Abstract
INTRODUCTION
Most adults who use electronic cigarettes (ECs) also smoke combustible cigarettes (CCs). Quitting ECs appears common among dual users but little is known regarding adult dual users’ motivations and methods to quit ECs or how this relates to quitting CCs.
METHODS
We used Amazon Mechanical Turk, a web-based crowd-sourcing service, to survey 366 US adults with a history of regular EC and CC use. This analysis examined motivations and methods to quit both products among a subset of 204 (55.7%) respondents with dual use and a history of one or more attempts to quit ECs.
RESULTS
Most respondents (95%) were using ECs at the time of this survey and had a lifetime median of five EC quit attempts. The most common motivations to quit ECs were health (74%), money/cost (45%), and to reduce risk of COVID-19 (25%). The most common EC quit methods were cutting down (68%), getting advice from a doctor (28%), quitting 'cold turkey' (24%), nicotine replacement therapy (24%), and switching to ECs with less nicotine (24%). Most motivations and methods to quit ECs and CCs were moderately to highly correlated, suggesting similarity in dual users’ approach to quitting the two products.
CONCLUSIONS
Dual users had a range of motivations and methods to quit ECs, most of which were similar to their motivations and methods to quit CCs. These findings support the need to develop treatment for adults motivated to quit ECs and demonstrate that dual users may currently engage in similar strategies to quit both products.
Keywords: electronic cigarettes, cigarette smoking, quit attempt, cessation, motivation to quit
INTRODUCTION
Electronic cigarette (EC) use has increased dramatically in the past decade1. Though initiating ECs to quit combustible cigarettes (CCs) is common1, most adults who use ECs continue smoking CCs1,2. Among those who continue to smoke CCs (i.e. dual users), motivation to stop using both products appears common3-5. Most US adults (62%) who use ECs plan to quit ECs at some point in the future6 and 45% of US adolescents who use ECs report seriously thinking about quitting ECs7. However, little is known regarding the specific reasons (i.e. motivations) and strategies used (i.e. methods) to quit ECs among adults who use both ECs and CCs.
Discontinuing EC use is common among adult CC smokers who try using ECs3-5. In a large national study of US adults, over half (56%) of current CC smokers who started using ECs later discontinued ECs4 and findings were similar in a European cohort study with a two year follow-up5. Much of the prior research on stopping ECs includes adults who tried and subsequently discontinued ECs in the absence of self-defined regular EC use or dependence4,8. Among adults with a history of regular EC use, the most common reasons for discontinuation were that ECs were not rewarding9,10 or useful9,11 enough to warrant continued use.
In contrast, people who quit nicotine products often do so because of competing contingencies (e.g. health concerns, stigma, or monetary cost) despite continued cravings or urges to smoke. For example, among adults who smoke CCs, quitting often requires motivation12, multiple failed attempts13, and treatment14 to overcome symptoms of CC dependence and withdrawal. Use of ECs can also produce dependence1 and withdrawal upon cessation15. Thus, among those who become dependent on ECs, quitting ECs could require some motivations or strategies similar to quitting CCs16. Though research on reasons for quitting ECs among adults is limited, one recent qualitative study identified health, financial cost, and freedom from addiction as common reasons for wanting to quit ECs among youth and young adults seeking EC cessation treatment17 but did not assess reasons for quitting among dual users per se. Further, little is known regarding the influence of the ongoing COVID-19 pandemic on motivations to quit ECs.
With regard to methods for quitting ECs, one recent analysis identified that approximately half of US adults with an attempt to quit ECs in the past year attempted to quit gradually (i.e. reduced to quit)6. Among adult EC users who attempted to quit all at once, common strategies included support from family or friends (25%) and counseling or self-help materials (11%). Though 55% of respondents were also current CC smokers, this study did not distinguish between EC cessation strategies used by dual users versus exclusive EC users6. Methods used to quit ECs could be limited by the lack of empirically supported EC cessation treatment options. A text-message based EC cessation service has engaged a large number of youth and young adult EC users3, for which a randomized controlled effectiveness trial is ongoing18. A non-randomized exploratory study offered a sample of dual users varenicline and found that self-selected use of the medication was a strong predictor of prolonged EC abstinence at a 6-month follow-up (OR=7.8)19. Examples of quitting ECs include a case study which documented the use nicotine replacement therapy (NRT) to quit ECs20 and another case study that described the use of motivational interviewing in combination with nicotine tapering to quit ECs21.
