Abstract
We describe herein a Cu(OTf)2 catalyzed oxidative arylation of a tertiary carbon-containing substrates including aryl malononitriles, 3-aryl benzofuran-2-ones, and 3-aryl oxindoles. In some cases, the nitrile groups of the aryl malononitriles undergo further reactions leading to lactones or imines. These reaction conditions are applicable for a range of arenes, including phenols, anilines, anisoles and heteroarenes. Mechanistic studies support the formation of a cationic intermediate via a two-electron oxidation.
Graphical Abstract

Quaternary carbon centers are frequently found in natural products and bioactive molecules, and their construction is still a challenging task.1 In one approach, all-carbon quaternary centers are constructed by cross-coupling between a tertiary alkyl halide and an organometallic reagent in the presence of a transition metal catalyst.2 Alternatively, these structures can be prepared by C-H activation reactions of one substrate and combining with either an organometallic reagent or an organic halide.3 In recent years, oxidative coupling has drawn considerable attention due to its more step and atom economical approach.4 This transformation avoids using pre-functionalized reagents such as organometallic reagents and organic halides and can undergo coupling with the selective cleavage of two C-H bonds. Although this transformation is highly attractive and efficient, challenges remain in the regioselective cleavage of specific C-H bonds.
Despite recent advancements, there are limited examples of oxidative arylation on tertiary carbon centers. ortho-Quinones have been used as electrophiles in the arylation of tertiary aryl cyanoacetates and dicarbonyl compounds.5,6 The Pietruszka group performed a similar arylation of 3-aryl oxindoles with Laccase as the catalyst (Scheme 1a).7 Later, the Li group and the Loh group independently reported oxidative arylations of 3-aryl oxindoles with anisoles and heteroarenes, but no phenols, using an iron catalyst and stoichiometric graphene oxides, respectively (Scheme 1b).8 More recently, the Kambe group reported a copper-catalyzed coupling between 3-aryl benzofuran-2-ones and heteroarenes9 (Scheme 1c), and the Oshima group reported an iron-catalyzed coupling between azlactones and heteroarenes.10 The nonoxidative arylation of aryl malononitriles has only been reported using diaryliodonium salts but was limited due to the nature of the iodonium species (Scheme 1d).11 Overall, the oxidative coupling of these species with phenols and anilines has not been documented and is the focus of this report (Scheme 1e).
Scheme 1.
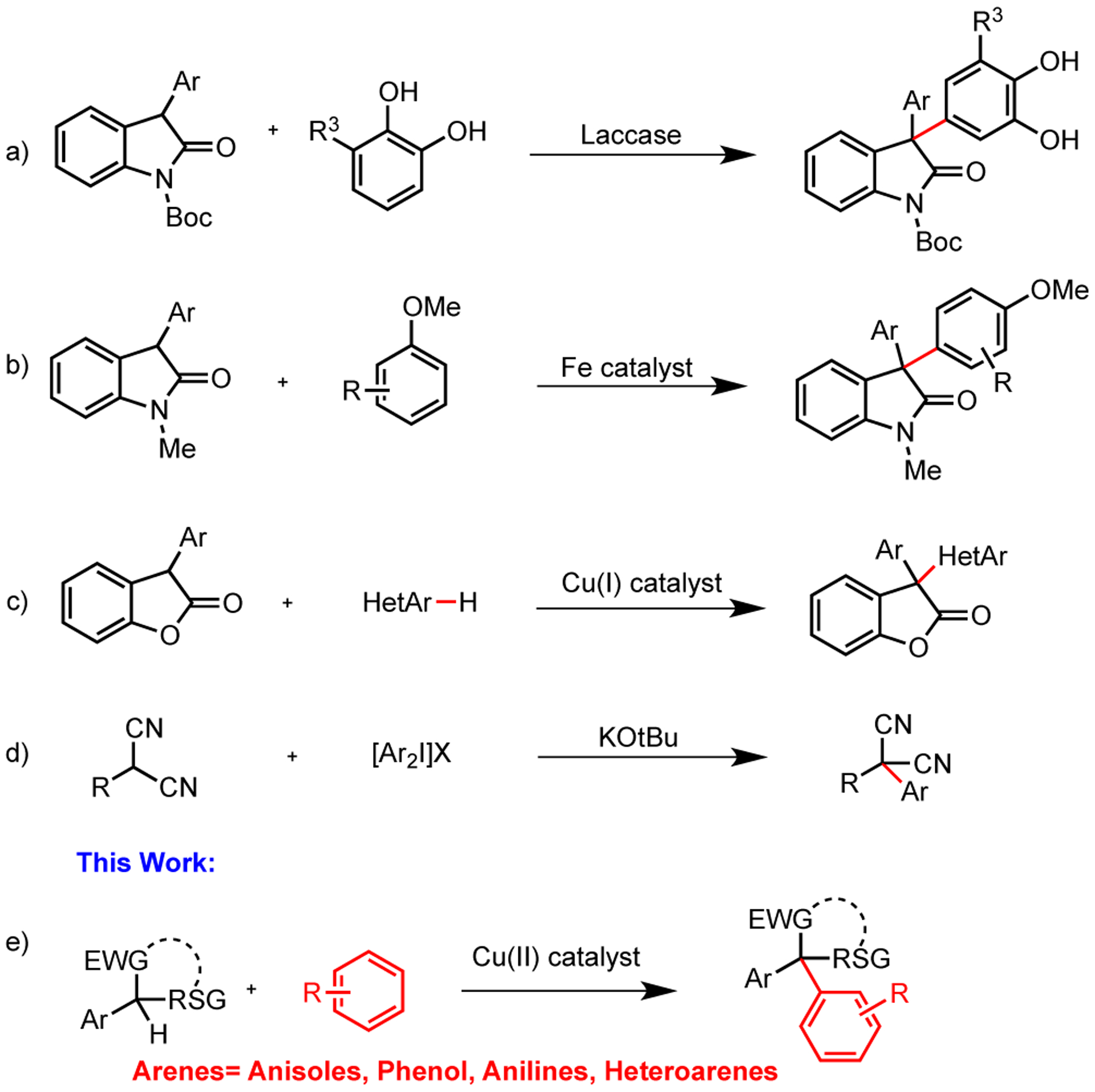
Previous Reports and This Work
Our group previously reported the palladium-catalyzed oxidative coupling of azlactones with toluene analogs through benzylic C-H activation.12 Recently, this reaction was also found to be effective for other hindered substrates like aryl malononitriles, aryl cyanoacetates, 3-aryl oxindoles, and 3-aryl benzofuranones.13 The reaction forms all carbon quaternary centers and is believed to proceed via oxidation of tertiary carbon containing substrates to form dimers (Scheme 2). These dimers are in equilibrium with the monomer captodative radicals in solution at elevated temperature.14 To take advantage of the unique ability of these substrates as radical precursors, we envisioned a novel oxidative coupling reaction of these substrates with arenes and heteroarenes (Scheme 2).
