Abstract
In 2007, common vampire bats were the source of the first outbreak of paralytic bovine rabies in Uruguay. The outbreak coincided in space and time with the fragmentation of native grasslands for monospecific forestry for wood and cellulose production. Using spatial analyses, we show that the increase in grassland fragmentation, together with the minimum temperature in the winter, accounts for the spatial pattern of outbreaks in the country. We propose that fragmentation may increase the connectivity of vampire bat colonies by promoting the sharing of feeding areas, while temperature modulates their home range plasticity. While a recent introduction of the virus from neighboring Brazil could have had an effect on outbreak occurrence, we show here that the distribution of rabies cases is unlikely to be explained by only an invasion process from Brazil. In accordance with previous modeling efforts, an increase in connectivity may promote spatial persistence of rabies virus within vampire bat populations. Our results suggest that land use planning might help to reduce grassland fragmentation and thus reduce risk of rabies transmission to livestock. This will be especially important in the context of climatic changes and increasing minimum temperatures in the winter.
Keywords: Spatial autoregressive models, Geographically weighted regression, Desmodus rotundus, Minimum mean temperature, Spillover, Uruguay
Introduction and Purpose
The combination of landscape change and increasing anthropogenic food availability for wildlife may influence wildlife demographics, foraging behavior, and immune responses, affecting the spatial and temporal dynamics of infectious diseases (Gottdenker et al. 2014; Becker et al. 2015). Species’ behavioral responses to landscape change in particular may be bounded by their ability to adapt physiologically to changing environmental conditions (Weiner 1992; Wong and Candolin 2015). In particular, low temperatures and associated heat loss might limit the energy budget of species and their ability to respond plastically (Weiner 1992). Hence, populations living close to the limit of the species distribution could exhibit lower plasticity to respond to various environmental changes.
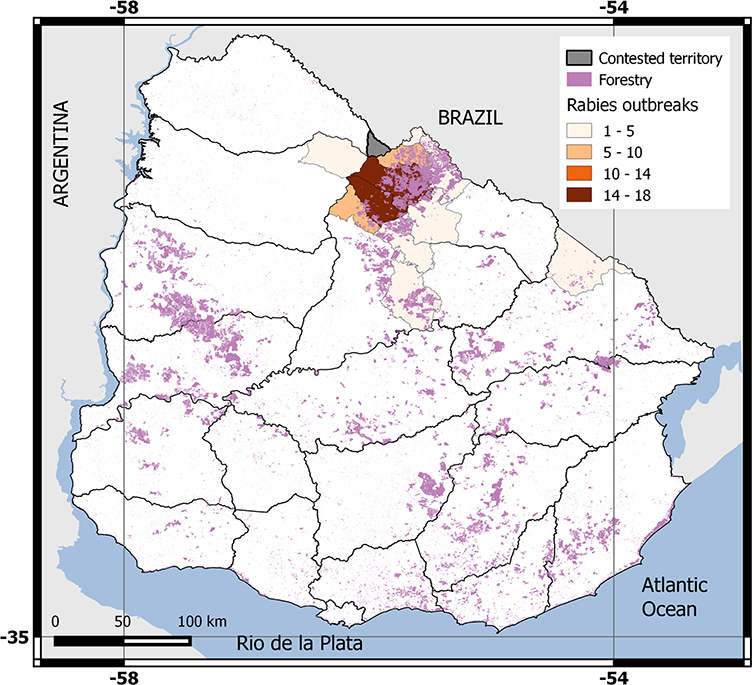
In Latin America, vampire bats (Desmodus rotundus) have replaced dogs as the main source of rabies infection, following successful control campaigns of canine rabies in many countries (Vigilato et al. 2013; Velasco-Villa et al. 2017). Vampire bats feed on vertebrate blood (primarily mammals), and this foraging ecology facilitates transmission of rabies virus through saliva. In 2007, an explosive outbreak of paralytic bovine rabies (i.e., 200 cattle deaths within two weeks) was detected for the first time in Uruguay (Botto Nuñez et al. 2019). Vampire bats were confirmed as the source of infection through virus isolation and evident feeding on livestock, and the outbreak cost the country ~ USD 2 million in immediate vaccination expenses (Guarino et al. 2013; Botto Nuñez et al. 2019). Bovine rabies outbreaks have continued to occur sporadically in the northeast region of the country (Fig. 1), suggesting that the virus continues to circulate. The last outbreak was recorded in March 2017 (Botto Nuñez et al. 2019). Rabies has not spread to other areas of Uruguay, despite abundant livestock and the presence of vampire bats throughout the country (Botto Nuñez et al. 2019). Uruguay presents a novel case-study as vampire bat-borne rabies spilled over into a livestock population that has robust surveillance. Given the conditions of the country, it is unlikely that a rabies outbreak would go undetected by animal health authorities, suggesting that the outbreak in 2007 was likely the first introduction of the virus (Botto Nuñez et al. 2019).
Figure 1.
Distribution of ranches with bovine rabies cases in Uruguay from 2007 to 2017, and distribution of exotic forestry areas in 2011. Data from the Uruguayan Ministry of Livestock, Agriculture and Fisheries (MGAP) and the Uruguayan National Environment Office (DINAMA).
Uruguay, in contrast with most other Latin American countries, has experienced an increase in forest cover over the past 30 years (Ministerio de Ganadería Agricultura y Pesca 2012; Hansen et al. 2013). However, rather than reforestation efforts, this change has been caused by monospecific exotic forestry for wood and cellulose production replacing highly diverse native grasslands (Silveira and Alonso 2009). This commercial exotic forestry fragments the native grasslands, replacing them with habitat that provides little or no food for vampire bats. Fragmentation of natural ecosystems has been often associated with pathogen emergence, persistence, and spillover (Schneider et al. 2001; Schneider et al. 2009; Gottdenker et al. 2014; de Thoisy et al. 2016; Rulli et al. 2017; Faust et al. 2018; Mastel et al. 2018). However, grassland fragmentation is underrepresented in the fragmentation literature (Fardila et al. 2017). Forest fragmentation has been associated with pathogen spillover through increased contact between reservoir and recipient hosts (e.g., edge effects) or by changes in abundances and diversity of reservoir species (e.g., dilution effects) (Rulli et al. 2017; Faust et al. 2018). However, the effects of fragmentation on reservoir host movement and subsequent disease dynamics have been less studied (Gottdenker et al. 2014). Moreover, habitat fragmentation is predicted to have strong effects on host movement and infection dynamics, particularly in highly mobile hosts such as bats, but quantifying such changes in natural systems is challenging (Hess 1996; Plowright et al. 2011; Becker et al. 2017; Becker et al. 2018c; Kessler et al. 2018). Thus, this grassland system provides an opportunity to test different effects of land-use change on pathogen spillover.
