Abstract
Background
Thoracic aortic endovascular repair (TEVAR) of uncomplicated type B aortic dissection (uTBAD) has favorable long-term outcomes but higher early adverse events compared with the optimal medical treatment. Recently, clinical evidence concerning vascular surgery indicates that elevated preoperative systemic inflammatory response predicts adverse clinical events. The aim of our study was to evaluate the relationship between preoperative neutrophil-to-lymphocyte ratio (NLR) and early outcomes of uTBAD patients undergoing TEVAR.
Results
216 patients diagnosed with uTBAD were included in this retrospective study between January 2015 and December 2018. The median (IQR) follow-up period was 21 (15–33) months. An early adverse event was defined as occurring within 2 years after the procedure. Median patient age was 60 (IQR, 48–68) years and 78.7 % were male. Early adverse events occurred in 24 patients (11.1 %). In the multivariable analysis, preoperative NLR (HR per SD, 1.98; 95 % CI, 1.14–3.44; P = 0.015) was associated with 2-year adverse events.
Conclusions
NLR is an independent predictive factor of early adverse events in uTBAD patients undergoing TEVAR.
Keywords: Neutrophil‐to‐lymphocyte ratio, Thoracic endovascular aortic repair, Uncomplicated type B aortic dissection, Systemic inflammatory response, Inflammatory markers
Background
Acute type B aortic dissection (TBAD), one kind of acute aortic syndromes, featured with sudden onset, rapid progression, and high mortality [1, 2]. Despite its name, “uncomplicated” TBAD (uTBAD) is still a problematic disorder that is associated with high long-term mortality when treated medically alone [3]. With the advantages in exclusion of primary entry tear and remodeling of affected aorta, thoracic endovascular aortic repair (TEVAR) was attempted in the uTBAD combined with optimal medical treatment [4]. Clinical researches based on retrospective cohort suggest that TEVAR is beneficial in long-term outcomes for uTBAD [3, 5].
However, there are still debates about the endovascular repair of uTBAD because of disappointing early outcomes compared with optimal medical treatment [6]. Recently, there has been a significant body of work which has investigated the use of systemic inflammatory marker neutrophil-to-lymphocyte ratio (NLR) to both prognosticate patients and to guide treatment [7–9]. In a cross-section study containing 1021 patients receiving major vascular surgery, elevated preoperative NLR (> 5) was an independent risk factor of 2-year mortality [10]. It would therefore be of great interest to clearly define the association with baseline NLR and early clinical outcomes in uTBAD patients undergoing TEVAR.
The study aimed to determine whether elevated baseline NLR is correlated to early poor outcomes of uTBAD patients after TEVAR.
Methods
Patient selection
A total of 216 consecutive patients at the Department of Vascular and Endovascular Institution (Changhai Hospital, Shanghai, China) who underwent TEVAR of their primary uTBAD between January 2015 and December 2018 were included in the current study.
Overall exclusion criteria were: patients who were < 18 years old; had aortic dissection secondary to iatrogenic injury, trauma, intramural hematoma or penetrating aortic ulcer; had genetic disease such as Marfan syndrome or Ehlers-Danlos syndrome [11].
Additionally, the following susceptible factors and clinical conditions that may affect baseline systemic inflammatory status or postoperative status were excluded: (1) Patients with proximal landing zone 1 demanding for a hybrid or multi-fenestration technique; (2) Any peripheral artery disease or coronary artery disease being treated with dual anti-platelet therapy; (3) Any malignant disease or end-stage disease like uremia; (4) Any chronic infectious disease being treated with antibiotics; (5) Any autoimmune disease being treated with glucocorticoid [12].
According to the criteria above, 33 patients were excluded, which composed of 5 cases diagnosed with Marfan Syndrome, 8 cases diagnosed with cancer, 12 cases treated with hybrid technique, 1 case with AMI treated with dual anti-platelet therapy, 3 cases treated with dialysis and 4 cases suspected with signs of infection.
Computed tomography angiography (CTA) examinations were performed before the operation to evaluate characteristics of aortic dissection. The follow-up period ended on February 1, 2020.
Medical management
Medical treatment aimed to relieve the stress of aortic adventitia and reduce the volume of false lumen [6]. Aggressive antihypertensive therapy by oral or intravenous route was used to keep the systolic blood pressure < 120 mmHg and the heart rate < 70 beat/min [6, 11]. Adequate analgesia was administered when patients still felt pain after antihypertensive therapy.
