Pseudomonas aeruginosa infection is one of the most difficult health care-associated infections to treat due to the ability of the organism to acquire a multitude of resistance mechanisms and express the multidrug resistance phenotype. Ceftolozane/tazobactam (C/T), a novel β-lactam/β-lactamase inhibitor combination, addresses an unmet medical need in patients with these multidrug-resistant P. aeruginosa infections.
KEYWORDS: Pseudomonas aeruginosa, ceftolozane/tazobactam, molecular characterization
ABSTRACT
This study established the in vitro activity of ceftolozane/tazobactam (C/T) and its genotypic resistance mechanisms by whole-genome sequencing (WGS) in 195 carbapenem-nonsusceptible Pseudomonas aeruginosa (CNSPA) clinical isolates recovered from Singapore between 2009 and 2020. C/T susceptibility rates were low, at 37.9%. Cross-resistance to ceftazidime/avibactam was observed, although susceptibility to the agent was slightly higher, at 41.0%. Whole-genome sequencing revealed that C/T resistance was largely mediated by the presence of horizontally acquired β-lactamases, especially metallo-β-lactamases. These were primarily disseminated in well-recognized high-risk clones belonging to sequence types (ST) 235, 308, and 179. C/T resistance was also observed in several non-carbapenemase-producing isolates, in which resistance was likely mediated by β-lactamases and, to a smaller extent, mutations in AmpC-related genes. There was no obvious mechanism of resistance observed in five isolates. The high C/T resistance highlights the limited utility of the agent as an empirical agent in our setting. Knowledge of local molecular epidemiology is crucial in determining the potential of therapy with novel agents.
IMPORTANCE Pseudomonas aeruginosa infection is one of the most difficult health care-associated infections to treat due to the ability of the organism to acquire a multitude of resistance mechanisms and express the multidrug resistance phenotype. Ceftolozane/tazobactam (C/T), a novel β-lactam/β-lactamase inhibitor combination, addresses an unmet medical need in patients with these multidrug-resistant P. aeruginosa infections. Our findings demonstrate geographical variation in C/T susceptibility owing to the distinct local molecular epidemiology. This study adds on to the growing knowledge of C/T resistance, particularly mutational resistance, and will aid in the design of future β-lactams and β-lactamase inhibitors. WGS proved to be a useful tool to understand the P. aeruginosa resistome and its contribution to emerging resistance in novel antimicrobial agents.
INTRODUCTION
Pseudomonas aeruginosa is one of the most common pathogens implicated in hospital-acquired infections (1). Aside from its intrinsic resistance to several antibiotics, its propensity to acquire resistance is responsible for its multidrug resistance profile, rendering the pathogen a therapeutic challenge (2). Carbapenems are the drugs of choice in the management of severe P. aeruginosa infections. Unfortunately, resistance to this class of agents has developed, resulting in carbapenem-nonsusceptible P. aeruginosa (CNSPA). Carbapenem nonsusceptibility rates in clinical P. aeruginosa isolates at Singapore General Hospital have hovered at approximately 8 to 10% since 2011 (3). This is congruent to the nation’s overall carbapenem resistance rate in P. aeruginosa clinical isolates derived from public hospitals (https://www.moh.gov.sg/resources-statistics/reports/one-health-report-on-antimicrobial-utilisation-and-resistance-2017). Additionally, carbapenem nonsusceptibility was detected in 24% of P. aeruginosa hospital-acquired infections in Singapore (1).
Ceftolozane/tazobactam (C/T) is a novel broad-spectrum new-generation cephalosporin/β-lactamase inhibitor combination that is highly active against P. aeruginosa. This novel agent has been designed to “escape” many of P. aeruginosa’s common resistance mechanisms, including AmpC hydrolysis, drug efflux, and OprD porin inactivation (2, 4, 5). Several studies have also demonstrated high C/T susceptibilities against CNSPA, supporting its empirical use in such infections, in which most other antibiotics are rendered ineffective (6–8). However, antibiotic susceptibilities are subject to geographical and institutional variations. The lack of local surveillance data has limited our understanding of the clinical utility of C/T in the local context. The objectives of this study were to establish the in vitro activity of C/T in a collection of CNSPA isolates recovered from Singapore and to characterize the genotypic profiles of C/T-nonsusceptible CNSPA.
