Abstract
Background
Radiotherapy is the mainstay of brain metastasis (BM) management. Radiation necrosis (RN) is a serious complication of radiotherapy. Bevacizumab (BV), an anti-vascular endothelial growth factor monoclonal antibody, has been increasingly used for RN treatment. We systematically reviewed the medical literature for studies reporting the efficacy and safety of bevacizumab for treatment of RN in BM patients.
Materials and methods
PubMed, Medline, EMBASE, and Cochrane library were searched with various search keywords such as “bevacizumab” OR “anti-VEGF monoclonal antibody” AND “radiation necrosis” OR “radiation-induced brain necrosis” OR “RN” OR “RBN” AND “Brain metastases” OR “BM” until 1st Aug 2020. Studies reporting the efficacy and safety of BV treatment for BM patients with RN were retrieved. Study selection and data extraction were carried out by independent investigators. Open Meta Analyst software was used as a random effects model for meta-analysis to obtain mean reduction rates.
Results
Two prospective, seven retrospective, and three case report studies involving 89 patients with RN treated with BV were included in this systematic review and meta-analysis. In total, 83 (93%) patients had a recorded radiographic response to BV therapy, and six (6.7%) had experienced progressive disease. Seven studies (n = 73) reported mean volume reductions on gadolinium-enhanced T1 (mean: 47.03%, +/− 24.4) and T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI images (mean: 61.9%, +/− 23.3). Pooling together the T1 and T2 MRI reduction rates by random effects model revealed a mean of 48.58 (95% CI: 38.32–58.85) for T1 reduction rate and 62.017 (95% CI: 52.235–71.799) for T2W imaging studies. Eighty-five patients presented with neurological symptoms. After BV treatment, nine (10%) had stable symptoms, 39 (48%) had improved, and 34 (40%) patients had complete resolution of their symptoms. Individual patient data was available for 54 patients. Dexamethasone discontinuation or reduction in dosage was observed in 30 (97%) of 31 patients who had recorded dosage before and after BV treatment. Side effects were mild.
Conclusions
Bevacizumab presents a promising treatment strategy for patients with RN and brain metastatic disease. Radiographic response and clinical improvement was observed without any serious adverse events. Further class I evidence would be required to establish a bevacizumab recommendation in this group of patients.
Keywords: Bevacizumab (BV), Radiation necrosis (RN), Dexamethasone (Dex), MRI imaging, Adverse events
Introduction
Brain metastasis (BM) is the most common adult intracranial disease, and it is diagnosed in approximately 20 to 30% of cancer patients [1–3]. The most common primary tumor metastasizing to the brain is lung cancer (up to 50%), followed by breast cancer (up to 25%), melanoma (up to 20%), and to a lesser extent, renal cell carcinoma, colorectal cancer, and others [1–4]. Nonetheless, the incidence and frequency of BM is growing as newer systemic and immunotherapeutic agents are entering the treatment paradigm of these primary cancers [5–9]. Patients are living longer and are more prone to experience BM in their lifetime.
Depending on various prognostic factors, management of BM may involve surgical resection and/or radiation therapy in the form of stereotactic radiosurgery (SRS), whole brain radiotherapy, or a combination of two [1, 10–13]. A surge has been witnessed in the use of radiosurgery in BM patients with the approval of various targeted and immunotherapeutic agents for the management of primary sites of systemic cancers [6–9, 14]. Targeted agents after SRS for the brain have also been continued and have prolonged survival outcomes for patients with BM [6, 7, 9, 15, 16]. Radiation therapy has long been associated with the development of radiation necrosis (RN) in patients with intracranial disease [17–21]. The rate of RN following radiotherapy or radiosurgery has been estimated at 10–15% [17–21]. RN is considered as a dose-limiting toxicity for SRS [20, 21]. An increased incidence of RN has also been reported with a combination of SRS and systemic agents [22, 23]. In fact, the benefits of synergism from a combination of radiation and targeted agents are weighed against RN toxicity [7, 22, 23]. Hence, the management of RN takes a center stage in patients with intracranial disease.
Corticosteroids have long been the mainstay of RN treatment. It inhibits the pro-inflammatory response that promotes radiation necrosis and provides symptomatic relief via edema reduction, but long-term use is associated with serious side effects [19]. Surgery has also been used for resectable progressive RN, which can relieve mass effects and it also provides an opportunity to study tissue samples for diagnosis. However, persistent edema may need close monitoring for weeks [19, 24]. Another treatment strategy employed is hyperbaric oxygen therapy (HBOT) [25]. It can increase oxygen concentration to stimulate angiogenesis, restore blood supply to necrotic lesions, and accelerate healing. It has also shown improvement in RN symptoms alone or in combination with Endostar (a recombinant endostatin product) [25]. Laser interstitial thermal therapy (LITT) has been demonstrated to relieve RN symptoms, reduce progression, and improve survival in patients with RN and brain metastases [26, 27]. It has also been used to complement RN surgery [24]. Bevacizumab (BV) has also made it a treatment paradigm for RN [28–30]. Recent clinical trials have shown encouraging results [31–33].
Bevacizumab, an anti-VEGF monoclonal antibody, has been evaluated for RN treatment [28–30]. Its use in RN stems from the fact that RN tissues have elevated levels of VEGF [34, 35]. Radiotherapy induces vasogenic edema and ischemia, resulting in hypoxia that leads to the induction of hypoxia-inducible factor 1α (HIF1α) [34–38]. HIF1α upregulates VEGF through astrocytes and endothelial cells [36, 38]. White matter around necrotic areas has been identified as the main VEGF up-regulating site [36]. Immunohistochemistry (IHC) of RN surgical samples has confirmed increased levels of VEGF in reactive astrocytes surrounding the core of necrotic tissue [37]. VEGF is an important regulator of angiogenesis, leading to increased vascular permeability, damage to the blood-brain barrier (BBB), and ensuing brain edema [39]. Bevacizumab reduces vascular permeability and alleviates blood-brain barrier damage and brain edema through its binding to VEGF [28, 35, 39].
Several studies have reported the efficacy of BV in the treatment of RN diagnosed in primary brain tumor, metastatic, and patients with nasopharyngeal carcinoma (NPC) [31–33, 40–58]. Two randomized controlled trials have shown its efficacy over placebo or corticosteroid-receiving patients, without any increase in toxicity in primary brain tumors and NPC patients [31, 32]. Recently, a prospective phase II clinical trial has revealed efficacy of BV in patients with metastatic brain disease who have RN [33]. However, the majority of studies had included patients without differentiating for their intracranial disease type [50–54]. Here, we conducted a systematic review to gather evidence of the clinical efficacy of BV for patients with metastatic brain disease who have RN.
Methods & materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were rigorously followed [59].
Inclusion criteria
Patients & study types
Studies reporting the efficacy of bevacizumab for radiation necrosis occurring in patients with brain metastases after undergoing radiotherapy for intracranial disease.
Types of interventions
Bevacizumab
Outcomes of interest
Outcomes of prime interest were: radiographic response; edematous volume reductions on magnetic resonance imaging (MRI); and clinical improvement such as improvement/resolution of neurological symptoms and signs, increase in Karnofsky Performance Status (KPS) score, and decrease in dosage or discontinuation of dexamethasone. The secondary outcomes of interest were recurrence and safety outcomes, including adverse events.
Search strategy
Databases
PubMed, Medline, EMBASE, and the Cochrane library were searched until 1st Aug 2020. Various search terms such as “bevacizumab” OR “Anti-VEGF monoclonal antibody” AND “Radiation necrosis” OR “Radiation induced brain necrosis” OR “RN” OR “RBN” AND “Brain metastases” OR “BM” etc., were employed. Language was restricted to English. Furthermore, references of the retrieved studies were also inspected for more relevant literature.
Study selection
Relevant studies obtained from databases were imported into Endnote X9.3 software for organization and screening. Duplicates were removed and titles and abstracts were thoroughly screened. Studies were selected according to the aforementioned inclusion criteria. In situations of discrepancies, other authors were consulted.
Data extraction
“The Cochrane Collaboration Data Collection form-RCTs and non-RCTs” was modified according to our requirements and used for recording data. The extracted data included general characteristics/attributes of the studies and participants and the main outcomes of interest. The characteristics of the studies recorded were the first author, publication year, period of recruitment, research design, institute of research, number of participants, and follow-up time. The recorded attributes of participants included age, sex, presenting symptoms, KPS, dexamethasone use, and adverse events.