TEVAR procedure
All patients received general anesthesia in order to administrate blood pressure and heart rate during the procedure. All procedures were performed with patients using total endovascular techniques under fluoroscopic guidance. The proximal landing zone was considered to be at least > 2 cm [13] If the landing zone was inadequate, reconstruction of the left subclavian artery (LSCA) was performed to preserve the blood of the brain and reduce the complications [14].
Follow‐up
Patients were advised to check up annually in our hospital. Health conditions of patients who did not return were contacted via telephone.
Baseline blood count
Samples of 216 patients’ complete blood count with differential 24 hours before the TEVAR procedure were accessed from the electronic medical record in Changhai Hospital.
Statistics analysis
Continuous variables were expressed as the mean ± standard deviation or median (with interquartile range, IQR); categorical variables were expressed as frequencies and percentages. A time-dependent receiver operating characteristic (ROC) curve was utilized to identify a cut-off value NLR associated with 2-year adverse events. Study cohort was then divided into two groups by cut-off value of NLR 4.8. Patients were then grouped into ‘low NLR’ (< 4.8) and ‘high NLR’ (≥ 4.8) groups to identify the differences of clinical characteristics and outcomes. Difference between the two groups were compared by Student’s t-test or Mann-Whitney U test for Continuous variables and a chi-square test for categorical variables. To analyze the relationship between variates and outcomes, univariate and multivariate Cox proportional hazards regression models was used. Variables found to be susceptive (P < 0.2) in univariate analysis were entered into a Cox regression multivariate model using a backwards conditional method. A P value < 0.05 was considered statistically significant and all estimates were two-tailed. All analyses were performed with R (http://www.R-project.org) and Empower Stats software (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
Definition of terms and outcomes
Phase of TBAD was according to the VIRTUE Registry and ESC guideline [4, 11]. “Uncomplicated” is a relatively stable status administrated by optimal medicine, on which the patients are expected to survive without severe comorbidities [11, 15]. The definition of procedure success was to exclude the primary entry tear in the premise that there was no type I or III endoleak at the end of the operation. Post-implantation syndrome (PIS) was defined as fever > 38 °C, WBC > 12.0/nl and CRP > 10 mg/dl within 3 days after TEVAR despite negative blood culture results [16]. The definition of an early adverse event was occurrence within 2 years after the procedure. The primary endpoint was early adverse events, which included all-cause mortality, type I/II endoleak, retrograde aortic dissection (RTAD), stent graft-induced new entry (SINE), paraplegia, major stroke, aortic rupture.
Results
Baseline characteristics
The median follow-up time was 21 months (interquartile range, IQR 15–33). The baseline characteristics of this cohort are documented in Table 1. Briefly, 50 % of patients were older than 60, 21.3 % were females. Additional characteristics of the patients included smoking in 27.8 %, alcohol use in 18.1 %, hypertension in 66.2 %, diabetes in 4.6 %, chronic obstructive pulmonary disease (COPD) in 3.7 %, renal insufficiency in 5.1 %, stroke in 4.6 %, and coronary artery disease (CAD) in 6.5 % (Table 1). For the inflammatory status, 48.1 % had high NLR and 51.9 % had low NLR (Table 1). Significant differences was shown between the high and low NLR group in median age (year) (54 vs. 62; P = 0.018), portion of partial thrombosis (%) (30.8 % vs. 16.1 %; P = 0.02), median systolic blood pressure on admission (mmHg) (148 vs. 140; P = 0.007), median serum creatinine before procedure (µmol/l) (81 vs. 76; P = 0.036), median platelet count before procedure (×109/l) (170 vs. 221; P < 0.001) (Table 1).
Table 1.
