Abstract
N6-methyladenosine (m6A) modification is a dynamic and reversible post-transcriptional modification and the most prevalent internal RNA modification in eukaryotic cells. YT521-B homology domain family 2 (YTHDF2) is a member of m6A “readers” and its role in human diseases remains unclear. Accumulating evidence suggests that YTHDF2 is greatly implicated in many aspects of human cancers and non-cancers through various mechanisms. YTHDF2 takes a great part in multiple biological processes, such as migration, invasion, metastasis, proliferation, apoptosis, cell cycle, cell viability, cell adhesion, differentiation and inflammation, in both human cancers and non-cancers. Additionally, YTHDF2 influences various aspects of RNA metabolism, including mRNA decay and pre-ribosomal RNA (pre-rRNA) processing. Moreover, emerging researches indicate that YTHDF2 predicts the prognosis of different cancers. Herein, we focus on concluding YTHDF2-associated mechanisms and potential biological functions in kinds of cancers and non-cancers, and its prospects as a prognostic biomarker.
Keywords: m6A, YTHDF2, Cancers, Non-cancers, Biological function, Up and downregulation, signaling pathways
Introduction
As far as we know, there are over 160 post-transcriptional modifications of RNA, which are characterized among all living organisms and produce a functional diversity that allows four basic ribonucleotide residues to obtain multiple functions [1]. Accumulating researches discovered a variety of RNA modifications in eukaryotic mRNAs, such as pseudouridine (Ψ), 5-methylcytosine (m5C), N1-methyladenosine (m1A) and m6A modifications [2]. m6A modification was first identified in mRNA-enriched RNA fractions in 1974 [3]. It was the methylation of the N6 position of adenosine bases and the most common internal RNA modification in eukaryotic cells [4]. With the application of available methods for detecting m6A, insights into the underlying mechanisms have been disclosed in recent decades. m6A RNA methylation is enriched in 3′untranslated regions (3′UTRs) [5, 6], and functions to modify kinds of RNAs, such as microRNAs (miRNAs) [7, 8], long non-coding RNAs (lncRNAs) [9] and messenger RNAs (mRNAs) [10].
m6A modification was recognized as a dynamic and reversible post-transcriptional modification in mammalian cells and maintained by multi-components, such as methyltransferase (MTase) complex and demethylase [11]. MTase complex referred to “writers”, including methyltransferase-like 3/14/16 (METTL3/14/16), KIAA1429, wilms tumor 1-associated protein (WTAP), RBM15 and RBM15B, which appended m6A sites and significantly influenced specific physiopathological processes [12]. Demethylase referred to as “erasers”, including alkB homolog 5 (ALKBH5) and fat mass and obesity-associated protein (FTO), acted to changeover the methylation [13, 14]. Besides, another group of m6A binding proteins, including YT521-B homology (YTH) domain family, IGF2BP1/2/3 and HNRNPA2B1, were termed as "readers" and functioned to recognize m6A sites [15–18].
Accumulating evidence showed that YTHDF2, as a member of m6A “readers”, played a significant role in multiple diseases, such as hematopathy and cancers [19–21]. Besides, significant progress had been made in fully understanding the role of m6A modifications in mRNA decay [22], pre- ribosomal RNA (rRNA) processing [23], and so on. These mechanisms were associated with diverse physiological and pathological processes, such as regulating inflammatory response [24], inducing pluripotent stem cells [25], adipogenesis [19]. Therefore, YTHDF2 might have a far-reaching influence on the development of human diseases, especially cancers.
Herein, we concluded the biological functions and the role of YTHDF2 in diverse diseases, especially cancers (Fig. 1), and hoped to reveal the underlying mechanisms.
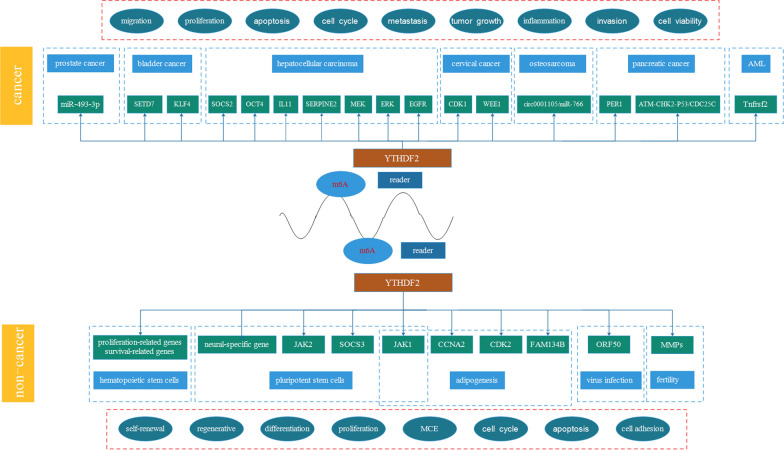
Fig. 1.