Scheme 2.
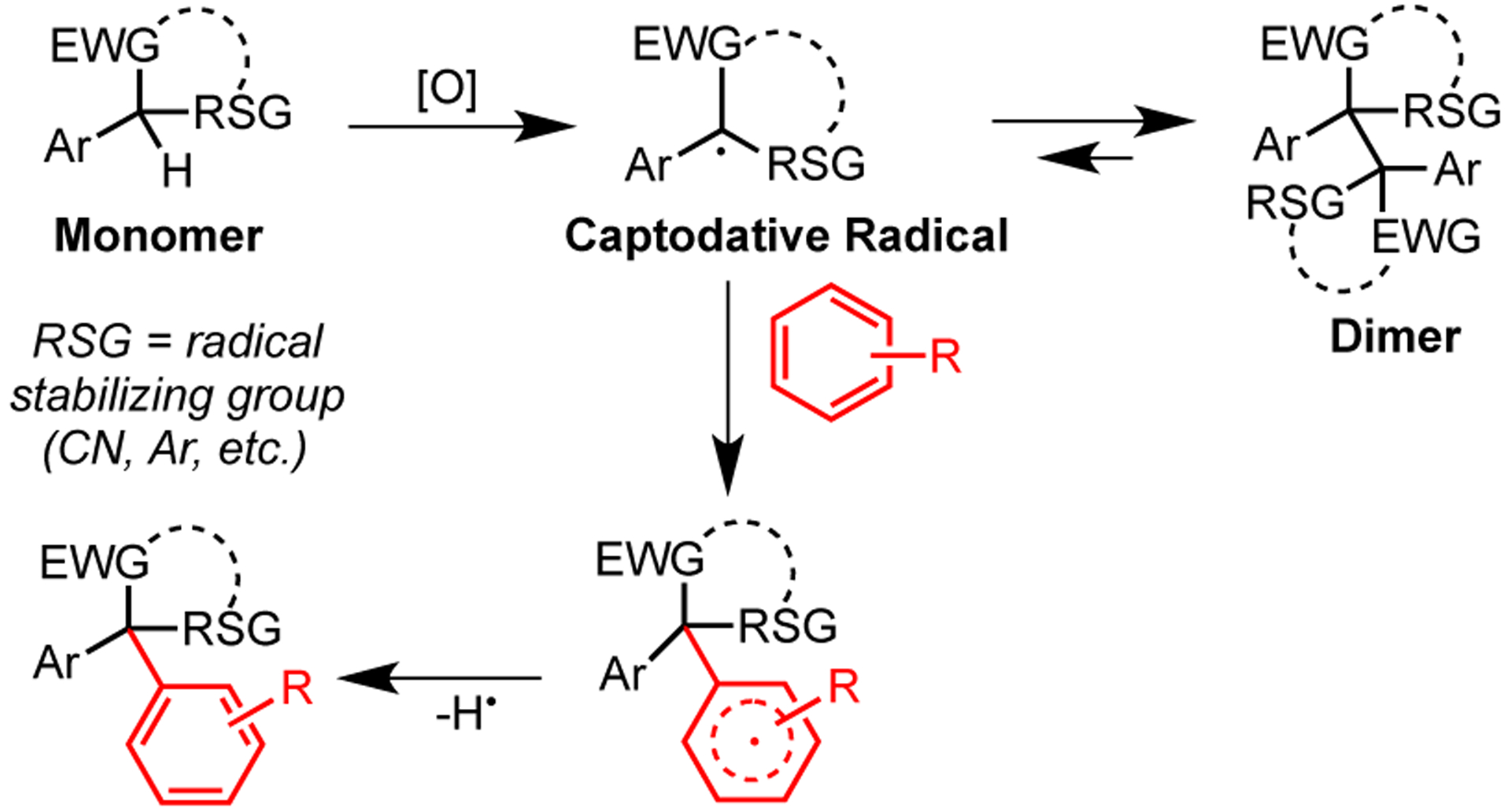
Hypothesis
The resultant adducts give rise to the structural assemblies found in biologically active natural products (Figure 1)15 In this work, we describe oxidative coupling of the tertiary carbon centers in aryl malononitriles, 3-arylbenzofuranones, and 3-aryl oxindoles with a range of arenes including phenols, anilines, and heteroarenes. Experimental and computational studies were also performed to investigate the reaction pathway.
Figure 1.