Furthermore, outcomes of interest, including radiographic response, RN volume reduction on MRI images, clinical improvement, and safety. Scrutiny and examination of eligible studies was accomplished with full text reading by two independent reviewers (M.K. and Z.Z).
Assessment of risk for bias
Quality assessment was carried out using the Reporting Checklist for Authors developed by The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group [60].
Statistical analysis
Descriptive statistics, including frequency, percentage, mean, median, range, and standard deviation, were calculated with Microsoft Excel for Mac 2019 v16.43. Mean reduction rates were directly extracted from the studies or indirectly via Engauge Digitizer. The weighted mean and standard deviation was estimated according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions version 6.0 [61, 62]. Pooled estimates (weighted mean and confidence interval) was obtained with Open Meta Analyst software, which uses the R package “metafor” for meta-analysis [63–65]. The pooled mean was estimated using a continuous random effects model with the DerSimonian-Laird method [66]. Heterogeneity was assessed using the I2 test. I2 values of 25, 50, and > 50% were considered as low, moderate, and high heterogeneity [67]. P < 0.05 was considered statistically significant.
Results
Overall, two prospective studies, seven retrospective studies, and three case reports involving 89 patients with RN treated with BV were obtained following the research strategy and study selection process [33, 48–58]. The PRISMA flow diagram for the same is illustrated in Fig. 1. Among them, 39 patients were male and 50 were female (Table 1). Lung (54, 61%) and breast (12, 14%) cancers constituted the main primary pathology for BM. All patients had developed RN after receiving radiation therapy to the brain [33, 48–58]. Stereotactic radiotherapy (SRT) (37, 33%), which is SRS delivered in fractions, was the main component of treatment delivered after BM development, followed by single-dose SRS (26, 23%) and whole-brain radiotherapy (WBRT) (22, 19%). SRS was also the main radiation strategy given as radiation boost after conventional radiotherapy (24, 21%) [33, 48–58]. The time duration from radiotherapy induction to RN development was reported in most studies as the time from RT to RN diagnosis and, in a few studies, as RT to BV induction (Table 1). The mean time from RT to RN diagnosis ranged between 6.5 and 19 months, and for RT to BV induction was between 4.6 and 11 months [33, 48–58]. All the studies had used various combinations of diagnostic procedures to determine the RN diagnosis, including MRI, magnetic resonance spectroscopy (MRS), methionine positron emission topography (MET-PET scans), and biopsy/pathology [33, 48–58]. Differentiation between disease progression and RN diagnosis was based on the imaging guidelines reported in previous studies [68–74]. The imaging characteristics are outlined in Table 2. Most common dose of BV used were 5–10 mg/kg [49–54, 57]. Other doses applied ranged from as low as 1 mg/kg to as high as 15 mg/kg [33, 48–58]. The timing of BV induction ranged from every 2 weeks to every 6 weeks [33, 48–58]. The mean number of treatment cycles completed by patients ranged from two to six cycles. Follow up time also varied from 3.3 to 22.7 months. The details are illustrated in Table 1.
Fig. 1.
PRISMA flow diagram of research strategy and study selection
Table 1.
General characteristics of the included studies
| Study | Study design & period | Location | No | Age | M | F | Primary pathology | Radiation | RN Diagnosis | RT to RN Diagnosis | RT to BV Tx | BV dosage | No. of cycles | Follow Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang, et al. (2012) [50] |
Retrospective Mar 2010 - Jan 2012 |
Huashan Hospital, Fudan University, Shanghai, China | 5 | 65 | 4 | 1 |
Colon 3 Lung 2 |
EBRT/SRS/FSRT | MRI, MRS, PET | 4.6 | 7.5 mg/kg q2 week | 2–6 | 6 | |
| Boothe, et al. (2013) [51] |
Retrospective 3-year |
Memorial Sloan- Kettering Cancer Center, New York, USA | 11 | 58 | 4 | 7 |
Breast 5 NSCLC 6 |
WBRT/SRS | MRI, biopsy, PET | 12.4 | 59.6 days |
7.5 mg/kg q3w (1) 10 mg/kg q2w (8) 15 mg/kg q4/6w (2) |
6 | 3.3 |
| Furuse, et al. (2013) [52] |
Retrospective Jan 2009 - Oct 2010 |
Osaka Medical College, Takatsuki, Osaka, Japan | 3 | 62 | 1 | 2 | Unknown | SRS | MRI, MET-PET | 11 (median) | 5 mg/kg q2w | 3 | 14.4 | |
| Yonezawa, et al. (2014) [53] |
Prospective Nonrandomized Jun 2010 - Sep 2011 |
Kizawa Memorial Hospital, Minokamo, Japan | 2 | 52.5 | 1 | 1 | Lung | WBRT/SRS/SRT | MRI, MET-PET | 19 | 5 mg/kg q2w | 6 | ||
| Sadraei, et al. (2015) [54] | Retrospective Jul 2007 - Jun 2012 | Cleveland Clinic, Cleveland, Ohio, USA | 17 | 55.7 | 5 | 12 | lung (9), breast (4), rectal (1), melanoma (1), NSTC (1), FT (1)), | WBRT/SRS | MRI, PET, biopsy |
16.9 10.1 |
5/7.5/10/15 mg/kg q2/3w | 6 | 8 | |
| Zhuang, et al. (2015) [55] |
Retrospective Jun 2011 - Dec 2014 |
Tianjin Cancer Hospital, Tianjin, China | 14 | 56 | 6 | 8 | Lung 11, Breast 1, Lymphoma 1, Gastric cancer 1 | RT | MRI, PET, pathology | 5 mg/kg q3-4w | 3 | 12 | ||
| Xiang-Pan, L., et al. (2015) [49] | Retrospective | Wuhan, China | 1 | 60 | 1 | Lung | WBRT/SRS | MRI | 12 | 7.5 mg/kg q3w | 2 | |||
| Alessandretti, et al. (2013) [48] | Retrospective | Hospital São José, São Paulo, Brazil | 2 | 49.5 | 2 | Melanoma | WBRT/SRS | MRI | 11.5 | 5,7.5 mg/kg q6/4w | ||||
| Zhuang, et al. (2019) [33] |
Prospective II CT Dec 2016 - Feb 2019 |
Tianjin Cancer Hospital, Tianjin, China | 21 | 55 (median, range 43–70) | 11 | 10 |
Lung 17 Breast 2 Kidney cancer 2 |
SRT | MRI | 17.6 | 1 mg/kg q3w | 3 | 22.7 | |
| Tanigawa, et al. (2019) [56] | Retrospective | Kagoshima University, Kagoshima, Japan | 4 | 61.25 | 2 | 2 | Lung | STI (stereotactic irradiation) | MRI | 7.75 | 15 mg/kg q3–4w | |||
| Ma, et al. (2017) [57] | Retrospective | Zhejiang University, Hangzhou, China | 2 | 62 | 2 | NSCLC | SRS | MRI | 6.5 | 5 mg/kg q2w/7.5 mg/kg q3w | 2.5 | 9 | ||
| Glitza, I. et al. (2017) [58] | Retrospective | The University of Texas MD Anderson Cancer and Baylor College of Medicine, Houston, Texas, USA | 7 | 57 | 5 | 2 | Melanoma | SRS/WBRT | Surgery, MRI, pathology | 8.14 | 5, 7.5, 10 mg/kg | 3.7 | ||
| This study | Systematic review | 89 | 39 | 50 | 1-15 mg/kg q2-6w | 2–6 |
Abbreviations: CT Clinical trial, No No of patients, M Male, F Female, WBRT Whole brain radiotherapy, SRS Stereotactic radiosurgery, SRT Stereotactic radiotherapy, EBRT External beam radiotherapy, RT Radiotherapy, FSRT Fractionated stereotactic radiotherapy, RN Radiation necrosis, BV Bevacizumab, Tx Treatment, NSCLC Non-small cell lung cancer, FT Fallopian tube, NSTC Non-seminomatous testicular cancer, MRI Magnetic resonance imaging, PET Positron, emission topography, q2w Every 2 weeks
Table 2.