The role of YTHDF2 in human cancers and non-cancers. YTHDF2 took a great part in prostate cancer, bladder cancer, hepatocellular carcinoma, cervical cancer, osteosarcoma, AML and pancreatic cancer through modulating miR-403-3p, SETD7, KLF4, SOCS2, OCT4, IL11, SERPINE2, MEK, ERK, EGFR, CDK1, WEE1, circ0001105, PER1 and ATM-CHK2-P53/CDC25C. The modulation processes were closely related with tumor cell migration, invasion, metastasis, proliferation, apoptosis, cell cycle, cell viability and inflammation. In addition, YTHDF2 played an important role in hematopoietic stem cells, pluripotent stem cells, adipogenesis, virus infection, and male and female fertility by regulating proliferation-related genes, survival-related genes, neural-specific gene, JAK1/2, SOCS3, CCNA2, CDK2, FAM134B, Tnfrsf2, ORF50 and MMPs. The regulation process were strongly related with cell self-renewal, regenerative, differentiation, proliferation, MCE, cell cycle, apoptosis and cell adhesion
The structure and mechanism of YTHDF2
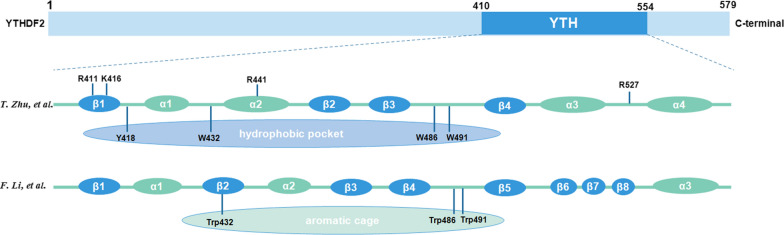
The structure of YTHDF2 provided great help in disclosing the specific molecular mechanisms of the recognition of m6A sites. Zhu et al. [26] discovered that YTHDF2 consisted of a C-terminal YTH domain, which targeted the m6A-containing RNA. There were five known YTH domain containing proteins in human genome, including YTHDF1-3 and YTHDC1-2, and YTH domain is highly conserved in these YTH domain-containing proteins [27]. YTH domain of YTHDF2 was a globular fold with four-stranded β-sheets (β1-β4), four α helices (α1-α4) and flanking regions on two sides. Besides, residues K416 and R527 of the above C-terminal YTH domain greatly participated in targeting RNA backbone and residues W432 and W486 within the hydrophobic pocket. All these specific bindings contributed to the specific recognition of m6A. However, Li et al. [28] declaimed in the same year that YTH domain of YTHDF2 showed a globular fold with a central core of eight β strands (β1-β8), three α helices (α1-α3) and two 310 helices. Additionally, the m6A mononucleotide was positioned in an aromatic cage, which consisted of residue Trp486 in β4-β5 loop, Trp432 in β2 strand and Trp491 in β4-β5 loop (Fig. 2).
Fig. 2.
The structure of YTH domain in YTHDF2
Furthermore, YTHDF2 recruited the CCR4–NOT deadenylase complex. The modulation relied on the communication between YTHDF2 and the superfamily homology domain of the CNOT1 subunit, thus inducing the decay of m6A-containing mRNAs [29]. Besides, in vitro and in vivo assays suggested that m6A modification significantly enhanced the phase separation of YTHDF2 [30]. Liquid-like phase separation (LLPS) of YTHDF2 might depend on binding m6A mRNAs .
The role of YTHDF2 in human cancers
In the field of cancer research, YTHDF2 had been found to greatly participate in the development of various cancers, including bladder cancer, hepatocellular carcinoma (HCC), gastric cancer, breast cancer, osteosarcoma, cervical cancer, prostate cancer, pancreatic cancer, acute myeloid leukemia (AML) and so on. It was interesting that YTHDF2 was up-regulated or down-regulated in different cancers, and played an oncogenic role or acted as a tumor suppresser (Table 1). Next, we will further discuss the specific role of YTHDF2 in multiple cancers.
Table 1.
Expression, role, biological function and potential targets of YTHDF2 in cancers
| Cancer | Expression | Role | Biological function | Target | Ref |
|---|---|---|---|---|---|
| Bladder Cancer | ↑ | Oncogene | Migration | SETD7 and KLF4 | [21] |
| Hepatocellular Carcinoma | − | Oncogene | SOCS2 | [33] | |
| − | Oncogene | CSC liver phenotype, metastasis | OCT4 | [34] | |
| ↓ | Tumor suppressor | Inflammation, vasculature remodeling, metastasis | IL11, SERPINE2 | [35] | |
| ↓ | Tumor suppressor | Proliferation, tumor growth | EGFR | [36] | |
| Cervical Cancer | ↑ | Oncogene | Proliferation, apoptosis, cell cycle | - | [38] |
| Gastric Cancer | ↑ | Oncogene | Proliferation, cell cycle, apoptosis | - | [40] |
| Osteosarcoma | ↓ | Tumor suppressor | Cell viability, invasion, tumor growth | [42] | |
| Pancreatic Cancer | − | Oncogene | Proliferation, migration, invasion Tumor growth, metastasis | PER1 | [44] |
| Prostate Cancer | ↑ | Oncogene | Proliferation, migration | miR-493-3p | [46] |
YTHDF2 dysregulation in bladder cancer
Bladder cancer is the most common malignant tumor of the urinary tract and the treatment of bladder cancer has not been improved significantly since 30 years ago [31]. Recently, researchers constantly pay attention to the biological role of m6A modification in bladder cancer. Luckily, it was found that YTHDF2 was up-regulated in bladder cancer and the up-regulated YTHDF2 advanced the progression of bladder cancer through cooperating with METTL3 and directly degrading the mRNAs of SETD7 and KLF4 in an m6A dependent manner [21].