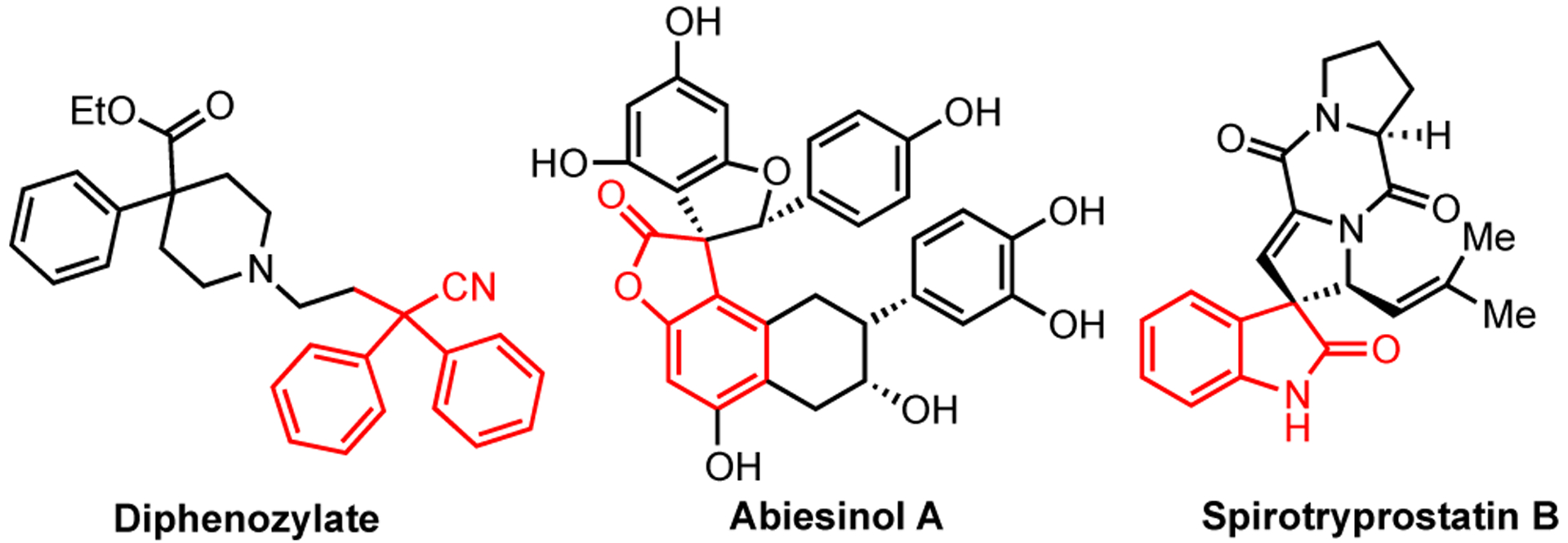
Selected bioactive compounds containing 3-aryl-substituted benzofuran-2-ones and oxindoles.
Our investigation commenced with 2-(4-methoxyphenyl) malononitrile 1a and m-cresol 2a as the coupling partners in the presence of 20 mol % CuCl2 and K2S2O8 in acetonitrile at 100 °C. After 24 h, the reaction furnished the oxidative coupling product in 65% (Table 1, entry 1). Use of 10 mol % Cu(OTf)2 instead provided the product in 92% isolated yield at 60 °C after 15 h (entry 2). In the absence of copper salt, the reaction was slow and only afforded 19% yield after 24 h (entry 3). When tert-butyl hydroperoxide (TBHP) was used as an oxidant, the yield decreased significantly to 30% (entry 4). We also observed that the yield of coupled product was lower when K2S2O8 or 2a was reduced to 1 equivalent relative to the aryl malononitrile (entries 5 and 6).
Table 1.
Development of Catalytic Arylationa
 | ||||
|---|---|---|---|---|
| entry | change to conditions | T (°C) | t (h) | yield (%)b |
| 1 | CuCl2 (20 mol %) instead of Cu(OTf)2 | 100 | 24 | 65 |
| 2 | none | 60 | 15 | 96 (92) |
| 3 | no copper | 60 | 14 | 19 |
| 4 | t-BuOOH instead of K2S2O8 | 60 | 15 | 30 |
| 5 | 1 equiv K2S2O8 | 60 | 15 | 56 |
| 6 | 1 equiv 2a | 60 | 15 | 67 |
Reaction condition: 1a (0.1mmol), 2a (0.25 mmol), Cu(OTf)2 (0.01 mmol), K2S2O8 (0.2 mmol), MeCN (0.75 mL), 60 °C for 15 h.
1H NMR yields using CH2Br2 as an internal standard. The value in parentheses is an isolated yield.
With these optimized conditions, we examined the substrate scope of aryl malononitriles with various arenes (Scheme 3). In our initial examination, different mono-substituted and di-substituted phenols were tested with aryl malononitriles. Several phenols worked well with the yield of the coupled products up to 98% (3a, 3c-3l). In addition, an anisole variant also coupled well (3b). Usually, the aryl malononitriles regioselectively coupled at the para-position of the phenols/anisole (3a-3l). When the para-position of the phenol was blocked, coupling occurred at the ortho-position and was accompanied by tandem coupling/cyclization to form benzofuran-2-one derivatives (3m-3o).16 The proximity of phenolic and nitrile group allows the nucleophilic addition of the phenolic oxygen to the electrophilic nitrile group.
Scheme 3.
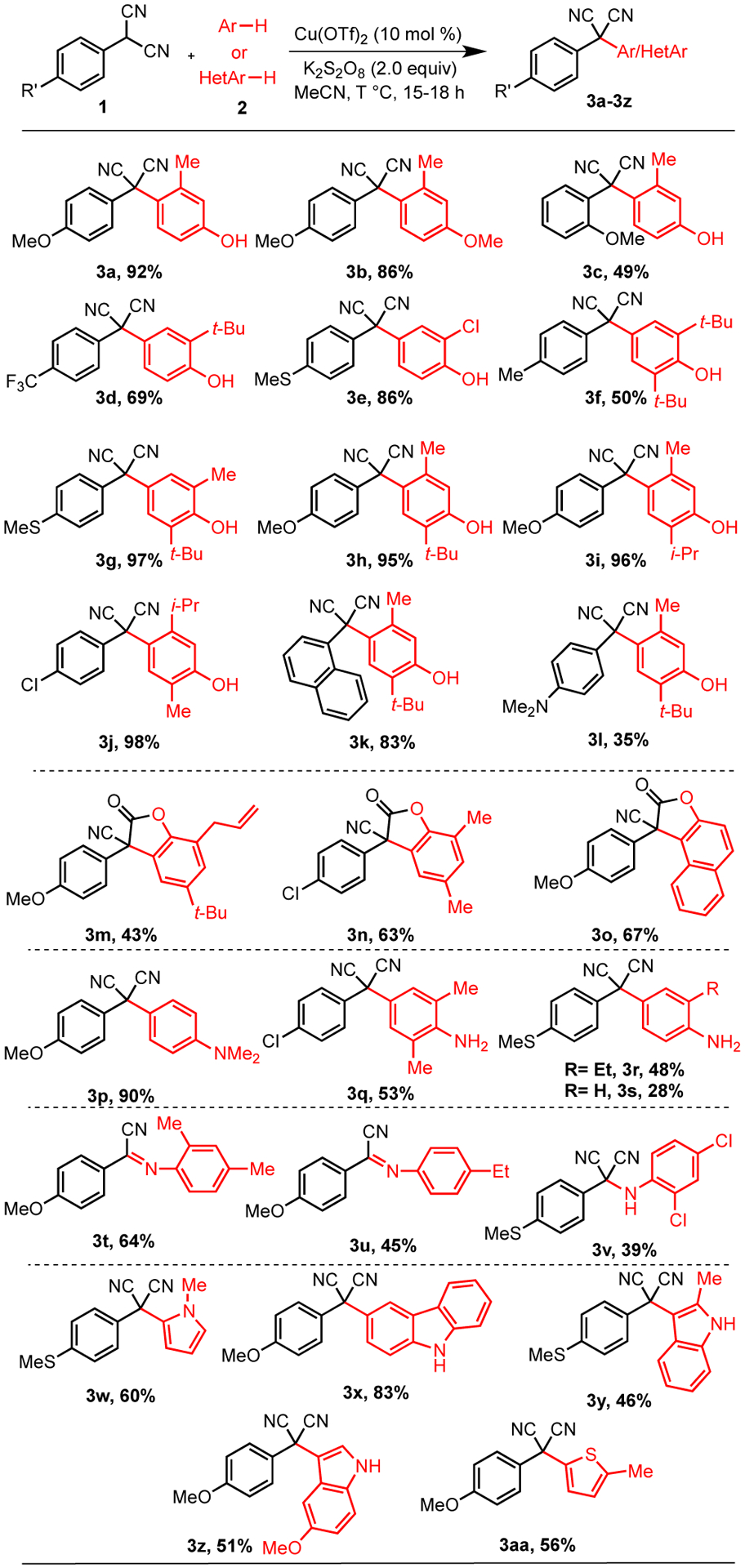
Oxidative Coupling of Aryl Malononitriles With Phenols, Anilines, and Heteroarenesa
aReaction conditions: 1 (0.1 mmol), 2 (0.25 mmol), Cu(OTf)2 (0.01 mmol), K2S2O8 (0.2 mmol), acetonitrile (0.75 mL) at 60 °C or 100 °C.