Imaging characteristics for diagnosis of radiation necrosis
| Imaging Technique | Characteristics |
|---|---|
| MRI |
-Contrast enhancement pattern (soap bubble or Swiss cheese pattern, etc.), -Location of enhancement (periventricular, corpus callosum, midline crossing, subependymal spread), -Multiplicity (single/multiple), -Distance from primary tumor site (ipsilateral/contralateral) |
| MRS |
-Decreased peaks in Cho, NAA and Cr, -Low Cho/Cr values -Elevated Lip-Lac/Cho |
| PET | -No uptake of radionuclides |
Abbreviations: MRI Magnetic resonance imaging, MRS Magnetic resonance spectroscopy, PET Positron emission topography
Measurement of MRI changes and calculation of reduction rate
Slight variations were noticed in methods for assessing the volume calculation and reduction rate on MRI images among the studies. Two studies estimated the area of lesion at the level of maximum bi-dimensional measurement according to McDonald’s criteria, and the difference was expressed as percent change from the baseline MRI profiles [50, 54, 75]. In some studies, the hyperintense area was manually outlined, measured, and summed across slices and was multiplied by the layer thickness to calculate the total lesion area, but the reduction rate was estimated differently [33, 51–53, 55]. Volume reduction was obtained by subtracting of post-treatment from pre-treatment volume, dividing post-treatment by pre-treatment volume, and the following formula: volume before BV – volume after BV / volume before BV [33, 51–53, 55]. Zhuang et al. calculated the edema index as: EI = volume of (edema + necrosis)/volume of necrosis [33, 55]. For T1 MRI, changes in the signals were measured in three different areas in the strengthening region of necrosis and compared to the white matter signal value of the same MRI to obtain a ratio that was used to express the reduction rate as the difference between pre- and post-treatment [33, 55]. We calculated the difference from the graphs available in their studies.
Patients characteristics
Ten studies reported individual patient characteristics and treatment-related data for 54 patients with RN [48–54, 56–58]. The details are outlined in Table 3. These patients consisted of 22 male and 32 female patients, and their average age was 58 years. The mean time from RT to RN diagnosis was 11.7 months and from RT to induction of BV treatment was 15.5 months [48–50, 52–54, 56–58]. BV dosage ranged from 5 mg/kg to as high as 15 mg/kg, every 2 weeks to every 6 weeks for an average of 5.7 treatments [48–50, 52–54, 56–58]. Three studies also provided treatment durations for each patient [48, 51, 57]. The mean BV treatment duration averaged at 3.29 months [48, 51, 57]. Neurological symptoms, such as headache, visual disturbances, seizures, limb weakness, etc., have been reported in nine studies [33, 48, 51–58]. Five studies reported adverse events after BV for individual patients [33, 54–56, 58]. Detailed information is provided in Table 3.
Table 3.
Individual characteristics and treatment outcomes for RN patients
| Study | Age | Sex | Primary histology | Radiation | Dose | RN site | Last RT to RN diagnosis | RT to BV Tx | BV dosage (mg/kg) | No of treatments | Treatment Duration (months) | Volume reduction on T1W-Gd-enhanced MRI | Volume reduction on T2W FLAIR MRI | Pre-Tx KPS | Post-Tx KPS | KPS increase | Pre-Tx Dex (mg) | Post-Tx Dex (mg) | Dex reduction (mg) | Presenting Symptoms | Improvement | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang, et al. (2012) [50] | 70 | M | colon | SRS | 17 Gy | L temporal | 6 | 0 | 0 | 30 | 30 | 0 | 15 | 12.5 | 2.5 | Improved | ||||||
| 71 | M | colon | EBRT | 36 Gy | L frontal | 4 | 65 | 10 | 40 | 80 | 40 | 15 | 5 | 10 | Improved | |||||||
| 71 | M | Lung | FSRT | 31.5 Gy/3f | L occipital | 7 | 87 | 78 | 50 | 90 | 40 | 15 | 0 | 15 | Improved | |||||||
| 67 | F | colon |
EBRT SRS |
39 Gy/10 f 16 Gy |
R frontal | 1 | 52 | 78 | 50 | 90 | 40 | 15 | 0 | 15 | Worsened | |||||||
| 46 | M | Lung |
EBRT SRS |
30 Gy/13 f 16 Gy |
L occipital | 5 | 50 | 30 | 60 | 80 | 20 | 12.5 | 5 | 7.5 | Improved | |||||||
| Boothe, et al. (2013) [51] | 58 | M | NSCLC |
WBRT SRS |
37.5 25/25 |
R frontal/L temporal | 10q2w | 2.3 | 38/64 | 3/36 | 8 | 0 | 8 | None | Improved | |||||||
| 50 | F | Breast | SRS | 30 | R occipital | 10q2w | 2.3 | 82 | 75 | 2 | 0.3 | 1.7 | Visual field disturbance, headaches | Stable | ||||||||
| 27 | F | Breast |
WBRT SRS |
37.5 18/21/21 |
L frontal/L temporal/R parietal | 10q2w | 1.4 | 38/64/91 | 70/67/63.5 | 4 | 0 | 4 | Seizures | Improved | ||||||||
| 79 | F | NSCLC | SRS | 18 | R parietal | 10q2w | 0.5 | 82 | 60 | 0.2 | 0 | 0.2 | Lower left leg weakness | Improved | ||||||||
| 67 | F | Breast |
WBRT SRS |
30 18 |
Cerebellum | 10q2w | 14.3 | 73 | 72 | Headaches, lower leg weakness | Stable | |||||||||||
| 54 | F | Breast |
WBRT SRS |
37.5 15 |
R frontal | 10q2w | 3.9 | 21 | 44.6 | Left arm weakness | Improved | |||||||||||
| 67 | M | NSCLC | SRS | 30 | R frontal | 15q6w | 2.8 | 10 | 3 | 24 | 0 | 24 | Seizures, left sided weakness | Improved | ||||||||
| 50 | F | Breast |
WBRT SRS |
35 21 |
R frontal | 7.5q3w | 1.4 | 91 | 46 | 20 | 0 | 20 | Fatigue, lethargy, facial asymmetry | Improved | ||||||||
| 67 | F | NSCLC | SRS | 21 | L occipital |
102w 4 w |
89 | 84.5 | 6 | 0 | 6 | Confusions, visual hallucinations | Stable | |||||||||
| 73 | M | NSCLC | SRS | 21 | L parietal | 102w | 96 | 54.6 | 8 | 2 | 6 | Seizure, right sided hemiparesis | Improved | |||||||||
| 63 | M | NSCLC | SRS | 21 | L occipital | 15q4w | 1.8 | 100 | 77 | 8 | 0 | 8 | Imbalance, right sided tinnitis | Resolved | ||||||||
| Furuse, et al. (2013) [52] | 57 | F | unknown | SRS | frontal | 5 | 73.4 | 40 | 60 | 20 | Improved | |||||||||||
| 74 | F | unknown | SRS | frontal | 47 | 74.4 | 60 | 60 | 0 | Resolved | ||||||||||||