YTHDF2 dysregulation in HCC
HCC, the main type of liver cancer, leads to the most common cancer-related death all over the world. Although surgical resection is the the best treatment for HCC, most patients are diagnosed at advanced stages with regional spread and metastasis, which are not eligible for surgical resection [32]. As a result, exploring the underlying mechanisms provides opportunities to improve the prognosis of HCC patients. Researches disclosed that YTHDF2 cooperated with METTL3 and took a part in HCC progression [33]. In detail, METTL3-mediated m6A modification repressed the suppressor of cytokine signaling 2 (SOCS2), which acted as a tumor suppressor in HCC via YTHDF2-mediated mRNA degradation. He et al. [34] discovered that YTHDF2 might function as an oncogene in the development of HCC. YTHDF2 advanced the cancer stem cell (CSC) liver phenotype and HCC lung metastases by adjusting the m6A methylation in the 5′UTR of OCT4 mRNA.
In contrast, some researches indicated that YTHDF2 severed as a tumor suppressor in HCC. For example, YTHDF2 was down-regulated in both hypoxic cells and HCC tissues, and inversely related to the m6A/A ratio [35]. Furthermore, hypoxia blocked the expression of YTHDF2 in a hypoxia-inducible factor-2α (HIF-2α)-dependent manner, and the deficiency of YTHDF2 promoted HCC cell growth, inflammation, metastasis and vasculature remodeling. Mechanistically, the above-mentioned functions were achieved by YTHDF2 through processing the decay of interleukin 11 (IL11) and serpin family E member 2 (SERPINE2) mRNAs. Zhong et al. [36] also declared that the inhibition of YTHDF2 was induced by hypoxia in HCC cells. And the expression of YTHDF2 was inversely correlated with HCC cell proliferation through activating MEK and ERK signaling pathways and destabilizing EGFR mRNA.
YTHDF2 dysregulation in cervical cancer
Cervical cancer is one of the most prevalent gynecological cancers across the world. Surgical resection and chemo-radiotherapy is the main treatment for cervical cancer [37]. However, the specific mechanisms underlying the development of cervical cancer remain unclear. The expression of YTHDF2 was up-regulated in the cervical cancer tissues. In detail, YTHDF2 knockdown restrained proliferation, promoted apoptosis, and arrested the cells at the S phase in cervical cancer cells [38]. YTHDF2 silence in HeLa cells was correlated with a mitotic entry delay. This regulation partly depended on the activation of Wee1-like protein kinase (WEE1), a negative regulator of the cell cycle, and the stability of YTHDF2, which is greatly associated with cyclin-dependent kinase 1 (CDK1) activity [22].
YTHDF2 dysregulation in gastric cancer
It is known that gastric cancer is one of the malignant cancers of the digestive tract [39]. In recent years, studies focus on the biological role of YTHDF2 in gastric cancer. YTHDF2 was found to be significantly up-regulated in gastric cancer tissues compared with that in normal tissues. In vitro assay revealed that YTHDF2 slience inhibited gastric cancer cell proliferation, arrested cell in G1 phase, and accelerated cell apoptosis [40].
YTHDF2 dysregulation in osteosarcoma
Osteosarcoma is one of the most common malignant bone tumors in childhood and adolescence and the survival rate for patients with metastatic or relapsed osteosarcoma remains low at approximately 20% in over 25 years [41]. Recently, studies constantly pay attention to the role of YTHDF2 in the progression of osteosarcoma. YTHDF2 was found to cooperate with a circRNA in the development of osteosarcoma. Firstly, YTHDF2 was discovered to be down-regulated in osteosarcoma tissues [42]. Also, the findings offered evidence that YTHDF2 took a part in the tumorigenesis and progression of osteosarcoma through adjusting the circ_0001105/miR-766 axis.