Subsequently, we investigated this reaction condition with several substituted and non-substituted anilines. N,N-Dimethylaniline generated the coupling product in excellent yield (3p). However, other anilines gave lower yields of the products (3q-3s). The lower yield may be due to the propensity of anilines to polymerize under oxidative conditions.17 para-Coupling of anilines was also observed similar to that of phenols. The site selectivity correlates well to the relative nucleophilicity of the available sites both here and with phenols.18
Unlike the para-substituted phenols where cyclization was observed, para-substituted anilines coupled via the nitrogen centers (3t-3v). When electron rich anilines were employed, subsequent E1-like elimination of HCN afforded the α-imino nitriles (3t-3u), which are useful in the synthesis of α-amino nitriles and α-amino acids. These α-iminonitriles are usually synthesized using toxic trimethyl silane or hydrogen cyanides.19 In accord with this hypothesis, electron-poor anilines, which would be less effective at stabilizing the cationic intermediate arising from cyanide loss in an E1-like mechanism, formed the α-aminonitrile instead (3v).20
Overall, a diverse range of outcomes were observed depending on the phenol or aniline used. para-Phenol coupling gave rise to a single C-C bond formation(3a-3l) while ortho-phenol coupling was accompanied by cyclization (3m-3o). Anilines are even more diverse with para-coupling (3p-3s), C-N coupling (3v), and C-N coupling accompanied by elimination(3t-3u).
Heteroarenes are common motifs in drug molecules and natural products. Owing to this central role, we aklso examined different heteroatom-containing arenes in these couplings. Nitrogen and sulfur derived heteroarenes were well tolerated with good to excellent yields (3w-3aa). On the other hand, the oxygen containing heteroarenes like furan were not effective in this reaction, which may arise from the sensitivity of furans to ring opening under oxidizing conditions.21 Regarding the scope of aryl malononitriles, the reaction proceeded well for substrates with both electron-withdrawing and electron-donating groups. The parent 2-phenylmalononitrile afforded low yield likely due to polymerization at the para-position from the radical intermediate.22 Even so, the reaction tolerates important functional groups like halides and alkenes (3e, 3j, 3m, 3n, 3q). The reaction performs well at larger scale (3.5 mmol) forming the product 3a in 83% yield.
Other tertiary carbon containing substrates including 3-aryl benzofuran-2-ones and 3-aryl oxindoles were investigated to establish scope of this process. These substrates are also known to undergo oxidative dimerization. As expected from this premise, these substrates were effective generating the corresponding products from phenols in moderate to excellent yields (Scheme 4, 5a-5e, 5j-5k), anilines (5f-5g, 5l), an anisole (5i) and heteroarenes (5h, 5m-5n). Several electron-donating and electron-withdrawing groups in these substrates as well were tolerated with a moderate to excellent yields.
Scheme 4.