| 55 | M | unknown | SRS | frontal | 49 | 77.5 | 80 | 90 | 10 | Stable | ||||||||||||
| Yonezawa, et al. (2014) [53] | 54 | M | Lung |
WBRT SRS |
30 20 |
15 | 55.9 | 88.9 | 60 | 70 | 10 | Seizure, motor weakness | Improved | |||||||||
| 51 | F | Lung | SRT | 30/5 f | 23 | 43.2 | 65 | 90 | 100 | 10 | Headache, numbness | Improved | ||||||||||
| Sadraei, et al. (2015) [54] | 61 | M | NSCLC | SRS | 18 Gy | R posterofrontal cingulate | 8 | 5q2w | 8 | 35.2 | 92 | 24 | 0.5 | 23.5 | Left sided weakness, gait problems | Improved | ||||||
| 46 | F | NSCLC |
RT SRS |
36.5 24 |
R cerebellar |
17 11 |
5q2w | 9 | 56.1 | 83.6 | Y | Improved | ||||||||||
| 62 | M | NSCLC | SRS | 18, 24, 24 | Frontal, L temporal |
16 4 |
7.5q3w | 3 | + 37.8 | + 74.1 | 16 | 4 | 12 | Y | Improved | |||||||
| 59 | F | NSCLC |
WBRT SRS |
44 24 |
R cerebellar |
6 5 |
30.8 | 58.9 | Y | Resolved | Proteinuria (bevacizumab held) grade 1 | |||||||||||
| 58 | F | NSCLC |
WBRT SRS |
40 24 |
R temporal L frontoparietal |
58 53 |
10q2w | 9 | 28.8 | 34.1 | 16 | 0 | 16 | Y | Resolved | UTI (requiring holding of 1 treatment) grade 2 | ||||||
| 46 | F | NSCLC |
WBRT SRS |
37.5 18 |
L occipital |
17 10 |
7.5q3w | 10 | 18.5 | 48.2 | 8 | 0 | 8 | Y | Improved | |||||||
| 58 | M | NSCLC |
WBRT SRS |
37.5 24 |
L parietal |
11 9 |
15q3w | 4 | 100 | 38.1 | 8 | 0 | 8 | Y | Improved | |||||||
| 63 | F | NSCLC | SRS | 18 | Bithalamic L midbrain | 18 | 7.5q3w | 4 | 76.9 | 52.9 | 8 | 0 | 8 | Y | No | |||||||
| 55 | F | Breast |
WBRT SRS |
37.5 24 |
L posterofrontal |
14 6 |
10q2w | 5 | 35.4 | 43.2 | 24 | 2 | 22 | Y | Improved | |||||||
| 58 | F | Breast |
WBRT SRS |
37.5 18 |
R frontal |
27 11 |
5q2w | 3 | 64.7 | 26.7 | 8 | 8 | 0 | Y | Improved | |||||||
| 52 | F | Breast |
WBRT SRS |
37.5 18 |
L cerebellar |
7 5 |
10q2w | 13 | 66.7 | 32.8 | 2 | 1 | 1 | Y | Improved | |||||||
| 58 | F | Melanoma |
WBRT SRS |
37.5 24 |
L frontal |
3 7 |
7.5q3w | 2 | 82.4 | 74.9 | 6 | 4 | 2 | Y | Resolved | DVT and PE grade 3 | ||||||
| 39 | F | Breast |
WBRT SRS |
37.5 24 |
L cerebellar |
14 8 |
10q2w | 7 | 25 | 77.3 | 8 | 0 | 8 | Y | Improved |
fatigue grade 2 |
||||||
| 57 | F | Fallopian tube | SRS | 20 | L parietal | 6 | 15q3w | 9 | 74.5 | 84.9 | 8 | 0 | 8 | Y | Resolved | |||||||
| 63 | M | Rectal |
WBRT SRS |
37.5 16 |
L frontal |
12 4 |
10q2w | 8 | 25.4 | 13.5 | 4 | 2 | 2 | Y | Resolved | |||||||
| 67 | F | NSCLC | SRS | 18 | L frontal | 3 | 10q2w | 8 | 22 | 53 | 4 | 0 | 4 | Y | Resolved | |||||||
| 45 | M | NSTC | SRS | 18 | R frontal | 5 | 5q2w | 4 | 32.2 | 46.2 | 4 | 0 | 4 | Y | Resolved | Hypertension grade 2 | ||||||
| Xiang-Pan, L., et al. (2015) [49] | 60 | F | lung |
WBRT SRS |
L temporal | 12 | 7.5q3w | ⇓ | ⇓ | Resolved | ||||||||||||
| Alessandretti, et al. (2013) [48] | 51 | F | melanoma |
WBRT SRS (3 lesions) |
17 |
5q2w/ 7.5q4w |
3 | ⇓ | ⇓ | 4 | 0 | 4 | severe drowsiness, unable to self-ambulate | Resolved | ||||||||
| 48 | F | melanoma |
SRS WBRT |
6 | 5q6w | ⇓ | ⇓ | 4 | 0 | 4 | partial seizures (facial tremor) | Resolved | ||||||||||
| Tanigawa, et al. (2019) [56] | 78 | M | Lung (adenocarcinoma) | STI (stereotactic irradiation) | 9.2 | 15q3–4w | ⇓ | ⇓ | Y | Resolved | Hypertension, proteinuria | |||||||||||
| 74 | M | Lung (adenocarcinoma) | STI (stereotactic irradiation) | 12.2 | 15q3–4w | ⇓ | ⇓ | Y | Resolved | Hypertension | ||||||||||||
| 49 | F | Lung (adenocarcinoma) | STI (stereotactic irradiation) | 5 | 15q3–4w | ⇓ | ⇓ | Y | Resolved | Oedema, hypertension, proteinuria | ||||||||||||
| 44 | F | Lung (adenocarcinoma) | STI (stereotactic irradiation) | 4.6 | 15q3–4w | ⇓ | ⇓ | Y | Resolved | proteinuria | ||||||||||||
| Ma, Y., et al. (2017) [57] | 58 | F | NSCLC | SRS | 11 | 5 mg/kg q2w | 4 weeks | ⇓ | ⇓ | speech disorder and weakness in the right arm | Improved | |||||||||||
| 66 | F | NSCLC | SRS | 2 | 7.5 mg/kg q3w | ⇓ | ⇓ | headache and fatigue | resolved | |||||||||||||
| Glitza, I. et al. (2017) [58] | 56 | M | Melanoma |
WB SRS |
30 18 |
L frontal | 4 | 7.5 | 4 | ⇓ | ⇓ | Memory loss, seizure | Improvement | |||||||||
| 71 | F | Melanoma | SRS | 20 | R frontal | 13 | 7.5 | 3 | ⇓ | ⇓ | Seizures, expressive aphrasia | Improvement | ||||||||||
| 64 | F | Melanoma | WB | 30 | R parietal | 4 | 7.5 | 5 | ⇓ | ⇓ | Weakness, gait disturbance, aphasia, memory loss | Resolution | Arthralgia, dysgeusia | |||||||||
| 52 | M | Melanoma |
SRS WB |
20/12/18 30 |
R frontal | 13 | 5 | 2 | Weakness, gait disturbance, cognitive deficit | worsened | ||||||||||||
| 65 | M | Melanoma | SRS | 20/16 | L temporal | 8 | 7.5 | 2 | None | worsened | ||||||||||||
| 37 | M | Melanoma | WB | 30 | Bifrontal | 8 | 10 | 6 | ⇓ | ⇓ | Behavioral changes, memory loss | improvement | ||||||||||
| 55 | M | Melanoma | SRS | 24/21 | R occipital/R frontal | 7 | 7.5 | 4 | ⇓ | ⇓ | Seizure, memory loss | Improvement | ||||||||||
| 58 +/−10.6 | M 22, F 32 | 11.7 | 15.5 | 5.7 | 3.29 | 57.4% | 56.2% | 56 | 75 | 23.75 | 10.4 | 1.6 | 9.08 |
Abbreviations: CT Clinical trial, No No of patients, M Male, F Female, WBRT Whole brain radiotherapy, SRS Stereotactic radiosurgery, SRT Stereotactic radiotherapy, EBRT External beam radiotherapy, RT Radiotherapy, FSRT Fractionated stereotactic radiotherapy, RN Radiation necrosis, BV Bevacizumab, Tx Treatment, NSCLC Non-small cell lung cancer, FT Fallopian tube, NSTC Non-seminomatous testicular cancer, MRI Magnetic resonance imaging, PET Positron, emission topography, q2w Every 2 weeks, Y Yes, R Right, L Left
Radiographic response
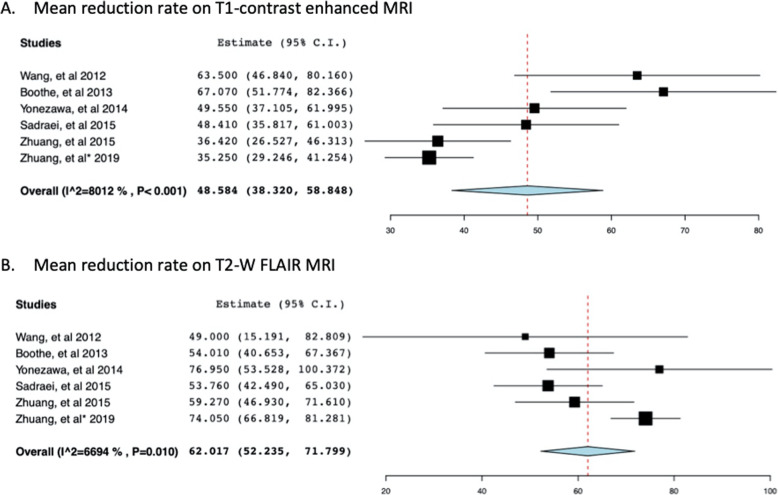
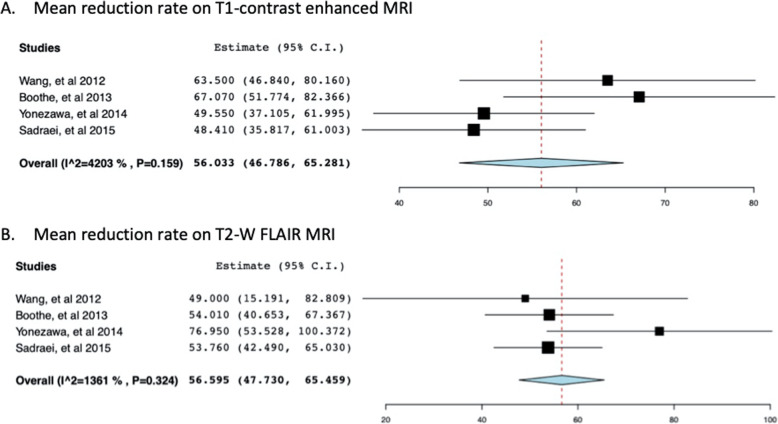
