Abstract
Cigarette smoke (CS) is one of the severe risk factors for the development of the pulmonary disease. However, the underlying mechanisms, especially the CS-induced the human bronchial epithelial cells (BEAS-2B) apoptosis related to endoplasmic reticulum stress (ERS) and autophagy, remains to be studied. This study aims to investigate the relationship between ERS and autophagy in apoptosis induced by CS condensate (CSC). BEAS-2B cells were stimulated with 0.02, 0.04 and 0.08 mg/ml CSC for 24 h to detect the ERS, autophagy and apoptosis. Then, ERS and autophagy of BEAS-2B cells were inhibited, respectively, by using 4-PBA and 3-MA, and followed by CSC treatment. The results showed that CSC decreased cell viability, increased cell apoptosis, elevated cleaved-caspase 3/pro-caspase 3 ratio and Bax expressions, but decreased Bcl-2 expressions. The GRP78 and CHOP expressions and LC3-II/LC3-I ratio were dose-dependently increased. The structure of the endoplasmic reticulum was abnormal and the number of autolysosomes was increased in BEAS-2B cells after CSC stimulation. The LC3-II/LC3-I ratio was decreased after ERS inhibition with 4-PBA, but GRP78 and CHOP expressions were enhanced after autophagy inhibition with 3-MA. CSC-induced apoptosis was further increased, Bax expressions and cleaved-caspase 3/pro-caspase 3 ratio were improved, but Bcl-2 expressions were decreased after 3-MA or 4-PBA treatment. In conclusion, the study indicates that ERS may repress apoptosis of BEAS-2B cells induced by CSC via activating autophagy, but autophagy relieves ERS in a negative feedback. This study provides better understanding and experimental support on the underlying mechanisms of pulmonary disease stimulated by CS.
Keywords: cigarette smoke condensate, BEAS-2B cells, endoplasmic reticulum stress, autophagy, apoptosis
Introduction
Smoking, one of the foremost risk factors for many disease etiologies, has a significant impact on respiratory health and can increase the mortality risk of chronic bronchitis and emphysema [1]. Cigarette smoke (CS), which is composed of gaseous and particulate matter containing more than 7000 chemical substances and at least 70 carcinogens, is also a hazardous form of indoor air pollution, causing 1.2 million premature deaths each year [2,3]. CS condensate (CSC) collected from burning cigarettes, has complex chemical constituents, mainly including polycyclic aromatic hydrocarbons [4]. These chemicals can cause damage to the cardiovascular system, nervous system and other systems, especially the respiratory system [5]. Thus, it’s meaningful for us to find out the molecular mechanisms of pulmonary damage caused by CS.
Epithelial cell apoptosis is considered to be the initial event of various pulmonary diseases [6]. Cell apoptosis may play an important role in lung diseases through two different ways. On the one hand, excessive apoptosis may lead to disease [7]. On the other hand, cells not cleared by apoptosis will extend the inflammation and prolong the repair process due to the fact that the release of toxic contents [8]. Therefore, apoptosis is the necessary physiological processes maintaining epithelial tissue homeostasis and clearing inflammatory cells.
Recent studies have showed that endoplasmic reticulum stress (ERS) is of great significance in the occurrence and development of lung diseases [9–11]. ERS is activated under the condition of cellular stress, such as energy deficiency, redox imbalance or exposure to toxicant [12]. Mounting evidences show that autophagy is a vital way to degrade the unfolded or misfolded proteins in the endoplasmic reticulum cavity when ERS activation [13,14]. ERS can cause the derivation of autophagy to restore homeostasis of the endoplasmic reticulum and promote cell survival [15,16]. In addition, some studies found that the regulation of autophagy on ERS may have a negative feedback [17], showing that autophagy may inhibit ERS to avoid the aggravation of intracellular stress. Simultaneously, ERS and autophagy are closely related to the occurrence of apoptosis. Excessive activation of autophagy caused by ERS can induce cell apoptosis, further aggravating cell damage [18,19]. Intriguingly, many studies have proved that autophagy can either inhibit apoptosis [20,21] or activate apoptosis when ERS occurs [22,23] to determine cell survival or death. However, the roles of autophagy and ERS in apoptosis of bronchial epithelial cells induced by CSC are not entirely clear.
In this study, an in vitro cellular model of CSC stimulation was established to study the relationship between ERS and autophagy in apoptosis of BEAS-2B cells. Cell viability was used to select the appropriate doses of CSC. The expression levels of essential proteins were detected to reflect autophagy and ERS. After inhibiting autophagy or ERS, the interaction roles of ERS, autophagy and apoptosis were obtained. This study provided theoretical support on the mechanisms of pulmonary disease.
Materials and Methods
Cell line and cell culture
The human bronchial epithelial (HBE) cell line BEAS-2B was obtained from Professor Yongjun Wu (School of Public Health, Zhengzhou University, China). The cells were cultured in RPMI-1640 medium (Solarbio, Beijing, China) with 10% fetal bovine serum (FBS; Gemini, California, USA) at 37°C in a saturated humidity atmosphere containing 5% CO2 and 95% air [24].
CSC preparation
CSC was prepared by using cigarettes with a tar content of 10 mg, utilizing a RM20H Rotary Smoking Machine (Borgwaldt KC, Germany) and a Cambridge filter pad (92 mm, Whatman, Maidstone, UK) to pump and catch respectively. Two hundred ninety-nine milligrams CSC were collected. CSC mixed with dimethylsulfoxide (DMSO) was extracted after shaking for 30 min. Subsequently, the supernatant was collected after standing for 30 min and filtered through 0.22 μm cellulose membranes to obtain sterile CSC solution, reaching a final concentration of 10 mg/ml, and stored at −80°C until used.