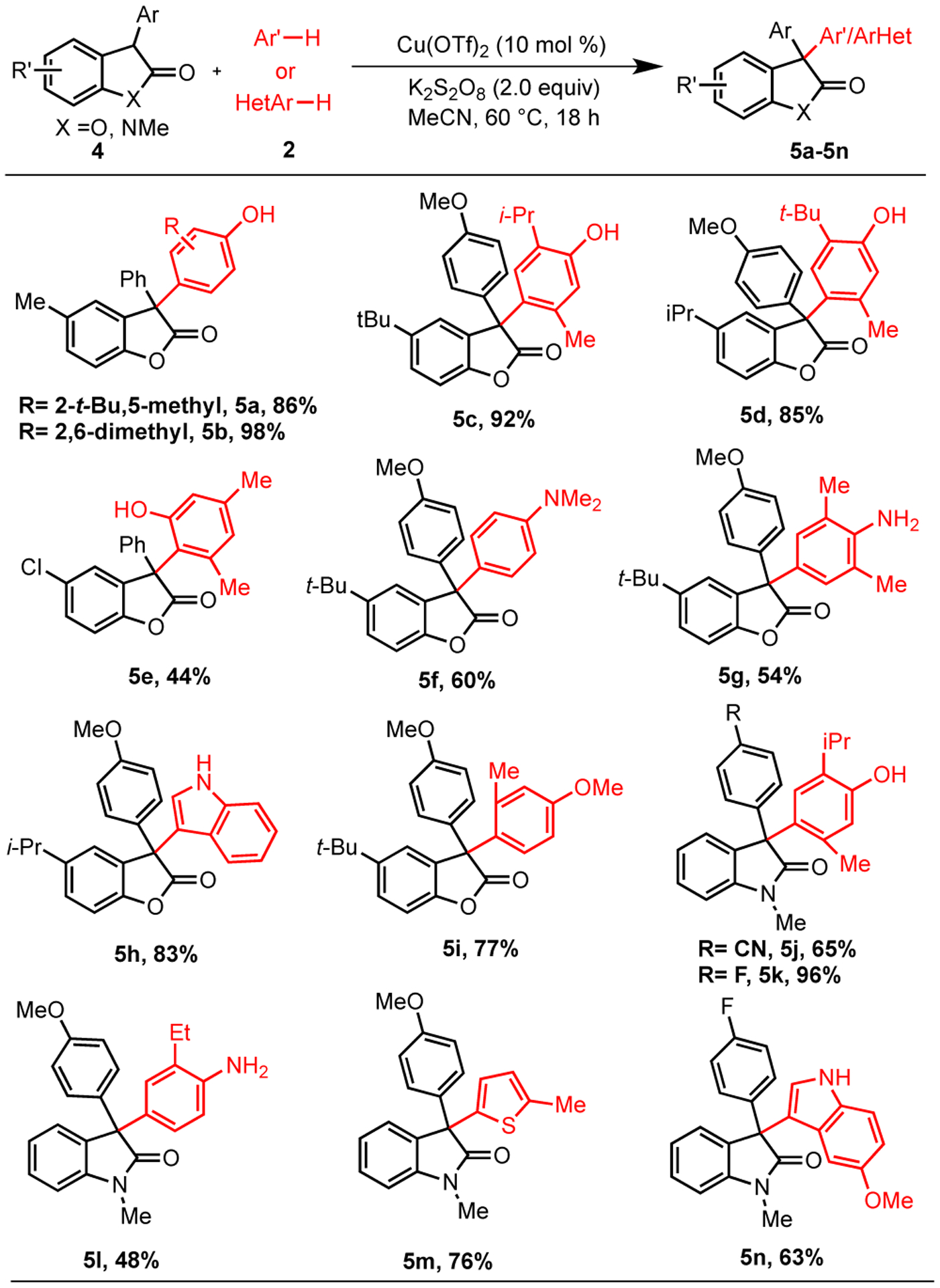
Substrate Scope of 3-Arylbenzofuran-2-ones and 3-Aryloxindoles with Phenols, Anilines and Heteroarenesa
aReaction conditions: 4 (0.1 mmol), 2 (0.25 mmol), Cu(OTf)2 (0.01 mmol), K2S2O8 (0.2 mmol), acetonitrile (0.75 mL) at 60 °C. a100 °C.
Relative to other oxidative coupling methods, the scope of the current method is distinct. For example, the copper catalyzed protocols9 and reactions of diaryliodonium salts11 would not accommodate phenols or anilines without protection. On the other hand, the diaryliodonium salts are effective in installing a phenyl group while the current method is not (predominantly dimer is observed when benzene is employed with <5% arylation).
To learn more about whether the malononitrile, benzofuran, and oxindole dimers are involved in the reaction course, the dimer 1a1, which is known to form readily upon oxidation, was subjected with 2-tert-butyl m-cresol under the standard reaction condition. The reaction formed 97% yield of the desired product (Scheme 5a).
Scheme 5.
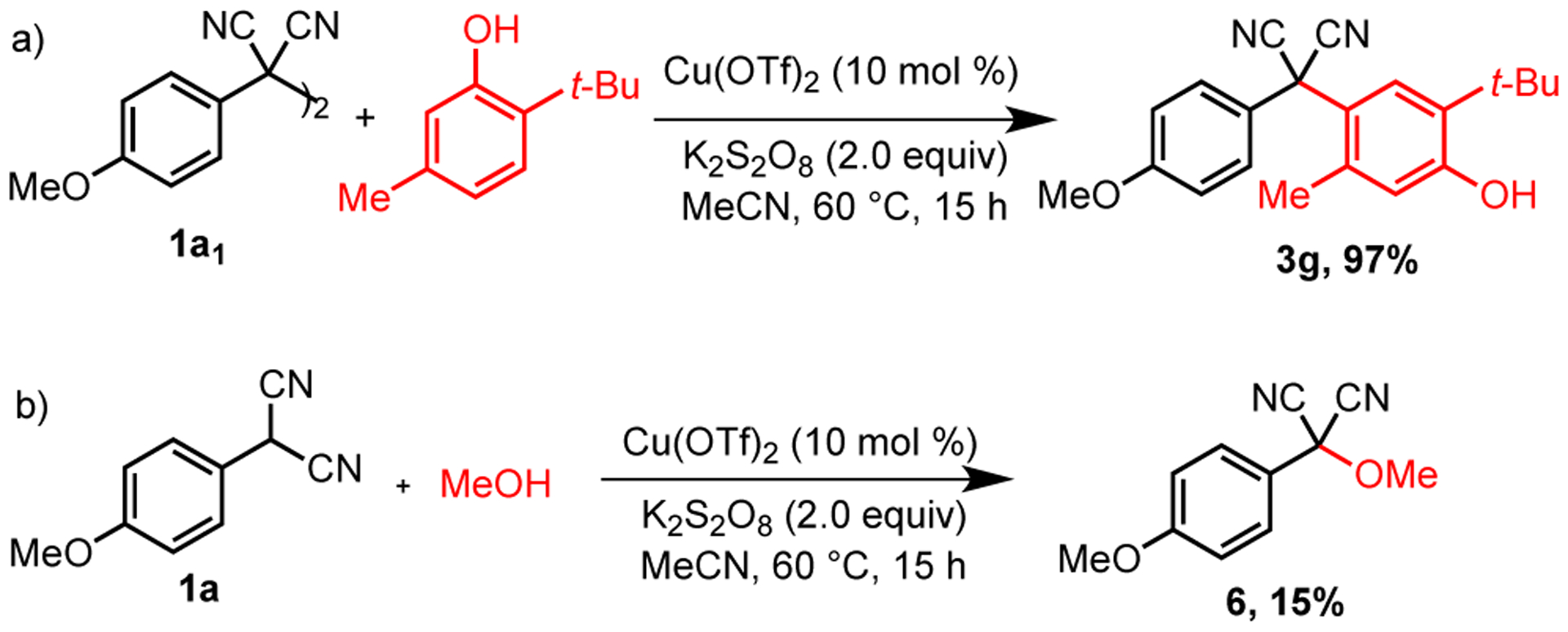
Reactions with Dimer and Methanol
To assess the reactive species under oxidative conditions the oxidation potentials of 2-(4-methoxyphenyl) malononitrile and its dimer 1a1 were measured. Due to the insolubility of 1a1 in acetonitrile, the oxidation potentials were obtained in dichloromethane and were found to be 0.075 V and 0.105 V vs. SCE (see Supporting Information), respectively. Since we used K2S2O8 (EOx= 2.01 V) as an oxidant, these results suggest that the neutral substrate can undergo two successive oxidations to form a cationic species. In order to investigate the feasibility of such a cationic intermediate in the reaction, a reaction of 1a with methanol in place of the arene partner was performed under standard conditions. The methoxy adduct 6 was isolated in 15% yield (Scheme 5b). Since alkanols are well known for trapping carbocation intermediates.23,24
Based on these results and the literature, a possible reaction pathway is outlined in Scheme 6. First the malononitrile (or benzofuran, oxindole) substrate oxidizes to form a dimer. This dimer remains in equilibrium with monomer radical in solution.14 Further oxidation of this captodative radical provides cationic intermediate B. Nucleophilic arene addition to B forms arenium intermediate C, which upon deprotonation generates the product.