Commercial forestry in the 2007 outbreak area increased more than tenfold in the previous 17 years (from 17,967 ha in 1990 to 256,874 ha in 2007 in Rivera and Tacuarembó Departments), and this is now the most forested territory in the country (Ministerio de Ganadería Agricultura y Pesca 2012). During the same period, livestock production in the area also increased (Dirección de Estadísticas Agropecuarias 2011; Dirección de Estadísticas Agropecuarias 2014a). Owing to this simultaneous increase in forestry area and livestock herds, domestic animals, the primary food source of vampire bats (Voigt and Kelm 2006a; Streicker and Allgeier 2016), are now concentrated in increasingly scattered grassland habitat patches. This increase in livestock density may have driven an increase in size of vampire bat colonies, as demonstrated in previous work in Latin America (Streicker et al. 2012; Delpietro et al. 2017; Becker et al. 2018a), while the concentration of this food source in fewer, separated patches could alter vampire bat foraging patterns and population connectivity. Specifically, this shifted distribution of livestock could increase foraging times of bats roosting within forest patches and facilitate more overlap of bats in these shared feeding patches between colonies (Delpietro et al. 2017; Botto Nuñez et al. 2019).
While rabies virus has typically been considered an epizootic pathogen that travels in spatial waves (Delpietro and Nader 1989; Johnson et al. 2014; Benavides et al. 2016), longitudinal studies of vampire bats in Perú suggest that the pathogen instead persists enzootically within this reservoir host (Streicker et al. 2012). Previous data-driven mathematical models from Perú also accordingly suggest that rabies cannot persist in isolated populations of vampire bats. Asynchronous connectivity and metapopulation dynamics facilitate persistence of rabies virus alongside frequent (non-lethal) immunizing exposures (Blackwood et al. 2013). Thus, increases in population connectivity and inter-colony interactions at shared feeding sites driven by these shifts in livestock distribution in Uruguay could facilitate rabies persistence.
The plasticity of changing foraging times, in response to changes in livestock distribution, could be constrained by temperature, as heat loss limits bat flight times (McNab 1973; Weiner 1992; Wong and Candolin 2015). Hence, the effects of changes in livestock distribution need to be disentangled from the effects of climate variables. Particularly, minimum temperature of the coldest month has been identified as an important limiting factor for the distribution of vampire bats (McNab 1973; Zarza et al. 2017; Hayes and Piaggio 2018). This finding has two main explanations: lowest temperatures in roost sites determine the energy expenditure necessary to maintain body functions, and air temperature determines heat loss during flight, limiting its duration and foraging distances (McNab 1973).
Here, we used an extensive and spatially explicit dataset from Uruguay to describe the spatial structure of rabies outbreaks across the country and to test the spatial association between rabies outbreaks and landscape transformation. Specifically, we aimed to examine how the distribution of bovine rabies outbreaks was associated with the fragmentation of grazing areas, the density of commercial afforestation, the concentration of livestock, and temperature. We predicted that (a) outbreak densities of rabies would be higher in more fragmented landscapes where livestock is concentrated in separated patches and (b) vampire bats’ ability to respond to fragmentation of livestock rearing areas would be limited by minimum winter temperatures.
Methods
Study Area
Uruguay is located in the southern cone of South America (Fig. 1). The climate is temperate with an annual rainfall of 1300 mm and mean temperature of 17°C, with latitudinal variations across the country (20°C and 1600 mm in the north, and 16°C and 985 mm in the coast) (PNUMA 2008). Uruguay is generally flat, with a median elevation of 104 meters above sea level, and with a maximum altitude of 514 m. Uruguay is included in the Uruguayense biogeographic region—a landscape that is dominated by subtropical grasslands, modified by agricultural activities in varying degrees (Evia and Gudynas 2000; PNUMA 2008).
Spatial Data
We obtained the distribution of 2007–2017 rabies outbreaks from the Uruguay Ministry of Livestock, Agriculture and Fisheries. We aggregated the rabies data to the counts of rabies-quarantined ranches (i.e., an individually owned livestock-rearing facility) per police precinct, which are the geostatistical units used in Uruguay (Uruguay Census and Statistics Bureau, INE).
Spatial data on the distribution of livestock were obtained from the National Agricultural Census, which is performed regularly (at least every 10 years) by the Uruguay Ministry of Livestock, Agriculture and Fisheries. The information is publicly available for 2011 at the scale of census tracts (Dirección de Estadísticas Agropecuarias 2011; 2014a; 2014b). The total number of cattle, horses, and sheep were recorded for each agricultural census tract, as these species are common prey of vampire bats (Bohmann et al. 2018) and are raised mostly free roaming in ranches, and so their density can be calculated for large areas. We did not include poultry in this analysis, as these are generally exploited as a secondary food source to domestic herbivores (Bobrowiec et al. 2015). As livestock species vary in size and thus blood available for vampire bats, we used livestock biomass to approximate prey availability (Becker et al. 2018a). Total livestock biomass was obtained by summing species counts multiplied by mean body mass (kg; Jones et al. 2009).
The distribution of forestry and grassland areas was obtained from the National Environment Bureau (Dirección Nacional de Medio Ambiente - DINAMA) for the years 2000 and 2011 (Alvarez et al. 2015; DINAMA 2017), based on interpretation of Landsat TM and ETM+ images following the Land Cover Classification System (Alvarez et al. 2015; Di Gregorio 2016). We used the categories Natural Herbaceous (He), Shrubs (Ar), Palm Groves (Pa), and Naturally Flooded Areas (ANi) as areas compatible with livestock rearing (see Supplementary Table S1 for the Land Cover Classification System legend used in Uruguay and the categories selected). We used the category Forestry Plantations (PF) to indicate non-native forests. Spatial data on average minimum temperature for the coldest month (BIO6) were obtained from WorldClim database version 2 (Fick and Hijmans 2017). All spatial data were projected using the WGS84 / UTM 21S (EPSG 32721) coordinate system and converted into rasters with cell sizes of ~100 km2 and then incorporated into a hexagonal grid of 500 km2 cells based on weighted averages. Although these data on spatial covariates range from 2000 to 2011 (and rabies data range from 2007 to 2017), the vast majority of rabies cases in Uruguay occurred through 2010 (Figure S1). Following processing spatial data, all analyses were conducted in R (R Core Team 2016).
Fragmentation Analysis
For each land-cover layer (years 2000 and 2011), we created one spatial variable for livestock areas and another for non-native forests. Four cell-based metrics were used to assess fragmentation of livestock areas, both for 2000 and 2011: proportion of landscape (pl), number of patches (n), mean patch size (mps), and effective mesh size (ems) (Jaeger 2000; Baldi et al. 2006; Baldi and Paruelo 2014). For each cell c of the grid, we used the following formulas:
where Ac is the area of the grid cell c, and Aic is the area of each of the nc grassland polygon inside the cell c. For non-native forests, we calculated the number of patches (fnp) and proportion of landscape (fpl) both for 2000 and 2011. We also calculated the recent change in the four fragmentation variables (i.e., n, pl, mps, ems) and the two forestry variables (i.e., fnp, fpl) from 2011 to 2000. As fragmentation variables exhibited collinearity (Figure S2), we performed a principal component analysis (PCA) with the 12 variables of livestock area fragmentation, for 2000, 2011 and the change between 2000 and 2011 (Table S2). The PCA was performed over the correlation matrix, as the scale of the variables included varied significantly, using the function princomp from the stats package. Using the spectral decomposition (i.e., using prcomp) rather than the singular decomposition (i.e., princomp) gave similar results, with only inverted loadings on the second component (Figure S4).