There is a dearth of empirically-supported treatments for EC cessation among adults and little is known about how dual users attempt to quit ECs on their own (i.e. in the absence of researcher or healthcare provider intervention). Further, it is unclear whether adult dual users have similar or different motivations for attempting to quit ECs versus CCs. We surveyed adults with a history of dual use (i.e. regular use of both ECs and CCs) who had attempted or succeeded at quitting ECs to improve understanding of motivations and methods to quit ECs and how this relates to quitting CCs.
METHODS
We recruited participants using Amazon Mechanical Turk, a web-based crowd-sourcing service. Eligibility criteria were: 1) aged ≥21 years, 2) live in the US, 3) a lifetime history of use of ECs containing nicotine on ≥15 days in a given month, 4) CC smoking on ≥15 days in a given month, and 5) one or more attempts to reduce or quit ECs in their lifetime. We elected to exclude people with EC and CC use on <15 days in a given month in order to ensure that included participants were regular users of nicotine products (i.e. ≥50% of days). Of the 593 who screened, 366 were eligible and completed the survey in April 2020. The analytic sample for this study comprised a subset of 204 (55.7%) respondents who reported a history of simultaneous regular use of both ECs and CCs and a history of one or more attempts to quit ECs (See Supplementary file Table S1 for a description of participants excluded from this analysis). The survey was originally designed to inform a tobacco treatment intervention for dual users that will be tested in an upcoming randomized controlled trial. This study was approved by the Institutional Review Board at the University of Vermont and all respondents consented to complete this survey.
Measures
Demographics and tobacco use
Respondents answered questions regarding demographics and past 30 days EC devices and flavors used, frequency of EC and CC use, and time to first EC or CC use after waking. Time to first CC is a validated and commonly used indicator of cigarette dependence22 and time to first EC appears to be a valid measure of EC dependence23. We assessed attempts to quit by asking: ‘How many times in your life have you intentionally [“quit vaping nicotine or quit using e-cigarettes” OR “quit smoking cigarettes”] for a day (24 hours) or more?’. We considered respondents to be currently abstinent from ECs or CCs if they responded ‘0’ to ‘During the past 30 days, on how many days did you [“use an e-cigarette/ vape containing nicotine” OR “smoke one or more cigarettes”]?’.
Motivations to quit
We assessed respondents’ motivations to quit ECs and CCs separately by asking: ‘What were your reasons for quitting or attempting to quit [“e-cigarettes/vapes containing nicotine” OR “smoking”]?’. We instructed respondents to ‘select all that apply’ from a list of common motivations to quit each product adapted from ongoing EC research24: a) health; b) money/ cost; c) didn’t like the taste or smell, d) difficulty obtaining [‘device, e-liquid, pods, cartridges’ OR ‘cigarettes’]; e) negative experience while using; f) I was embarrassed by my [‘e-cigarette/vape use’ OR ‘smoking’]; g) to reduce the risk of harm from coronavirus (COVID-19); h) I started or increased e-cigarette use or vaping [offered as a motivation to quit CCs only], i) to perform better in school, work, sports, etc.; j) for another person, like a family member or friend or partner; k) I started or increased other tobacco use; l) I didn’t like [‘e-cigarettes or vaping’ OR ‘smoking cigarettes’] anymore; and m) freedom from addiction.
Methods to quit
We assessed methods for quitting each product separately by asking: ‘What methods have you used to quit [“e-cigarettes/vapes containing nicotine” OR “smoking cigarettes”]’?. We instructed respondents to ‘select all that apply’ from a list of common methods adapted from prior research25: a) cut down on my [‘e-cigarette/vape use’ OR ‘smoking’] before quitting; b) switched to pods, liquids or e-juice with less nicotine before quitting (asked for quitting ECs only); c) switched to e-cigarettes/vapes containing nicotine (asked for quitting CCs only); d) got advice from my doctor on quitting; e) used nicotine patch, gum or lozenge; f) used bupropion or Zyban; g) used varenicline or Chantix; h) called a telephone quitline; i) used an app on my phone, smart-watch, tablet, or computer; j) read written materials on quitting; i) went to individual or group counseling for help with quitting; k) ‘cold turkey’ (quit abruptly without help); l) switched to a different tobacco product; and m) other. In addition, we instructed respondents to select the single method that was ‘most effective to help you quit’.