The baseline preoperative characteristics of uTBAD patients receiving TEVAR grouped by NLR value < 4.8 and ≥ 4.8
| Variable | Overall | Low NLR (< 4.8) |
High NLR (≥ 4.8) |
Standardized difference | P value |
|---|---|---|---|---|---|
| N (%) | 216 | 112 (51.9 %) | 104 (48.1 %) | ||
| Age (year) | 60 (48–68) | 62 (50–70) | 54 (46–66) | 0.32 | 0.018 |
| Age group (year) | 0.27 | 0.146 | |||
| < 40 | 20 (9.3 %) | 10 (8.9 %) | 10 (9.6 %) | ||
| 40–60 | 88 (40.7 %) | 39 (34.8 %) | 49 (47.1 %) | ||
| ≥ 60 | 108 (50.0 %) | 63 (56.3 %) | 45 (43.3 %) | ||
| Male | 170 (78.7 %) | 83 (74.1 %) | 87 (83.7 %) | 0.24 | 0.087 |
| BMI | 24.9 (3.9) | 24.9 (4.0) | 25.0 (3.8) | 0.04 | 0.764 |
| Smoking | 60 (27.8 %) | 30 (26.8 %) | 30 (28.9 %) | 0.05 | 0.736 |
| Drinking | 39 (18.1 %) | 20 (17.9 %) | 19 (18.3 %) | 0.01 | 0.937 |
| Hypertension | 143 (66.2 %) | 73 (65.2 %) | 70 (67.3 %) | 0.05 | 0.741 |
| Diabetes | 10 (4.6 %) | 5 (4.5 %) | 5 (4.8 %) | 0.02 | 0.904 |
| COPD | 8 (3.7 %) | 3 (2.7 %) | 5 (4.8 %) | 0.11 | 0.408 |
| Renal insufficiency | 11 (5.1 %) | 8 (7.1 %) | 3 (2.9 %) | 0.2 | 0.155 |
| Coronary artery diseases | 14 (6.5 %) | 9 (8.0 %) | 5 (4.8 %) | 0.13 | 0.336 |
| Stroke | 10 (4.6 %) | 4 (3.6 %) | 6 (5.8 %) | 0.10 | 0.442 |
| Dissection morphology | 0.04 | 0.747 | |||
| Confined in thoracic aorta | 56 (25.9 %) | 28 (25.0 %) | 28 (26.9 %) | ||
| Extended to abdominal aorta | 160 (74.1 %) | 84 (75.0 %) | 76 (73.1 %) | ||
| False lumen patency | 0.38 | 0.021 | |||
| Patent false lumen | 156 (72.2 %) | 90 (80.4 %) | 66 (63.5 %) | ||
| Partial thrombosis | 50 (23.2 %) | 18 (16.1 %) | 32 (30.8 %) | ||
| Complete thrombosis | 10 (4.6 %) | 4 (3.6 %) | 6 (5.8 %) | ||
| Symptoms on admission | 0.09 | 0.81 | |||
| Chest/back pain | 156 (72.2 %) | 79 (70.6 %) | 77 (74.0 %) | ||
| Abdominal pain | 52 (24.1 %) | 29 (25.9 %) | 23 (22.1 %) | ||
| Other symptoms | 8 (3.7 %) | 4 (3.6 %) | 4 (3.9 %) | ||
| Location of the primary entry tear | 0.16 | 0.231 | |||
| > 2 cm from the LSCA | 169 (78.2 %) | 84 (75.0 %) | 85 (81.7 %) | ||
| ≤ 2 cm from the LSCA | 47 (21.8 %) | 28 (25.0 %) | 19 (18.3 %) | ||
| Maximum diameter of thoracic aorta (mm) |
40.7 (35.2–44.3) |
39.7 (35.0–44.1) | 40.8 (36.5–44.3) | 0.09 | 0.517 |
| Maximum diameter of abdominal aorta (mm) |
28.7 (26.6–32.9) |
28.5 (26.5–32.4) | 29.4 (26.6–32.9) | 0.16 | 0.248 |
| SBP on admission (mmHg) | 143 (132–154) | 140 (130–150) | 148 (136–157) | 0.37 | 0.007 |
| DBP on admission (mmHg) | 80 (75–89) | 82 (75–89) | 80 (75–88) | 0.04 | 0.77 |
| Temperature preoperatively (℃) |
36.6 (36.4–36.8) |
36.6 (36.4–36.9) |
36.6 (36.4–36.8) |
0.24 | 0.077 |
| Creatinine preoperatively (µmol/l) | 79 (64–91) | 76 (59–86) | 81 (65–95) | 0.29 | 0.036 |
| Platelet count preoperatively (×109/l) | 200 (152–272) | 221 (169–302) | 170 (149–225) | 0.42 | 0.003 |
Categorical variables are reported as frequency and percentage; continuous variables are reported as median (25th–75th percentile)
uTBAD uncomplicated type B aortic dissection, TEVAR thoracic endovascular aortic repair, NLR neutrophil-to-lymphocyte ratio, BMI body mass index, COPD chronic obstructive pulmonary disease, LSCA left subclavian artery, SBP systolic blood pressure, DBP diastolic blood pressure
Medical managements
Details of medical treatment was shown in Table 2. There was no difference between the two groups except calcium-channel blockers (high NLR group vs. low NLR group, 84.6 % vs. 69.6 %, P = 0.009).