YTHDF2 dysregulation in pancreatic cancer
Pancreatic cancer is also a prevalent malignancy. On account of tumor metastasis and recurrence and lack of effective treatments, the clinical outcomes of pancreatic cancer remain poor [43]. One research found that YTHDF2 participated in the progression of pancreatic cancer via collaborating with ALKBH5, a pivotal demethylase of m6A [44]. The loss of ALKBH5 post-transcriptionally decreased the expression of PER1 in YTHDF2-dependent manner, and facilitated cell migration, invasion, proliferation and tumor growth in pancreatic cancer. Moreover, the up-regulation of PER1 contributed to reactivating the ATM-CHK2-P53/CDC25C signaling pathway, which greatly suppressed tumor cell growth.
YTHDF2 dysregulation in prostate cancer
In America, prostate cancer is the second leading cause of death. The main treatment of prostate cancer contains surgical removal of the prostate, chemo-radiotherapy and hormonal therapy. However, the 5-years survival rate declines significantly for patients with metastatic prostate cancer [45]. In the study of YTHDF2, researchers found that YTHDF2 was frequently overexpressed in prostate cancer and YTHDF2 knockdown elevated m6A level and retained tumor cell proliferation and migration with the up-regulated miR-493-3p [46].
YTHDF2 dysregulation in acute myeloid leukemia (AML)
AML is one of the most common and fatal forms of hematopoietic malignancies. With standard chemotherapies, only a small number of patients with AML survive more than 5 years [47]. In 2006, Nguyen et al. [48] first identified YTHDF2 in AML with reciprocal 21q22/RUNX1 translocations. AML was an aggressive clonal disorder, which blocked myeloid differentiation and self-renewing of leukemic stem cells (LSCs) [49]. Until 2019, Paris et al. [20] declared that YTHDF2 was overexpressed in human AML and the up-regulation of YTHDF2 played an important role in the development of LSC and the initiation and propagation of AML partly through shortening the half-life of multiple m6A transcripts which were closely related with LSC function. For instance, the tumor necrosis factor receptor Tnfrsf2 was closely related to the apoptosis of LSCs. On the other hand, the deficiency of YTHDF2 was proved to enhance hematopoietic stem cells (HSCs) activity and sensitize AML cells to tumor necrosis factor (TNF). As a result, YTHDF2 was supposed to be a novel and promising target in the treatment of hematological malignancy.
YTHDF2 dysregulation with cancer prognosis
Based on current researches, it was found that the expression of YTHDF2 was closely associated with cancer prognosis. For example, up-regulated YTHDF2 indicated a poor prognosis in patients with cervical cancer [38]. And down-regulated YTHDF2 predicted more aggressive tumor phenotypes and a worse prognosis of osteosarcoma [42]. However, the relationship remains controversial even in the same cancer. He et al. [34] found that up-regulated YTHDF2 indicated poor overall survival and recurrence-free survival of HCC patients. However, Hou et al. [35] discovered that the down-regulation of YTHDF2 was significantly connected with poor TNM stage classification, overall survival and recurrence-free survival of HCC patients.
The role of YTHDF2 in stem cells
YTHDF2 dysregulation in HSCs
Hematopoietic stem cells were essential for life-long hematopoiesis and contributed to a subtle the balance between self-renewal and differentiation [50, 51]. The transplantation of human umbilical cord blood (hUCB) HSCs had been applied in hematologic, immune, and genetic diseases. However, HSCs in hUCB were scarce, which greatly limited their extensive application [52]. In 2018, Li et al. [53, 54] declared that YTHDF2 knockout in mice specifically increased the number of HSCs with no defects in their progenitor or lineage cells. In the further functional assay, it was found that the down-regulated YTHDF2 expanded functional hUCB HSCs without influencing lineage commitment, and YTHDF2 modulated hUCB HSCs self-renewal by m6A-dependent mRNA decay. In the same year, Wang et al. [55] revealed that YTHDF2 took a part in reading and processing m6A modification in HSCs. Furthermore, the deficiency of YTHDF2 decreased the degradation of mRNAs of proliferation/survival-related genes under hematopoietic stresses, thus boosting the regenerative capacity of HSCs.
YTHDF2 dysregulation in pluripotent stem cells (PSCs)
Embryonic stem cells (ESCs) hold unprecedented promise for the treatment of disease, organ transplantation and so on. Induced PSCs (iPSCs) resembled ESCs in multiple biological processes [56]. However, on account of ethical issues, iPSCs failed to be extensively used. Later, researchers uncovered that porcine iPSCs (piPSCs) might be potential alternative stem cells, due to their great similarity in the human genome and physiological characteristics [57]. Recent studies disclosed YTHDF2 was overexpressed in iPSCs [58]. Further mechanism exploration found that YTHDF2 in iPSCs acted to destabilize a set of m6A-modified transcripts, which was closely connected with neural development and contributed to the loss of pluripotency and inhibited the expression of neural-specific genes.
