Abstract
Background
The genotoxicity of cisplatin (CP) as a platinum-based antineoplastic agent due to its oxidative stress induction was well known. In this research, we examined 4-hydroxychalcone (4-HCH) as a natural food that presents flavonoid effects on reactive oxygen species (ROS) production and CP-induced in vivo genotoxicity.
Method and materials
Cytotoxicity of CP and 4-HCH was measured on human embryonic kidney 293 cells with MTT assay. Then, intracellular ROS content at IC50 concentration of CP was measured with 2′,7′-dichlorofluorescein diacetate (DCFDA) dye. Finally, 4-HCH was administered intraperitoneally at 10 and 40 mg/kg/BW doses as a pre and post-treatment schedule in a mice model of CP genotoxicity (7 mg/kg). Acridine-orange-stained bone marrow cells were quantified for micronucleus presence examination.
Results
The calculated IC50 of CP and 4-HCH were reported around 19.4 and 133.6 μM, respectively, on HEK293 cells. Also, it was observed that 4-HCH at 0.2, 2 and 10 μM concentrations did not show obvious cytotoxicity. The fluorimetry confirmed that pre-treatment with 10 μM and co-treatment with 2 μM of 4-HCH could attenuate the CP-induced ROS production (P < 0.05 and P < 0.01, respectively). Also, the lowest micronucleated cells were seen in 10 mg/kg 4-HCH-treated group after CP exposure (39 ± 7.9, P < 0.0001).
Discussion
Our results demonstrated the antigenotoxic action of 4-HCH in CP-treated mice bone marrow cells for the first time in both concentrations of 10 and 40 mg/kg especially in the form of co-treatment. Further studies required clinical application of this compound in a combination of CP to attenuate the normal cells’ genotoxicity side effects.
Keywords: 4-hydroxychalcone, cisplatin, micronucleus test, oxidative stress, DCFDA
Introduction
cis-Diamminedichloroplatinum(II) (CP), the first platinum compound approved by FDA in 1978, is one of the most potent agents available for cancer chemotherapy [1]. It is used in various types of cancer treatment regimen including head and neck, cervical, breast, lung, ovarian, gastric and bladder with around 90% cure rate [2]. However, using the platinum-based agents is often associated with several toxic side effects, including, hepatotoxicity [3], cardiotoxicity [4]and more importantly nephrotoxicity [5].
The molecular mechanism of CP (CP) toxicity is associated with its biological activation by the sequential thermal exchange of two chloride groups with hydroxyl ligands [6]. After activation, CP that acts as a potent nucleophilic agent, which attacks DNA targets leading to the formation of inter/intra cross-links [7], protein adducts and strand breaks, [8] resulted in inhibition of replication, transcription and cell death [9,10].
On the other hand, oxidative stress is also involved in CP-induced genotoxicity. It is capable of generating reactive oxygen species (ROS) such as superoxide and hydroxyl radicals [11] and suppressed antioxidant defensive system including enzymes, non-enzymatic molecules and reduced glutathione [12,13]. Several normal tissue malignancies have been reported based on the broad mechanism of action and genotoxic activity of this antitumor drug.
Chalcones are naturally presented aromatic ketones with an α,β-unsaturated carbonyl group between aryl rings [14]. This class of open-chain flavonoids is present in grains, roots, fruits, tea, vegetables, flowers and wines, which are routinely used [15]. These compounds have been suggested to have a wide range of pharmacological effects, such as antiosteoporosis, anticonvulsant, anti-inflammatory, antimalarial, antimicrobial, larvicidal, antimutagenic and anticancer with a tyrosinase inhibitory activity [16–21]. In addition, the potent antioxidant activity due to the presence of the reactive keto-vinylene group in their structure has also been shown in [22].