Scheme 6.
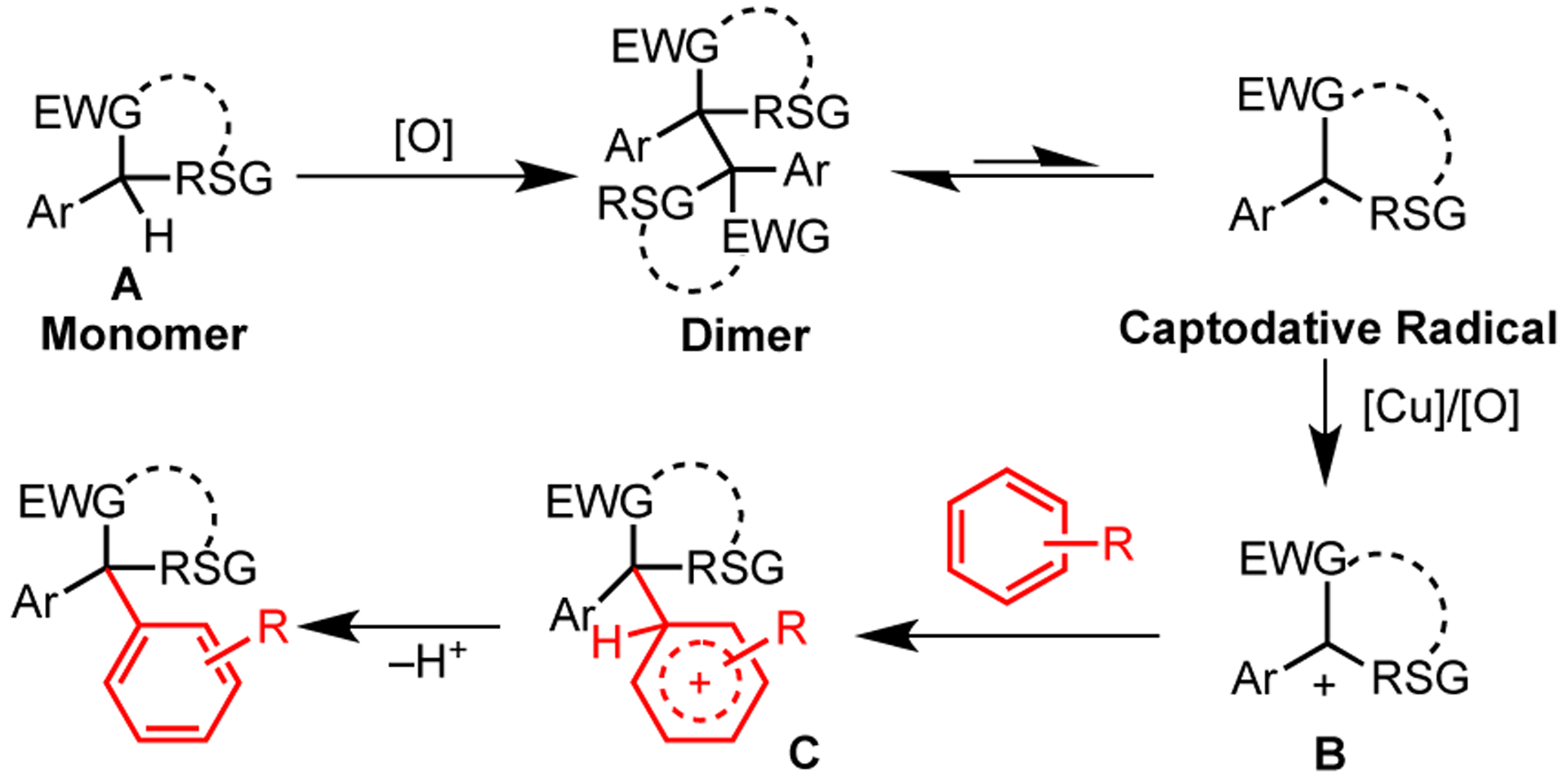
Proposed Mechanism
Based on the qualitative relative rates (para-methoxyarylmalonontirle reacts faster than the para-CF3 variant), it appears that the oxidation, not deprotonation, steps are rate limiting. Since oxidizability drives reactivity, shifting reactivity toward less substituted or more electron-poor systems will either require much stronger oxidants or a different mechanism. For example, alkyl malononitriles and oxindoles with hydrogen/alkyl in the place of the α-aryl subsitutent produced no product. Cyanoacetates also proceeded in low yield. These substrates are less capable of either stabilizing the captodative radical or carbocation intermediates in B.