Radiographic response was defined as any reduction observed in the RN or edema volume on MRI images (Gd-enhanced T1 and T2-FLAIR) [33, 48–58]. Radiographic response was 93% (n = 83) after BV therapy induction. Six (6.7%) patients experienced progression of RN or failed to respond to bevacizumab [33, 48–58]. Seven studies involving 73 patients with RN reported a mean volume reduction on T1-enhanced and T2-FLAIR MRI images (Table 4) [33, 50–55]. The weighted mean reduction in volume on T1 Gd-enhanced MRI was 47.03% (+/− 24.4), and on FLAIR imaging was 61.9% (+/− 23.3). The average decrease in volume reduction for each study is given in Table 4. The mean volume reduction for studies ranged between 35 and 63.5% on enhanced MRI and 49 and 75.1% on FLAIR MRI images [33, 50–55]. Pooling together the T1 and T2 MRI reduction rates by random effects model revealed a mean of 48.58 (95% CI: 38.32–58.85) for the T1 reduction rate and 62.017 (95% CI: 52.235–71.799) for T2W imaging studies (Fig. 2). Significant heterogeneity was revealed for both comparisons (I2 = 80%, p < 0.001; I2 = 66.9%, p = 0.01, respectively). We undertook sensitivity analysis by excluding the studies reported by Zhuang et al. as the method for data calculation differed from other studies [33, 55]. Heterogeneity was lost upon excluding the studies suggesting that the difference in calculation method may have been the contributing factor (Fig. 3). Analysis of individual patient data revealed a 57.4% mean volume reduction on T1 enhanced and 56.2% on flair imaging, for 41 patients (Table 3) [48–54, 56–58]. The extent of volume reduction on MRI images has not been reported in some studies [48, 49, 56–58].
Table 4.
Radiographic responses and MRI changes after treatment with bevacizumab
| Studies | No of patients | Radiographic responses | T1 Gd enhancement volume reduction (mean) | T2 FLAIR volume reduction (mean) |
|---|---|---|---|---|
| Wang, et al. (2012) [50] | 5 | 4 (80%) | 63.5% | 49% |
| Furuse, et al. (2013) [52] | 3 | 100% | 75.1% | |
| Boothe, et al. (2013) [51] | 11 | 100% | 67.1% | 54.1% |
| Alessandretti, et al. (2013) [48] | 2 | 100% | ||
| Yonezawa, et al. (2014) [53] | 2 | 100% | 49.5% | 76.9% |
| Xiang-Pan, et al. (2015) [49] | 1 | 100% | ||
| Sadraei, et al. (2015) [54] | 17 | 16 (95.8%) | 52% | 53.7% |
| Zhuang, et al. (2015) [55] | 14 | 13 (92.9%) | 36% | 59% |
| Ma, Y., et al. (2017) [57] | 2 | 100% | ||
| Glitza, I. et al. (2017) [58] | 7 | 5 (71%) | ||
| Zhuang, et al. (2019) [33] | 21 | 20 (95.2%) | 35% | 74% |
| Tanigawa, et al. (2019) [56] | 4 | 100% | ||
| This study | 89 | 83 (93%) | Mean: 47.03% (+/− 24.4) | Mean: 61.78% (+/− 23.2) |
Fig. 2.
Forest plot of meta-analysis of mean reduction rate on T1-contrast enhanced MRI (a) and T2W FLAIR MRI (b) after bevacizumab (BV) treatment for radiation necrosis (RN) in patients with brain metastases
Fig. 3.
Sensitivity analysis of studies that used comparatively similar methods for estimation of reduction rates. Results are shown as forest plot of meta-analysis of mean reduction rate on T1-contrast enhanced MRI (a) and T2W FLAIR MRI (b)
Clinical improvement
Clinical improvement was measured in terms of improvement reported in neurological symptoms, KPS, and/or weaning of dexamethasone dosage [33, 48–58]. Overall, 85 patients presented with neurological symptoms because of RN, such as headaches, limb weaknesses, cognitive functions, and gait problems (Table 3) [33, 48–58]. After BV treatment, nine (10%) patients had stable symptoms, 39 (46%) patients had improved, and 34 (40%) patients had complete resolution of their symptoms [33, 48–56]. The symptoms worsened in three patients [50, 58]. Individual patient data was available for 54 patients [48–54, 56]. The KPS score was reported in 10 patients from three studies [50, 52, 53]. Improvement in KPS was observed in eight (80%) patients [50, 52, 53]. Dexamethasone discontinuation or reduction in dosage was observed in 30 (97%) of 31 patients who had recorded dosage before and after BV treatment [48, 50, 51, 54, 56]. The mean dose reduction for these patients was 9.08 mg (Table 3).
Recurrence
Only one study (n = 14) reported a recurrence rate [55]. The recurrence rate was very high: 10 of the 13 responding patients had RN recurrence. Sadraei et al. also reported that four patients had RN recurrence, but the type of intracranial disease (primary brain tumor, NPC, or BM) was not identified [54]. A single patient in the study by Wang et al. also had recurrence with no evidence of intracranial disease type [50].
Adverse events
Overall, five studies (n = 63) reported adverse events occurring in 14 (22%) patients after bevacizumab treatment (Table 5) [33, 54–56, 58]. A retrospective study reported grade 1 side effects in two (14%) patients. Adverse events reported were mild allergy and hypertension [55]. Hypertension resolved spontaneously. Similar side effects (mild allergy, hypertension) in two (9.5%) patients were reported in a prospective clinical trial conducted by the same group [33]. Side effects reported for individual patients were available in the study by Sadraei et al. [54]. One patient with non-small cell lung carcinoma (NSCLC) reported grade 1 proteinuria, for which bevacizumab treatment was withheld. Similarly, the other NSCLC patient reported a grade 2 urinary tract infection that also required withholding one dose of BV treatment. Of the 17 patients with RN, five (29%) patients (two with NSCLC, one with melanoma, one with breast cancer, and one with NSTC) reported side effects after BV treatment. Grade 3 deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in melanoma patients. The patient with breast cancer reported grade 2 fatigue, and the NTSC patient experienced grade 2 hypertension. All the participants in the case series (n = 4) reported by Tanigawa et al. experienced side effects involving hypertension, edema, and proteinuria [56]. Only one patient had experienced side effects such arthralgia and dysgeusia in the study by Glitza, I. et al. [58]. Adverse events were not reported in the remaining studies [48–53, 57].
Table 5.