Autocorrelation Analysis and Spatial Modeling
To explore the existence of simple spatial trends and to test for spatial stationarity needed to assess spatial autocorrelation, we fit a linear model for rabies outbreak density with the x and y coordinates of the centroids of the grid cells and the xy interaction, as the predictors. As a significant effect was detected, residuals were then used as the response variable for all remaining analyses.
Because rabies data could show spatial autocorrelation (i.e., nearby areas have similar outbreak densities), we calculated a global index on the model residuals via Geary’s C using the spdep package (Bivand et al. 2013; Bivand and Piras 2015; Brunsdon and Comber 2015). This analysis used a Monte Carlo randomization to assess statistical significance and row-standardized spatial weights for the neighbors list.
To next test how landscape changes were associated with the distribution of rabies outbreaks, we fit a spatial autoregressive model (SAR) (Brunsdon and Comber 2015) for the residuals of the linear model, using the spdep package, because an ordinary least squares linear model is not appropriate for spatially autocorrelated data (Bivand et al. 2013; Bivand and Piras 2015). We included livestock biomass, the amount of fragmentation in livestock areas, the recent changes in fragmentation, non-native forest land cover, and the mean minimum temperature of the coldest month as predictors. We used a forward selection process, including one variable in each step, based on the improvement of Akaike’s information criterion (AIC). We used a difference of two units in AIC as the minimum improvement to include a variable (Burnham and Anderson 2002) and used an intercept-only model as a null model. We assessed the degree of spatial dependence in the model by estimating the value of λ in the fitted model using the spdep package (Bivand et al. 2013; Bivand and Piras 2015; Brunsdon and Comber 2015). To assess whether the model accounted for autocorrelation, we tested the residuals for Geary’s C (Jetz et al. 2005; Brunsdon and Comber 2015).
To investigate the local variation of the effect of the environmental variables, we next fit a geographically weighted regression (GWR) (Lichstein et al. 2002; Jetz et al. 2005) for the residuals of the linear model, using the spgwr package (Bivand and Yu 2017) and the same predictors used for the SAR model (i.e., recent changes in fragmentation, non-native forest land cover, and the mean minimum temperature of the coldest month). This method allows estimating coefficients that vary across space. The varying coefficients can account for local effects not included in the model. Again, we followed a forward selection process (and considered an intercept-only model), but to avoid overfitting, we considered the AIC value for the global model (i.e., with stationary coefficients for each variable) for the selection. We also tested residuals for Geary’s C to assess whether the top model accounted for autocorrelation.
To obtain the final predictions from the spatial modeling, we added the predictions from the linear regression to the predictions of each of the spatial models (i.e., fitted(LM) + fitted(SAR) and fitted(LM) + fitted(GWR), as both SAR and GWR were fitted using the residuals of the linear regression as the response variable. All data and R scripts to reproduce our analyses are available in a Zenodo repository: https://doi.org/10.5281/zenodo.3735667
Results
Fragmentation Analysis
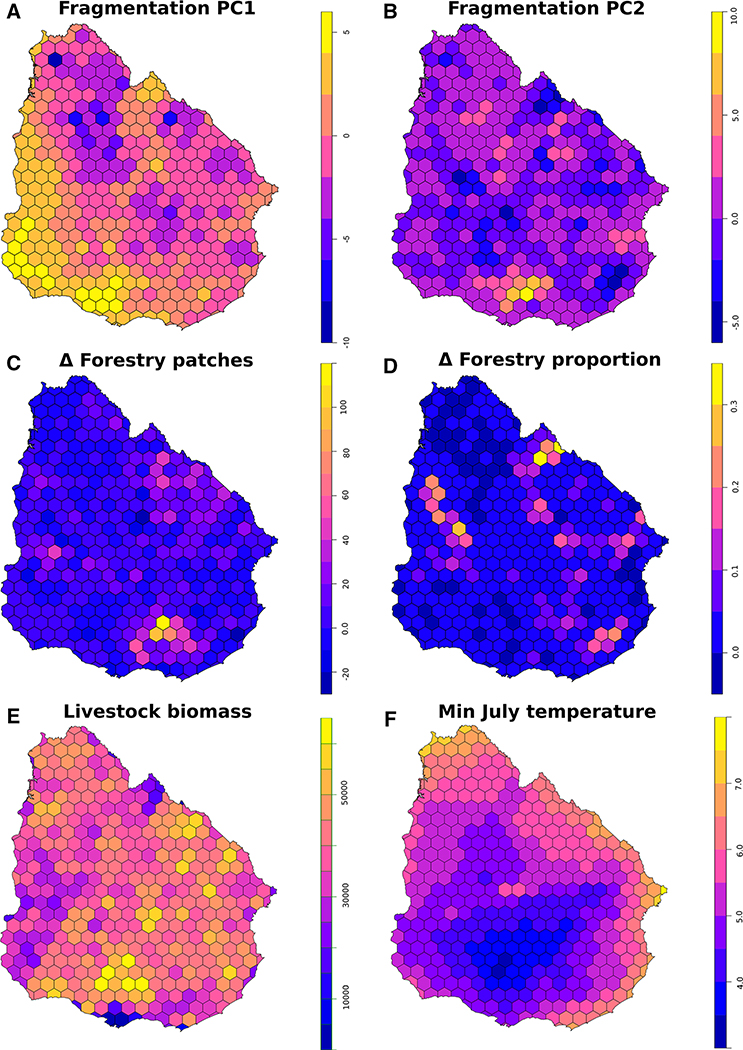
The first principal component (pc.frag1) explained the 46.74% of the total variance (Figure S3) and was related to fragmentation status. pc.frag1 increased with the increase in number of patches or with the decrease in proportion of landscape covered by livestock areas, the mean patch size, and the effective mesh size. The second principal component (pc.frag2) explained the 20.94% of the total variance and was associated with recent change in fragmentation. Negative values in the pc.frag2 are indicative of increased fragmentation from 2000 to 2011. Both components showed positive and significant spatial autocorrelation, being greater for the second component (pc.frag1: C = 0.25, p value < 0.01; pc.frag2: C = 0.52, p value < 0.01). Fragmentation was more intense in the western and southwestern region (related to intensive crop production, mainly soybean, wheat, corn, and sorghum) and in the northeastern region (related to commercial afforestation) (Fig. 2a, b). Recent changes in forest coverage were related to increased fragmentation in the northeastern region (Fig. 2a–d).
Figure 2.