In summary, we successfully developed simple and mild conditions to construct quaternary carbon centers via oxidative arylation catalyzed by Cu(OTf)2. The reaction condition performs well with a range of substrates including aryl malononitriles, 3-arylbenzofuranones, and 3-aryloxindoles undergoing addition to several classes of arenes including phenols, anisole, anilines, and heteroarenes. Mechanistic experiments suggest the formation of tertiary carbocation intermediates which initiate SEAr reactions.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the NSF (CHE1764298) and the NIH (R35 GM131902) for financial support of this research. Partial instrumentation support was provided by the NIH and NSF (1S10RR023444, 1S10RR022442, CHE-0840438, CHE-0848460, 1S10OD011980, CHE-1827457). Dr. Charles W. Ross III (UPenn) is acknowledged for obtaining accurate mass data.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectral data (PDF)
REFERENCES
- 1.(a) Büschleb M; Dorich S; Hanessian S; Tao D; Schenthal KB; Overman LE Synthetic Strategies toward Natural Products Containing Contiguous Stereogenic Quaternary Carbon Atoms. Angew. Chemie., Int. Ed 2016, 55, 4156–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Peterson EA; Overman LE Contiguous Stereogenic Quaternary Carbons: A Daunting Challenge in Natural Products Synthesis. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 11943–11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zultanski SL; Fu GC Nickel-Catalyzed Carbon-Carbon Bond-Forming Reactions of Unactivated Tertiary Alkyl Halides: Suzuki Arylations. J. Am. Chem. Soc 2013, 135, 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Thuy-Boun PS; Villa G; Dang D; Richardson P; Su S; Yu JQ Ligand-Accelerated Ortho -C-H Alkylation of Arylcarboxylic Acids Using Alkyl Boron Reagents. J. Am. Chem. Soc 2013, 135, 17508–17513. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li J; Warratz S; Zell D; De Sarkar S; Ishikawa EE; Ackermann L N-Acyl Amino Acid Ligands for Ruthenium(II)-Catalyzed Meta-C-H Tert-Alkylation with Removable Auxiliaries. J. Am. Chem. Soc 2015, 137, 13894–13901. [DOI] [PubMed] [Google Scholar]; (c) Paterson AJ; St John-Campbell S; Mahon MF; Press NJ; Frost CG Catalytic Meta-Selective C-H Functionalization to Construct Quaternary Carbon Centres. Chem. Commun 2015, 51, 12807–12810. [DOI] [PubMed] [Google Scholar]; (d) Leitch JA; McMullin CL; Paterson AJ; Mahon MF; Bhonoah Y; Frost CG Ruthenium-Catalyzed Para-Selective C−H Alkylation of Aniline Derivatives. Angew. Chemie., Int. Ed 2017, 56, 15131–15135. [DOI] [PubMed] [Google Scholar]
- 4.For reviews on CDC reactions, see:; (a) Li C Exploring C - C Bond Formations Beyond. Accounts 2009, 42, 335–344. [DOI] [PubMed] [Google Scholar]; (b) Lyons TW; Sanford MS Palladium-Catalyzed Ligand-Directed C - H Functionalization Reactions. Chem. Rev 2010, 110, 1147–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wencel-Delord J; Dröge T; Liu F; Glorius F Towards Mild Metal-Catalyzed C-H Bond Activation. Chem. Soc. Rev 2011, 40, 4740–4761. [DOI] [PubMed] [Google Scholar]; (d) Kozlowski MC Oxidative Coupling in Complexity Building Transforms. Acc. Chem. Res 2017, 50, 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Bogle KM; Hirst DJ; Dixon DJ An Organocatalytic Oxidative Coupling Strategy for the Direct Synthesis of Arylated Quaternary Stereogenic Centers. Org. Lett 2007, 9, 4901–4904. [DOI] [PubMed] [Google Scholar]; b) Bogle KM; Hirst DJ; Dixon DJ An Oxidative Coupling for the Synthesis of Arylated Quaternary Stereocentres and Its Application in the Total Synthesis of Powelline and Buphanidrine. Tetrahedron, 2010, 66, 6399–6410. [Google Scholar]
- 6.Maeno Z; Yamamoto M; Mitsudome T; Mizugaki T; Jitsukawa K Oxidative Cross-Coupling Reaction of Catechols with Active Methylene Compounds in an Aqueous Medium Using an AlPO4-Supported Ru Catalyst. Catal. Sci. Technol 2018, 8, 5401–5405. [Google Scholar]
- 7.Pietruszka J; Wang C Laccase-Catalyzed 3-Arylation of 3-Substituted Oxindoles. ChemCatChem, 2012, 4, 782–785. [Google Scholar]
- 8.a) Wu HR; Huang HY; Ren CL; Liu L; Wang D; Li CJ FeIII-Catalyzed Cross-Dehydrogenative Arylation (CDA) between Oxindoles and Arenes under an Air Atmosphere. Chem. - A Eur. J 2015, 21, 16744–16748. [DOI] [PubMed] [Google Scholar]; b) Wu H; Qiu C; Zhang Z; Zhang B; Zhang S; Xu Y; Zhou H; Su C; Loh KP Graphene-Oxide-Catalyzed Cross-Dehydrogenative Coupling of Oxindoles with Arenes and Thiophenols. Adv. Synth. Catal 2020, 362, 789–794. [Google Scholar]
- 9.Tang Z; Liu Z; Tong Z; Xu Z; Au CT; Qiu R; Kambe N Cu-Catalyzed Cross-Dehydrogenative Coupling of Heteroaryl C(Sp2)-H and Tertiary C(Sp3)-H Bonds for the Construction of All-Carbon Triaryl Quaternary Centers. Org. Lett 2019, 21, 5152–5156. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji T; Tanaka T; Tanaka T; Yazaki R; Ohshima T Catalytic Aerobic Cross-Dehydrogenative Coupling of Azlactones En Route to α,α-Disubstituted α-Amino Acids. Org. Lett 2020, 22, 4164–4170. [DOI] [PubMed] [Google Scholar]
- 11.Han J; Qian X; Xu B; Wang L Potassium Tert -Butoxide Mediated Arylation of 2-Substituted Malononitriles Using Diaryliodonium Salts. Synlett. 2017, 28, 2139–2142. [Google Scholar]