Cell viability
Cell viability was assessed by using Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China) assays. BEAS-2B cells were seeded in a 96-well plate (Corning, NY, USA) at a density of 5 × 103/well and treated with nine different concentrations (0, 0.02, 0.04, 0.06, 0.08, 0.10, 0.12, 0.14 and 0.16 mg/ml) of CSC for 24 h when cells were growing in the logarithmic phase. The timing of exposure was chosen based on the previous work from others [25,26]. Each well was rinsed with cold 1 × Phosphate buffer solution (PBS) before the addition of 10 μl CCK-8. After incubation for 4 h (37°C, 5% of CO2), absorbance at 450 nm was measured by a microplate reader (ELx808; BioTek, Winooski, VT, USA).
Preparation of inhibitors
4-phenylbutyrate (4-PBA; Sigma, Louis, MO, USA) and 3-methyladenine (3-MA; MedChemExpress, USA) were used to prepare the mother liquor, respectively. Twenty millimoles per liter 4-PBA (5 ml) were prepared with serum-free RPMI-1640 medium (Solarbio, Beijing, China). Then, 20 mmol/l NaOH (5 ml) was prepared. After 4-PBA and NaOH were completely dissolved respectively, these two solutions were mixed as 10 mmol/l mother liquor of 4-PBA and stored at 4°C. According to the manufacturer’s instructions, 10 mmol/l mother liquor of 3-MA (10 ml) was prepared with serum-free RPMI-1640 medium (Solarbio, Beijing, China).
Cell apoptosis
The apoptosis of BEAS-2B cells was detected by using a commercial AnnexinV-FITC/propidium iodide (PI) kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions [27]. The cell culture medium in the six-well plate was sucked out into the centrifuge tube first. Then, BEAS-2B cells of each well were digested with trypsin not containing EDTA, sucked out into the centrifuge tube in the previous step, centrifuged at 1000 g for 5 min, washed twice with PBS and resuspended and counted. Cells (1 × 105) were taken, centrifuged at 1000 g for 5 min to collect cell precipitation. After the addition of Annexin V-FITC and PI, the cells were further incubated in darkness at room temperature for 15 min and analyzed by flow cytometry (BD Accuri C6, Becton-Dickinson, Franklin Lakes, NJ, USA).
The examination of endoplasmic reticulum and autolysosome by transmission electron microscope
After treatment with CSC, BEAS-2B cells were fixed with electron microscope fixative for 2–4 h. The cells were postfixed in 1% osmic acid and 0.1 mol/l phosphate buffer (PB) for 2 h, followed by staining with uranium-lead double dyeing (uranium acetate saturated alcohol solution and lead citrate). Endoplasmic reticulum and autolysosome were observed by utilizing a transmission electron microscope (TEM).
Western blot
Total protein was extracted by using RIPA lysis buffer and Phenylmethyl sulfonyl fluoride (PMSF) on ice for 30 min and centrifuged at 12 000 g for 10 min. The protein concentration was assayed by using the bicinchoninic acid protein assay kit (Cwbio Biotech, China). An equivalent amount of protein was isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (PVDF), which were then blocked in 5% skim milk at 37°C for 2 h and incubated at 4°C overnight with different primary antibodies. Primary antibodies included rabbit anti-LC3A/B antibody (Cell Signaling Technology, USA), mouse anti-CHOP antibody (Cell Signaling Technology, USA), rabbit anti-GRP78 Bip antibody (Abcam, USA), mouse anti-β-actin antibody (Servicebio, Wuhan, China), rabbit anti-Caspase 3 antibody (Cell Signaling Technology, USA), rabbit anti-Bax antibody (Cell Signaling Technology, USA), rabbit anti-Bcl-2 antibody (Cell Signaling Technology, USA) and rabbit anti-GAPDH antibody (Goodhere, Hangzhou, China). After washing in tris-buffered saline and Tween 20 (TBST) for three times, the PVDF membranes were then incubated with secondary antibodies (goat anti-rabbit IgG antibody; goat anti-mouse IgG antibody) (Beyotime, Shanghai, China) at 37°C for 1 h. The membranes were rinsed with TBST for three times again, followed by visualization using enhanced chemiluminescence (ECL) kit (Cwbio Biotech, China).
Statistical analyses
Each experiment was performed three times and the data were displayed as means ± standard deviation (SD). Statistical analyses and graphs were presented by using GraphPad Prism 5.0. P values < 0.05 were considered statistically significant. Comparisons between different groups were carried out by one-way analysis of variance (one-way ANOVE) and the least significant difference test.
Results
CSC inhibited cell viability of BEAS-2B cells
To detected cell viability of BEAS-2B cells after CSC stimulation, BEAS-2B cells were treated with 0, 0.02, 0.04, 0.06, 0.08, 0.10, 0.12, 0.14 and 0.16 mg/ml CSC for 24 h. CCK-8 analysis indicated that cell viability was inhibited after CSC stimulation. There was a dose-dependent relationship between CSC concentrations and cell viability. Cell viability was gradually attenuated with the increasing CSC concentrations (Fig. 1). Cell viability was decreased to around 50% when BEAS-2B cells were stimulated with 0.10 mg/ml CSC.
Figure 1.