(This study was presented in part at the 29th European Congress of Clinical Microbiology & Infectious Diseases, Amsterdam, Netherlands, 13 to 16 April 2019 [P1334].)
RESULTS AND DISCUSSION
Antimicrobial susceptibility profiles.
A total of 195 CNSPA isolates were included in the study. Only 74 (37.9%) isolates were susceptible (inhibited at <8 mg/liter). Table 1 shows the susceptibility patterns for various antibiotics against CNSPA. C/T demonstrated better activity than the other β-lactams, with the exception of ceftazidime/avibactam (CZA), which had a slightly higher susceptibility rate (41.0%). Only 66 (33.8%) isolates were susceptible to both C/T and CZA. Considerably higher susceptibility rates were observed for the non β-lactam antibiotics, such as amikacin (58.0%). Resistance remained rare for polymyxin B (3.1%).
TABLE 1.
Activities of antimicrobial agents against 195 clinical isolates of carbapenem-nonsusceptible Pseudomonas aeruginosa
| Antimicrobial agent | MIC (mg/liter) |
Susceptibilitya |
||||
|---|---|---|---|---|---|---|
| 50% | 90% | Range | ||||
| % S | % I | % R | ||||
| Ceftolozane/tazobactamb | ≥128/4 | ≥128/4 | ≤0.5/4 to ≥128/4 | 37.9 | 3.1 | 59.0 |
| Other β-lactam agents | ||||||
| Imipenem | 32 | ≥64 | 1 to ≥64 | 3.6 | 8.7 | 87.7 |
| Meropenem | 32 | ≥64 | ≤0.25 to ≥64 | 13.3 | 8.2 | 78.5 |
| Doripenem | 32 | ≥64 | ≤0.25 to ≥64 | 16.4 | 8.7 | 74.9 |
| Aztreonam | 32 | ≥128 | 4 to ≥128 | 13.9 | 22.0 | 64.1 |
| Cefepime | ≥128 | ≥128 | ≤1 to ≥128 | 17.4 | 10.8 | 71.8 |
| Piperacillin/tazobactam | 128/4 | ≥256/4 | 4 to ≥256/4 | 13.9 | 8.7 | 77.4 |
| Ceftazidime/avibactamb | 32/4 | ≥128/4 | 1/4 to ≥128/4 | 41.0 | 59.0 | |
| Other classes | ||||||
| Amikacin | 8 | ≥128 | ≤1 to ≥128 | 58.0 | 6.1 | 35.9 |
| Gentamicinc | 35.4 | 2.0 | 62.6 | |||
| Levofloxacin | 32 | ≥64 | ≤0.25 to ≥64 | 18.0 | 8.7 | 73.3 |
| Polymyxin B | 1 | 2 | ≤0.25 to ≥32 | 96.9 | 3.1 | |
S, susceptible; I, intermediate; R, resistant.
Susceptibility was determined using gradient MIC test strips.
Susceptibility was determined using disk diffusion or Vitek routinely at the microbiology laboratory.
Whole-genome sequencing (WGS) revealed carbapenemase production among 86 of the 195 isolates (44.1%); all 86 were nonsusceptible to C/T, as expected (C/T MIC50, ≥128/4 mg/liter; MIC90, ≥128/4 mg/liter). Hence, all 74 C/T-susceptible CNSPA isolates were observed in the 109 remaining non-carbapenemase-producing isolates, resulting in a susceptibility rate of 67.9% (C/T MIC50, 2/4 mg/liter; MIC90, ≥128/4 mg/liter) in this cohort.
The low C/T susceptibility rate (37.9%) is in contrast to several other studies conducted elsewhere, in which moderate to high susceptibility rates (ranging from 67 to 88%) were observed in multidrug-resistant or carbapenem-resistant P. aeruginosa (9–11). Even among non-carbapenemase-producing CNSPA isolates, moderate C/T susceptibility (67.9%) was observed. This suggests that C/T has limited utility as an empirical agent for suspected P. aeruginosa hospital-acquired infections in our setting, and susceptibility testing for the agent or knowledge of carbapenemase status is imperative prior to its use. Notably, most of the C/T-nonsusceptible isolates were recovered prior to the introduction of C/T into our institution, even among non-carbapenemase-producing CNSPA, substantiating that drivers of C/T resistance are likely not limited to C/T usage.
Genomic profiles of 121 C/T-nonsusceptible P. aeruginosa isolates.