Hydroxychalcones are compounds with two benzene or phenol aromatic rings linking to an unsaturated side chain. In the past decades, a large number of reports have been published on the beneficial effects of hydroxy chalcones especially due to their antioxidant and antitumor activities [23,24]. 4-HCH is an alpha, beta-unsaturated ketone with the core structure of chalcone and one hydroxyl substituent on the four positions of the A ring. Also, it can inhibit the angiogenesis by affecting vascular endothelial growth factor and basic fibroblast growth factor intracellular signaling pathways [25]. These agents can act as chemopreventive and chemotherapeutic drugs with several different mechanisms [23,24,26].
The aim of the present study was to investigate the administration effects of 4-hydroxy chalcone on CP-induced genotoxicity. For this purpose, we performed the micronucleus assay in mice treated with CP and 4-hydroxy chalcone bone marrow cells.
Material and Methods
Chemicals
4-Hydroxy chalcone was purchased from Indofine (Hillsborough, NJ, USA). CM-H2DCFDA was supplied from Invitrogen (Waltham, Massachusetts, USA), CP and all the other commercially available reagents were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
Cell culture
Human embryonic kidney 293 cells or HEK293 cells were obtained from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran) and were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and antibiotics (100 μg/ml streptomycin, 100 U/ml penicillin G) at 37°C in a humidity-controlled incubator with 5% CO2. The cells were subcultured at 50–80% confluences using 0.05% trypsin-0.02% EDTA [27].
Cell survival
This assay is based on a direct relationship between the viable cells and absorbance provided by the enzymatic reduction phenomenon of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye. The effect of CP and 4-hydroxy chalcone on cell viability was evaluated by MTT assay as performed earlier [28]. Selective doses viz. 0.2, 2, 10, 20, 30, 50, 100 and 200 μM of CP and 4-hydroxy chalcone were prepared in the final volume of 100 μL media. After 48 h of exposure, 10 μL of MTT solution (5 mg/ml stock solution) was added in each well and re-incubated for 3 h at 37°C until formazan blue crystal developed. Media were discarded from each well, and 100 μL of DMSO was added to dissolve formazan crystals for 10 min at 37°C. The absorbance was recorded at 540 nm by ELISA microplate reader and relative percentage cell viability was calculated.
ROS measurement
Cytosolic reactive oxygen species (ROS) was evaluated using the 2′,7′-dichlorofluorescein diacetate (DCFDA) dye. The cells in positive-control wells (only CP treatment), pre-treatment (exposed to the safe dose of 4-HCH 18 h, before CP exposure) and co-treatment (co-exposure to CP and 4-HCH) were washed after 60 min, incubated with DCFDA for 30 minute and processed as shown in Fig. 1. The fluorescent intensity was analyzed using the Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, USA) at 485 nm excitation and 528 nm emission. Finally, the relative florescence unit (CFI) was calculated based on the unstained cells and positive-control florescent emission [29,30].
Figure 1.
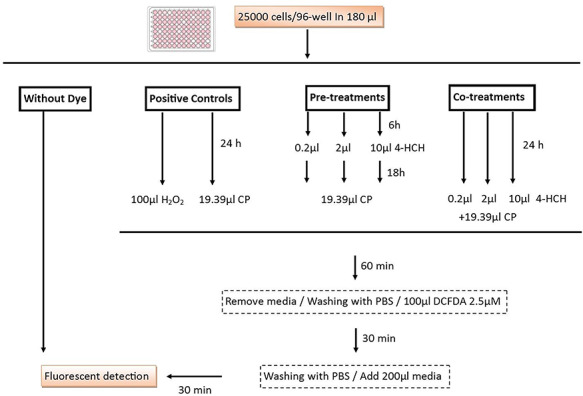
Graphical abstract representing the cells preparation for fluorescent detection of intracellular reactive oxygen species using the 2′,7′-dichlorofluorescein diacetate (DCFDA) dye. CP (CP) and hydrogen peroxide were chosen as a positive control and HEK293 cells were pre- and post-treated with 0.2, 2 and 10 μM of 4-HCH.