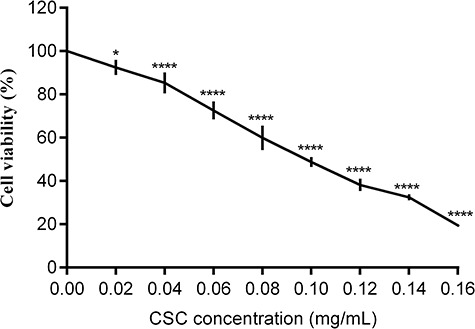
CSC reduced cell viability of BEAS-2B cells. BEAS-2B cells were exposed with 0, 0.02, 0.04, 0.06, 0.08, 0.10, 0.12, 0.14 and 0.16 mg/ml CSC for 24 h. Cell viability was measured by using CCK-8 assay. The values were presented as means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicated significant differences from the control (DMSO) group
CSC changed cell morphology and induced BEAS-2B cell apoptosis
Three doses of CSC (0.02, 0.04 and 0.08 mg/ml) were selected for the following experiments, ensuring that cell viability surpassed 50%. To investigate the adverse effect on BEAS-2B cells after CSC treatment, the changes of cell morphology were observed by utilizing inverted microscope (Fig. 2A). In the blank group, BEAS-2B cells were in the natural shape of long fusiform with antennae connected between cells. The culture supernatant was clean and free of impurities in the blank group. Cells of DMSO group had normal morphology without obvious changes compared to the blank group. Compared with DMSO group, BEAS-2B cells with CSC treatment appeared aberrant morphologic changes, such as irregular configuration, enlargement of cells, and reduced antennae connected between cells. To further find out the effects of CSC on apoptosis, the apoptosis rate and expression levels of apoptosis-related proteins (Bax, Bcl-2 and caspase 3) were both measured. The results of apoptosis showed that there was not statistically significant between DMSO group and blank group (Fig. 2B). But cell apoptosis rates were gradually enhanced with the increasing CSC concentrations. The apoptosis rates in the CSC group were significantly higher than that in DMSO group. The western blot results showed that the expression levels of Bax and the cleaved-caspase 3/pro-caspase 3 ratio were risen, but the expression levels of Bcl-2 was dropped with the CSC concentration increasing (Fig. 2C). Thus, CSC had cytotoxicity to BEAS-2B cells. Cell morphology was changed and cell apoptosis was induced after CSC treatment.
Figure 2.
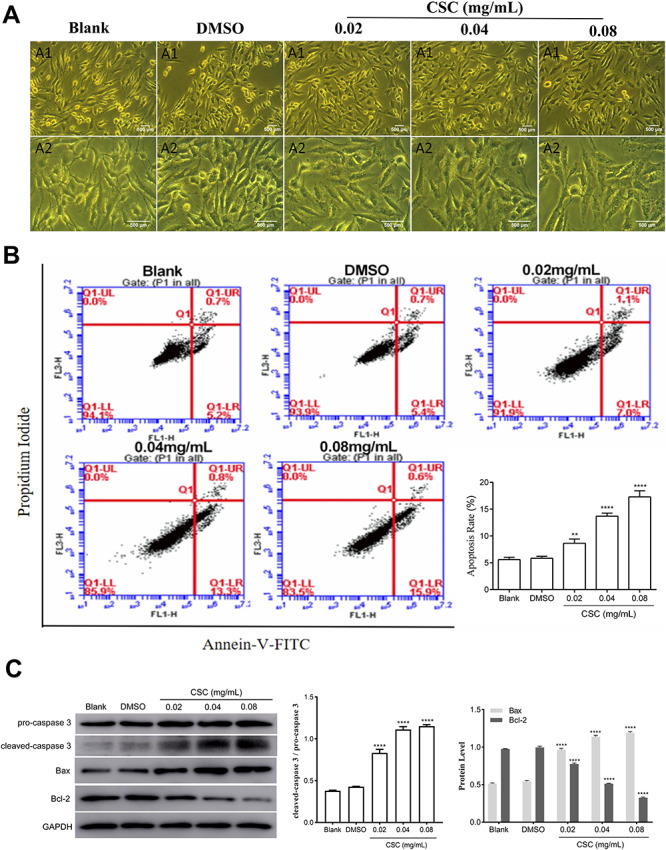
CSC changed cell morphology and promoted cell apoptosis of BEAS-2B cells. (A) The changes in cell morphology were observed by utilizing inverted microscope. A1: cell morphology under 10 × 10 magnification; A2: cell morphology under 10 × 20 magnification. (B) Apoptosis was detected by flow cytometry. The values were presented as means ± SD of three independent experiments. (C) The expression levels of caspase 3, Bax and Bcl-2 were detected by western blot. Quantitative analysis of the proteins was performed. The values were presented as means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicated significant differences from the control (DMSO) group
CSC induced ERS in BEAS-2B cells
ERS plays a vital role in the occurrence and progress of the various pulmonary disease. As shown in Fig. 3A, structural abnormalities appeared in the endoplasmic reticulum of BEAS-2B cells induced by CSC stimulation. The morphology and structure of the endoplasmic reticulum in the blank group and DMSO group were normal without apparent damage, but the structure appeared obvious swelling and expansion in the CSC group. Increased expression level of Glucose-regulated protein 78 (GRP78) is a hallmark protein of ERS. C/EBP homologous protein (CHOP) is a key protein in the process of apoptosis induced by ERS [28]. CHOP, mainly existing in the cytoplasm, is expressed very low. When cells are under stress, the expression of CHOP is greatly increased and accumulates in the nucleus [29]. To investigate whether ERS is correlated with CSC exposure on BEAS-2B cells, the expression levels of GRP78 and CHOP protein were detected. The results of western blot revealed that the expression levels of GRP78 and CHOP were increased after CSC exposure compared to DMSO group (Fig. 3B–D). These results concluded that CSC induced ERS in BEAS-2B cells after CSC treatment.
Figure 3.