Spatial distribution of predictive variables used for modeling the density of ranches with rabies cases. A. Fragmentation PC1 (pc.frag1), positive values indicate higher fragmentation of livestock areas. B. Fragmentation PC2 (pc.frag2), recent changes in fragmentation of livestock areas, negative values indicate increased fragmentation from 2000 to 2011. C. Δ forestry patches (c.fnp), change in number of exotic forestry patches between 2000 and 2011. D. Δ forestry proportion (c.fpl), change in proportion of landscape covered by exotic forestry between 2000 and 2011. E. Livestock biomass (liv.biom), considering cattle, horses, and sheep. F. Minimum Jul temperature (temp_jul), average minimum temperature of the coldest month,
Autocorrelation Analysis and Spatial Modeling
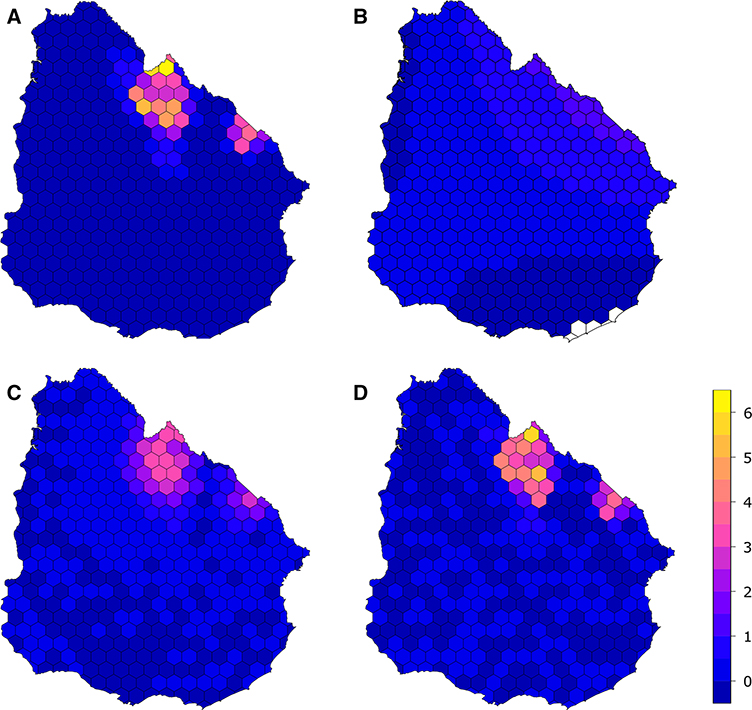
The density of ranches with rabies cases (Fig. 3a) was significantly predicted by longitude, latitude, and their interaction in our initial linear model (adjusted R2 = 0.19, p < 0.01, Table S3). Fitted values suggested an underlying northeast-to-southwest gradient in bovine rabies outbreaks, with higher expected values in the northeast and decreasing values in the northeast-to-southwest direction (Fig. 3B, Figure S5). We accordingly found positive and significant autocorrelation (C = 0.38, p < 0.01) in the detrended residuals (Figure S6, S7). This suggests additional spatial structure in the outbreaks beyond the latitudinal–longitudinal gradient where areas with higher density of cases are clustered. In subsequent spatial models, we used these residuals as our response variable.
Figure 3.
Measured and estimated rabies density: a Calculated density of ranches with rabies cases in the regular grid, from the data from official veterinary services. b Distribution of fitted values from the linear regression showing a northeast-to-southwest trend on the density of ranches with rabies cases. There are higher expected densities in the northeastern region of the country and decreasing values in the northeast-to-southwest direction (see also Figure S5 for an enhanced color scale for these predicted values). c Predicted density of ranches with rabies cases in the regular grid, combining the predictions of the simultaneous autoregressive model and the linear model. d Predicted density of ranches with rabies cases in the regular grid, combining the predictions of the Geographically Weighted Regression and the Linear model. Color scale is the same for the four maps.
We next fitted a SAR model to the detrended density of ranches with rabies cases (linear model residuals). The final model included the change in number of forestry patches (c.fnp) and the mean minimum temperature of the coldest month (temp_jul; wi = 0.98). This model showed an improvement from the model containing only the change in number of forestry patches (c.fnp; ΔAIC = 8.22, wi = 0.02; Table 1) and showed a discrete but significant positive spatial effect (λ = 0.88, p < 0.01). Residuals showed no significant spatial autocorrelation (C = 0.96, p = 0.15, Figure S8), implying that spatial structure was accounted for by the SAR. Combining the fitted values from the SAR and the linear model for simple trends accounted for a large portion of the variability in the original density of ranches with rabies cases (R2 = 0.76; Fig. 3, A and C, Figure S12 A, Table S5).
Table 1.
Forward selection process for the SAR models. Estimated coefficients and p values (p) are presented for each variable (starred p values are significant at 0.05 level), and differences in Akaike information criteria values in comparison with the best ranking model (ΔAIC), and Akaike weights (wi) are shown for each model. The first step, considering an intercept-only model, is used as null model.
| Variables | Estimate | p | ΔAIC | wi |
|---|---|---|---|---|
| Step 1: detrended_residuals~1 | 14.44 | 0.00 | ||
| Intercept | 0.00 | |||
| Step 2: detrended_residuals ~ c.fnp | 8.22 | 0.02 | ||
| Intercept | − 0.04 | |||
| c.fnp | 0.01 | *<0.01 | ||
| Step 3: detrended_residuals ~ c.fnp * temp_jul | 0.00 | 0.98 | ||
| Intercept | − 0.27 | |||
| c.fnp | − 0.04 | *<0.01 | ||
| temp_jul | 0.04 | 0.68 | ||
| c.fnp:temp_jul | 0.01 | *<0.01 | ||
The final GWR model included the first component of the fragmentation PCA (pc.frag1), the change in number of commercial forestry patches (c.fnp), the change in proportion of landscape covered by forestry (c.fpl), and mean minimum temperature of the coldest month (temp_jul) (Table 2, Figure S9). The differences in AIC and Akaike weights were much larger when evaluating the weighted models than when comparing the global regression models: the Akaike weights were 0.78 and 1.00 for the best model in the global regression and the weighted regression, respectively (Table 2). There was no trend in the values of the fitted coefficients in relation to the values of each variable (Table S4, Figure S10). A trend in the fitted coefficients would have suggested an unaccounted process, linking the explanatory variable with the disease density. While there was some spatial heterogeneity in the distribution of residuals, these were not concentrated in the area of the outbreaks. Residuals again showed no significant spatial autocorrelation (C = 1.13, p = 0.99, Figure S11). When combined with the predictions from the linear model, the GWR offered a better prediction of the distribution of the density of ranches with rabies cases (Figure 3, A and D; R2 = 0.94, Figure S12 B, Table S5).
Table 2.
Forward variable selection process for the GWR models. The global component of the four fitted GWR is shown. Differences AIC values and Akaike weights (wi) are shown for each model, in comparison with the best ranking model, both from the global regression (ΔAIC[GR]; wi [GR]) and from the GWR model (ΔAIC[GWR]; wi [GWR]). Global estimated coefficients are presented for all included variables, along with the p values (p; starred p values are significant at 0.05 level). Response variable: residuals from the linear model (detrended_residuals). The first step, considering an intercept-only model, is used as null model.