Additional quitting measures
We assessed change in CC smoking during respondents’ last EC quit attempt by asking: ‘Which of the following is true about the last time you quit e-cigarettes/vapes containing nicotine?’: a) I didn’t smoke cigarettes before or after I quit e-cigarettes/ vapes; b) I smoked cigarettes about the same before and after I quit e-cigarettes/vapes; c) My cigarette smoking decreased when I quit cigarettes/vapes; d) My cigarette smoking increased when I quit e-cigarettes/vapes; and e) I don't know’. We used the same language to ask about change in EC use the last time respondents quit CCs. We assessed respondents’ perception of their EC use when quitting CCs by asking: ‘How did using e-cigarettes/vapes affect quitting cigarette smoking? (0=made it much easier to quit smoking; 10=made it much harder to quit smoking)’.
Statistical analysis
We used descriptive statistics to summarize demographic information, tobacco use characteristics, and methods and motivators for quitting each product. In addition, we calculated point-biserial correlations between motivations and methods to quit ECs and CCs. There were no missing data for methods or motivations to quit ECs. With regard to CCs, nine respondents were missing data for methods used to quit and none were missing data for motivations to quit CCs. We conducted all analyses using SPSS (IBM Corp., Armonk, NY, USA). Missing data were handled with listwise deletion per SPSS default procedures.
RESULTS
Respondents were mostly White, male, who were in their mid thirties (Table 1). All had attempted to quit both ECs and CCs and few (<5%) achieved abstinence from either product. At the time of this survey, respondents were primarily non-daily users of ECs and CCs and most used tobacco flavored rechargeable pod-based ECs in the past 30 days (Table 1). On the days they used ECs or CCs, over a quarter of respondents vaped or smoked within 30 minutes of waking.
Table 1.
Participant characteristics (N=204)
| Characteristics | % (95% CI) |
|---|---|
| Female | 30.9 (24.9–37.6) |
| Age (years), Mean ± SD | 35.7 ± 10.5 |
| Marital status | |
| Married or living as if married | 71.1 (64.4–76.9) |
| Widowed, divorced, separated | 5.4 (3.0–9.5) |
| Never married | 23.5 (18.2–29.9) |
| Education level | |
| <High school | 4.4 (2.3–8.3) |
| High school graduate or GED | 9.8 (6.0–14.2) |
| Some college or Associate’s degree | 17.6 (13.0–23.5) |
| Bachelor’s or advanced degree | 68.1 (61.4–74.2) |
| Hispanic ethnicity | 23.0 (17.7–29.4) |
| Racea | |
| American Indian/Alaska Native | 3.9 (2.0–7.7) |
| Asian | 2.0 (0.7–5.1) |
| Black/African American | 11.3 (7.6–16.4) |
| Native Hawaiian/Pacific Islander | 1.0 (0.2–3.9) |
| White | 84.8 (79.2–89.1) |
| Employment | |
| Full-time ≥35 h/week | 91.7 (87.0–94.8) |
| Part-time <35 h/week | 5.9 (3.4–10.2) |
| Currently not working for pay | 2.5 (0.7–5.2) |
| Median age of first use (years) | |
| ECs | 20 |
| CCs | 18 |
| Median number of lifetime quit attempts | |
| ECs | 5 |
| CCs | 5 |
| Current 30-day abstinence | |
| Abstained from ECs only | 2.9 (1.3–6.4) |
| Abstained from CCs only | 2.9 (1.3–6.4) |
| Abstained from both ECs and CCs | 2.0 (0.7–5.1) |
| Current dual users (n=194) | |
| Days of use in past 30 days, Mean ± SD | |
| ECs | 17.7 ± 8.9 |
| CCs | 18.9 ± 9.4 |
| EC device used in past 30 daysa | |
| Rechargeable or pod-based device | 73.7 (67.0–79.5) |
| Disposable device | 17.0 (12.3–23.0) |
| Modular device with refillable tank | 6.7 (3.9–11.2) |
| Other or not sure | 2.6 (1.1–6.1) |
| EC flavor used in past 30 daysa | |
| Tobacco | 66.5 (59.5–72.8) |
| Menthol | 46.4 (39.4–53.5) |
| Mint | 42.3 (35.5–49.4) |
| Clove or spice | 8.8 (5.5–13.7) |
| Fruit | 24.2 (18.7–30.8) |
| Chocolate | 25.8 (20.1–32.4) |
| Alcoholic drink | 15.5 (11.0–21.3) |
| Non-alcoholic drink | 6.7 (3.9–11.2) |
| Candy or sweets | 14.9 (10.6–20.7) |
| Other | 4.6 (2.4–8.7) |
| Used within 30 minutes of waking | |
| ECs | 31.4 (25.2–38.4) |
| CCs | 27.8 (21.9–34.6) |
Respondents could select one or more options.