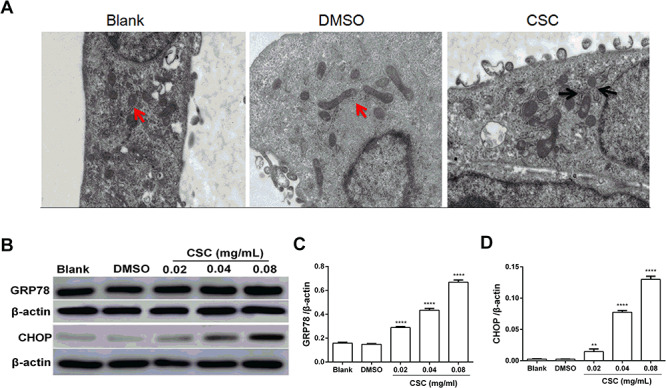
CSC induced ERS in BEAS-2B cells. (A) BEAS-2B cells treated with 0.04 mg/ml CSC for 24 h were observed by TEM. The red arrow showed normal morphology of endoplasmic reticulum, and the black arrow showed swelling and dilation of the endoplasmic reticulum. (B) The expression levels of GRP78 and CHOP were detected by western blot. (C, D) Quantitative analysis of the proteins was performed. The values were presented as means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicated significant differences from the control (DMSO) group
CSC activated autophagy in BEAS-2B cells
Autophagy is an important catabolic process to maintain cell homeostasis. To analysis whether CSC could induce autophagy, autolysosomes in BEAS-2B cells stimulated with CSC were measured. The number of autolysosomes apparently enhanced in CSC group compared to blank group and DMSO group (Fig. 4A). Microtubule-associated protein 1 light chain 3 (LC3), a pivotal protein involved in the formation of autophagosome, is an essential indicator of autophagy [30]. Non-lipidized LC3-I combining with phosphatidylethanolamine on autophagosome membrane transforms into lipidized LC3-II in the process of autophagy formation, which is considered as a sign of autophagosome formation [31–33]. So the ratio of LC3-II and LC3-I reflects the occurrence of autophagy. Western blot analysis showed that the ratio of LC3-II and LC3-I was significantly increased with the concentration of CSC increasing (Fig. 4B and C), indicating that CSC induced autophagy of BEAS-2B cells.
Figure 4.
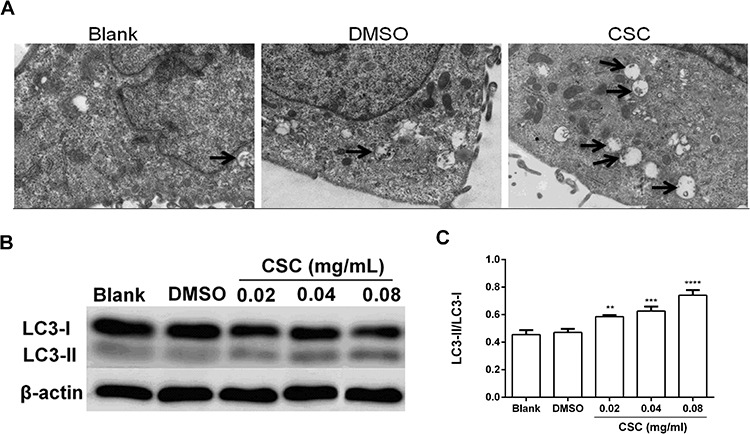
CSC induced autophagy in BEAS-2B cells. (A) BEAS-2B cells treated with 0.04 mg/ml CSC for 24 h were observed by TEM. The black arrow showed autolysosome. (B) The expression levels of LC3-I and LC3-II were detected by western blot. (C) Quantitative analysis of the proteins was performed. The values were presented as means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicated significant differences from the control (DMSO) group
CSC-induced autophagy in BEAS-2B cells was attenuated after inhibiting ERS
To explore whether autophagy is induced by ERS after CSC treatment, 4-phenylbutyrate (4-PBA), an inhibitor of ERS, was applied to restrain ERS. 4-PBA, a low molecular weight fatty acid and a non-toxic pharmacological compound, can be used as a chemical chaperone to stabilize the peptide structure, improve the folding ability of the cavity and transport abnormal proteins [34,35]. BEAS-2B cells were pretreated with 4-PBA (1, 5 and 10 mmol/l) to select optimal restrain concentration by detecting the expression level of GRP78. As shown in Fig. 5A, the expression level of GRP78 in CSC+ 5 mmol/l 4-PBA group was significantly declined compared to the CSC group. Therefore, 5 mmol/l 4-PBA was chosen as the optimal restrain concentration. The results showed that the expression levels of GRP78 and CHOP in CSC+ 4-PBA group were lower than that in the CSC group (Fig. 5B). The ratio of LC3-II and LC3-I in CSC+ 4-PBA group was declined compared to the CSC group (Fig. 5B). It can be inferred from the results that autophagy of BEAS-2B cells induced by CSC was repressed after inhibiting ERS, evincing that ERS may be involved in the induction of autophagy.
Figure 5.
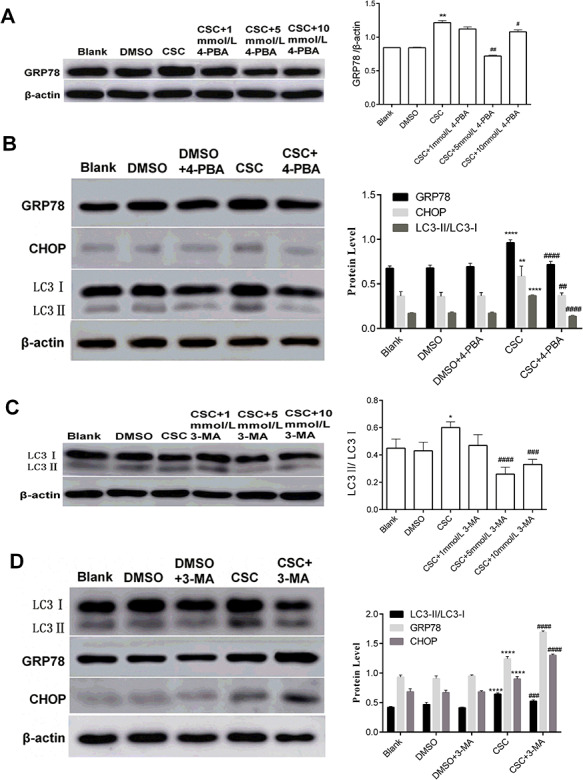
The relationship between autophagy and ERS in BEAS-2B cells stimulated by CSC. BEAS-2B cells were treated with 4-PBA or 3-MA, and followed by 0.04 mg/ml CSC treatment. (A) The expression level of GRP78 was examined by western blot after inhibitor (4-PBA) treatment. (B, D) Western blot analysis of protein expression levels (GRP78, CHOP, LC3-I, and LC3-II) after 4-PBA (5 mmol/l) or 3-MA (5 mmol/l) pretreatment and their quantitative analysis were performed. (C) The expression levels of LC3-I and LC3-II were examined by western blot after inhibitor (3-MA) treatment. The values were presented as means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicated significant differences from the control (DMSO) group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 indicated significant differences from the CSC group
CSC-induced ERS in BEAS-2B cells was enhanced after inhibiting autophagy
To further find out the relationship between autophagy and ERS, 3-methyladenine (3-MA), an inhibitor of autophagy, was used to suppress autophagy. 3-MA has been widely used in the inhibition of autophagy [36]. BEAS-2B cells were pretreated with 3-MA (1, 5 and 10 mmol/l) to select restrain concentration by detecting the expression levels of LC3-I and LC3-II protein (Fig. 5C). The result showed that the ratio of LC3-II and LC3-I in CSC+ 5 mmol/l 3-MA group was apparently dropped compared to CSC group. Therefore, 5 mmol/l 3-MA was chosen as the appropriate restrain concentration. Compared to the CSC group, the increase of the ratio of LC3-II and LC3-I was reversed after 3-MA treatment, but the expression levels of GRP78 and CHOP in CSC+ 3-MA group were improved (Fig. 5D). In short, ERS was enhanced via restraining autophagy in BEAS-2B cells after CSC treatment, indicating that ERS could be alleviated through autophagy. These results indicated that autophagy may have a negative feedback regulation on ERS.