Animal exposure
Healthy, young male adult (8–10 weeks) Swiss mice, weighing 18–22 g, obtained from the animal facilities of the Isfahan University of Medical Science, Faculty of Pharmacy and Pharmaceutical Sciences, were brought to the laboratory five days before the experiments and housed in plastic cages (40 × 30 × 16 cm), lined with wood shavings, kept at 25 ± 3°C and 55 ± 10% humidity, with a 12-h light–dark cycle. Standard food pellets and water were provided ad libitum. All animal studies were carried out according to the approved guidelines of the Iranian Institutional Animal Ethics Committee (IR.MUI.RESEARCH.REC.1397.158).
As shown in Fig. 2 Thirty-five mice were randomly divided into seven groups. The animals in Group 1 received phosphate buffer saline as vehicle intraperitoneally (IP) for five consecutive days, while Group 2 received 7 mg/kg/BW CP in a single IP administration (negative control). Group 3 received 40 mg/kg/BW 4-HCH for five consecutive days. The animals in Groups 4 and 5 received 10 and 40 mg/kg BW 4-hydroxy chalcone, respectively, for five consecutive days (pretreatment). On the last day, the animals in both groups received a single dose of 7 mg/kg BW CP IP. The animals in Groups 6 and 7 were treated with a single dose of 7 mg/kg BW CP IP. After 6 and 12 h, the animals in Group 6 received 10 mg/kg BW 4-hydoxychalcone, whereas the animals in Group 7 received 40 mg/kg BW 4-hydroxy chalcone (post-treatment). All the animals were euthanized by cervical dislocation after ketamine–xylazine injection (100–10 mg/kg).
Figure 2.
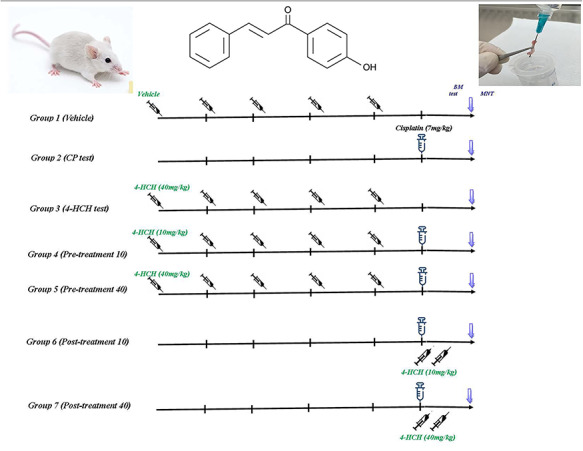
Animal experiment was done with seven groups consisting five male mice in each group. CP (CP) as a genotoxic drug (7 mg/kg/bw) administrated intraperitoneally at day 5 except in Groups 1 and 3. Group 1 received vehicle for five consecutive days. Groups 3–7 received 4-HCH (10 and 40 mg/kg/bw) intraperitoneally as a pre- and post-treatment schedule. All of subjects were euthanized at the end of day 6 and both femoral bone marrows were used for micronucleus assay.
Bone marrow micronucleus test
The micronucleus test was done according to a previously published protocol [31]. Briefly, 5 μL of the femoral of bone marrow cells was flushed out with fetal bovine serum and smeared on an acridine orange-coated glass slides and stored at room temperature for 30 min. Five slides per each animal were coded for blind count and analysis. Two thousand polychromatic erythrocytes per animal were observed using an Olympus fluorescent microscope (Olympus, Tokyo, Japan) with blue excitation filter. The frequencies of the micronucleated polychromatic erythrocytes (MNPCE) and the normochromatic erythrocytes were counted based on the OECD 474 guideline in vivo MNT assay protocol.
Statistical analysis
The statistical significance of the data was tested by using one-way analysis of variance test (ANOVA) with Tukey’s multiple comparison post hoc test using Graph Pad Prism 8 (Graph Pad Software, Inc., CA, USA). Values of P ≤ 0.05 were considered as statistically significant.