A brief summary of the genomic characteristics of all 195 CNSPA isolates is presented in Table 2. C/T nonsusceptibility was detected in 22 sequence types (STs) (21 known STs and 1 novel ST) in the 121 C/T-nonsusceptible CNSPA isolates. C/T resistance in the high-risk clones of ST235 (46/121 [38.0%]) and ST308 (33/121 [27.3%]) were the most prevalent. ST175 P. aeruginosa, the international high-risk clone with AmpC hyperproduction plus OprD inactivation which has been associated with C/T resistance, was not found in our study (12). In contrast, the ST types in C/T-susceptible isolates were even more widely distributed (58 different STs).
TABLE 2.
Genotypic characteristics of 195 CNSPA isolates
| Parameter | C/T-nonsusceptible isolates (n = 121) |
C/T-susceptible isolates (n = 74) | |
|---|---|---|---|
| Carbapenemase producers (n = 86) | Non-carbapenemase producers (n = 35) | ||
| No. of STs | 12 | 14 (13 + 1 new) | 58 (55 + 3 new) |
| Known STsa | 233, 235, 244, 308, 316, 357, 621, 773, 823, 964, 3440, 3444 | 155, 179, 235, 244, 252, 274, 313, 316, 357, 664, 815, 1076, 1666 | 11, 17, 27, 111, 155, 207, 235, 244, 245, 253, 266, 274, 292, 298, 314, 357, 389, 408, 446, 463, 471, 485, 508, 553, 560, 564, 569, 606, 620, 645, 697, 708, 773, 792, 815, 840, 882, 1076, 1247, 1649, 1930, 2013, 2021, 2033, 2069, 2326, 2476, 2651, 3078, 3311, 3439, 3442, 3443, 3445, 3446 |
| Harbors acquired β-lactamase | 10 | 24 | 5 |
| AmpC and regulator alterationb | 10 | 6 | |
| PBP3 alterationb | 3 | 1 | |
| Ceftazidime/avibactam susceptible | 4 | 10 | 66 |
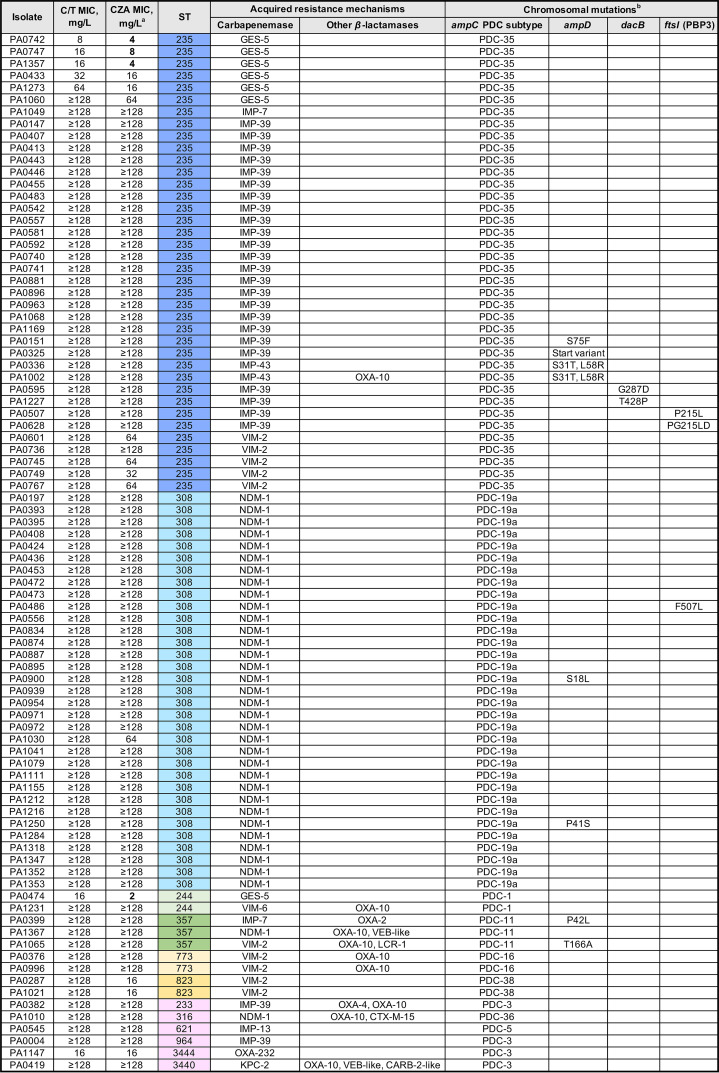
We analyzed the resistance mechanisms for the 121 C/T-nonsusceptible isolates. Figures 1 and 2 depict the isolates’ characteristics and the potential mechanisms responsible for C/T nonsusceptibility in 86 carbapenemase-producing and 35 non-carbapenemase-producing CNSPA isolates, respectively. C/T nonsusceptibility can be explained primarily by the presence of horizontally acquired carbapenemases in a large proportion of the C/T-nonsusceptible isolates (86/121 [71.1%]). The predominant types of genes detected were metallo-β-lactamases: blaNDM (35 isolates), blaIMP (31 isolates), and blaVIM (11 isolates). Carbapenem-hydrolyzing blaGES-5 accounted for the remaining isolates, with the exception of two isolates which harbored blaKPC-2 and blaOXA-232.