Li et al. [59] demonstrated that YTHDF2 modulated neural stem/progenitor cell (NSPC) self-renewal, proliferation and differentiation capabilities and the generation of neurons via facilitating m6A-mediated degradation of neural development-specific mRNAs. In addition, METTL3 deficiency weakened self-renewal ability and induced differentiation of piPSCs through inhibiting YTHDF1-mediated JAK2 translation and blocking YTHDF2-dependent SOCS3 mRNA decay. These modulations eventually suppressed the activation of JAK2–STAT3 pathway and the transcription of KLF4 and SOX2 [25]. YTHDF2 was also declared to participate in the regulation of bone marrow stem cells (BMSCs) differentiating into adipocytes. METTL3 knockdown in porcine BMSCs (pBMSCs) inhibited adipogenesis and enhanced YTHDF2-dependent JAK1 mRNA stability [60]. JAK1 modulated adipogenesis via mediating signal transducer and activator of transcription (STAT)5 expression and activity, thus regulating the CCAAT/enhancer binding protein (C/EBP)βtranscription.
YTHDF2 dysregulation in mesenchymal stem cells (adipogenesis)
M6A modification also greatly participated in adjusting adipogenesis, but the specific role of m6A on typical genes remained unclear [61]. Later, YTHDF2 was found to significantly participate in the recognition and degradation of methylated mRNAs, thus decreasing the expression of Cyclin A2 (CCNA2) and cyclin dependent kinase 2 (CDK2), which played essential roles in the mitotic clonal expansion (MCE) at the early stage of adipocyte differentiation [19]. CCNA2 and CDK2 were key cell cycle regulators, thereby promoting the cell cycle and suppressing adipogenesis of preadipocytes [62]. Moreover, YTHDF2 recognized and bound the m6A site of FAM134B to decrease the mRNA lifetime and inhibit the protein expression [63]. FAM134B was a cis-Golgi transmembrane protein and recognized to be greatly involved in preadipocytes adipogenic differentiation and lipid deposition.
The role of YTHDF2 in human non-cancers
YTHDF2 dysregulation in virus infection
Increasing studies indicated that m6A-related genes had pro- and anti-viral effects in distinct viral life cycles [64–66]. In 2018, Hesser et al. [67] discovered m6A level was up-regulated in cells infected with Kaposi’s sarcoma-associated herpes virus (KSHV). Mechanically, YTHDF2, which post-transcriptionally controlled the major viral lytic transactivator ORF50 abundance, mediated viral gene expression, lytic entry and virion production in KSHV-positive cell lines. However, the effect of m6A on KSHV infection relied on cell types.
YTHDF2 dysregulation with human longevity
Cardelli et al. [68] identified a locus that was closely related to human longevity. It was up to the chromosomal regions with the highest density of Alu elements, in 1p35. Then they found the locus corresponded to a (TG)n microsatellite in the YTHDF2 gene, and suggested that this locus played a potential role in human longevity.
YTHDF2 dysregulation with infertility
Male fertility relied on the mitosis of spermatogonia, which was a complex and highly controlled process [69]. YTHDF2 knockout in mouse spermatogonia acted to down-regulate matrix metallopeptidases (MMPs) and affected cell adhesion and proliferation through the m6A/mRNA degradation pathway [70]. As for female fertility, researchers claimed that YTHDF2, expressed at all stages of mammalian gametogenesis, was a pivotal factor in mammalian egg quality. In detail, the deficiency of YTHDF2 led to female infertility, as YTHDF2 was essentially needed for oocyte competence and regulated transcript dosage post-transcriptionally during oocyte maturation [71, 72].
Discussion
In this review, we discussed the specific structure of YTHDF2 and the underlying mechanisms. In addition, YTHDF2 was dysregulated in human cancers, including bladder cancer, HCC, gastric cancer, breast cancer, osteosarcoma, cervical cancer, prostate cancer, pancreatic cancer, acute myeloid leukemia (AML) and so on. Although surgical treatment, chemoradiotherapy, immunotherapy and targeted therapy have significantly improve the prognosis of cancer patients, the underlying mechanisms of cancers remain unclear. This review summarized the current researches and concluded that YTHDF2 played an oncogenic role or acted as a tumor suppresser by regulating tumor cell proliferation, survival, growth, apoptosis, cell cycle, migration, invasion, metastasis, cell viability and so on,. These multiple effects were achieved through diverse pathways, such as collaborating with miRNAs or circRNAs, communicating with METTL3, and recognizing and decaying corresponding mRNA. However, the relevant reasons why YTHDF2 played opposite roles in different cancers were still unclear. Further researches might focus on the upstream molecular regulation mechanisms to explore the causes of YTHDF2 disorders in human cancers. Besides, regarding that YTHDF2 influenced various signaling pathways through recognizing m6A-modified sites of mRNAs, we’d better pay close attention to the most advantageous signaling pathways and get rid of those interfering ones. It was also declared that YTHDF2 was dysregulated in human non-cancers. For example, YTHDF2 took a great part in hematopoietic stem cell self-renewal and differentiation, inducing pluripotent stem cells, regulating adipogenesis, mediating viral gene expression, modulating male and female fertility. Further exploration might also focus on the detailed mechanisms regarding human non-cancers.