Results
Cytotoxicity assay
The effect of different concentration of CP on cell survival in HEK293 cells are shown in Fig 3a. The percent cell survival data indicate that at 0.2 and 2 μM concentrations. CP did not have any cytotoxic effects on HEK293 cells as compared to the control group. As CP concentration was increased (30, 50, 100 and 200 μM), further dropped in the percent of cell survival was observed as compared to the control group (P < 0.01). The half-maximal inhibitory concentration (IC50) of CP in our experiment was obtained at 19.4 μM concentration for further studies.
Figure 3.
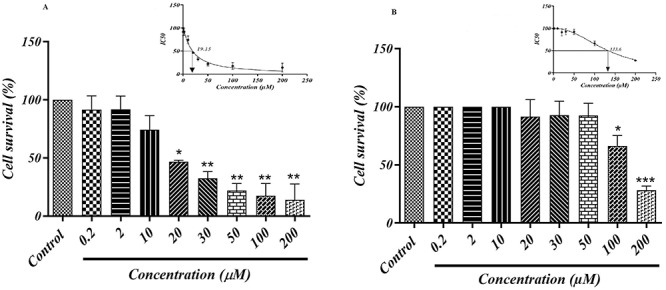
MTT assay of (A) CP and (B) 4-HCH in HEK293 cells. Values are expressed as means ± SD from three independent experiments. *Significant difference compared to the control group (P < 0.05), **(P < 0.01) and ***(P < 0.001). IC50 value for each item was calculated with GraphPad prism software.
It was observed that 4-HCH at 0.2, 2 and 10 μM concentration did not have any cytotoxic effect on HEK293 cells, so we selected these three optimum doses for further studies. However, significant cell reduction was observed at 100 μM (P < 0.05) and 200 μM (P < 0.001) as compared to the control group; with this regards, the IC50 of 4-HCH was obtained around 133.6 μM (Fig. 3b).
Intracellular ROS
The measured intracellular ROS was presented as CFI values in Fig. 4. As it was shown, CP at IC50 concentrations (19.15 μM) significantly increased free radical production at the first hour of cellular exposure in comparison with the normal control (P < 0.0001). On the whole, adding the 4-HCH to cells showed a dose-dependent reduction in CFI value; however, the statistically significant effects were observed at pre-treatment with 10 μM (P < 0.05) and co-treatment with 2 and 10 μM of 4-HCH (P < 0.01). In general, the stronger protective effect against produced ROS was observed in co-treated groups with 4-HCH.
Figure 4.
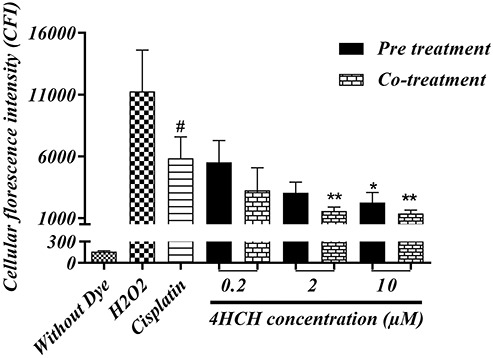
HEK293 intracellular ROS measurement via 2′,7′-dichlorofluorescein diacetate dye. Cells were exposed to the H2O2 as a positive ROS inducer control. CP at an IC50 concentration (19.15 μM) was used in mentioned groups. All values are presented as mean ± SD from three independent experiments. *Significant difference compared to the CP (positive control) group (P < 0.05), **(P < 0.01). # CP group as compared to the normal cells **(P < 0.0001).