FIG 1.
Mechanisms of ceftolozane/tazobactam (C/T) resistance in 86 carbapenemase-producing CNSPA isolates. a, Bold values indicate ceftazidime/avibactam (CZA) susceptibility. b, The main chromosomal mutations (ampC, ampR, dacB, and ftsI) leading to amino acid substitutions compared to the reference wild-type comparator amino acid sequences from Pseudomonas aeruginosa PAO1 are shown. The list of nonsynonymous variations were refined to include only those more likely to be involved in the C/T-resistant phenotype, i.e., (i) mutations with known effect on resistance according to published evidence and (ii) mutations with predicted functional impact (i.e., deleterious) and not identified in wild-type/susceptible isolates. There were no mutations found in ampR in this set of isolates. PDC, Pseudomonas-derived cephalosporinase; ST, sequence type.
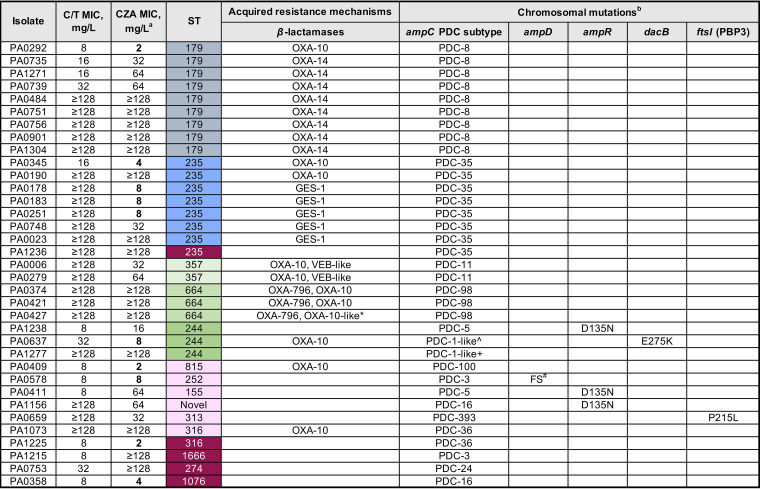
FIG 2.
Mechanisms of C/T resistance in 35 non-carbapenemase-producing CNSPA isolates. a, Bold values indicate CZA susceptibility. b, The main chromosomal mutations (ampC, ampR, dacB, and ftsI) leading to amino acid substitutions compared to the reference wild-type comparator amino acid sequences from Pseudomonas aeruginosa PAO1 are shown. The list of nonsynonymous variations was refined to include only those more likely to be involved in the C/T-resistant phenotype, i.e., (i) mutations with known effect on resistance according to published evidence and (ii) mutations with predicted functional impact (i.e., deleterious) and not identified in wild-type/susceptible isolates. *, G439C amino acid substitution in OXA-10; ^, A163T amino acid substitution in AmpC; +, ΔK74-E75 in AmpC; #, frameshift (FS) at position 149.
In the 35 non-carbapenemase-producing C/T-nonsusceptible CNSPA isolates, horizontally acquired extended-spectrum β-lactamases (ESBLs) were frequently observed (24/35 [68.6%]). Notably, blaOXA-14, the extended-spectrum variant of blaOXA-10 which has been associated with C/T resistance (13), was detected in eight isolates, all of which were ST179. blaVEB, blaGES-1, blaOXA-10, and other blaOXA variants were also detected. C/T appeared to have variable activity in P. aeruginosa with secondary ESBLs. Various ESBLs such as those encoded by blaGES and blaVEB have been shown to inactivate C/T (14).