Conclusion
In conclusion, YTHDF2 has great potential for clinical application by acting as a novel diagnostic/prognostic biomarker. However, further researches are still needed to expound the specific role of YTHDF2 in human cancers and non-cancers.
Acknowledgements
Not applicable.
Abbreviations
- YTHDF2
YT521-B homology (YTH) domain family 2
- rRNA
Pre-ribosomal RNA
- m1A
N1-methyladenosine
- m6A
N6-methyladenosine
- m5C
5-methylcytosine
- 3’UTRs
3’untranslated regions
- miRNAs
MicroRNAs
- mRNAs
Messenger RNAs
- lncRNAs
Long non-coding RNAs
- MTase
Methyltransferase
- METTL3/14/16
Methyltransferase-like 3/14/16
- WTAP
Wilms tumor 1 (WT1)-associated protein
- FTO
Fat mass and obesity-associated protein
- ALKBH5
alkB homolog 5
- m6A-RNA
m6A-containing RNA
- SH
Superfamily homology
- LLPS
Liquid-like phase separation
- HCC
Hepatocellular carcinoma
- SOCS2
Suppressor of cytokine signaling 2
- OS
Overall survival
- RFS
Recurrence-free survival
- CSC
Cancer stem cell
- HIF-2α
Hypoxia-inducible factor-2α
- IL11
Interleukin 11
- SERPINE2
Serpin family E member 2
- WEE1
Wee1-like protein kinase
- CDK1
Cyclin-dependent kinase 1
- AML
Aacute myeloid leukemia
- LSCs
Leukemic stem cells
- TNF
Tumor necrosis factor
- HSCs
Hematopoietic stem cells
- hUCB
Human umbilical cord blood
- ESCs
Embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- piPSCs
Porcine induced pluripotent stem cells
- NSPC
Neural stem/progenitor cell
- BMSCs
Bone marrow stem cells
- pBMSCs
Porcine BMSCs
- STAT
Signal transducer and activator of transcription
- C/EBP
CCAAT/enhancer binding protein
- EGCG
Epigallocatechin gallate
- CCNA2
Cyclin A2
- CDK2
Cyclin dependent kinase 2
- MCE
Mitotic clonal expansion
- FAM134B
Sequence similarity 134 member B
- KSHV
Kaposi’s sarcoma-associated herpes virus
- MMPs
Matrix metallopeptidases
Authors’ contributions:
Conceptualization, JW and AL; methodology, JW; validation, JW; formal analysis, JW; investigation, JW; resources, JW; data curation, JW; writing—original draft preparation, JW; writing—review and editing, AL; supervision, AL; project administration, AL; funding acquisition, JW. All authors read and approved the final manuscript.
Funding
This research was funded by the Medical Research Grant of Jiangsu Commission of Health, grant number M2020010 to Jinyan Wang, the Priority Academic Program Development of Jiangsu Higher Education Institutions, grant number JX10231802 to Jinyan Wang, Postgraduate Research and Practice Innovation Program of Jiangsu Province, grant number SJCX17_0387 to Jinyan Wang, the Science Foundation of Jiangsu Health vocational college, grant number JKC201948 to Jinyan Wang, the Science and Technology Development Fund of Nanjing Medical University, grant number NMUB2019235 to Jinyan Wang, the Research and development fund of Kangda College of Nanjing Medical University, grant number KD2020KYJJZD006 to Jinyan Wang.