Micronucleus test
The ratio of micronucleated polychromatic erythrocytes (MNPCE) in 2000 polychromatic erythrocytes (PCE) was shown in Fig. 5. As expected, the negative-control group (Vehicle) had low MNPCE frequency (15.96 ± 5.32), and the CP-treated control group demonstrated a significant jump in MNPCE frequency as compared to the negative control group (125.6 ± 6.28, P < 0.0001), which is supporting the genotoxicity of the CP with single 7-mg/kg IP dose administration. However, measured MNPCE cells in 4-HCH-treated subjects confirmed the absence of obvious genotoxic effect at 40 mg/kg IP administration (21.85 ± 4.52). As it was shown in pre- and post-treated groups, the frequency of micronucleated cells was significantly decreased in comparison with CP control group (P < 0.0001), in which the lowest MN cells were seen in 10-mg/kg-treated subjects with 4-HCH after CP exposure (39 ± 7.9).
Figure 5.
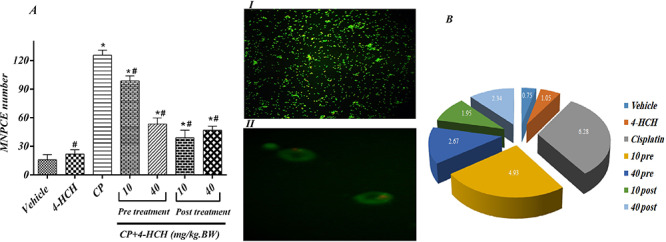
Effects of treatments with different doses of the 4-HCH (4HCH) on the number of MNPCE (A) and the frequency of MNPCE per 2000 PCE in bone marrow cells of mice (B) were shown. Orange–red micronuclei were indicated with white arrow in part I with 10X and II with 100X magnification.
CP: received 7 mg/kg BW CP. All drugs were administered intraperitoneally (IP). *Significant difference compared to the vehicle group (P < 0.001) and # significance in comparison with CP positive control group (P < 0.001).
Discussion
CP is an antineoplastic agent, which is a prime choice for the treatment of different cancers such as head and neck, ovarian, cervical and lung through platinum-based chemotherapy. Its exposure is frequently associated with increased free radicals and subsequently different types of damages to a cellular genetic material, which can cause subsequent malignancies in normal cells [32]. It has been proved that CP undergoes aquation inside the cells, and the platinum atom covalently binds to the N7 position of purines nucleotides of DNA leading to mutation, intra-strand and inter-strand cross-linking and strand breaks [33]. Charles et al. suggested that some natural products such as arbutin, chalcones, epicatechin, myricetin and sakuranetin induced non-homologous end-joining and DNA double-strand break repair, which is the major pathway used by higher eukaryotic cells to repair double-strand breaks and genomic instabilities [24].
The MTT assay showed that CP can reduce the HEK293 cells survival in a concentration-dependent manner. About 80% of cells were destroyed after 50 μM CP exposure, which confirmed the high cytotoxic potency of CP in HEK293 cells. Our finding is in collaboration with previous studies on CP cytotoxicity on various cell lines [34,35]. Which previous works suggested that CP cytotoxicity is probably because of increased free radicals such as ROS generation, induction of endoplasmic reticulum (ER) stress, inflammatory response and autophagy activation [36].
Flavonoids such as chalcones and hydroxy chalcones have been showed to be remarkable potential anticancer agents due to their ability to scavenge free radicals and so have the capacity to inactivate carcinogens [37]. 4-Hydroxy chalcone is one of the most potent chalcones in terms of free radicals scavenging based on its chemical structure and also is one of the safest one due to its slight cytotoxic properties [38]. It was known that chalcones with a benzene B-ring and polyphenol A-ring may have a less cytotoxic effect than those with phenols on both A- and B-rings. In our study, the absence of significant cytotoxic effect of 4-hydroxy chalcone in HEK293 cells up to 100 μM concentration was observed and it was suggested that this natural occurring flavonoid is safe in selected optimum doses for further studies.