| Variables | Estimate | p | ΔAIC [GR] | wi [GR] | ΔAIC [GWR] | wi [GWR] |
|---|---|---|---|---|---|---|
| Step 1: detrended_residuals ~ 1 | 35.41 | 1.60×10−8 | 162.46 | 5.28×10−36 | ||
| Intercept | 0.00 | |||||
| Step 2: detrended_residuals ~ pc.frag1 | 15.09 | 4.13×10−4 | 282.37 | 4.83×10−62 | ||
| Intercept | 0.00 | |||||
| pc.frag1 | 0.08 | *<0.01 | ||||
| Step 3: detrended_residuals ~ pc.frag1 + c.fnp | 7.14 | 0.02 | 255.74 | 2.92×10−56 | ||
| Intercept | − 0.06 | |||||
| pc.frag1 | 0.08 | *<0.01 | ||||
| c.fnp | 0.01 | *<0.01 | ||||
| Step 4: detrended_residuals ~ pc.frag1 + c.fnp + c.fpl | 2.75 | 0.20 | 228.05 | 3.02×10−50 | ||
| Intercept | − 0.10 | |||||
| pc.frag1 | 0.08 | *<0.01 | ||||
| c.fnp | 0.01 | *<0.01 | ||||
| c.fpl | 1.92 | *0.01 | ||||
| Step 5: detrended_residuals ~ pc.frag1 + c.fnp + c.fpl + temp_jul | 0.00 | 0.78 | 0.00 | 1.00 | ||
| Intercept | − 0.62 | |||||
| pc.frag1 | 0.08 | *<0.01 | ||||
| c.fnp | 0.01 | *<0.01 | ||||
| c.fpl | 1.95 | *0.01 | ||||
| temp_jul | 0.10 | *0.03 | ||||
Discussion
Rabies virus has been present in the regions neighboring Uruguay (i.e., southern Brazil and northern Argentina) for more than a century, yet vampire bat rabies was not detected in Uruguay until 2007 (Botto Nuñez et al. 2019). Given robust surveillance and historical records of vampire bats, the absence of rabies spillover in Uruguay cannot be explained by failure to detect the disease nor by the absence of the bat reservoir; a recent historical review suggests that contemporary changes in the ecology of rabies may better explain this recent phenomenon of emergence (Botto Nuñez et al. 2019). The largest expansion of commercial afforestation in the northeast region of the country coincides with the emergence of rabies in the area in space and time and could potentially explain the recent outbreaks, which we tested in our spatial analyses.
We hypothesized that concentration of livestock in separated patches might increase contact of previously unconnected vampire bat colonies, increasing pathogen transmission and facilitating pathogen persistence (Botto Nuñez et al. 2019). We in turn quantified the process of patch isolation, as represented by the mesh size and the number of patches, rather than more commonly used measures in epidemiological studies such as edge size, which is important as a proxy for the rate of contact between patches and matrix (Olivero et al. 2017; Rulli et al. 2017; Faust et al. 2018). Fragmentation metrics are diverse, and usually the effects of habitat loss and changes in habitat configuration are not disentangled (Fahrig 2003). In our system, contact rates between the reservoir host (vampire bats) and the recipient hosts (livestock) are not driven by edge length, as vampire bats are already using livestock as their main (and perhaps, in many areas, only) food source (Voigt and Kelm 2006b; Bobrowiec et al. 2015; Becker et al. 2018b; Bohmann et al. 2018; Botto Nuñez et al. 2019). In contrast with other studies of the relationship between infectious disease emergence and habitat fragmentation, fragmentation of livestock rearing areas in our system is not related to decreased availability or abundance of food or roosts (Olivero et al. 2017; Rulli et al. 2017; Faust et al. 2018). Instead, the direct effect of fragmentation in our system is a change in the spatial distribution of food sources, impacting the foraging strategies of bats and hence the connectivity and contacts between colonies. Thus, a novel approach to fragmentation (in relation to emergence of infectious diseases) was implemented to represent this different proposed mechanism.
We summarized the livestock area fragmentation process in two principal components that described overall fragmentation and recent changes in fragmentation. Both components showed significant spatial structure. Western and southwestern fragmentation areas were mainly related to cropland intensification (particularly soybean production), while northern areas were related to commercial afforestation (Fig. 2a), which has increased recently (more negative values of pc.frag2, Fig. 2b) (PNUMA 2008). The commercial afforestation process was highly stimulated by policies promoting and providing economic incentives to forestry ventures (PNUMA 2008).
We detected a spatial trend likely related to invasion of rabies from Brazil, as higher predicted values of rabies outbreaks were observed close to the northeastern border and decreased in the southwestern direction; however, this did not fully explain the spatial distribution of rabies cases (Fig. 3a, b). While an introduction of the virus from Brazil is likely, as outbreaks north of the border have been occurring for more than a century (Teixeira et al. 2008; Barbosa de Lucena 2009), this alone does not explain why cases in Uruguay only occurred in this area, why they only occurred after 2007, and why pathogen transmission has not expanded since 2007. Rabies cases in Uruguay probably occurred in two independent invasions, both close to the Brazilian border (Figs. 1, 3a), but neither expanded south, despite the presence of bats and livestock throughout Uruguay (Botto Nuñez et al. 2019). This could be further confirmed by phylogeographic analyses if sequences from both foci are made available.
One important spatial measure missing in our study is the local abundance of vampire bats, which is currently unavailable for Uruguay. Although presence and absence data can often be useful for estimating occupancy (which can serve as a surrogate for abundance in some contexts; MacKenzie and Nichols 2004), vampire bats are habitat generalists (Greenhall et al. 1983) and are present throughout Uruguay (González and Martínez-Lanfranco 2010). In lieu of spatial data on vampire bat abundance, livestock abundance might be a useful approximation, as livestock biomass indicates food availability that, along with roosts, limits vampire bat colony size. There is an ongoing survey of vampire bat roosts in Uruguay, but surveillance is higher in or near areas where rabies outbreaks have occurred. Mark–recapture studies would also be useful to estimate vampire bat colony sizes and connectivity.
The SAR model was able to capture the spatial structure of the rabies outbreaks, as the residuals from the model did not show spatial autocorrelation (Figure S8). The positive and significant λ estimate also showed positive spatial dependence in the rabies outbreaks with the two predictive variables included in the final model: the recent change in proportion of landscape occupied by forestry and the average minimum temperature in the coldest month. It is interesting to note that rabies outbreaks did not occur in areas with the largest livestock populations (Botto Nuñez et al. 2019). Livestock populations in Uruguay are large enough to sustain large populations of vampire bats, even in the relatively low-density areas; this could explain the lack of a significant effect of livestock in our analysis (González and Martínez-Lanfranco 2010; Botto Nuñez et al. 2019). In contrast, the minimum temperature in the winter had significative effects (both SAR and GWR models, Tables 1 and 2). While temperature variation is moderate within the country, Uruguay is on the edge of the limit of the tolerance range for the species (McNab 1973; Greenhall et al. 1983). Winter temperatures might influence the length of foraging flights, affecting the ability of vampire bats to modify their home ranges in response to resource’s fragmentation.
The GWR model was used mainly as a descriptive analysis to explore the effect of allowing regression coefficients to vary spatially. The results showed significant effects of fragmentation level, the recent changes in forestry, and the coldest temperatures. Allowing spatial variation in the coefficients allowed splitting the regional effects and accounted for unobserved variables, such as the potential influx of the virus from the north. While the fitting process for the GWR model does not guarantee that the model accounted for the global spatial autocorrelation in the response variable, the final model fitted showed no spatial structure in the residuals (Figure S11).