Availability of data and material
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boccaletto P. et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res, 2018. 46(D1): D303-d307. [DOI] [PMC free article] [PubMed]
- 2.Sánchez-Vásquez E, et al. Emerging role of dynamic RNA modifications during animal development. Mech Dev. 2018;154:24–32. doi: 10.1016/j.mod.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maity, A. and B. Das, N6-methyladenosine modification in mRNA: machinery, function and implications for health and diseases. 2016. 283(9): p. 1607–30. [DOI] [PubMed]
- 5.Ke S, et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29(19):2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 7.Han J, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18(1):110. doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H, et al. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J Exp Clin Cancer Res. 2019;38(1):163. doi: 10.1186/s13046-019-1159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S. ALKBH5-mediated m(6)A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Stem Cells Int. 2020;20:34. doi: 10.1186/s12935-020-1105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Xu B, Shi J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene. 2020;722:144076. doi: 10.1016/j.gene.2019.144076. [DOI] [PubMed] [Google Scholar]
- 11.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29(13):1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613. doi: 10.1016/j.biopha.2019.108613. [DOI] [PubMed] [Google Scholar]
- 13.Jin, D. and J. Guo. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. 2020. 19(1): p. 40. [DOI] [PMC free article] [PubMed]
- 14.Huang Y, et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell. 2019;35(4):677–691.e10. doi: 10.1016/j.ccell.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y, et al. Multivalent m(6)A motifs promote phase separation of YTHDF proteins. Cell Res. 2019;29(9):767–769. doi: 10.1038/s41422-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller S, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, H., et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. 2018. 20(3): p. 285–295. [DOI] [PMC free article] [PubMed]
- 18.Alarcón CR, et al. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162(6):1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu R, et al. Epigallocatechin gallate targets FTO and inhibits adipogenesis in an mRNA m(6)A-YTHDF2-dependent manner. Int J Obes (Lond) 2018;42(7):1378–1388. doi: 10.1038/s41366-018-0082-5. [DOI] [PubMed] [Google Scholar]
- 20.Paris J, et al. Targeting the RNA m(6)A Reader YTHDF2 Selectively Compromises Cancer Stem Cells in Acute Myeloid Leukemia. Genome Biol. 2019;25(1):137–148.e6. doi: 10.1016/j.stem.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie, H., et al. METTL3/YTHDF2 m(6) A axis promotes tumorigenesis by degrading SETD7 and KLF4 mRNAs in bladder cancer. 2020. 24(7): p. 4092–4104. [DOI] [PMC free article] [PubMed]
- 22.Fei Q. YTHDF2 promotes mitotic entry and is regulated by cell cycle mediators. Anal Chem. 2020;18(4):e3000664. doi: 10.1371/journal.pbio.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai X, et al. YTHDF2 Binds to 5-Methylcytosine in RNA and Modulates the Maturation of Ribosomal RNA. Protein Cell. 2020;92(1):1346–1354. doi: 10.1021/acs.analchem.9b04505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu R, et al. m6A Reader YTHDF2 Regulates LPS-Induced Inflammatory Response. Hepatology, 2019. 20(6). [DOI] [PMC free article] [PubMed]
- 25.Wu R, et al. m(6)A methylation controls pluripotency of porcine induced pluripotent stem cells by targeting SOCS3/JAK2/STAT3 pathway in a YTHDF1/YTHDF2-orchestrated manner. Cell Death Dis. 2019;10(3):171. doi: 10.1038/s41419-019-1417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu T, et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. RNA. 2014;24(12):1493–1496. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma C, Liao S, Zhu Z. Crystal structure of human YTHDC2 YTH domain. Biochem Biophys Res Commun. 2019;518(4):678–684. doi: 10.1016/j.bbrc.2019.08.107. [DOI] [PubMed] [Google Scholar]
- 28.Li F, et al. Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res. 2014;24(12):1490–1492. doi: 10.1038/cr.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang G, et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Cell Res. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, et al. Binding to m(6)A RNA promotes YTHDF2-mediated phase separation. 2020. 11(4): p. 304–307. [DOI] [PMC free article] [PubMed]
- 31.Pardo JC, et al., Moving towards Personalized Medicine in Muscle-Invasive Bladder Cancer: Where Are We Now and Where Are We Going? Int J Mol Sci, 2020. 21(17). [DOI] [PMC free article] [PubMed]
- 32.Li R, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. J Cell Physiol. 2019;18(1):18. doi: 10.1186/s12943-019-0948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. 2018. 67(6): p. 2254–2270. [DOI] [PubMed]
- 34.He C, Zhang C. YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. PLoS Biol, 2020. [DOI] [PubMed]
- 35.Hou J, et al. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Cell Res. 2019;18(1):163. doi: 10.1186/s12943-019-1082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L, et al. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019;442:252–261. doi: 10.1016/j.canlet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, S., et al., FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylation. 2018. 57(5): p. 590–597. [DOI] [PubMed]
- 38.Li Z, et al. Knockdown of YTH N(6)-methyladenosine RNA binding protein 2 (YTHDF2) inhibits cell proliferation and promotes apoptosis in cervical cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36(3):255–263. [PubMed] [Google Scholar]
- 39.Liu, T. and S. Yang, Dysregulated N6-methyladenosine methylation writer METTL3 contributes to the proliferation and migration of gastric cancer. 2020. 235(1): p. 548–562. [DOI] [PubMed]