Effects of ERS or autophagy inhibition on the CSC-induced apoptosis in BEAS-2B cells
To explore the association of ERS and autophagy on apoptosis, cell apoptosis rates were measured after 4-PBA or 3-MA treatment, respectively. Compared to CSC group, cell apoptosis rate was increased in CSC+ 4-PBA group and CSC+ 3-MA group, indicating that the inhibition of ERS or autophagy may promote apoptosis of BEAS-2B cells induced by CSC (Fig. 6A). Meanwhile, in CSC+ 4-PBA group and CSC+ 3-MA group, the expression levels of Bax and cleaved-caspase 3/pro-caspase 3 ratio were increased, but the expression levels of Bcl-2 were decreased compared to the CSC group (Fig. 6B). These results revealed that ERS and autophagy may suppress apoptosis, indicating that ERS and autophagy may display a cellular protective effect to keep cells from dying in the process of cytotoxicity induced by CSC.
Figure 6.
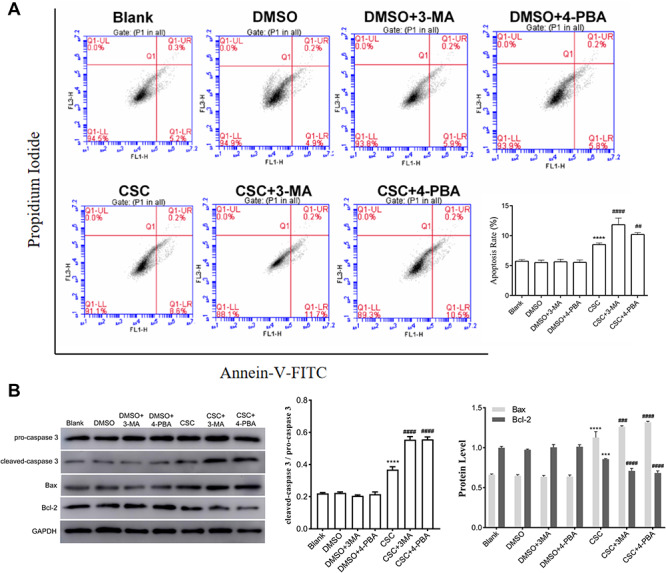
Effects of ERS or autophagy inhibition on the CSC-induced apoptosis in BEAS-2B cells. BEAS-2B cells were treated with 4-PBA or 3-MA, and then followed by 0.04 mg/ml CSC treatment. (A) Cell apoptosis was detected by flow cytometry. Quantitative analysis of apoptosis was performed. (B) The expression levels of caspase 3, Bax and Bcl-2 were detected by western blot. Quantitative analysis of the proteins was performed. The values were presented as means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicated significant differences from the control (DMSO) group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 indicated significant differences from the CSC group
Discussion
At present, studies on the relationship between ERS and autophagy in apoptosis of HBE cells caused by CS are still unclear and need to be further studied. A more explicit understanding of the relationships will be needed to find out the effects of CS on lung irritation. This study evaluated the interaction between ERS and autophagy, as well as its effects on apoptosis of BEAS-2B cells, and obtained a diagram based on the results (Fig. 7). This research provides a better understanding and experimental support on the mechanisms of pulmonary diseases stimulated by CS.
Figure 7.
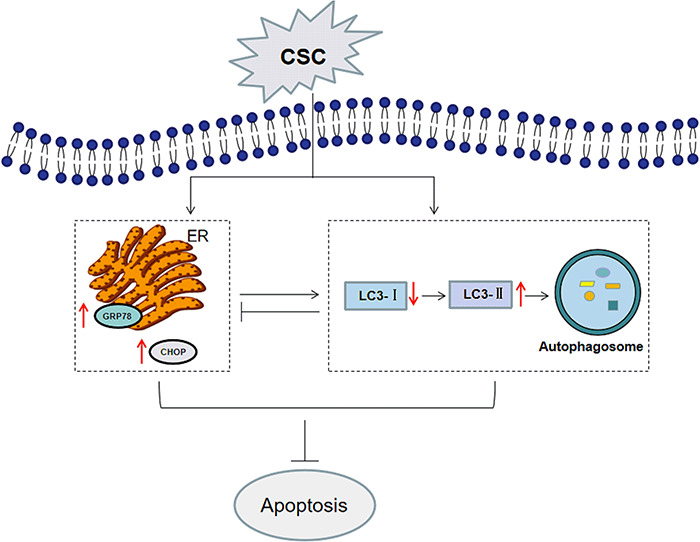
Proposed mechanisms of CSC-induced apoptosis in BEAS-2B cells. CSC treatment activated ERS and autophagy. CSC-induced ERS suppression led to the restraint of autophagy, which demonstrated that ERS induced autophagy. But CSC-induced autophagy suppression promoted ERS, which further indicated autophagy alleviated ERS in a negative feedback. CSC-induced apoptosis of BEAS-2B cells was further increased after autophagy or ERS inhibition, showing that apoptosis induced by CSC may be restrained by promoting autophagy or ERS
CS first contacts bronchial epithelial cells which is the first barrier contacting with the outside world, resisting external stimulation and maintaining airway homeostasis in the process of inhalation [37]. Caspase 3 plays a central role in the execution phase of cell apoptosis [38]. Bax belongs to the BCL2 protein family and functions as an apoptotic activator, but Bcl-2 encodes an integral outer mitochondrial membrane protein that can block the apoptotic death of some cells [39]. Mounting studies reveals that CSC induces apoptosis of various cell types, such as HBE cells, ovarian cells and human placenta choriocarcinoma cells [40–42]. This study found that cell viability was inhibited and caspase 3, Bax and Bcl-2 expressions were regulated to affect cell apoptosis after CSC stimulation in BEAS-2B cells, which was consistent with the findings of others [25,43].
