fatigue and focused on neural mechanisms pertaining to fatigue reduction, and grip strength was included as a manipulation check. Nevertheless, such a pattern of results suggests that exercise training might induce selective effects on the CNS (based on neuroimaging metrics), leading to select functional changes among persons with MS, as opposed to broad, nonspecific effects on brain and function. This is consistent with a recently proposed conceptual framework for explaining why exercise training might improve cognition and mobility among persons with MS.30
The examination of exercise as a neuroplasticity-inducing behavior in persons with MS is clearly in its infancy. A substantially larger body of evidence is needed in order to provide firmer conclusions on the neural mechanisms that might underlie exercise-related changes in function in this population. Based on the pattern of results of the current systematic review, one potential starting point for growing the field involves the generation of stronger hypotheses pertaining to specific exercise-related mechanisms of action for inducing specific functional changes. Such an approach is consistent with the recently proposed Rehabilitation Treatment Specification System,31 as well as the principles of adaptation and specificity outlined in the American College of Sports Medicine guidelines for exercise prescription.32 Namely, to better characterize exercise-related neuroplasticity in persons with MS, researchers must provide rationale for the inclusion of specific exercise interventions (and comparators) for improving specific outcomes based on hypothesized mechanisms of action in select groups of participants. This can allow for better contextualization of neuroplasticity as a true brain-behavior relationship (ie, inclusion of analyses on potential associations among exercise-related changes in neuroimaging and functional outcomes).
Indeed, one focal approach has been suggested for systematically improving the quality, scope, and translation of research for understanding the effects of exercise training on the CNS for improving functioning among persons with MS (ie, the PRIMERS conceptual framework30). The PRIMERS conceptual framework proposes that the physiological regulation of exercise behavior requires the rapid and efficient processing and integration of multisensory input. Those multisensorial demands incrementally increase with chronic, progressive exercise training, and with repeated bouts of exercise, communication within and across key neural networks becomes more efficient in order to keep up with the incrementally increasing demands associated with the physiological regulation of exercise. PRIMERS then hypothesizes that the enhanced efficiency of communication at the brain-systems level results in adaptations over time in functions that rely on those neural networks.30 That framework involves using multiple paradigms for understanding the putative mechanisms of exercise-related adaptations on CNS functioning among persons with MS. This includes adopting acute and chronic exercise paradigms, the use of multiple neuroimaging modalities, examining the downstream effects on functioning based on exercise effects on certain neural networks, as well as examining exercise as a possible neuroprotective behavior over time in this population.30 Adopting such a systematic approach could provide a springboard for accumulating generalizable evidence evaluating the possibility of exercise training-related neuroplasticity among persons with MS. If such evidence is positive, this could increase the likelihood that clinicians might recommend exercise to patients as an approach for managing the disease. Beyond PRIMERS,30 another critical aspect for future research in this area involves the systematic examination of the sensitivity and specificity of outcomes for capturing exercise-related neuroplasticity, in particular, among persons with MS, as is being done for generalized rehabilitation in this population.14
There are several other important limitations in this area. One particularly glaring limitation involves the lack of adequately powered RCTs. The majority of selected papers involved small samples that did not involve significance testing for testing efficacy, given possible reductions in degrees of freedom. The lack of adequately powered RCTs in this area further might be a by-product of challenges associated with securing extramural funding.33 Performing (possibly underpowered) early-stage trials does not minimize the possibility of type I error, but such pilot research is important for reducing type II error and avoiding the premature dismissal of possibly beneficial interventions.34 Of note, the publication of such preliminary research in this area should lead to the eventual publication of larger, more stringent RCTs that might allow for better evaluations of exercise training as a possible neuroplasticity-inducing behavior in persons with MS. Another overall limitation pertains to the lack of focus on persons with progressive MS and/or severe ambulatory disability in exercise trials that examine neural mechanisms of exercise-related functional adaptations. We note that this concern has been raised across several review papers.1,2
Conclusions
To date, it has been clearly established that exercise training is associated with a myriad of functional benefits among persons with MS, yet the neural mechanisms of those effects have not been adequately examined using an established framework of neuroplasticity. Indeed, such functional changes resulting from exercise training in MS are purportedly due to neuroplasticity. However, when characterizing exercise training as a possible neuroplasticity-inducing behavior based on a well-established conceptual model,10 the overall evidence base is not robust, as there are insufficient data to draw such conclusions. This is a product of a very small number of published papers that do not sufficiently examine hypothesized mechanisms of action, perhaps based on research questions that do not appropriately conceptualize exercise-induced neuroplasticity as a true brain-behavior relationship. Future research efforts might consider examining specific neural changes that would be expected to result from exercise prescriptions that are specifically designed to induce certain functional changes among persons with MS. Such a research approach is entirely consistent with well-established models of neuroplasticity.10