- 40.Zhang J, et al. Knockdown of YTH N(6)-methyladenosine RNA binding protein 2 (YTHDF2) inhibits proliferation and promotes apoptosis in MGC-803 gastric cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33(12):1628–1634. [PubMed] [Google Scholar]
- 41.Wang JY, et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother. 2020;121:109627. doi: 10.1016/j.biopha.2019.109627. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, et al. Circular RNA circ_0001105 Inhibits Progression and Metastasis of Osteosarcoma by Sponging miR-766 and Activating YTHDF2 Expression. Onco Targets Ther. 2020;13:1723–1736. doi: 10.2147/OTT.S234668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, et al. Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic cancer promotes cancer proliferation by activating the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2019;38(1):497. doi: 10.1186/s13046-019-1470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo X, et al. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer. 2020;19(1):91. doi: 10.1186/s12943-020-01158-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, et al. RNA m(6)A Methyltransferase METTL3 Promotes The Growth Of Prostate Cancer By Regulating Hedgehog Pathway. J Hematol Oncol. 2019;12:9143–9152. doi: 10.2147/OTT.S226796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, et al. Downregulation of N(6)-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N(6)-methyladenosine levels. Oncotarget. 2018;9(3):3752–3764. doi: 10.18632/oncotarget.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31(1):127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen TT, et al. Identification of novel Runx1 (AML1) translocation partner genes SH3D19, YTHDf2, and ZNF687 in acute myeloid leukemia. Genes Chromosomes Cancer. 2006;45(10):918–932. doi: 10.1002/gcc.20355. [DOI] [PubMed] [Google Scholar]
- 49.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dzierzak E, Bigas A. Blood Development: Hematopoietic Stem Cell Dependence and Independence. Cell Stem Cell. 2018;22(5):639–651. doi: 10.1016/j.stem.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci. 2012;1266:138–150. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 53.Li, Z., et al., Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. 2018. 28(9): p. 904–917. [DOI] [PMC free article] [PubMed]
- 54.Huang S, et al. Author Correction: Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. Oncogene. 2018;28(10):1042. doi: 10.1038/s41422-018-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, H., et al., Loss of YTHDF2-mediated m(6)A-dependent mRNA clearance facilitates hematopoietic stem cell regeneration. 2018. 28(10): p. 1035–1038. [DOI] [PMC free article] [PubMed]
- 56.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Secher JO, et al. Initial embryology and pluripotent stem cells in the pig–The quest for establishing the pig as a model for cell therapy. Theriogenology. 2016;85(1):162–171. doi: 10.1016/j.theriogenology.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Heck AM, et al. YTHDF2 destabilizes m(6)A-modified neural-specific RNAs to restrain differentiation in induced pluripotent stem cells. ACS Chem Biol. 2020;26(6):739–755. doi: 10.1261/rna.073502.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li, M., et al., Ythdf2-mediated m(6)A mRNA clearance modulates neural development in mice. 2018. 19(1): p. 69. [DOI] [PMC free article] [PubMed]
- 60.Yao Y, et al. METTL3 inhibits BMSC adipogenic differentiation by targeting the JAK1/STAT5/C/EBPβ pathway via an m(6)A-YTHDF2-dependent manner. Faseb j. 2019;33(6):7529–7544. doi: 10.1096/fj.201802644R. [DOI] [PubMed] [Google Scholar]
- 61.Merkestein M, et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat Commun. 2015;6:6792. doi: 10.1038/ncomms7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu R, et al. FTO regulates adipogenesis by controlling cell cycle progression via m(6)A-YTHDF2 dependent mechanism. Cell Res. 2018;1863(10):1323–1330. doi: 10.1016/j.bbalip.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Cai M, et al. Loss of m(6) A on FAM134B promotes adipogenesis in porcine adipocytes through m(6) A-YTHDF2-dependent way. IUBMB Life. 2019;71(5):580–586. doi: 10.1002/iub.1974. [DOI] [PubMed] [Google Scholar]
- 64.Lichinchi G, et al. Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe. 2016;20(5):666–673. doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martínez-Pérez, M., et al., Arabidopsis m(6)A demethylase activity modulates viral infection of a plant virus and the m(6)A abundance in its genomic RNAs. 2017. 114(40): p. 10755–10760. [DOI] [PMC free article] [PubMed]
- 66.Gonzales-van Horn, S.R. and P. Sarnow, Making the Mark: The Role of Adenosine Modifications in the Life Cycle of RNA Viruses. Cell Host Microbe, 2017. 21(6): p. 661–669. [DOI] [PMC free article] [PubMed]
- 67.Hesser, C.R. and J. Karijolich, N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi's sarcoma-associated herpesvirus infection. 2018. 14(4): p. e1006995. [DOI] [PMC free article] [PubMed]
- 68.Cardelli M, et al. A polymorphism of the YTHDF2 gene (1p35) located in an Alu-rich genomic domain is associated with human longevity. J Gerontol A Biol Sci Med Sci. 2006;61(6):547–556. doi: 10.1093/gerona/61.6.547. [DOI] [PubMed] [Google Scholar]
- 69.Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–187. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- 70.Huang T, et al. YTHDF2 promotes spermagonial adhesion through modulating MMPs decay via m(6)A/mRNA pathway. PLoS Pathog. 2020;11(1):37. doi: 10.1038/s41419-020-2235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanova I, et al. The RNA m(6)A Reader YTHDF2 Is Essential for the Post-transcriptional Regulation of the Maternal Transcriptome and Oocyte Competence. Mol Cell. 2017;67(6):1059–1067.e4. doi: 10.1016/j.molcel.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie L, Zhao BS, He C. "Gamete On" for m(6)A: YTHDF2 Exerts Essential Functions in Female Fertility. J Cell Mol Med. 2017;67(6):903–905. doi: 10.1016/j.molcel.2017.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.