ERS and autophagy are closely related to apoptosis. For one thing, some studies indicate that ERS plays a vital role in apoptosis [44]. Some people found that ERS induced the expression of HMG-CoA reductase degradation protein 1 (HRD1), an idiocratic ubiquitin ligase on the endoplasmic reticulum, to protect alveolar type II epithelial cells from apoptosis induced by CS extract [45]. One study has found that CHOP was involved in ERS-induced apoptosis in human carcinoma cells [46]. Some researchers stated that ERS and apoptosis induced by methamphetamine (MA) were via PERK-eIF2α-CHOP signaling in the chronic pulmonary injury [47]. One previous study found that cell apoptosis was prevented and the expressions of caspase 3 and Bax were inhibited after inhibiting ERS [48]. Their another research also revealed that the inhibition of ERS or CHOP-targeting small interfering RNA (siRNA) ameliorated cell apoptosis during hypoxia/reoxygenation (H/R) injury, which was different from our experimental results [49]. Our study found that CSC-induced apoptosis of BEAS-2B cells was increased after ERS inhibition, showing that apoptosis induced by CSC may be suppressed by promoting ERS to enhance cell survival. For another, autophagy plays an important role in the regulation of apoptosis [50,51]. Autophagy existing as a vital catabolic process and a kind of protective mechanism can maintain cell homeostasis by degrading organelles, proteins and macromolecules that are delivered to lysosomes. However, autophagy can inhibit apoptosis to protect cells or enhance apoptosis to promote cell death. One study found that carvedilol attenuated liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells [52]. Other findings revealed that treatment with melatonin significantly inhibited H2O2-induced retinal pigment epithelial cell damage, increased the autophagy effect and decreased the apoptotic rate [53]. Some studies found that autophagy exerted a protective effect in pulmonary infection and inflammatory diseases [54]. However, studies also showed that inhibition of autophagy inhibited apoptosis, and decreased the caspase 3 and Bax expressions caused by palmitic acid, which was not consistent with our experimental results [48]. Our study found that CSC-induced apoptosis of BEAS-2B cells was further increased after autophagy inhibition, showing that apoptosis induced by CSC may be restrained by promoting autophagy to keep cells from dying and play a cellular protective role in the whole process.
The relationship between ERS and autophagy has been a hot research field. Endoplasmic reticulum can be affected by microvariation inside and outside the cells. When cell homeostasis is out of balance, ERS appearances to activate the unfolded protein response signaling pathway to resist stress and maintain cell survival. When ERS activation, autophagy can degrade the unfolded or misfolded proteins in the endoplasmic reticulum cavity to relieve cellular stress and restore homeostasis [55]. It has been confirmed that autophagy will be up-regulated in response to ERS. So, 4-PBA was used to verify whether ERS induced autophagy of BEAS-2B cells in this study. If the concentration of CSC is too low, it has little effect on the cell viability. If the concentration of CSC is too high, it is not conducive to the follow-up experiments, so 0.04 mg/ml CSC was chosen as a proper concentration in CSC+ 4-PBA group and CSC+ 3-MA group. In this study, BEAS-2B cells were treated with 4-PBA, and followed by 0.04 mg/ml CSC treatment. The result showed that 4-PBA pretreatment attenuated the CSC-induced autophagy, indicating that the inhibition of ERS attenuated autophagy of BEAS-2B cells induced by CSC, which demonstrated that ERS was involved in the induction of autophagy. In addition, increasing findings put emphasis on the effects of autophagy on ERS. Some people have found that autophagy played a crucial role in maintaining ER and calcium homeostasis in T lymphocytes [56]. Others have stated that autophagy may play an important role in alleviating ERS [57,58]. Therefore, 3-MA was applied to inhibit autophagy of BEAS-2B cells to explore the relationship between ERS and autophagy. We found that the expression levels of GRP78 and CHOP were improved after 3-MA pretreatment, showing that the inhibition of autophagy promoted the expressions of ERS-related proteins, which further indicated that autophagy alleviated ERS. One study has found that autophagy was inhibited after inhibiting the ERS, which was consistent with our finding. But the expression levels of ERS-related molecules after inhibiting autophagy were different from our results. The expression of CHOP was increasing, but the expression of GRP78 was dropped, which was inconsistent with the results of this study [26].