Adverse events reported with bevacizumab treatment
| Studies | Patients | Symptoms |
|---|---|---|
| Sadraei, et al. (2015) [54] | 5 (29%) |
Grade 1: proteinuria (1). Grade 2: hypertension (1), fatigue (1), urinary tract infection (1). Grade 3: DVT/pulmonary embolism (1) |
| Zhuang, et al. (2015) [55] | 2 (14%) | Grade 1: mild allergy (1), hypertension (1) |
| Zhuang, et al. (2019) [33] | 2 (9.5%) | Grade 1: mild allergy (1), hypertension (1) |
| Tanigawa, et al. (2019) [56] | 4(100%) | Hypertension (3), proteinuria (3), edema (1) |
| Glitza, I. et al. (2017) [58] | 1(14%) | Arthralgia (1), dysgeusia (1) |
Discussion
We retrieved studies evaluating the efficacy of BV in the management of RN in patients who had received radiation therapy for brain metastases [33, 48–58]. Most patients showed a reduction in the edema and RN volume by over 50% on MRI images until their last follow-up [33, 48–56]. In some studies, edema volume reduction was over 70% in patients with BM [52, 53]. Radiographic responses corresponded with improvements in clinical outlook. Neurological symptoms were stabilized, improved, or completely resolved upon BV induction (Table 3). Several studies have reported a similar efficacy data for BV in patients with primary brain tumors (gliomas and glioblastoma), and NPC [31, 32, 45–47]. In a study by Wang et al., there were patients with other primary brain tumors who demonstrated a similar efficacy in reducing edema volume (T1 post-gd: 61%, T2 FLAIR: 57%), and showed improvement in neurological symptoms (100%) [50]. Fursue, et al. study, as well, had eight patients who had RN with primary brain tumors, other than the three BM patients [52]. A mean edema volume reduction rate of 45% was revealed for these patients. In addition to BM patients, seven other patients (five primary brain tumors and two arteriovenous malformations (AVM) patients) were also included in the study by Sadraei et al. [54]. The study reported an average reduction of 47.4 and 50.7% on both MRI images (T1W and FLAIR), respectively. Gonzalez et al. conducted a retrospective study showing radiographic and neurological symptom improvement in eight patients who had RN with primary brain tumors after being treated with BV (dosage: 5 mg/kgq 2 w /7.5 mg/kgq 3 w) [45]. Average reduction changes of 48 and 60% on post-contrast T1 and FLAIR MRI images were exhibited after a mean of 8.1 weeks from BV treatment start, respectively. In a separate retrospective study by Torcuator et al., six patients with RN diagnosed using biopsy and treated with BV also demonstrated significant reductions in both MRI images (T1 post-gd: 79%, T2 FLAIR: 49%) [46]. Li, et al., in their study comprising 50 NPC patients, though with a slightly lower response rate of 76.0% (38/50), had reported a significant decrease in edema volume reduction on FLAIR images (72.6%, p < 0.001) [47].
All these studies, however, constitute a low-level clinical evidence for the efficacy of BV therapy [45–47, 50, 52, 54, 56–58]. Zhuang et al. conducted a prospective clinical trial involving 21 patients who had RN with brain metastatic disease [33]. All patients, except for one, showed radiographic improvement. There is class I evidence for patients with primary brain and NPC tumors [31, 32]. Levin et al., in a randomized placebo-controlled trial, using a bevacizumab dose of 7.5 mg/kg every 3 weeks for seven patients with biopsy-proven RN with primary brain tumors, showed an average percentage change of 59 and 63% in RN volume on T1W and FLAIR images, respectively [31]. A recently concluded RCT involving 58 NPC patients treated with bevacizumab revealed a 65.5% (38/58) response rate [32]. The mean percentage change in RN volume observed on T1 post-gd and T2W FLAIR MRI were 25.5 and 51.8%, respectively. The mean change between before and after bevacizumab treatment was significant for both detected MRI images. Both these studies have reported significant differences in the radiographic responses and RN volume reduction rates observed on both MRI images between bevacizumab and placebo/corticosteroids, suggesting a better outcome for bevacizumab [31, 32].
In our systematic review, one study reported a very high RN recurrence rate (77%) in BM patients [55]. Other studies have failed to report recurrence of such a magnitude. Other than the two studies mentioned in the Results section, there are few other studies that also have cases of RN recurrence [50, 54]. Two patients in the RCT conducted by Levin et al. reported RN recurrence in glioma patients [31]. NPC patients from two other studies have also shown a moderate rate of recurrence [32, 47]. A recurrence rate of 39.5% was observed in a retrospective study of 50 NPC patients [47]. A similar recurrence rate (36.8%) was also demonstrated in the RCT of 58 NPC patients conducted by Xu et al. [32]. The underlying mechanism has not been exclusively investigated in these patients. Apparently, all three kinds of intracranial diseases (primary brain tumors, metastatic, or NPC) have registered RN recurrence [31, 32, 47, 55]. Recurrence was slightly higher in BM patients as reported, but the study had a low level of evidence. Hence, no conclusions could be drawn about the relationship between RN recurrence and the underlying intracranial disease type. Zhuang et al. identified a correlation between RN recurrence and duration after the initial BV withdrawal [55]. Further, Li et al. indicated that duration from induction of radiation therapy RN diagnosis and BV intervention as predictive factors for RN recurrence [47]. Further investigations are required to establish any underlying cause of RN recurrence. Another important aspect of RN recurrence is its diagnosis. Pathology is the standard for diagnosing RN or recurrence [76–79]. However, almost all of these studies relied on imaging criteria reported in previous studies for the diagnosis of RN and recurrence [31, 32, 47, 55, 76–79]. For example, in a case report, re-enlargement of RBN after being on BV for 8 months was attributed to recurrence of lung cancer as resected specimen revealed necrotic areas with viable tumor cells [80]. Hence, an accurate recurrence rate could only be determined with pathology, which could be further examined by larger comprehensively organized trials.
In this systematic review, clinical improvement was observed in a majority of the patients; however, some patients did not show any clinical improvement or experienced symptomatic worsening and progression. Medical literature also reveals similar examples. In the study by Gronier et al., no clinical improvement was observed in all three participants with malignant brain tumors after BV therapy (10 mg/kg per month) [81]. One patient had experienced lymphopenia after one perfusion of bevacizumab; the other had developed a transient ischemic attack and a corneal ulcer. Side effects reported in our review were mild, and only one grade 3 pulmonary embolism was described [33, 54–58]. Several other investigations have also highlighted similar low-grade adverse events [31, 32, 46, 50, 53]. In the retrospective study of Torcuator, et al. (n = 6), only one patient experienced mild fatigue after BV treatment [46]. Grade 2 AEs, including hypertension, fatigue, and proteinuria, were observed in 18% (3/17) of participants of the study by Wang, et al. [50]. However, the patients’ primary intracranial diseases were not identified. In the study by Yonezawa, et al., 33% (3/11) of participants had also shown grade 1 or 2 side effects such anemia, leukopenia, neutropenia, and lymphocytopenia [53]. More importantly, the class I evidence in this regard has shown the safety of BV therapy in primary brain tumors and NPC patients [31, 32]. Levin et al. reported that six (55%) patients experienced side effects [31]. Three of these adverse events were considered serious, including aspiration pneumonia, pulmonary embolus secondary to DVT, and superior sagittal sinus thrombosis. The other three patients showed ischemic changes due to small vessel thrombosis [31]. Another RCT conducted by Xu et al. reported 40 grade 1 or 2 adverse events experienced by 58 patients with NPC [32]. Only one grade 3 adverse event of ischemic stroke was observed. Furthermore, a similar portfolio was revealed for the corticosteroid-treated group, suggesting that BV treatment may not increase the toxicity experienced by patients with RN [32].
From the literature, it appears that bevacizumab was able to elicit therapeutic efficacy at any prescribed dose or frequency [31–33, 40–56]. The initial doses used were 5, 7.5, 10, and 15 mg/kg every 2 weeks to every 6 weeks. All doses were tolerated and were not associated with any increase in toxicity. It has been suggested that BV efficacy is associated with its anti-angiogenic effects rather than the dose [33]. In a case report, BV at a dose as low as 3 mg/kg was shown to be effective [48]. In a prospective clinical trial, patients were exposed to ultra-low doses of BV at 1 mg/kg [33]. Radiographic responses were observed in 20 of the 21 patients. Such a versatile dosing profile makes this treatment reachable to a broader population, as it is an expensive treatment. To date, exact cost-benefit relationship evaluation has not been adequately addressed for bevacizumab therapy [29]. It may cost around 4800 to 19,200 U.S. dollars (USD) for a single four to eight-week course of 5 to 10 mg/kg, administered every other week at a cost of 600 USD per 100 mg [82, 83]. An increase of 2.4 months in survival, a 20% improvement in a patient’s quality of life, or a linear combination of the two was required for bevacizumab treatment to be considered cost-effective according to a basic hypothetical calculation using 10,000 USD cost for a course of BV therapy and a quality-adjusted-life-year (QALY) threshold of 50,000 USD [84]. Hence, further studies are needed to establish a dose requirement for achieving the maximum benefit and to make the bevacizumab treatment cost-effective.