We noted that the distribution of these exogenous β-lactam resistance elements was limited primarily to three main clones, ST235 (n = 46), ST308 (n = 33), and ST179 (n = 9), which accounted for 72.7% of the C/T-nonsusceptible isolates. Within each clone, there was little or no intraclonal variation. This suggests that multidrug resistance, including C/T resistance, is contributed primarily by a limited number of clones which have gained a strong foothold in our setting, although P. aeruginosa organisms of other diverse STs could also acquire these ESBLs/carbapenemases over time, resulting in broad-spectrum resistance.
As ceftolozane is neither affected by efflux pumps nor transported via OprD, resistance is driven primarily by acquisition of ESBLs, AmpC hyperproduction, AmpC structural modifications, or mutations in PBP3 (15, 16). Since we observed a number of C/T-nonsusceptible isolates without any carbapenemases/ESBLs or harboring only narrow-spectrum beta-lactamases like blaOXA-10, we analyzed the chromosomal genes related to AmpC and its expression (ampC and the regulator genes ampD, ampR, and dacB), as well as the ftsI gene (encoding PBP3), which is the target binding site of C/T. Our analysis revealed that most of the 121 C/T-nonsusceptible CNSPA isolates harbored single nucleotide polymorphisms (SNPs) resulting in nonsynonymous AmpC amino acid substitutions. The number of amino acid substitutions ranged from 0 to 5 (median, 5). This is congruent to the high sequence polymorphism of AmpC reported for P. aeruginosa (16). Classification of the isolates based on Pseudomonas-derived cephalosporinase (PDC) subtypes showed a total of 17 different subtypes. The PDC-35 subtype was the most prevalent; it was detected solely in the 46 ST235 isolates. This was followed by PDC-19a, which was found exclusively in the 33 ST308 isolates. The majority of these AmpC amino acid substitutions were unlikely to be associated with C/T nonsusceptibility, as they were either similarly found in the susceptible strains in our study or have been described for wild-type strains elsewhere. We did not detect any SNPs implicated in C/T nonsusceptibility which had been described in literature previously (4, 16, 17). However, we did observe potentially deleterious variants (A163T in PA0637 and a 2-amino-acid deletion, K74-E75, in PA1277) in two isolates (Fig. 2). Deleterious SNPs in the other ampD, ampR, and dacB regulator genes and PBP3 variants were infrequently observed, occurring in only approximately 16% of the isolates. There were five nonsusceptible isolates (highlighted in dark purple in Fig. 2) which did not appear to have any ESBLs/carbapenemases or alterations in AmpC or PBP3. The identified deleterious SNPs in this study have not been reported in the literature, and thus, their role in mediating C/T resistance requires further validation.
Although the aim of this study did not include a detailed investigation of the mechanisms of CZA resistance, we noted that there were differential susceptibilities in the two agents. Cross-resistance was high due to the high prevalence of metallo-β-lactamases, which both agents were inactive against. In contrast to tazobactam, avibactam was designed to have potent activity against class C β-lactamases and have a slightly broader anti β-lactamases activity (inclusive of KPC and OXA-48) (14, 17). This could explain the observation of the 14 (11.6%) isolates among the 121 C/T-nonsusceptible CNSPA isolates which remained susceptible to CZA (Table 2). These isolates primarily harbored GES and OXA β-lactamases (Fig. 1 and 2), which can be inhibited by avibactam. Additionally, 8 (10.8%) of the 74 C/T-susceptible CNSPA were resistant to CZA, which had moderate MICs near the breakpoint (16 mg/liter). We postulate that resistance in these isolates may be attributed to drug efflux and/or decreased cell permeability in the presence of low levels of AmpC overexpression which may still be overcome by C/T (18–20). Nevertheless, due to the slight difference in the activities of the two agents, there may be a role for each agent, depending on the molecular epidemiology of the setting.
Concluding remarks.
CNSPA is a major treatment challenge due to a lack of available effective agents. Novel agents such as the AmpC-stable C/T are introduced in a bid to expand the armamentarium against these difficult-to-treat organisms. In this study, we assessed the rates of in vitro susceptibility to C/T and the molecular mechanisms mediating C/T resistance in CNSPA recovered from a large tertiary hospital in Singapore where C/T has only recently (January 2019) been introduced into its formulary.