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Motl RW, Sandroff BM, Kwakkel G, et al. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017;16:848–856. [DOI] [PubMed] [Google Scholar]
- 2.Motl RW, Sandroff BM. Exercise as a countermeasure to declining central nervous system function in multiple sclerosis. Clin Ther. 2018;40:16–25. [DOI] [PubMed] [Google Scholar]
- 3.Motl RW, Pilutti LA. Is physical exercise a multiple sclerosis disease modifying treatment? Expert Rev Neurother. 2016;16:951–960. [DOI] [PubMed] [Google Scholar]
- 4.Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klaren RE, Motl RW, Woods JA, Miller SD. Effects of exercise in experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis). J Neuroimmunol. 2014;274:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci Biobehav Rev. 2013;37(9 pt B):2268–2295. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Leone A, Amedi A, Fregni F, et al. The plastic human brain cortex. Ann Rev Neurosci. 2005;28:377–401. [DOI] [PubMed] [Google Scholar]
- 11.Meredith CN, Frontera WR, Fisher EC, et al. Peripheral effects of endurance training in young and old subjects. J Appl Physiol (1985). 1989;66:2844–2849. [DOI] [PubMed] [Google Scholar]
- 12.Prosperini L, Di Filippo M. Beyond clinical changes: rehabilitation-induced neuroplasticity in MS. Mult Scler. 2019;25:1348–1362. [DOI] [PubMed] [Google Scholar]
- 13.Negaresh R, Motl RW, Zimmer P, Mokhtarzade M, Baker JS. Effects of exercise training on multiple sclerosis biomarkers of central nervous system and disease status: a systematic review of intervention studies. Eur J Neurol. 2019;26:711–721. [DOI] [PubMed] [Google Scholar]
- 14.Rocca MA, Preziosa P, Filippi M. Application of advanced MRI techniques to monitor pharmacologic and rehabilitative treatment in multiple sclerosis: current status and future per-spectives. Exp Rev Neurother. 2019;19:835–866. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard C, Shephard RJ. Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement. Human Kinetics; 1994. [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prosperini L, Fanelli F, Petsas N, et al. Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology. 2014;273:529–538. [DOI] [PubMed] [Google Scholar]
- 19.Leavitt VM, Cirnigliaro C, Cohen A, et al. Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase. 2013;20: 695–697. [DOI] [PubMed] [Google Scholar]
- 20.Sandroff BM, Johnson CL, Motl RW. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology. 2017;59:61–67. [DOI] [PubMed] [Google Scholar]
- 21.Sandroff BM, Wylie GR, Sutton BP, Johnson CL, DeLuca J, Motl RW. Treadmill walking exercise training and brain function in multiple sclerosis: preliminary evidence setting the stage for a network-based approach to rehabilitation. Mult Scler J Exp Transl Clin. 2018;4:2055217318760641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjølhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356–1365. [DOI] [PubMed] [Google Scholar]
- 23.Tavazzi E, Bergsland N, Cattaneo D, et al. Effects of motor rehabilitation on mobility and brain plasticity in multiple sclerosis: a structural and functional MRI study. J Neurol. 2018;265:1393–1401. [DOI] [PubMed] [Google Scholar]
- 24.Feys P, Moumdjian L, van Halewyck F, et al. Effects of an individual 12-week community-located “start-to-run” program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler. 2019;25:92–103. [DOI] [PubMed] [Google Scholar]
- 25.Huiskamp M, Moumdjian L, van Asch P, et al. A pilot study of the effects of running training on visuospatial memory in MS: A stronger functional embedding of the hippocampus in the default-mode network [published online July 18, 2019]? Mult Scler. doi: 10.1177/1352458519863644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orban A, Garg B, Sammi MK, et al. Effect of high-intensity exercise on multiple sclerosis function and phosphorus magnetic resonance spectroscopy outcomes. Med Sci Sports Exerc. 2019;51:1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbar N, Sandroff BM, Wylie GR, et al. Progressive resistance exercise training and changes in resting-state functional connectivity of the caudate in persons with multiple sclerosis and severe fatigue: a proof-of-concept study. Neuropsychol Rehabil. 2020;30:54–66. doi: 10.1080/09602011.2018.1449758 [DOI] [PubMed] [Google Scholar]
- 28.Pilutti LA, Platta ME, Motl RW, Latimer-Cheung AE. The safety of exercise training in multiple sclerosis: a systematic review. J Neurol Sci. 2014;343:3–7. [DOI] [PubMed] [Google Scholar]
- 29.Prosperini L, Fortuna D, Gianni C, Leonardi L, Marchetti MR, Pozzilli C. Home-based balance training using the Wii balance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair. 2013;27:516–525. [DOI] [PubMed] [Google Scholar]
- 30.Sandroff BM, Motl RW, Reed WR, Barbey AK, Benedict RHB, DeLuca J. Integrative CNS plasticity with exercise in MS: the PRIMERS (PRocessing, Integration of Multisensory Exercise-Related Stimuli) conceptual framework. Neurorehabil Neural Repair. 2018;32:847–862. [DOI] [PubMed] [Google Scholar]
- 31.Hart T, Dijkers MP, Whyte J, et al. A theory-driven system for the specification of rehabilitation treatments. Arch Phys Med Rehabil. 2019;100:172–180. doi: 10.1016/j.apmr.2018.09.109 [DOI] [PubMed] [Google Scholar]
- 32.American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. 7th ed Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 33.Sandroff BM, DeLuca J. Will behavioral treatments for cognitive impairment in multiple sclerosis become standards-of-care? [published online February 28, 2019] Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 34.Mohr DC, Spring B, Freedland KE, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78:275–284. [DOI] [PubMed] [Google Scholar]