CC: combustible cigarette. EC: electronic cigarette. GED: General Education Development. SD: standard deviation. All participants from the USA and completed the Amazon Mechanical Turk survey for this study in April 2020.
Motivations to quit
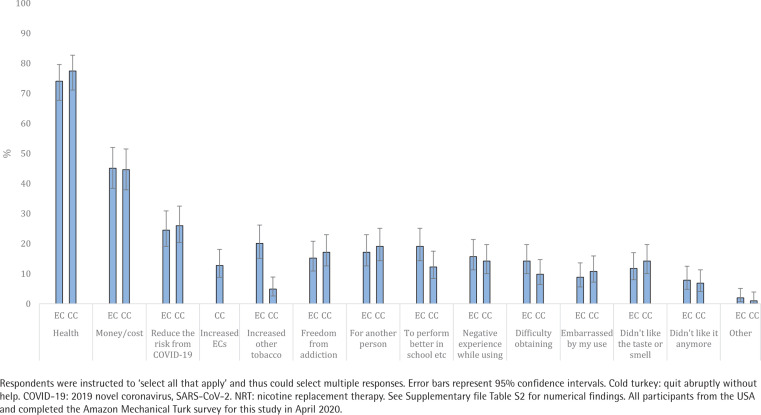
Respondents endorsed a median of two reasons (of the 13 response options) for quitting ECs. The most common were: 1) health, 2) money/cost, 3) to reduce the risk of harm from COVID-19, 4) increased use of other tobacco, and 5) to perform better at work or school (Figure 1 and Supplementary file Table S2). Similarly, respondents endorsed a median of two reasons (of the 14 response options) for quitting CCs. The most common were: 1) health, 2) money/cost, 3) to reduce the risk of harm from COVID-19, 4) freedom from addiction, and 5) for another person. There were moderate to large correlations between quitting ECs and CCs for 12 of the 13 reasons that applied to both products (rpb range: 0.35–0.65; Supplementary file Table S3). The most highly correlated motivations for quitting were to reduce risk of harm from COVID-19 (rpb=0.65, p<0.001), for another person (rpb=0.61, p<0.001), and money/cost (rpb=0.57, p<0.001). See Table 2 for the motivations to quit endorsed by the 10 respondents who achieved 30-day abstinence from ECs and the 10 respondents who achieved 30-day abstinence from CCs.
Figure 1.
Motivations for quitting e-cigarettes (EC) and combustible cigarettes (CC) among dual users (N=204)
Table 2.
Motivations and methods to quit electronic cigarettes (ECs) and combustible cigarettes (CCs) among the respondents abstinent from ECs or CCs for the past 30 days with a history of dual use
| ECs (n=10) | CCs (n=10) | |
|---|---|---|
| Motivation to quit | ||
| Health | 6 | 9 |
| Money/cost | 6 | 6 |
| Reduce the risk from COVID-19 | 0 | 1 |
| Increased ECs | 0 | 1 |
| Increased other tobacco | 3 | 0 |
| Freedom from addiction | 0 | 4 |
| For another person | 0 | 1 |
| To perform better in school etc. | 0 | 0 |
| Negative experience while using | 1 | 1 |
| Difficulty obtaining | 0 | 0 |
| Embarrassed by my use | 3 | 2 |
| Didn’t like the taste or smell | 2 | 0 |
| Didn’t like it anymore | 3 | 1 |
| Other | ||
| Method used to quit | ||
| Cut down before quitting | 5 | 7 |
| Cold turkey* | 7 | 2 |
| Got advice from doctor | 1 | 2 |
| Switched to ECs | 0 | 2 |
| Switched to low nicotine pods | 0 | 0 |
| Used NRT | 1 | 3 |
| Read written material | 0 | 1 |
| Used an app | 1 | 1 |
| Went to counseling | 0 | 2 |
| Switched to different tobacco | 3 | 0 |
| Used bupropion | 0 | 0 |
| Used varenicline | 0 | 0 |
| Called a quitline | 0 | 0 |
| Other | 0 | 0 |
Respondents were instructed to ‘select all that apply’ and thus could select multiple responses.