The observed high C/T nonsusceptibility rates in our CNSPA, together with cross-resistance to CZA, the other novel β-lactamase inhibitor combination, signify a severe therapeutic challenge in CNSPA infections. Our results also affirm the limited use of C/T as an empirical agent in our setting, reserving the agent for culture-directed indications. Aside from polymyxin B and amikacin, which are often associated with toxicities, there are no safer and tolerable options for our multidrug-resistant CNSPA, prompting the urgent need to explore the use of other novel agents, such as cefiderocol or combination therapy, to fill the gaps in the armamentarium against CNSPA in our setting (21).
The high nonsusceptibility rates may be corroborated by our molecular findings. There is a high prevalence of well-established multidrug-resistant carbapenemase-producing P. aeruginosa high-risk clones (ST235, ST308, and ST179) among the C/T-nonsusceptible isolates. Additionally, diverse STs can also acquire ESBLs/carbapenemases, leading to reduced β-lactam susceptibilities. The role of constitutive AmpC variants leading to structural modifications and/or hyperproduction in mediating C/T resistance appeared to be minimal in our population. The ampC gene in P. aeruginosa is highly polymorphic, and mutations did not necessarily translate to changes in C/T phenotype.
A limitation of this study is that we did not measure the change in expression levels in AmpC. AmpC hyperproduction mediated by mutations in other unstudied genes may have been responsible for C/T resistance. Though they appear to be rare, we are not aware of the true proportion of AmpC hyperproducers in our population. However, more importantly, C/T resistance in strains not producing carbapenemases/acquired ESBLs predates the introduction of C/T into our clinical practice. This highlights that C/T resistance could result independently of C/T use, which could be due to the rampant use of other β-lactam antibiotics that are able to induce or derepress AmpC production (22). There is also a possibility that C/T resistance is independent of AmpC or β-lactamase activity. Mechanisms driving C/T resistance still need to be further explored.
Although C/T has been reported to be highly active against P. aeruginosa and retained susceptibility in CNSPA elsewhere in the world, susceptibility is not universal. The prevalence of C/T resistance is related to the molecular epidemiology of P. aeruginosa, which can vary temporally and geographically. In our setting, where prevalence of acquired β-lactamases is high, the utility of C/T is limited. Knowledge of the molecular epidemiology and genotypes is important in evaluating the place of therapy with novel agents.
MATERIALS AND METHODS
Collection of bacterial isolates.
Nonduplicate clinical CNSPA isolates, which exhibited nonsusceptibility to at least one carbapenem (doripenem, meropenem, imipenem), collected at the Singapore General Hospital (SGH) Pharmacy Research Laboratory between 2009 and 2020 from various culture sites (blood [53.5%], lower respiratory specimens [16.1%], skin and soft tissue [11.6%], urine [5.8%], and other sites, including bone, gastrointestinal tract, etc. [13.0%]), were studied. Isolates were randomly selected for testing from the laboratory’s repository, which comprised CNSPA isolates collected from an informal carbapenem-nonsusceptible Gram-negative pathogen surveillance study of hospital inpatients initiated in 2015. Isolates from prior to 2015 were collected via convenience sampling or were submitted to the laboratory for antibiotic combination testing.
These isolates were subjected to genus identification and confirmation as per the institution’s microbiology laboratory routine procedures, i.e., using Vitek GNI+ cards with the Vitek 2 instrument (bioMérieux, Hazelwood, MO) and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) system (Bruker Daltonik, Germany), if necessary. All isolates were preserved in Microbank cryovials (Pro-Lab Diagnostics, Richmond Hill, ON, Canada) at −80°C and subcultured twice on Trypticase soy agar-5% sheep blood plates (BD, Sparks, MD) before experimental testing.
Antibiotic susceptibilities.
Susceptibilities to meropenem, imipenem, doripenem, cefepime, piperacillin/tazobactam, levofloxacin, amikacin, and polymyxin B were determined using customized 96-well broth microdilution plates (TREK Diagnostics, East Grinstead, UK) in accordance with the manufacturer’s recommendations. Gradient MIC test strips were used to determine ceftazidime/avibactam (bioMérieux, Marcy l’Etoile, France) and ceftolozane/tazobactam (Liofilchem, Roseto degli Abruzzi, Italy) susceptibilities. Ceftazidime susceptibility was not routinely tested in this institution, as the agent was reserved primarily for the treatment of melioidosis. All MICs were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (23). P. aeruginosa ATCC 27853 was used as the quality control strain.