Berndtsson et al. (2007) found that CP can induce DNA damage, whereas superoxide anion formation reaches its highest level after 3 h of administration, suggesting that the protective agent must be used at the same time in order to neutralize the generated free radicals [39]. It was previously observed that maltol as a natural antioxidant could protect HEK293 cells from cytotoxicity, ROS generation and apoptosis induced by CP through the AMPK-mediated PI3K/Akt and p53 signaling pathways in form of co-treatment [40]. Also, it was reported that CP-induced oxidative stress in HEK293 and cholangiocarcinoma cells can completely be suppressed by N-acetylcysteine as a precursor of glutathione and superoxide scavenger in a form of co-treatment [41].
One of the most important mechanisms of tumor induction is free-radicals-mediated damages to the cells such as DNA mutation, chromosomal aberrations and formation of micronuclei, which can lead to cancerous cells, aging or cell death [42]. It was observed that increased intracellular ROS due to CP exposure was attenuated with 4-HCH at concentrations as low as 2 μM in form of co-treatment, also in both pretreatment and co-treatment of 10 μM concentration. This finding is in accordance with the previous studies, which were reported that lower concentrations of chalcones including phloretin or quercetin can significantly reduce ROS generation due to CP exposure [43]. It was suggested that chemical keto-vinylene reactive group in the backbone structure of chalcones and its derivatives such as 4-hydroxy chalcone can neutralize ROS and their toxic effect to cells [44]. On the other hand, Perjési et al. found that Quercetin and some synthetic chalcones have the most antioxidant activity at the first 10 min of incubation and this activity decrease significantly after 240 min of incubation [45]. These time-basing evidence may justify why in our experiment we observed a stronger protective effect of co-treatment groups than pre-treatment groups of 4-HCH in order to reverse ROS generation induced by CP.
In addition to free radical’s generation, CP-produced platinated tubulin in the cells, which could not assemble into microtubules and subsequently cause strong tubulin aggregation, resulted in micronucleus formation [46]. As it was expected in accordance with the previous discussed findings, CP showed a strong genotoxic effect through a significant increase in the frequency of bone marrow MNPCE. On the other hand, recent findings showed that chalcones, 4-hydroxyderricin, and xanthoangelol and also sulfonamide chalcone blocked the formation of micronucleus induced by genotoxic agents [47]. It was observed that 4-HCH treatment even though in a lower concentration such as 10 mg/kg.BW 24 h after CP exposure could significantly decrease the induced genotoxicity.
Conclusion
Our results demonstrated the antigenotoxic action of 4-hydroxy chalcone in CP-treated mice bone marrow cells for the first time in both concentrations of 10 and 40 mg/kg especially in the form of co-treatment. Further studies required clinical application of this compound in a combination of CP to attenuate the normal cells’ genotoxicity side effects.
Highlights
The measured IC50 of CP and 4-HCH were 19.4 μM 4 and 133.6 μM on HEK293 cells.
2 μM of 4-HCH could attenuate the CP-induced ROS production.
Treatment with 10 mg/kg of 4-HCH significantly lowered the CP-induced MN cells.
Time dependency of antioxidant treatment in CP genotoxicity.
Acknowledgments
We thank our colleagues specially Dr. Mehdi Eftekhari who helped us in this research.
Conflict of interest statement
The authors state that they have no conflict of interests.
Funding
This work was supported by the Isfahan University of Medical Sciences and Pharmaceutical Research Center (grant number 397312).