Cold turkey: quit abruptly without help.
COVID-19: 2019 novel coronavirus, SARS-CoV-2. NRT: nicotine replacement therapy. All participants from the USA and completed the Amazon Mechanical Turk survey for this study in April 2020.
Methods used to quit
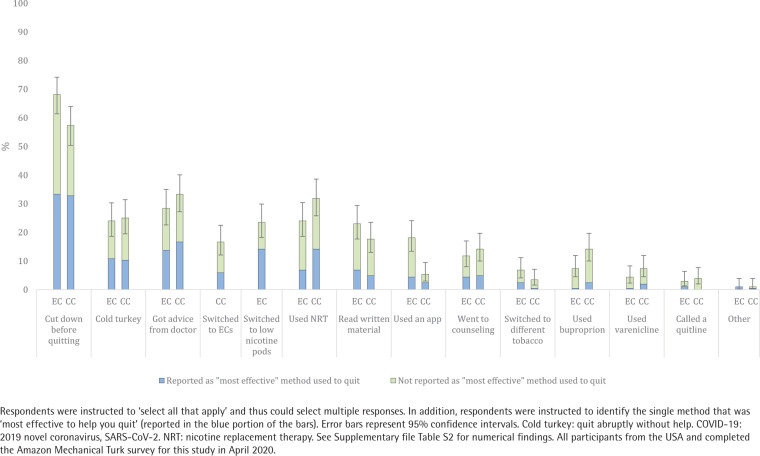
Respondents endorsed a median of two strategies (of the 13 response options) used to quit ECs. The most common were: 1) cutting down to quit (i.e. reduction), 2) quitting ‘cold turkey’ (i.e. quit abruptly without aid), 3) getting advice from a doctor, 4) NRT, and 5) switching to ECs with less nicotine (Figure 2 and Supplementary file Table S2). Respondents reported that the most effective methods to quit ECs were cutting down first (35.3%), quitting ‘cold turkey’ (15.8%), and switching to ECs with less nicotine (14.2%; Figure 2 and Supplementary file Table S2). Similarly, respondents endorsed a median of two strategies (of the 13 response options) used to quit CCs. The most common were: 1) cutting down to quit, 2) getting advice from a doctor, 3) quitting ‘cold turkey’, 4) NRT, and 5) switching to ECs or reading written material. Respondents reported the most effective methods to quit CCs were cutting down first (36.8%), getting advice from a doctor (17.7%), and using NRT (16.2%; Figure 2 and Supplementary file Table S2). There were moderate to large correlations between 10 of the 12 strategies used to quit that applied to both ECs and CCs (rpb range: 0.35–0.60; Supplementary file Table S4). The most highly correlated methods used to quit were reading written material (rpb=0.60, p<0.001), using bupropion (rpb=0.58, p<0.001), and cutting down to quit (rpb=0.58, p<0.001). See Table 2 for the methods to quit endorsed by the 10 respondents who achieved 30-day abstinence from ECs and the 10 respondents who achieved 30-day abstinence from CCs.
Figure 2.
Methods used to quit e-cigarettes (EC) and combustible cigarettes (CC) among dual users (N=204)
Additional quitting outcomes
During respondents’ most recent attempt to quit ECs, 34.8% (95% CI: 28.5–41.6) decreased their CC smoking, 36.3% (95% CI: 29.9–43.1) did not change their CC smoking, 20.1% (95% CI: 15.1– 26.2) increased their CC smoking, and 8.3% (95% CI: 5.2–13.0) maintained abstinence from CCs. During respondents’ most recent attempt to quit CCs, 36.8% (95% CI: 30.4–43.6) decreased their EC use, 33.8% (95% CI: 27.6–40.6) did not change their EC use, 17.6% (95% CI: 13.0–23.5) increased their EC use, and 5.9% (95% CI: 3.4–10.1) maintained abstinence from ECs. When asked how using ECs affected quitting CCs, respondents reported a mean difficulty of 6.1 (SD=2.6) where: 0=ECs made it much easier to quit CCs; and 10=ECs made it much harder to quit CCs.