DNA preparation and whole-genome sequencing.
Genomic DNAs were extracted and purified from overnight bacterial cultures with the DNeasy blood and tissue kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol. Paired-end whole-genome sequencing (WGS) was performed on the genomic DNAs using the MiSeq/HiSeq systems (Illumina Inc., CA), with a resultant coverage of at least 100-fold. Raw sequences were assessed for quality using FastQC (v0.11.3, Babraham Institute), followed by removal of adaptors and poor-quality bases using Trimmomatic (24, 25). Trimmed sequences were then assembled de novo using SPAdes software (26).
Genotypic profiling.
Acquired resistance genes were identified using the SRST2 package (v0.2.0), which mapped raw short reads to the ARG-ANNOT database (27, 28). Selected chromosomal gene targets related to C/T susceptibility were analyzed by aligning assembled sequences to the PAO1 reference genome (GenBank accession no. AE004091.2), and variants were called with the pipeline Snippy (v4.6.0) (available at https://github.com/tseemann/snippy). The Protein Variation Effect Analyzer (PROVEAN) software tool was used to predict the impact of identified amino acid substitutions on protein biological function, i.e., whether the amino acid substitution was neutral or deleterious (http://provean.jcvi.org/index.php) (29). Sequence types (STs) were identified using the Basic Local Alignment Search Tool (BLAST) against the PubMLST database (https://pubmlst.org/paeruginosa/).
Ethics statement.
This study is exempted from review by the Singhealth Centralised Institutional Review Board, as it is a retrospective study involving archival bacterial isolates, which does not fall under the Human Biomedical Research Act. No identifiable data were collected.
Accession number(s).
Whole-genome sequences of the C/T-nonsusceptible CNSPA are available in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA656645.
ACKNOWLEDGMENTS
We thank the laboratory staff at the Singapore General Hospital Microbiology Laboratory for assisting in the collection of the isolates.
Andrea Lay-Hoon Kwa received unrestricted funding for investigator-initiated research from Pfizer, Inc., and Merck Sharp & Dohme Corp. This study was supported by funding from the National Medical Research Council, Singapore (NMRC/CG/C005/2017, NMRC/CG/M011/2017, NMRC/TA/0056/2017, and NMRC/MOH324 000018-00).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Cai Y, Venkatachalam I, Tee NW, Tan TY, Kurup A, Wong SY, Low CY, Wang Y, Lee W, Liew YX, Ang B, Lye DC, Chow A, Ling ML, Oh HM, Cuvin CA, Ooi ST, Pada SK, Lim CH, Tan JWC, Chew KL, Nguyen VH, Fisher DA, Goossens H, Kwa AL, Tambyah PA, Hsu LY, Marimuthu K. 2017. Prevalence of healthcare-associated infections and antimicrobial use among adult inpatients in Singapore acute-care hospitals: results from the First National Point Prevalence Survey. Clin Infect Dis 64:S61–S67. doi: 10.1093/cid/cix103. [DOI] [PubMed] [Google Scholar]
- 2.Livermore DM 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 3.Teo JQ, Cai Y, Lim TP, Tan TT, Kwa AL. 2016. Carbapenem resistance in Gram-negative bacteria: the not-so-little problem in the little red dot. Microorganisms 4:13. doi: 10.3390/microorganisms4010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabot G, Bruchmann S, Mulet X, Zamorano L, Moya B, Juan C, Haussler S, Oliver A. 2014. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother 58:3091–3099. doi: 10.1128/AAC.02462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buehrle DJ, Shields RK, Chen L, Hao B, Press EG, Alkrouk A, Potoski BA, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 60:3227–3231. doi: 10.1128/AAC.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupper M, Sutherland C, Nicolau DP. 2017. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible Pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob Agents Chemother 61:e00875-17. doi: 10.1128/AAC.00875-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kresken M, Korber-Irrgang B, Korte-Berwanger M, Pfennigwerth N, Gatermann SG, Seifert H, German Carbapenem Resistance Study Group . 2020. Dissemination of carbapenem-resistant Pseudomonas aeruginosa isolates and their susceptibilities to ceftolozane-tazobactam in Germany. Int J Antimicrob Agents 55:105959. doi: 10.1016/j.ijantimicag.2020.105959:105959. [DOI] [PubMed] [Google Scholar]