The underlying mechanisms between ERS and autophagy in apoptosis are very complex, and more studies are needed. Some people have found that cyclosporine-induced both apoptosis and autophagy in malignant glioma cells via induction of ER stress. They found that cell death increased after inhibiting autophagy, indicating that ERS-induced autophagy could reduce cell damage and play a protective role [59]. And other researchers found that ERS was attenuated and cell apoptosis was ameliorated after activating autophagy, while suppressed autophagy could aggravated ERS and apoptosis, which was consistent with our results [49]. However, autophagy induced by ERS not only plays a protective role, but also promotes cell death in previous studies [60,61]. One study has revealed that autophagic cell death in glioma cells was found after ERS [61]. Therefore, the relationship between autophagy and ERS in the apoptosis remains to be more studied. In this study, we found that ERS induced autophagy and inhibited apoptosis of BEAS-2B cells after CSC treatment, and autophagy relieved ERS and inhibited apoptosis. These results demonstrated that ERS can inhibit apoptosis by activating autophagy, and autophagy could negatively regulate ERS, which was consistent with others [26,62].
Given all that, CSC-induced autophagy of BEAS-2B cells was mitigated after inhibiting ERS, while CSC-induced ERS was elevated after inhibiting autophagy. Simultaneously, cell apoptosis was increased when ERS or autophagy were repressed, respectively. In conclusion, results from our work reveal that ERS may repress apoptosis of BEAS-2B cells induced by CSC via activating autophagy, but autophagy relieves ERS in a negative feedback, which may play a cellular protective effect.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81172717 and 81402712).
Contributor Information
Qi Yu, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Sa Yang, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Zhongqiu Li, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Yonghang Zhu, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Zhenkai Li, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Jiatong Zhang, Department of Disease Control and Prevention, Hospital of Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Chunyang Li, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Feifei Feng, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Wei Wang, Department of Occupational and Environmental Health, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Qiao Zhang, Department of Toxicology, College of Public Health, Zhengzhou University, 100 Kexue Ave, Zhongyuan District, Zhengzhou 450001, China.
Conflict of interest statement
There are no conflicts of interest to declare.
References
- 1. Bierut LJ 2018 langley award for basic research on nicotine and tobacco: bringing precision medicine to smoking cessation. Nicotine Tob Res 2020;22:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Courtney R The health consequences of Smoking-50 years of progress: a report of the surgeon general. 2014 Drug Alcohol Rev 2015;34:694–5. [Google Scholar]
- 3. Aghapour M, Raee P, Moghaddam SJ et al. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol 2018;58:157–69. [DOI] [PubMed] [Google Scholar]
- 4. Dusautoir R, Zarcone G, Verriele M et al. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J Hazard Mater 2020;401:123417. [DOI] [PubMed] [Google Scholar]
- 5. Sculier JP, Berghmans T, Meert AP. Update in lung cancer and mesothelioma 2009. Am J Respir Crit Care Med 2010;181:773–81. [DOI] [PubMed] [Google Scholar]
- 6. Kuwano K Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol 2007;4:419–29. [PubMed] [Google Scholar]
- 7. Shetty SK, Tiwari N, Marudamuthu AS et al. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol 2017;187:1016–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006;129:1673–82. [DOI] [PubMed] [Google Scholar]
- 9. Nemmar A, Al-Salam S, Yuvaraju P et al. Chronic exposure to water-pipe smoke induces alveolar enlargement, DNA damage and impairment of lung function. Cell Physiol Biochem 2016;38:982–92. [DOI] [PubMed] [Google Scholar]
- 10. Lakshmanan AP, Thandavarayan RA, Palaniyandi SS et al. Modulation of AT-1R/CHOP-JNK-Caspase12 pathway by olmesartan treatment attenuates ER stress-induced renal apoptosis in streptozotocin-induced diabetic mice. Eur J Pharm Sci 2011;44:627–34. [DOI] [PubMed] [Google Scholar]
- 11. Alam S, Li ZJ, Atkinson C et al. Alpha(1)-antitrypsin confers a Proinflammatory phenotype that contributes to chronic obstructive pulmonary disease. Am J Resp Crit Care 2014;189:909–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotamisligil GS Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez A, Ordonez R, Reiter RJ et al. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res 2015;59:292–307. [DOI] [PubMed] [Google Scholar]
- 14. Shen SY, Zhang Y, Wang Z et al. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci 2014;10:212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith M, Wilkinson S. ER homeostasis and autophagy. Essays Biochem 2017;61:625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ktistakis NT ER platforms mediating autophagosome generation. BBA-Mol Cell Biol L 2020;1865:158433. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Zhu G, Gou X et al. Negative feedback loop of autophagy and endoplasmic reticulum stress in rapamycin protection against renal ischemia-reperfusion injury during initial reperfusion phase. FASEB J 2018;17:fj201800299R. [DOI] [PubMed] [Google Scholar]
- 18. Gong J, Wang XZ, Wang T et al. Molecular signal networks and regulating mechanisms of the unfolded protein response. J Zhejiang Univ Sci B 2017;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu P, Xue J, Zhang ZJ et al. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis 2017;8:3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang X, Yuan Y, Jiang L et al. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: involvement of PARK2-dependent mitophagy. Autophagy 2014;10:1801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hou W, Han J, Lu CS et al. Autophagic degradation of active caspase-8 a crosstalk mechanism between autophagy and apoptosis. Autophagy 2010;6:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomar D, Prajapati P, Sripada L et al. TRIM13 regulates caspase-8 ubiquitination, translocation to autophagosomes and activation during ER stress induced cell death. Biochim Biophys Acta 2013;1833:3134–44. [DOI] [PubMed] [Google Scholar]