DISCUSSION
Most of the adult dual users in our sample reported multiple motivations to quit ECs and most motivations to quit ECs and CCs were moderately or highly correlated. The similarities between motivations to quit is noteworthy given the differences between products (e.g. combusted vs non-combusted), but not wholly unexpected given that both products deliver nicotine. Health concerns were the most common motivation to quit ECs, which is consistent with prior research on youth and young adults seeking EC cessation treatment17 but differs from prior studies that found lack of satisfaction was a primary reason for discontinuing EC use9,11. In contrast, we found less than 10% reported motivation to quit because they ‘didn’t like’ ECs anymore. Differences in findings are likely because we assessed motivations to quit among dual users with a history of regular EC use. Much of the prior research assessed discontinuation among participants with past but not current EC use, many of whom no longer found ECs enjoyable. The large correlation between quitting ECs and CCs due to health concerns identified in this study could reflect the growing misperception that ECs are equally or more harmful than CCs26. In addition to health concerns, over 40% reported cost was a motivation to quit ECs, which is consistent with prior research on EC discontinuation9 and quitting17. Finally, though the effects of vaping on risk of harm from COVID-19 are unclear27, nearly a quarter of respondents reported that they were motivated to quit ECs to reduce risk of harm from COVID-19. While this motivation to quit overlaps with general health concerns, our findings suggest the coronavirus pandemic may represent a novel influence on nicotine and tobacco use among dual users28.
Respondents used a variety of strategies to quit ECs and most tried more than one method of quitting. Cutting down to quit was most commonly endorsed as the single most effective strategy to quit ECs and used by the majority of dual users in this study. This is consistent with national data on adult EC users6 and CC smokers29 suggesting reduction is among the most common strategies used to quit each product. Thus, reduction-based interventions could be an acceptable form of treatment for people who use both ECs and CCs. Over a quarter of respondents reported getting advice from a doctor to quit, which is consistent with prior research on the importance of medical advice when quitting CCs30. This finding highlights the need for research to inform physicians who are tasked with providing cessation advice to adult dual users. Further, nearly a quarter reported switching to low nicotine ECs to quit, which is noteworthy given the U.S. Food and Drug Administration’s authority to regulate the nicotine content of tobacco products, including ECs31. Prior research demonstrates that switching to low nicotine CCs decreases CC smoking and dependence32 but more research is needed to identify the effects of switching to low nicotine ECs. Nearly a quarter of respondents reported quitting ‘cold turkey’ (i.e. stopping abruptly without help), which may reflect the current lack of available evidence-based treatments for EC cessation. However, a similar number of respondents reported using NRT to quit ECs despite the current lack of evidence to support NRT for EC cessation. There were medium to large correlations between most strategies used to quit ECs and CCs suggesting that dual users with a history of attempts to quit both products may take similar approaches to quitting each product. Future research is needed to assess whether dual users view ECs versus CCs as their ‘primary’ nicotine product and whether this influences strategies used to quit one or both products.
During respondents’ most recent attempt to quit ECs, over one-third decreased their CC smoking and over 20% increased their CC smoking. Similarly, over one-third decreased their EC use and nearly 20% increased EC use during their last attempt to quit CCs. Most (95%) respondents failed to quit ECs, thus the effects of successfully quitting versus continuing ECs on CC smoking remain unclear. The effects of EC cessation on CC smoking could be influenced by how (e.g. quitting ECs with or without treatment) and when (e.g. quitting ECs before, during, or after quitting CCs) dual users quit. Though the effects of quitting ECs are unclear, motivation to quit ECs appears common6,7. Thus treatment is needed to maximize potential benefits (e.g. abstinence from all nicotine and tobacco products) and reduce potential harms (e.g. compensatory CC smoking) among dual users who intend to quit ECs.