Temperature is a major determinant of the distribution of vampire bats due to their poor thermoregulatory capacity and low tolerance for periods of fasting. Lacking fat storage, vampire bats cannot deal with a series of cold nights where energy invested in foraging flight is not compensated by energy obtained from feeding (McNab 1969; McNab 1973; Greenhall et al. 1983). Uruguay is in the southern limit of the species’ distribution, and while Desmodus rotundus is present throughout the country, the cold temperatures might limit the distance they can fly to feed in the winter. For instance, if food sources become patchier, vampire bats roosting in higher temperatures could modify (expand) their home ranges. By contrast, those colonies roosting in places with lower temperatures during winter nights might exhibit limited plasticity in home ranges, as the energy needed for foraging is inversely related to temperature. Thus, colder temperatures in the winter might modulate the effect of patchiness of food sources on connectivity. The area where the outbreaks occurred has recently fragmented livestock areas as well as the highest minimum temperatures in the winter (Fig. 2). During the 20th century, there was an overall increase of 0.8°C, where minimum temperatures increased all year round (PNUD 2007). A regional study also concluded that in the period 1960–2000 and based on daily minimal temperatures, there was a consistent trend of increase in temperature of coldest nights and an increase in the number of warm nights (Vincent et al. 2005). This trend is more evident in coastal areas (Vincent et al. 2005). For the next decades, the temperature is expected to keep rising with a total increase of 1.0–2.5°C for 2050 (in relation to the 2000) (Vincent et al. 2005). In this context, and combined with increased land use change and fragmentation of native grasslands (Baldi and Paruelo 2014), understanding how temperature affects plasticity of wildlife to respond to other environmental changes is crucial for predicting potential pathogen spillover risks.
In this work, we showed that the spatial distribution of rabies outbreaks can be explained by a combination of landscape fragmentation and climatic conditions. The recent changes in landscape structure in Uruguay, explained by changes in land use practices, can explain the occurrence of recent outbreaks in the early 2000 s. However, temperature might limit the expansion of rabies into other areas of Uruguay. Our results show that an understudied fragmentation process, such as grassland fragmentation, might have an important effect on the dynamics of infectious diseases. Processes that affect movement of individuals across roosts, such as culling activities, have been proposed to facilitate persistence of rabies virus in vampire bat colonies (Streicker et al. 2012; Blackwood et al. 2013). Similarly, changes in the distribution of food sources can facilitate persistence by increasing contact among colonies. Our results also suggest that physiological limits imposed by temperature affect how species respond to landscape change, modulating how fragmentation may affect the spatial spread of pathogens. More work is needed to disentangle the effects of temperature from the effects of habitat fragmentation. In particular, field studies on how vampire bat home ranges vary across fragmentation and temperature gradients are needed to understand the effects of both variables on vampire bat connectivity and ultimately on rabies virus persistence and spillover risk. Such work would also link habitat and disease data on identical timescales and provide greater inference into associations between environmental change and rabies dynamics in bats and livestock.
Conclusion
The effects of temperature in combination with fragmentation are major factors in determining the spatial pattern of rabies outbreaks in Uruguay. Even when the effects of virus invasion were removed, fragmentation and temperature explained the spatial structure of disease. These results are consistent with our hypotheses that fragmentation could enhance connectivity among vampire bat colonies and promote viral persistence by increasing the rate of immigration of infected individuals. This increased ability of the virus to persist would likely facilitate virus transmission to livestock. The congruence between the conceptual results from both models provided stronger support for the effects of fragmentation on disease emergence. While more experimental support is needed for causal inference and directing policy (such as field-based estimations of vampire bat home ranges across a gradient of fragmented areas), our results suggest that land use planning might help to reduce fragmentation of livestock rearing areas when planning new forestry ventures to help reduce the risk of rabies to livestock. In particular, incorporating metrics of fragmentation of natural grasslands into environmental impact assessment would account for habitat disturbance. Given our results, future work should also analyze landscape fragmentation in the context of climate change, as temperature modulates the responses bat behavior to the fragmentation of food sources.
Supplementary Material
Acknowledgments
GBN was funded by a Fulbright Graduate Scholarship, Bat Conservation International (Student Research Scholarship for Global Bat Conservation Priorities), and American Society of Mammalogists (Latin American Student Field Research Award). RKP was supported by the National Science Foundation (DEB-1716698), the Defense Advanced Research Projects Agency (DARPA D16AP00113), the US National Institutes of General Medical Sciences IDeA Program (P20GM103474 and P30GM110732), and the Strategic Environmental Research and Development Program (RC-2633). This material is based upon work that is supported by the National Institute of Food and Agriculture, US Department of Agriculture, Hatch project under accession number 0185725. The views, opinions, and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the US Government. We want to thank the collaboration of Alvaro Soutullo and Marcel Achkar (UDELAR) during the early stages of this research, Angel Segura (UDELAR) for his valuable comments on the data analysis, Nathan Justice (MSU) for his help with the processing of some of the spatial layers and Devin Jones (MSU) for the help with the manuscript editing. The Uruguay’s Ministry of Livestock (MGAP) provided the data on rabies quarantined ranches through a formal request under the Free Access to Public Information Act (Law 18.831) in January 2017 (Exp. 2016/7/1/1/14253).
Footnotes
Electronic supplementary material: The online version of this article (https://doi.org/10.1007/s10393-020-01486-9) contains supplementary material, which is available to authorized users.