References
- 1. Kelland L The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573–84. [DOI] [PubMed] [Google Scholar]
- 2. Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans 2010;39:8113–27. [DOI] [PubMed] [Google Scholar]
- 3. Al-Majed AA Carnitine deficiency provokes cisplatin-induced hepatotoxicity in rats. Basic Clin Pharmacol Toxicol 2007;100:145–50. [DOI] [PubMed] [Google Scholar]
- 4. Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA et al. Events the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol Res 2006;53:278–86. [DOI] [PubMed] [Google Scholar]
- 5. De Jongh FE, Van Veen RN, Veltman SJ et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer 2003;88:1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenberg B, Vancamp L, Trosko JE, Mansour VH. Platinum Compounds: a New Class of Potent Antitumour Agents. Nature 1969;222:385–6. [DOI] [PubMed] [Google Scholar]
- 7. Furuta T, Ueda T, Aune G et al. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res 2002;62:4899–902. [PubMed] [Google Scholar]
- 8. Woźniak K, Błasiak J. Recognition and repair of DNA-cisplatin adducts. Acta Biochim Pol 2002;49:583–96. [PubMed] [Google Scholar]
- 9. Mello JA, Lippard SJ, Essigmann JM. DNA Adducts of cis-Diamminedichloroplatinum(II) and Its Trans Isomer Inhibit RNA Polymerase II Differentially in Vivo. Biochemistry 1995;34:14783–91. [DOI] [PubMed] [Google Scholar]
- 10. Attia SM Mutat Res Toxicol Environ Mutagen 2012;741:22–31. [DOI] [PubMed] [Google Scholar]
- 11. Yoshida M, Fukuda A, Hara M et al. Melatonin prevents the increase in hydroxyl radical-spin trap adduct formation caused by the addition of cisplatin in vitro. Life Sci 2003;72:1773–80. [DOI] [PubMed] [Google Scholar]
- 12. Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol 2010;62:45–52. [DOI] [PubMed] [Google Scholar]
- 13. Antunes LMG, Araújo MCP, Darin JDC, Maria de Lourdes PB. Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat Res Toxicol Environ Mutagen 2000;465:131–7. [DOI] [PubMed] [Google Scholar]
- 14. Goodarzi M, Saeys W, De Araujo MCU et al. Binary classification of chalcone derivatives with LDA or KNN based on their antileishmanial activity and molecular descriptors selected using the Successive Projections Algorithm feature-selection technique. Eur J Pharm Sci 2014;51:189–95. [DOI] [PubMed] [Google Scholar]
- 15. Mondal R, Das Gupta A, Mallik AK. Synthesis of flavanones by use of anhydrous potassium carbonate as an inexpensive, safe, and efficient basic catalys. Tetrahedron Lett 2011;52:5020–4. [Google Scholar]
- 16. Gutierrez RMP, Muñiz-Ramirez A, Sauceda JV. The potential of chalcones as a source of drugs. African J Pharm Pharmacol 2015;9:237–57. [Google Scholar]
- 17. Seo WD, Ryu YB, Curtis-Long MJ et al. Evaluation of anti-pigmentary effect of synthetic sulfonylamino chalcone. Eur J Med Chem 2010;45:165–79. [DOI] [PubMed] [Google Scholar]
- 18. Torigge T, Arisawa M, Itoh S et al. Anti-mutagenic chalcones: Antagonizing the mutagenicity of benzo(a)pyrene on Salmonella typhimurium. Biochem Biophys Res Commun 1983;112:833–42. [DOI] [PubMed] [Google Scholar]
- 19. Nerya O, Musa R, Khatib S et al. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004;65:1389–95. [DOI] [PubMed] [Google Scholar]
- 20. Xue Y, Zheng Y, Zhang L et al. Theoretical study on the antioxidant properties of 2'-hydroxychalcones: H-atom vs. electron transfer mechanism. J Mol Model 2013;19:3851–62. [DOI] [PubMed] [Google Scholar]
- 21. Edenharder R, Grünhage D. Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. Mutat Res Toxicol Environ Mutagen 2003;540:1–18. [DOI] [PubMed] [Google Scholar]
- 22. Anto RJ, Sukumaran K, Kuttan G et al. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett 1995;97:33–7. [DOI] [PubMed] [Google Scholar]
- 23. Orlikova B, Tasdemir D, Golais F et al. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr 2011;6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charles C, Nachtergael A, Ouedraogo M et al. Mutat Res Toxicol Environ Mutagen 2014;768:33–41. [DOI] [PubMed] [Google Scholar]