Limitations
Findings are from a sample of Mechanical Turk workers with a history of attempts to quit, and thus may not be representative of dual users in the US33. The survey assessed past 30 days EC and CC use and thus we were unable to test tobacco use or dependence as predictors of motivations or methods associated with prior quit attempts. The language used to assess motivations to quit in this survey differed from some prior surveys that assessed reasons for discontinuation9,11 and thus may not be directly comparable. We assessed health risks as a motivation to quit ECs and CCs independently but did not ask about perceived risks of one product relative to the other. The misperception that ECs are equally or more harmful than CCs could have influenced respondents’ motivations to quit. Given the relatively small sample size, we were unable to test differences between age groups or EC device. We did not collect information regarding why participants initiated EC and CC use or which product participants used first. This survey relied on retrospective recall and thus is subject to recall bias. Findings are cross-sectional and do not identify the longitudinal effects of quitting ECs.
CONCLUSIONS
This survey identified a range of motivations and methods to quit ECs among adult dual users and highlights the need for longitudinal research on attempts to quit ECs. Most respondents had more than one reason and tried more than one strategy to quit. Health was the most common motivation to quit ECs and cutting down to quit was the most common method. Most reasons and strategies to quit ECs and CCs were moderately to highly correlated, suggesting a similarity in dual users’ views on quitting the two products despite the fact the CCs are combusted and ECs are not. Broadly, these findings support the need to develop treatment for adult dual users motivated to quit ECs and demonstrate dual users could engage in similar strategies to quit both products.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Catherine Peasley-Miklus, Julia West, and Sara Lepine for their help creating and distributing this survey.
CONFLICTS OF INTEREST
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none was reported.
FUNDING
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103644. The authors were supported by T32 DA 7242 (EMK) and P20GM103644 (EMK, ACV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
REFERENCES
- 1.Glasser AM, Collins L, Pearson JL, et al. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am J Prev Med. 2017;52(2):e33–e66. doi: 10.1016/j.amepre.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman BN, Rostron B, Johnson SE, et al. Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013-2014. Tob Control. 2017;26(e2):e117–e126. doi: 10.1136/tobaccocontrol-2016-053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham AL, Jacobs MA, Amato MS. Engagement and 3-Month Outcomes From a Digital E-Cigarette Cessation Program in a Cohort of 27 000 Teens and Young Adults. Nicotine Tob Res. 2020;22(5):859–860. doi: 10.1093/ntr/ntz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepper JK, Ribisl KM, Emery SL, Brewer NT. Reasons for starting and stopping electronic cigarette use. Int J Environ Res Public Health. 2014;11(10):10345–10361. doi: 10.3390/ijerph111010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzoli L, Flacco ME, Ferrante M, et al. Cohort study of electronic cigarette use: effectiveness and safety at 24 months. Tob Control. 2017;26(3):284–292. doi: 10.1136/tobaccocontrol-2015-052822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen RL, Steinberg ML. Interest in Quitting E-cigarettes Among Adults in the United States. Nicotine Tob Res. 2020;22(5):857–858. doi: 10.1093/ntr/ntz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TT, Nahhas GJ, Carpenter MJ, et al. Intention to Quit Vaping Among United States Adolescents. JAMA Pediatr. 2021;175(1):97–99. doi: 10.1001/jamapediatrics.2020.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biener L, Song E, Sutfin EL, Spangler J, Wolfson M. Electronic Cigarette Trial and Use among Young Adults: Reasons for Trial and Cessation of Vaping. Int J Environ Res Public Health. 2015;12(12):16019–16026. doi: 10.3390/ijerph121215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong HH, Borland R, Cummings KM, et al. Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction. 2019;114 Suppl 1(Suppl 1):35–48. doi: 10.1111/add.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17(2):127–133. doi: 10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etter JF, Bullen C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 12.McCaul K, Hockemeyer J, Johnson R, Zetocha K, Quinlan K, Glasgow R. Motivation to quit using cigarettes: A review. Addict Behav. 