References
- Alvarez A, Blum A, Gallego F (2015) Atlas de cobertura del suelo del Uruguay Cobertura de suelo y cambios 2000–2011. DINAMA—FAO, Montevideo [Google Scholar]
- Baldi G, Guerschman JP, Paruelo JM (2006) Characterizing fragmentation in temperate South America grasslands. Agriculture, Ecosystems & Environment 116:197–208. 10.1016/j.agee.2006.02.009 [DOI] [Google Scholar]
- Baldi G, Paruelo JM (2014) Land-use and land cover dynamics in South American temperate grasslands. Ecology and Society 13:6 [Google Scholar]
- Barbosa de Lucena R (2009) Doenças de bovinos no Sul do Brasil: 6.706 casos. Universidade Federal de Santa Maria [Google Scholar]
- Becker DJ, Chumchal MM, Bentz AB, Platt SG, Czirják GÁ, Rainwater TR, Altizer S, Streicker DG (2017) Predictors and immunological correlates of sublethal mercury exposure in vampire bats. Royal Society Open Science 4:170073 10.1098/rsos.170073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Czirják GÁ, Volokhov DV, Bentz AB, Carrera JE, Camus MS, Navara KJ, Chizhikov VE, Fenton MB, Simmons NB, Recuenco SE, Gilbert AT, Altizer S, Streicker DG (2018) Livestock abundance predicts vampire bat demography, immune profiles, and bacterial infection risk. Philosophical Transaction of the Royal Society B 373:20170089 10.1098/rstb.2017.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Hall RJ, Forbes KM, Plowright RK, Altizer S (2018) Anthropogenic resource subsidies and host –parasite dynamics in wildlife. Philosophical Transactions of the Royal Society B. 10.1098/rstb.2017.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Snedden CE, Altizer S, Hall RJ (2018) Host dispersal responses to resource supplementation determine pathogen spread in wildlife metapopulations. American Naturalist. 10.1086/699477 [DOI] [PubMed] [Google Scholar]
- Becker DJ, Streicker DG, Altizer S (2015) Linking anthropogenic resources to wildlife-pathogen dynamics: A review and meta-analysis. Ecology Letters 18:483–495. 10.1111/ele.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, Valderrama W, Streicker DG (2016) Spatial expansions and travelling waves of rabies in vampire bats. Proceedings of the Royal Society B: Biological Sciences 283:1–26. 10.1098/rspb.2016.0328 [DOI] [Google Scholar]
- Bivand R, Hauke J, Kossowski T (2013) Computing the jacobian in gaussian spatial autoregressive models: An illustrated comparison of available methods. Geographical Analysis 45:150–179. 10.1111/gean.12008 [DOI] [Google Scholar]
- Bivand R, Piras G (2015) Comparing implementations of estimation methods for spatial econometrics. Journal of Statistical Software. 10.18637/jss.v063.i18 [DOI] [Google Scholar]
- Bivand R, Yu D (2017) spgwr: Geographically Weighted Regression. R package version 0.6–32. [Google Scholar]
- Blackwood JC, Streicker DG, Altizer S, Rohani P (2013) Resolving the roles of immunity, pathogenesis, and immigration for rabies persistence in vampire bats. Proceedings of the National Academy of Sciences of the United States of America 110:20837–20842. 10.1073/pnas.1308817110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowiec PED, Lemes MR, Gribel R (2015) Prey preference of the common vampire bat (Desmodus rotundus, Chiroptera) using molecular analysis. Journal of Mammalogy 96:54–63. 10.1093/jmammal/gyu002 [DOI] [Google Scholar]
- Bohmann K, Gopalakrishnan S, Nielsen M, LdosSB Nielsen, Jones G, Streicker DG, Gilbert MTP (2018) Using DNA metabarcoding for simultaneous inference of common vampire bat diet and population structure. Molecular Ecology Resources 18:1050–1063. 10.1111/1755-0998.12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto Nuñez G, Becker DJ, Plowright RK (2019) The emergence of vampire bat rabies in Uruguay within a historical context. Epidemiology and Infection 147:1–8. 10.1017/S0950268819000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsdon C, Comber L (2015) An introduction to R for spatial analysis and mapping, London: SAGE Publications Ltd [Google Scholar]
- Burnham KP, Anderson DR (2002) Model selection and multi-model inference A practical information-theoretic approach, 2nd Editio Springer, New York [Google Scholar]
- de Thoisy B, Bourhy H, Delaval M, Pontier D, Dacheux L, Darcissac E, Donato D, Guidez A, Larrous F, Lavenir R, Salmier A, Lacoste V, Lavergne A (2016) Bioecological drivers of rabies virus circulation in a neotropical bat community. PLOS Neglected Tropical Diseases 10:e0004378 10.1371/journal.pntd.0004378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpietro HA, Nader AJ (1989) Rabies of herbivores transmitted by vampire bats in north-eastern Argentina. Revue scientifique et technique 8:189–198 [DOI] [PubMed] [Google Scholar]
- Delpietro HA, Russo RG, Carter GG, Lord RD, Delpietro GL (2017) Reproductive seasonality, sex ratio and philopatry in Argentina’s common vampire bats. Royal Society Open Science 4:160959 10.1098/rsos.160959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio A (2016) Land Cover Classification System: Classification concepts. Software version 3. [Google Scholar]
- DINAMA (2017) Cobertura del Suelo. Dirección Nacional de Medio Ambiente Medio Ambiente. http://sit.mvotma.gub.uy/websdatos/cobertura.html. Accessed 24 Mar 2018
- Dirección de Estadísticas Agropecuarias (2011) Censo General Agropecuario, Montevideo: Resultados definitivos [Google Scholar]
- Dirección de Estadísticas Agropecuarias (2014a) Censo General Agropecuario 2011. http://redatam.org/binury/RpWebEngine.exe/Portal?BASE=CGA2011&lang=esp. Accessed 28 Mar 2017
- Dirección de Estadísticas Agropecuarias (2014b) Censo General Agropecuario 2011 - Microdatos. http://www2.mgap.gub.uy/portal/page.aspx?2.diea.diea-censo-2011.O.es.0. Accessed 2 Mar 2018
- Evia G, Gudynas E (2000) Ecología del paisaje en Ururguay Aportes para la conservación de la diversidad biológica. Dirección Nacional de Medio Ambiente; Junta de Andalucía Consejería de Medio Ambiente, Montevideo [Google Scholar]
- Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annual Review of Ecology Evolution and Systematics 34:487–515. 10.1146/annurev.ecolsys.34.011802.132419 [DOI] [Google Scholar]
- Fardila D, Kelly LT, Moore JL, McCarthy MA (2017) A systematic review reveals changes in where and how we have studied habitat loss and fragmentation over 20 years. Biological Conservation 212:130–138. 10.1016/j.biocon.2017.04.031 [DOI] [Google Scholar]
- Faust CL, McCallum HI, Bloomfield LSP, Gottdenker N, Dobson AP, Gillespie TR, Torney CJ, Plowright RK (2018) Pathogen spillover during land conversion. Ecology Letters 21:471–483. 10.1111/ele.12904 [DOI] [PubMed] [Google Scholar]
- Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37:4302–4315. 10.1002/joc.5086 [DOI] [Google Scholar]
- González EM, Martínez-Lanfranco JA (2010) Mamíferos de Uruguay. Guía de campo e introducción a su estudio y conservación. Vida Silvestre—Museo Nacional de Historia Natural, Ediciones de la Banda Oriental, Montevideo [Google Scholar]
- Gottdenker NL, Streicker DG, Faust CL, Carroll CR (2014) Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth. 10.1007/s10393-014-0941-z [DOI] [PubMed] [Google Scholar]
- Greenhall AM, Joermann G, Schmidt U, Seidel MR (1983) Desmodus rotundus. Mamm Species 202:1–63 [Google Scholar]
- Guarino H, Castilho JG, Souto J, RdeN Oliveira, Carrieri ML, Kotait I (2013) Antigenic and genetic characterization of rabies virus isolates from Uruguay. Virus Research 173:415–420. 10.1016/j.virusres.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A, Egorov A, Chini L, Justice CO, Townshend JRG (2013) High-resolution global maps of 21st-century forest cover change. Science 80(134):850–854 [DOI] [PubMed] [Google Scholar]