- 12.Curto JM; Kozlowski MC Chemoselective Activation of Sp 3 vs Sp 2 C − H Bonds with Pd(II). J. Am. Chem. Soc 2015, 137, 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong G; Nahide PD; Neelam UK; Amadeo P; Vijeta A; Curto JM; Hendrick CE; Vangelder KF; Kozlowski MC Bonds : Oxidative Coupling To Form Quaternary Centers. ACS Catal. 2019, 9, 3716–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenette M; Aliaga C; Font-Sanchis E; Scaiano JC Bond Dissociation Energies for Radical Dimers Derived from Highly Stabilized Carbon-Centered Radicals. Org. Lett 2004, 6, 2579–2582. [DOI] [PubMed] [Google Scholar]
- 15.(a) Lindsay-Scott PJ; Gallagher PT Synthesis of Heterocycles from Arylacetonitriles: Powerful Tools for Medicinal Chemists. Tetrahedron Lett. 2017, 58, 2629–2635. [Google Scholar]; (b) Wada SI; Hitomi T; Tokuda H; Tanaka R Anti-Tumor-Initiating Effects of Spiro-Biflavonoids from Abies Sachalinensis. Chem. Biodivers 2010, 7, 2303–2308. [DOI] [PubMed] [Google Scholar]; (c) Meyers C; Carreira EM Total Synthesis of (−)-Spirotryprostatin B**. Angew. Chem., Int. Ed 2003, 42, 694–696. [DOI] [PubMed] [Google Scholar]; (d) Bin Cui C; Kakeya H; Osada H Spirotryprostatin B, a Novel Mammalian Cell Cycle Inhibitor Produced by Aspergillus Fumigatus. J. Antibiot. (Tokyo) 1996, 49, 832–835. [DOI] [PubMed] [Google Scholar]; (e) Fleming FF; Yao L; Ravikumar PC; Funk L; Shook BC Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem 2010, 53, 7902–7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For other methods of preparation of benzofuranones see; (a) Smith BV; Bealor MD Condensation of Some Keto Esters with 2,4- and 2,6-Xylenols. J. Org. Chem 1962, 27, 3092–3096. [Google Scholar]; (b) Hajipour AR; Khorsandi Z Cobalt-Catalyzed C-H Activation/C[Sbnd]O Formation: Synthesis of Benzofuranones. Tetrahedron Lett. 2020, 61, 151396. [Google Scholar]; (c) Yang M; Jiang X; Shi WJ; Zhu QL; Shi ZJ Direct Lactonization of 2-Arylacetic Acids through Pd(II)-Catalyzed C-H Activation/C-O Formation. Org. Lett 2013, 15, 690–693. [DOI] [PubMed] [Google Scholar]
- 17.(a) Tang SJ; Wang AT; Lin SY; Huang KY; Yang CC; Yeh JM; Chiu KC Polymerization of Aniline under Various Concentrations of APS and HCl. Polym. J 2011, 43, 667–675. [Google Scholar]; (b) Tran HD; D’Arcy JM; Wang Y; Beltramo PJ; Strong VA; Kaner RB The Oxidation of Aniline to Produce “Polyaniline”: A Process Yielding Many Different Nanoscale Structures. J. Mater. Chem 2011, 21, 3534–3550. [Google Scholar]
- 18.Nieves-Quinones Y; Paniak TJ; Lee YE; Kim SM; Tcyrulnikov S; Kozlowski MC Chromium-Salen Catalyzed Cross-Coupling of Phenols: Mechanism and Origin of the Selectivity. J. Am. Chem. Soc 2019, 141, 10016–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Fontaine P; Chiaroni A; Masson G; Zhu J One-Pot Three-Component Synthesis of α-Iminonitriles by IBX/TBAB-Mediated Oxidative Strecker Reaction. Org. Lett 2008, 10, 1509–1512. [DOI] [PubMed] [Google Scholar]; (b) Seo HA; Cho YH; Lee YS; Cheon CH Formation of Amides from Imines via Cyanide-Mediated Metal-Free Aerobic Oxidation. J. Org. Chem 2015, 80, 11993–11998. [DOI] [PubMed] [Google Scholar]
- 20.For other methods of preparation of aminonitriles see; (a) Laskar DD; Prajapati D; Sandhu JS Lithium Bromide Catalyzed Ehrlich-Sachs Reactions Using Solvent-Free Conditions. Synth. Commun 2001, 31, 1427–1432. [Google Scholar]; (b) Gualtierotti JB; Schumacher X; Wang Q; Zhu J Synthesis of Iminonitriles by Oxone/Tbab-Mediated One-Pot Oxidative Three-Component Strecker Reaction. Synth. 2013, 45, 1380–1386. [Google Scholar]; (c) Seo HA; Cho YH; Lee YS; Cheon CH Formation of Amides from Imines via Cyanide-Mediated Metal-Free Aerobic Oxidation. J. Org. Chem 2015, 80, 11993–11998. [DOI] [PubMed] [Google Scholar]
- 21.Gutnov A Furan Ring as a Surrogate for Carboxy Group (Microreview). Chem. Heterocycl. Compd 2016, 52, 87–89. [Google Scholar]
- 22.(a) De Jongh HAP; De Jongh CRHI; Mijs WJ Oxidative Carbon-Carbon Coupling. I. The Oxidative Coupling of «-Substituted Benzyl Cyanides J. Org. Chem 1971, 36, 3160–3168; [Google Scholar]; (b) Kozlowski MC; DiVirgilio ES; Malolanarasimhan K; Mulrooney CA Oxidation of chiral α-phenylacetate derivatives: formation of dimers with contiguous quaternary stereocenters versus tertiary alcohols, Tetrahedron: Asymmetry, 2005,16,3599–3605. [Google Scholar]
- 23.(a) Shono T; Hamaguchi H; Matsumura Y Electroorganic Chemistry. XX. Anodic Oxidation of Carbamates. J. Am. Chem. Soc 1975, 97, 4264–4268. [Google Scholar]; (b) Xiang J; Shang M; Kawamata Y; Lundberg H; Reisberg SH; Chen M; Mykhailiuk P; Beutner G; Collins MR; Davies A; Del Bel M; Gallego GM; Spangler JE; Starr J; Yang S; Blackmond DG; Baran PS Hindered Dialkyl Ether Synthesis with Electrogenerated Carbocations. Nature, 2019, 573, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The formation of methoxy radical by oxidation with K2S2O8 cannot be ruled out:; Bartlett PD; Cotman JD The Kinetics of the Decomposition of Potassium Persulfate in Aqueous Solutions of Methanol. J. Am. Chem. Soc 1949, 71, 1419–1422. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