Several observations limit the results of our study. As a systematic review, the incorporated data comes from heterogeneous populations, diverse treatment centers, and a variety of research designs used for investigations. Moreover, the time period in which the case reports/studies were undertaken also varied. We included case reports and some retrospective studies [48–56]. Retrospective studies are prone to selection bias, recall bias, or misclassification bias and are subject to confounding [85]. Most of these studies mainly constitute class III level evidence, except for two prospective studies [48–56]. The types of radiation also differed from patient to patient. Moreover, pathology reports are used as standard for the diagnosis of RN; however, these studies mostly used imaging studies for RN diagnosis [48–56]. Some of the studies reported global adverse events/recurrence rates without differentiating between tumor types; however, they also contained participants other than BM patients [50, 53, 54]. Nonetheless, we presented the recurrence rates in results and side effects in the Discussion section to construct a better recurrence rate/adverse event profile for the readers. The follow-up for different studies also varied. The likelihood of only BV-responding patients being included in the study may also be prone to publication bias.
Conclusions
According to our results, bevacizumab can be considered safe and efficacious for BM patients diagnosed with RN. However, the level of evidence presented was low, making our bevacizumab efficacy results inconclusive. Furthermore, several dimensions of BV treatment for RN were less clarified and should be investigated in future trials. These include the diagnosis standard used for RN, impact of type/dose/fractionation of radiation therapy used on RN, patterns, and underlying mechanism of recurrence. The pending results of a phase II trial (NCT02490878) of BV plus corticosteroids versus corticosteroids plus placebo for radiation necrosis after radiosurgery for brain metastases will further define the role of bevacizumab in the management of radiation necrosis.
Acknowledgements
None
Authors’ contributions
MK and GL wrote the manuscript. MK, ZZ and SA performed the data search and data analysis. All authors corrected and proofed the final text. All authors read and approved the final manuscript.
Funding
The Natural Science Foundation of Shenzhen (No.JCYJ20170307095828424); Shenzhen Health and Family Planning System Research Project(No.SZBC2017024) were providing support for this work.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Khan, Email: drkhan_onco@outlook.com.
Zhihong Zhao, Email: xczhaozhihong@163.com.
Sumbal Arooj, Email: sumbal_arooj@outlook.com.
Guixiang Liao, Email: liaoguixiang@163.com.
References
- 1.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23(25):6207–6219. doi: 10.1200/jco.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 2.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/jco.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 4.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117(11):2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 6.Khan M, Arooj S, Li R, Tian Y, Zhang J, Lin J, et al. Tumor primary site and histology subtypes role in radiotherapeutic management of brain metastases. Front Oncol. 2020;10:781. doi: 10.3389/fonc.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M, Zhao Z, Arooj S, Liao G. Impact of tyrosine kinase inhibitors (TKIs) combined with radiation therapy for the management of brain metastases from renal cell carcinoma. Front Oncol. 2020;10:1246. doi: 10.3389/fonc.2020.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. SRS in combination with Ipilimumab: a promising new dimension for treating melanoma brain metastases. Technol Cancer Res Treat. 2018;17:1533033818798792. doi: 10.1177/1533033818798792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperduto PW, Deegan BJ, Li J, Jethwa KR, Brown PD, Lockney N, et al. Effect of targeted therapies on prognostic factors, patterns of care, and survival in patients with renal cell carcinoma and brain metastases. Int J Radiat Oncol Biol Phys. 2018;101(4):845–853. doi: 10.1016/j.ijrobp.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol. 2010;96(1):45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Khan M, Lin J, Liao G, Li R, Wang B, Xie G, et al. Comparison of WBRT alone, SRS alone, and their combination in the treatment of one or more brain metastases: review and meta-analysis. Tumour Biol. 2017;39(7):1010428317702903. doi: 10.1177/1010428317702903. [DOI] [PubMed] [Google Scholar]
- 13.Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Whole brain radiation therapy plus stereotactic radiosurgery in the treatment of brain metastases leading to improved survival in patients with favorable prognostic factors. Front Oncol. 2019;9:205. doi: 10.3389/fonc.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbour AB, Jacobs CD, Williamson H, Floyd SR, Suneja G, Torok JA, et al. Radiation therapy practice patterns for brain metastases in the United States in the stereotactic radiosurgery era. Adv Radiat Oncol. 2020;5(1):43–52. doi: 10.1016/j.adro.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallet AV, Dhermain F, Le Rhun E, Noël G, Kirova YM. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol. 2017;28(12):2962–2976. doi: 10.1093/annonc/mdx408. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AG, Ruiz J, Hughes R, Page BR, Isom S, Lucas JT, et al. Impact of systemic targeted agents on the clinical outcomes of patients with brain metastases. Oncotarget. 2015;6(22):18945–18955. doi: 10.18632/oncotarget.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485–502. doi: 10.1016/j.jocn.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Chung C, Bryant A, Brown PD, et al. Cochrane Database Syst Rev. 2018;7(7):Cd011492. doi: 10.1002/14651858.CD011492.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JA, Bennett EE, Xiao R, Kotecha R, Chao ST, Vogelbaum MA, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96(5):1060–1069. doi: 10.1016/j.ijrobp.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Loganadane G, Dhermain F, Louvel G, Kauv P, Deutsch E, Le Péchoux C, et al. Brain radiation necrosis: current management with a focus on non-small cell lung cancer patients. Front Oncol. 2018;8:336. doi: 10.3389/fonc.2018.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juloori A, Miller J, Parsai S, Kotecha R, Ahluwalia M, Mohammadi A, et al. Overall survival and response to radiation and targeted therapies among patients with renal cell carcinoma brain metastases. J Neurosurg. 2019;132:1–9. doi: 10.3171/2018.8.JNS182100. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro-Oncol. 2017;133(2):357–368. doi: 10.1007/s11060-017-2442-8. [DOI] [PubMed] [Google Scholar]
- 24.Ali FS, Arevalo O, Zorofchian S, Patrizz A, Riascos R, Tandon N, et al. Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21(8):66. doi: 10.1007/s11912-019-0818-y. [DOI] [PubMed] [Google Scholar]
- 25.Xing S, Fan Z, Shi L, Yang Z, Bai Y. Successful treatment of brain radiation necrosis resulting from triple-negative breast cancer with Endostar and short-term hyperbaric oxygen therapy: a case report. Onco Targets Ther. 2019;12:2729–2735. doi: 10.2147/OTT.S190409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neuro-Oncol. 2019;142(2):309–317. doi: 10.1007/s11060-019-03097-z. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Low J, Mrugala MM. Neuro-oncologists have spoken - the role of bevacizumab in the inpatient setting. A clinical and economic conundrum. Neurooncol Pract. 2019;6(1):30–36. doi: 10.1093/nop/npy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ET, Huberman M, Lu X-Q, Mahadevan A. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26(34):5649–5650. doi: 10.1200/jco.2008.19.1866. [DOI] [PubMed] [Google Scholar]
- 29.Lubelski D, Abdullah KG, Weil RJ, Marko NF. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neuro-Oncol. 2013;115(3):317–322. doi: 10.1007/s11060-013-1233-0. [DOI] [PubMed] [Google Scholar]
- 30.Delishaj D, Ursino S, Pasqualetti F, Cristaudo A, Cosottini M, Fabrini MG, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9(4):273–280. doi: 10.14740/jocmr2936e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Rong X, Hu W, Huang X, Li Y, Zheng D, et al. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101(5):1087–1095. doi: 10.1016/j.ijrobp.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang H, Zhuang H, Shi S, Wang Y. Ultra-low-dose Bevacizumab for cerebral radiation necrosis: a prospective phase II clinical study. Onco Targets Ther. 2019;12:8447–8453. doi: 10.2147/OTT.S223258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Chung YG, Kim CY, Kim HK, Lee HK. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19(6):879–886. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Cancer Netw. 2008;6(5):515–522. doi: 10.6004/jnccn.2008.0039. [DOI] [PubMed] [Google Scholar]