- 25. Aliomrani M, Sepand MR, Mirzaei HR et al. Effects of phloretin on oxidative and inflammatory reaction in rat model of cecal ligation and puncture induced sepsis. DARU J Pharm Sci 2016;24:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabzevari O, Galati G, Moridani MY et al. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem Biol Interact 2004;148:57–67. [DOI] [PubMed] [Google Scholar]
- 27. Selvaraj V, Bodapati S, Murray E et al. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int J Nanomedicine 2014;9:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Meerloo J, Kaspers GJL, Cloos J. Cell sensitivity assays: the MTT assay In: Cancer Cell Culture. USA: Springer, 2011, 237–45. [DOI] [PubMed] [Google Scholar]
- 29. Chen X, Zhong Z, Xu Z et al. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic Res 2010;44:587–604. [DOI] [PubMed] [Google Scholar]
- 30. Szychowski KA, Rybczyńska-Tkaczyk K, Leja ML et al. Tetrabromobisphenol A (TBBPA)-stimulated reactive oxygen species (ROS) production in cell-free model using the 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) assay-limitations of method. Environ Sci Pollut Res 2016;23:12246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayashi M, Morita T, Kodama Y et al. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat Res Lett 1990;245:245–9. [DOI] [PubMed] [Google Scholar]
- 32. Florea A-M, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yimit A, Adebali O, Sancar A, Jiang Y. Nat Commun 2019;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alraouji NN, Al-Mohanna FH, Ghebeh H et al. Tocilizumab potentiates cisplatin cytotoxicity and targets cancer stem cells in triple-negative breast cancer. Mol Carcinog 2020;59:1041–51. [DOI] [PubMed] [Google Scholar]
- 35. Luo H, Wang X, Ge H et al. Inhibition of ubiquitin-specific protease 14 promotes connexin 32 internalization and counteracts cisplatin cytotoxicity in human ovarian cancer cells. Oncol Rep 2019;42:1237–47. [DOI] [PubMed] [Google Scholar]
- 36. Choi Y-M, Kim H-K, Shim W et al. PLoS One 2015;10:e0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bandgar BP, Gawande SS, Bodade RG et al. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg Med Chem 2010;18:1364–70. [DOI] [PubMed] [Google Scholar]
- 38. Vogel S, Ohmayer S, Brunner G, Heilmann J. Natural and non-natural prenylated chalcones: synthesis, cytotoxicity and anti-oxidative activity. Bioorg Med Chem 2008;16:4286–93. [DOI] [PubMed] [Google Scholar]
- 39. Berndtsson M, Hägg M, Panaretakis T et al. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer 2007;120:175–80. [DOI] [PubMed] [Google Scholar]
- 40. Mi X, Hou J, Wang Z et al. Mediated PI3K/Akt and p53 signaling pathways. Sci Rep 2018;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tusskorn O, Prawan A, Senggunprai L et al. Naunyn Schmiedebergs Arch Pharmacol 2013;386:1009–16. [DOI] [PubMed] [Google Scholar]
- 42. Barzilai A, Yamamoto K-I. DNA Repair (Amst) 2004;3:1109–15. [DOI] [PubMed] [Google Scholar]
- 43. Li N, Sun C, Zhou B et al. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer. PLoS One 2014;9:e100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belsare DP, Pal SC, Kazi AA et al. Int J ChemTech Res 2010;2:1080–9. [Google Scholar]
- 45. Perjési P, Rozmer Z. Kinetic analysis of some chalcones and synthetic chalcone analogues on the Fenton-reaction initiated deoxyribose degradation assay. Open Med Chem J 2011;5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tulub AA, Stefanov VE. Cisplatin stops tubulin assembly into microtubules. A new insight into the mechanism of antitumor activity of platinum complexes. Int J Biol Macromol 2001;28:191–8. [DOI] [PubMed] [Google Scholar]
- 47. Maronpot RR Toxicological assessment of Ashitaba Chalcone. Food Chem Toxicol 2015;77:111–9. [DOI] [PubMed] [Google Scholar]