- 9.Skoglund E, Ledesma KR, Lasco TM, Tam VH. 2017. Ceftolozane/tazobactam activity against meropenem-non-susceptible Pseudomonas aeruginosa bloodstream infection isolates. J Glob Antimicrob Resist 11:154–155. doi: 10.1016/j.jgar.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Mirza HC, Hortaç E, Koçak AA, Demirkaya MH, Yayla B, Güçlü AÜ, Başustaoğlu A. 2020. In vitro activity of ceftolozane-tazobactam and ceftazidime-avibactam against clinical isolates of meropenem-non-susceptible Pseudomonas aeruginosa: a two-centre study. J Glob Antimicrob Resist 20:334–338. doi: 10.1016/j.jgar.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Karlowsky JA, Lob SH, Raddatz J, DePestel DD, Young K, Motyl MR, Sahm DF. 3 April 2020. In vitro activity of imipenem/relebactam and ceftolozane/tazobactam against clinical isolates of Gram-negative bacilli with difficult-to-treat resistance and multidrug-resistant phenotypes—SMART United States 2015–2017. Clin Infect Dis doi: 10.1093/cid/ciaa381. [DOI] [PubMed] [Google Scholar]
- 12.Del Barrio-Tofino E, Lopez-Causape C, Cabot G, Rivera A, Benito N, Segura C, Montero MM, Sorli L, Tubau F, Gomez-Zorrilla S, Tormo N, Dura-Navarro R, Viedma E, Resino-Foz E, Fernandez-Martinez M, Gonzalez-Rico C, Alejo-Cancho I, Martinez JA, Labayru-Echverria C, Duenas C, Ayestaran I, Zamorano L, Martinez-Martinez L, Horcajada JP, Oliver A. 2017. Genomics and susceptibility profiles of extensively drug-resistant Pseudomonas aeruginosa isolates from Spain. Antimicrob Agents Chemother 61:e01589-17. doi: 10.1128/AAC.01589-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraile-Ribot PA, Cabot G, Mulet X, Perianez L, Martin-Pena ML, Juan C, Perez JL, Oliver A. 2018. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother 73:658–663. doi: 10.1093/jac/dkx424. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz de la Rosa JM, Nordmann P, Poirel L. 2019. ESBLs and resistance to ceftazidime/avibactam and ceftolozane/tazobactam combinations in Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother 74:1934–1939. doi: 10.1093/jac/dkz149. [DOI] [PubMed] [Google Scholar]
- 15.Castanheira M, Mills JC, Farrell DJ, Jones RN. 2014. Mutation-driven beta-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother 58:6844–6850. doi: 10.1128/AAC.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrazeg M, Jeannot K, Ntsogo Enguene VY, Broutin I, Loeffert S, Fournier D, Plesiat P. 2015. Mutations in beta-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother 59:6248–6255. doi: 10.1128/AAC.00825-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papp-Wallace KM, Mack AR, Taracila MA, Bonomo RA. 2020. Resistance to novel beta-lactam-beta-lactamase inhibitor combinations: the “price of progress.” Infect Dis Clin North Am doi: 10.1016/j.idc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, Adkin R, Chaudhry A, Pike R, Staves P, Woodford N, BSAC Resistance Surveillance Standing Committee . 2017. Activity of ceftolozane/tazobactam against surveillance and 'problem' Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 72:2278–2289. doi: 10.1093/jac/dkx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int J Antimicrob Agents 34:402–406. doi: 10.1016/j.ijantimicag.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Wang J, Wang R, Cai Y. 2020. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist 22:18–27. doi: 10.1016/j.jgar.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Doi Y 2019. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis 69:S565–S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torrens G, Hernandez SB, Ayala JA, Moya B, Juan C, Cava F, Oliver A. 2019. Regulation of AmpC-driven beta-lactam resistance in Pseudomonas aeruginosa: different pathways, different signaling. mSystems 4:e00524-19. doi: 10.1128/mSystems.00524-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CLSI. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed CLSI, Wayne, PA. [Google Scholar]
- 24.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews S 2010. FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom. [Google Scholar]
- 26.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inouye M, Dashnow H, Raven L-A, Schultz MB, Pope BJ, Tomita T, Zobel J, Holt KE. 2014. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain J-M. 2014. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi Y, Chan AP. 2015. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31:2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]