- 36.Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet (London, England) 2009;374(9701):1639–1651. doi: 10.1016/s0140-6736(09)61299-x. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Engelbach JA, Yuan L, Cates J, Gao F, Drzymala RE, et al. Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain. Clin Cancer Res. 2014;20(10):2695–2702. doi: 10.1158/1078-0432.ccr-13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18(1):21. doi: 10.1186/s12943-019-0950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remon J, Le Pechoux C, Caramella C, Dhermain F, Louvel G, Soria JC, et al. Brain Radionecrosis treated with Bevacizumab in a patient with resected squamous cell carcinoma of the lung. J Thorac Oncol. 2017;12(1):e1–e3. doi: 10.1016/j.jtho.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 41.Jeyaretna DS, Curry WT, Jr, Batchelor TT, Stemmer-Rachamimov A, Plotkin SR. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol. 2011;29(7):e159–e162. doi: 10.1200/jco.2010.31.4815. [DOI] [PubMed] [Google Scholar]
- 42.Tye K, Engelhard HH, Slavin KV, Nicholas MK, Chmura SJ, Kwok Y, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neuro-Oncol. 2014;117(2):321–327. doi: 10.1007/s11060-014-1391-8. [DOI] [PubMed] [Google Scholar]
- 43.Benoit A, Ducray F, Cartalat-Carel S, Psimaras D, Ricard D, Honnorat J. Favorable outcome with bevacizumab after poor outcome with steroids in a patient with temporal lobe and brainstem radiation necrosis. J Neurol. 2011;258(2):328–329. doi: 10.1007/s00415-010-5747-5. [DOI] [PubMed] [Google Scholar]
- 44.Arratibel-Echarren I, Albright K, Dalmau J, Rosenfeld MR. Use of Bevacizumab for neurological complications during initial treatment of malignant gliomas. Neurologia. 2011;26(2):74–80. doi: 10.1016/j.nrl.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neuro-Oncol. 2009;94(1):63–68. doi: 10.1007/s11060-009-9801-z. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Huang X, Jiang J, Hu W, Hu J, Cai J, et al. Clinical variables for prediction of the therapeutic effects of Bevacizumab monotherapy in nasopharyngeal carcinoma patients with radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2018;100(3):621–629. doi: 10.1016/j.ijrobp.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Alessandretti M, Buzaid AC, Brandão R, Brandão EP. Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep Oncol. 2013;6(3):598–601. doi: 10.1159/000357401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang-Pan L, Yuxin C, Xiao-Fei W, Na L, Tang-Peng X, Xiao-Tao X, et al. Bevacizumab alleviates radiation-induced brain necrosis: a report of four cases. J Cancer Res Ther. 2015;11(2):485–487. doi: 10.4103/0973-1482.140782. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wang E, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. 2012;17(1):25. doi: 10.1186/2047-783X-17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-Oncology. 2013;15(9):1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furuse M, Nonoguchi N, Kawabata S, Yoritsune E, Takahashi M, Inomata T, et al. Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET. Jpn J Clin Oncol. 2013;43(3):337–341. doi: 10.1093/jjco/hys231. [DOI] [PubMed] [Google Scholar]
- 53.Yonezawa S, Miwa K, Shinoda J, Nomura Y, Asano Y, Nakayama N, et al. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J Neuro-Oncol. 2014;119(1):101–109. doi: 10.1007/s11060-014-1453-y. [DOI] [PubMed] [Google Scholar]
- 54.Sadraei NH, Dahiya S, Chao ST, Murphy ES, Osei-Boateng K, Xie H, et al. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol. 2015;38(3):304–310. doi: 10.1097/COC.0b013e31829c3139. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, et al. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. 2016;6:24364. doi: 10.1038/srep24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanigawa K, Mizuno K, Kamenohara Y, Unoki T, Misono S, Inoue H. Effect of bevacizumab on brain radiation necrosis in anaplastic lymphoma kinase-positive lung cancer. Respirol Case Rep. 2019;7(7):e00454. doi: 10.1002/rcr2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Y, Zheng C, Feng Y, Xu Q. Bevacizumab for the treatment of Gammaknife radiosurgery-induced brain radiation necrosis. J Craniofac Surg. 2017;28(6):e569–ee71. doi: 10.1097/scs.0000000000003874. [DOI] [PubMed] [Google Scholar]
- 58.Glitza IC, Guha-Thakurta N, D'Souza NM, Amaria RN, McGovern SL, Rao G, et al. Bevacizumab as an effective treatment for radiation necrosis after radiotherapy for melanoma brain metastases. Melanoma Res. 2017;27(6):580–584. doi: 10.1097/cmr.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 59.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 61.Altman DG, Machin D, Bryant TN, Gardner MJ. BMJ Books ISBN 0 7279 1375 1. 2000. Statistics with confidence second edition; pp. 28–31. [Google Scholar]
- 62.Higgins JPT, Li T, Deeks JJ. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Chapter 6: choosing effect measures and computing estimates of effect, Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019): Cochrane; 2019. Available from https://training.cochrane.org/handbook/current/chapter-06#section-6-5-2 (table 6.5.2a).
- 63.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36. 10.18637/jss.v036.i03.
- 65.Wallace B, Trikalinos T, Lau J, Trow P, Schmid C. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2011;49. 10.18637/jss.v049.i05.
- 66.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 67.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 69.Van Tassel P, Bruner JM, Maor MH, Leeds NE, Gleason MJ, Yung WK, et al. MR of toxic effects of accelerated fractionation radiation therapy and carboplatin chemotherapy for malignant gliomas. AJNR Am J Neuroradiol. 1995;16(4):715–726. [PMC free article] [PubMed] [Google Scholar]
- 70.Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26(8):1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 71.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30(2):367–372. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddy K, Westerly D, Chen C. MRI patterns of T1 enhancing radiation necrosis versus tumour recurrence in high-grade gliomas. J Med Imaging Radiat Oncol. 2013;57(3):349–355. doi: 10.1111/j.1754-9485.2012.02472.x. [DOI] [PubMed] [Google Scholar]
- 73.Rock JP, Hearshen D, Scarpace L, Croteau D, Gutierrez J, Fisher JL, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51(4):912–919. doi: 10.1097/00006123-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Takenaka S, Asano Y, Shinoda J, Nomura Y, Yonezawa S, Miwa K, et al. Comparison of (11)C-methionine, (11)C-choline, and (18)F-fluorodeoxyglucose-PET for distinguishing glioma recurrence from radiation necrosis. Neurol Med Chir (Tokyo) 2014;54(4):280–289. doi: 10.2176/nmc.oa2013-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/jco.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 76.Miyatake S-I, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo) 2015;55(1):50–59. doi: 10.2176/nmc.ra.2014-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–11846. doi: 10.3390/ijms150711832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah R, Vattoth S, Jacob R, Manzil FFP, O’Malley JP, Borghei P, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. RadioGraphics. 2012;32(5):1343–1359. doi: 10.1148/rg.325125002. [DOI] [PubMed] [Google Scholar]
- 79.Chernov MF, Hayashi M, Izawa M, Usukura M, Yoshida S, Ono Y, et al. Multivoxel proton MRS for differentiation of radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases. Brain Tumor Pathol. 2006;23(1):19–27. doi: 10.1007/s10014-006-0194-9. [DOI] [PubMed] [Google Scholar]
- 80.Furuuchi K, Nishiyama A, Yoshioka H, Yokoyama T, Ishida T. Reenlargement of radiation necrosis after stereotactic radiotherapy for brain metastasis from lung cancer during bevacizumab treatment. Respir Investig. 2017;55(2):184–187. doi: 10.1016/j.resinv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Gronier S, Bourg V, Frenay M, Cohen M, Mondot L, Thomas P, et al. Bevacizumab for the treatment of cerebral radionecrosis. Rev Neurol (Paris) 2011;167(4):331–336. doi: 10.1016/j.neurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;76(1):87–93. doi: 10.1212/WNL.0b013e318204a3af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fleming T, editor. Red book: pharmacy’s fundamental reference. 111. Thomson: Belmont; 2007. [Google Scholar]
- 84.Shiroiwa T, Sung Y-K, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 85.Sedgwick P. Retrospective cohort studies: advantages and disadvantages. BMJ. 2014;348:g1072. doi: 10.1136/bmj.g1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.