- 23. Deegan S, Saveljeva S, Logue SE et al. Deficiency in the mitochondrial apoptotic pathway reveals the toxic potential of autophagy under ER stress conditions. Autophagy 2014;10:1921–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Zhang Y, Meng L et al. LncRNA-ENST00000501520 promotes the proliferation of malignant-transformed BEAS-2B cells induced with coal tar pitch mediated by target genes. Environ Toxicol 2019;34:869–77. [DOI] [PubMed] [Google Scholar]
- 25. Park EJ, Park YJ, Lee SJ et al. Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicol Lett 2019;303:55–66. [DOI] [PubMed] [Google Scholar]
- 26. He BM, Chen Q, Zhou DB et al. Role of reciprocal interaction between autophagy and endoplasmic reticulum stress in apoptosis of human bronchial epithelial cells induced by cigarette smoke extract. IUBMB Life 2019;71:66–80. [DOI] [PubMed] [Google Scholar]
- 27. Li Z, Wang W, Meng L et al. Identification and analysis of key lncRNAs in malignant-transformed BEAS-2B cells induced with coal tar pitch by microarray analysis. Environ Toxicol Pharmacol 2020;79:103376. [DOI] [PubMed] [Google Scholar]
- 28. Marciniak SJ Endoplasmic reticulum stress in lung disease. Eur Respir Rev 2017;26:170018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 2004;11:381–9. [DOI] [PubMed] [Google Scholar]
- 30. Levine B Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 2005;120:159–62. [DOI] [PubMed] [Google Scholar]
- 31. Martinez J, Malireddi RK, Lu Q et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol 2015;17:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Martens S, Nakamura S, Yoshimori T. Phospholipids in autophagosome formation and fusion. J Mol Biol 2016;428:4819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reggiori F, Ungermann C. Autophagosome maturation and fusion. J Mol Biol 2017;429:486–96. [DOI] [PubMed] [Google Scholar]
- 34. Ayala P, Montenegro J, Vivar R et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol 2012;92:97–104. [DOI] [PubMed] [Google Scholar]
- 35. Kolb PS, Ayaub EA, Zhou W et al. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol 2015;61:45–52. [DOI] [PubMed] [Google Scholar]
- 36. Heckmann BL, Yang X, Zhang X, Liu J. The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br J Pharmacol 2013;168:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D'Anna C, Cigna D, Di Sano C et al. Exposure to cigarette smoke extract and lipopolysaccharide modifies cytoskeleton organization in bronchial epithelial cells. Exp Lung Res 2017;43:347–58. [DOI] [PubMed] [Google Scholar]
- 38. Lakhani SA, Masud A, Kuida K et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 2006;311:847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alves S, Neiri L, Chaves SR et al. N-terminal acetylation modulates Bax targeting to mitochondria. Int J Biochem Cell Biol 2018;95:35–42. [DOI] [PubMed] [Google Scholar]
- 40. Song C, Luo B, Gong L. Resveratrol reduces the apoptosis induced by cigarette smoke extract by upregulating MFN2. PLoS One 2017;12:e0175009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim CW, Go RE, Hwang KA et al. Effects of cigarette smoke extracts on apoptosis and oxidative stress in two models of ovarian cancer in vitro. Toxicol in vitro 2018;52:161–9. [DOI] [PubMed] [Google Scholar]
- 42. Lee HM, Choi KC. Cigarette smoke extract and isoprene resulted in the induction of apoptosis and autophagy in human placenta choriocarcinoma JEG-3 cells. Environ Toxicol 2018;33:178–90. [DOI] [PubMed] [Google Scholar]
- 43. Ji M, Zhang Y, Li N et al. Nicotine component of cigarette smoke extract (CSE) decreases the cytotoxicity of CSE in BEAS-2B cells stably expressing human cytochrome P450 2A13. Int J Environ Res Public Health 2017;14:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Puthalakath H, O'Reilly LA, Gunn P et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007;129:1337–49. [DOI] [PubMed] [Google Scholar]
- 45. Tan SX, Jiang DX, Hu RC et al. Endoplasmic reticulum stress induces HRD1 to protect alveolar type II epithelial cells from apoptosis induced by cigarette smoke extract. Cell Physiol Biochem 2017;43:1337–45. [DOI] [PubMed] [Google Scholar]
- 46. Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 2004;279:45495–502. [DOI] [PubMed] [Google Scholar]
- 47. Gu YH, Wang Y, Bai Y et al. Endoplasmic reticulum stress and apoptosis via PERK-eIF2alpha-CHOP signaling in the methamphetamine-induced chronic pulmonary injury. Environ Toxicol Pharmacol 2017;49:194–201. [DOI] [PubMed] [Google Scholar]
- 48. Yang L, Guan G, Lei L et al. Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperones 2018;23:1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan G, Yang L, Huang W et al. Mechanism of interactions between endoplasmic reticulum stress and autophagy in hypoxia/reoxygenationinduced injury of H9c2 cardiomyocytes. Mol Med Rep 2019;20:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang K Autophagy and apoptosis in liver injury. Cell Cycle 2015;14:1631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakahira K, Porras MAP, Choi AMK. Autophagy in pulmonary diseases. Am J Resp Crit Care 2016;194:1196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meng D, Li Z, Wang G et al. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed Pharmacother 2018;108:1617–27. [DOI] [PubMed] [Google Scholar]
- 53. Chang CC, Huang TY, Chen HY et al. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid Med Cell Longev 2018;2018:9015765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang K, Chen Y, Zhang P et al. Protective features of autophagy in pulmonary infection and inflammatory diseases. Cells 2019;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010;40:280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jia W, Pua HH, Li QJ et al. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J Immunol 2011;186:1564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yin JJ, Li YB, Wang Y et al. The role of autophagy in endoplasmic reticulum stress-induced pancreatic beta cell death. Autophagy 2012;8:158–64. [DOI] [PubMed] [Google Scholar]
- 58. Fan T, Huang Z, Wang W et al. Proteasome inhibition promotes autophagy and protects from endoplasmic reticulum stress in rat alveolar macrophages exposed to hypoxia-reoxygenation injury. J Cell Physiol 2018;233:6748–58. [DOI] [PubMed] [Google Scholar]
- 59. Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B. Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine a-induced cell death. Oncogene 2013;32:1518–29. [DOI] [PubMed] [Google Scholar]
- 60. Ding WX, Ni HM, Gao W et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 2007;282:4702–10. [DOI] [PubMed] [Google Scholar]
- 61. Rubiolo JA, Lopez-Alonso H, Martinez P et al. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell Signal 2014;26:419–32. [PubMed] [Google Scholar]
- 62. Boya P, Gonzalez-Polo RA, Casares N et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005;25:1025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