2006;31:42–56. doi: 10.1016/j.addbeh.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Chaiton M, Diemert L, Cohen JE, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6(6):e011045. doi: 10.1136/bmjopen-2016-011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD000146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes JR, Peters EN, Callas PW, et al. Withdrawal Symptoms From E-Cigarette Abstinence Among Former Smokers: A Pre–Post Clinical Trial. Nicotine Tob Res. 2020;22(5):734–739. doi: 10.1093/ntr/ntz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez S, Kaufman P, Pelletier H, et al. Is vaping cessation like smoking cessation? A qualitative study exploring the responses of youth and young adults who vape e-cigarettes. Addict Behav. 2020;113:106687. doi: 10.1016/j.addbeh.2020.106687. [DOI] [PubMed] [Google Scholar]
- 17.Amato MS, Bottcher MM, Cha S, Jacobs MA, Pearson JL, Graham AL. "It's really addictive and I'm trapped:" A qualitative analysis of the reasons for quitting vaping among treatment-seeking young people. Addict Behav. 2021;112:106599. doi: 10.1016/j.addbeh.2020.106599. [DOI] [PubMed] [Google Scholar]
- 18.Graham AL, Jacobs MA, Amato MS, Cha S, Bottcher MM, Papandonatos GD. Effectiveness of a Quit Vaping Text Message Program in Promoting Abstinence Among Young Adult E-Cigarette Users: Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2020;9(5):e18327. doi: 10.2196/18327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajek P, Peerbux S, Phillips-Waller A, Smith C, Pittaccio K, Przulj D. Are ‘dual users’ who smoke and use e-cigarettes interested in using varenicline to stop smoking altogether, and can they benefit from it? A cohort study of UK vapers. BMJ Open. 2019;9(3):e026642. doi: 10.1136/bmjopen-2018-026642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver B, Ripley-Moffitt C, Greyber J, Goldstein AO. Successful use of nicotine replacement therapy to quit e-cigarettes: lack of treatment protocol highlights need for guidelines. Clin Case Rep. 2016;4(4):409–411. doi: 10.1002/ccr3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahr M, Kelsh SE, Blower N. Pharmacist assisted vape taper and behavioral support for cessation of electronic nicotine delivery system use. Clin Case Rep. 2020;8(1):100. doi: 10.1002/ccr3.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerstrom KO. Time to first cigarette; the best single indicator of tobacco dependence? Monaldi Arch Chest Dis. 2003; 59(1):91–94. [PubMed] [Google Scholar]
- 23.Piper ME, Baker TB, Benowitz NL, Smith SS, Jorenby DE. E-cigarette Dependence Measures in Dual Users: Reliability and Relations With Dependence Criteria and E-cigarette Cessation. Nicotine Tob Res. 2020;22(5):756–763. doi: 10.1093/ntr/ntz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klemperer EM, West JC, Peasley-Miklus C, et al. Prevalence and reasons for reducing or quitting vaping in young adults: Findings from the PACE Vermont Study. Poster presented at: Society for Research on Nicotine and Tobacco Conference; New Orleans, LA. 2020. [Google Scholar]
- 25.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 26.Majeed BA, Weaver SR, Gregory KR, et al. Changing perceptions of harm of e-cigarettes among US adults, 2012–2015. Am J Prev Med. 2017;52(3):331–338. doi: 10.1016/j.amepre.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majmundar A, Allem JP, Cruz TB, Unger JB. Public health concerns and unsubstantiated claims at the intersection of vaping and COVID-19. Nicotine Tob Res. 2020;22(9) doi: 10.1093/ntr/ntaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemperer EM, West JC, Peasley-Miklus C, Villanti AC. Change in tobacco and electronic cigarette use and motivation to quit in response to COVID-19. Nicotine Tob Res. 2020;22(9) doi: 10.1093/ntr/ntaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid J, Hammond D, Tariq U, Burkhalter R, Rynard V, Douglas O. Tobacco Use in Canada: Patterns and Trends. Waterloo, ON: Propel Centre for Population Health Impact, University of Waterloo; 2019. https://uwaterloo.ca/tobacco-use-canada/sites/ca.tobacco-use-canada/files/uploads/files/tobacco_use_in_canada_2019.pdf. Accessed June 17, 2019. [Google Scholar]
- 30.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. journal;2013(5):Cd000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Drug Administration, HHS Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. Fed Regist. 2016;81(90):28973. [PubMed] [Google Scholar]
- 32.Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters K, Christakis DA, Wright DR. Are Mechanical Turk worker samples representative of health status and health behaviors in the U.S.? PLoS One. 2018;13(6):e0198835. doi: 10.1371/journal.pone.0198835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.