- Hayes MA, Piaggio AJ (2018) Assessing the potential impacts of a changing climate on the distribution of a rabies virus vector. PLoS ONE 13:e0192887 10.1371/journal.-pone.0192887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G (1996) Disease in metapopulation models: Implications for conservation. Ecology 77:1617–1632. 10.2307/2265556 [DOI] [Google Scholar]
- INE Mapas Vectoriales. Instituto Nacional de Estadística. http://www.ine.gub.uy/web/guest/vectoriales. Accessed 2 Mar 2018
- Jaeger JAG (2000) Landscape division, splitting index, and effective mesh size: New measures of landscape fragmentation. Landscape Ecology 15:115–130. 10.1023/A:1008129329289 [DOI] [Google Scholar]
- Jetz W, Rahbek C, Lichstein JW (2005) Local and global approaches to spatial data analysis in ecology. Global Ecology and Biogeography 14:97–98. 10.1111/j.1466-822X.2004.00129.x [DOI] [Google Scholar]
- Johnson N, Aréchiga-Ceballos N, Aguilar-Setien A (2014) Vampire bat rabies: ecology, epidemiology and control. Viruses 6:1911–1928. 10.3390/v6051911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orme CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Connolly C, Cutts MJ, Foster JK, Grenyer R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J, Teacher A, Bininda-Emonds ORP, Gittleman JL, Mace GM, Purvis A (2009) PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90:2648 10.1890/08-1494.1 [DOI] [Google Scholar]
- Kessler MK, Becker DJ, Peel AJ, Justice NV, Lunn T, Crowley DE, Jones DN, Eby P, Cecilia AS (2018) Changing resource landscapes and spillover of henipaviruses. Annals of the New York Academy of Sciences 1429:78–99. 10.1111/nyas.13910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein JW, Simons TR, Shriner SA, Franzrebb KE (2002) Spatial autocorrelation and autoregressive models in ecology. Ecological Monograph 72:445–463. 10.1890/0012-9615(2002)072[0445:saaami]2.0.co;2 [DOI] [Google Scholar]
- MacKenzie DI, Nichols JD (2004) Occupancy as a surrogate for abundance estimation. Animal Biodiversity and Conservation 27:461–467 [Google Scholar]
- Mastel M, Bussalleu A, Paz-Soldán VA, Salmón-Mulanovich G, Valdés-Velásquez A, Hartinger SM (2018) Critical linkages between land use change and human health in the Amazon region: a scoping review. PLoS ONE 13:e0196414 10.1371/journal.pone.0196414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab BK (1973) Energetics and the distribution of vampires. Journal of Mammalogy 54:131–144. 10.2307/1378876 [DOI] [Google Scholar]
- McNab BK (1969) The economics of temperature regulation in neotropical bats. Comparative Biochemistry and Physiology 31:227–268. 10.1016/0010-406X(69)91651-X [DOI] [PubMed] [Google Scholar]
- Ministerio de Ganadería Agricultura y Pesca (2012) Dirección General Forestal. http://www.mgap.gub.uy/portal/page.aspx?2,dgf,dgf-principal,O,es,0. Accessed 15 Sep 2016
- Olivero J, Fa JE, Real R, Márquez AL, Farfán MA, Vargas JM, Gaveau D, Salim MA, Park D, Suter J, King S, Leendertz SA, Sheil D, Nasi R (2017) Recent loss of closed forests is associated with Ebola virus disease outbreaks. Scientific Reports 7:14291 10.1038/s41598-017-14727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P (2011) Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proceedings of the Royal Society B: Biological Sciences 278:3703–3712. 10.1098/rspb.2011.0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PNUD (2007) Uruguay: El cambio climático aquí y ahora. Montevideo [Google Scholar]
- PNUMA (2008) GEO Uruguay Informe del estado del ambiente. Programa de Naciones Unidas para el Medio Ambiente; Centro Latinoamericano de Ecología Social, Montevideo [Google Scholar]
- R Core Team (2016) R: A language and environment for statistical computing. [Google Scholar]
- Rulli MC, Santini M, Hayman DTS, D’Odorico P (2017) The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Scientific Reports 7:1–8. 10.1038/srep41613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MC, Aron J, Santos-Burgoa C, Uieda W, Ruiz-Velazco S (2001) Common vampire bat attacks on humans in a village of the Amazon region of Brazil. Cad Saúde Pública 17:1531–1536. 10.1590/S0102-311X2001000600038 [DOI] [PubMed] [Google Scholar]
- Schneider MC, Romijn PC, Uieda W, Tamayo H, da Silva DF, Belotto A, da Silva JB, Leanes LF (2009) Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Revista Panamericana de Salud Pública 25:260–269. 10.1590/S1020-49892009000300010 [DOI] [PubMed] [Google Scholar]
- Silveira L, Alonso J (2009) Runoff modifications due to the conversion of natural grasslands to forests in a large basin in Uruguay. Hydrological Processes 23:320–329. 10.1002/hyp [DOI] [Google Scholar]
- Streicker DG, Allgeier JE (2016) Foraging choices of vampire bats in diverse landscapes: potential implications for land-use change and disease transmission. Journal of Applied Ecology 53:1280–1288. 10.1111/1365-2664.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicker DG, Recuenco S, Valderrama W, Gomez Benavides J, Vargas I, Pacheco V, Condori Condori RE, Montgomery J, Rupprecht CE, Rohani P, Altizer S (2012) Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proceedings of the Royal Society B: Biological Sciences 279:3384–3392. 10.1098/rspb.2012.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira TF, Holz CL, Caixeta SPMB, Dezen D, Cibulski SP, da Silva JR, Rosa JCA, Schmidt E, Ferreira JC, Batista HBCR, Caldas E, Cláudia Franco A, Roehe PM (2008) Diagnóstico de raiva no Rio Grande do Sul, Brasil, de 1985 a 2007. Pesqui Vet Bras 28:515–520. 10.1590/S0100-736X2008001000012 [DOI] [Google Scholar]
- Velasco-Villa A, Mauldin MR, Shi M, Escobar LE, Gallardo-Romero NF, Damon I, Olson VA, Streicker DG, Emerson G (2017) The history of rabies in the Western Hemisphere. Antiviral Research 146:221–232. 10.1016/j.anti-viral.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilato MAN, Cosivi O, Knöbl T, Clavijo A, Silva HMT (2013) Rabies update for Latin America and the Caribbean. Emerging Infectious Diseases 19:678–679. 10.3201/eid1904.121482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent LA, Peterson TC, Barros VR, Marino MB, Rusticucci M, Ramirez E, Alves LM, Ambrizzi T, Berlato MA, Grimm AM, Marengo JA, Molion L, Muncunill DF, Rebello E, Anunciaçao YMT, Quintana J, Santos JL, Baez J, Coronel G, Garcia J, Trebejo I, Bidegain M, Haylock MR, Karoly D (2005) Observed trends in indices of daily temperature extremes in South America 1960–2000. Journal of Climate 18:5011–5023. 10.1175/jcli3589.1 [DOI] [Google Scholar]
- Voigt CC, Kelm DH (2006) Host preference of the common vampire bat (desmodus rotundus; chiroptera) assessed by stable isotopes. Journal of Mammalogy 87:1–6. 10.1644/05-MAMM-F-276R1.1 [DOI] [Google Scholar]
- Weiner J (1992) Physiological limits to sustainable energy budgets in birds and mammals: Ecological implications. Trends in Ecology & Evolution 7:384–388 [DOI] [PubMed] [Google Scholar]
- Wong BBM, Candolin U (2015) Behavioral responses to changing environments. Behavioral Ecology 26:665–673. 10.1093/beheco/aru183 [DOI] [Google Scholar]
- Zarza H, Martínez-Meyer E, Suzán G, Ceballos G (2017) Geographic distribution of Desmodus rotundus in Mexico under current and future climate change scenarios: Implications for bovine paralytic rabies infection. Vet Mex OA 4:1–16. 10.21753/vmoa.4.3.390 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.