Table 2.
The details of medical managements grouped by NLR value < 4.8 and ≥ 4.8
| Variable | Overall | Low NLR (< 4.8) | High NLR (≥ 4.8) | Standardized difference | P value |
|---|---|---|---|---|---|
| Alpha-blockers | 166 (76.9 %) | 87 (77.7 %) | 79 (76.0 %) | 0.04 | 0.765 |
| Beta-blockers | 140 (64.8 %) | 69 (61.6 %) | 71 (68.3 %) | 0.14 | 0.306 |
| ARBs | 78 (36.1 %) | 43 (38.4 %) | 35 (33.7 %) | 0.1 | 0.469 |
| ACEIs | 32 (14.8 %) | 15 (13.4 %) | 17 (16.4 %) | 0.08 | 0.542 |
| CCBs | 166 (76.9 %) | 78 (69.6 %) | 88 (84.6 %) | 0.36 | 0.009 |
| Statins | 86 (39.8 %) | 44 (39.3 %) | 42 (40.4 %) | 0.02 | 0.869 |
Values are n (%)
ARBs angiotensin II receptor blockers, ACEIs angiotensin-converting enzyme inhibitors, CCBs calcium-channel blockers, NLR neutrophil-to-lymphocyte ratio
TEVAR procedure
27 adjunctive stents were implanted to reconstruct the LSCA. Details of the procedure were shown in Table 3.
Table 3.
The details of TEVAR procedure grouped by NLR value < 4.8 and ≥ 4.8
| Variable | Overall | Low NLR (< 4.8) |
High NLR (≥ 4.8) |
Standardized difference | P value |
|---|---|---|---|---|---|
| Length of hospital stay (days) | 10 (8–14) | 10 (7–14) | 10 (9–15) | 0.24 | 0.076 |
| Timing of procedure | 0.05 | 0.713 | |||
| Acute phase | 126 (58.3 %) | 64 (57.1 %) | 62 (59.6 %) | ||
| Subacute phase | 90 (41.7 %) | 48 (42.9 %) | 42 (40.4 %) | ||
| ASA Classification | 0.24 | 0.205 | |||
| I | 68 (31.5 %) | 33 (29.4 %) | 35 (33.7 %) | ||
| II | 140 (64.8 %) | 77 (68.8 %) | 63 (60.6 %) | ||
| III | 8 (3.7 %) | 2 (1.8 %) | 6 (5.7 %) | ||
| Length of procedure (minutes) | 95(75–110) | 95(80–122) | 85(72–105) | 0.31 | 0.025 |
| Types of stentgraft | |||||
| Cook Zenith | 93 (43.1 %) | 48 (42.9 %) | 45 (43.3 %) | 0.4 | 0.044 |
| Gore TAG | 54 (25.0 %) | 33 (29.4 %) | 21 (20.2 %) | ||
| Medtronic Valiant | 55 (25.5 %) | 21 (18.8 %) | 34 (32.7 %) | ||
| Microport Hercules | 14 (6.4 %) | 10 (8.9 %) | 4 (3.9 %) | ||
| Adjunctive stents | 27 (12.5 %) | 15 (13.4 %) | 12 (11.5 %) | 0.06 | 0.681 |
Values are median (25th–75th percentile) or n (%)
TEVAR thoracic endovascular aortic repair, NLR neutrophil-to-lymphocyte ratio, ASA American Society of Anesthesiologists
Early adverse events
There were no in-hospital adverse events in either group. PIS occurred in 8 (3.7 %) of 216 patients with no difference between the two groups (4.8 % for high NLR group and 2.7 % for low NLR group, P = 0.41). None of the patients above suffered from the adverse events within 2-year follow up.
Early adverse events occurred in 24 patients (11.1 %), which included one mortality due to stroke, 13 cases of type I /II endoleak, 5 cases of retrograde aortic dissection, 4 cases of aortic rupture (two died and the others survived after re-intervention) and one case of stent graft-induced new entry. The overall probability of freedom from adverse events in the high NLR group at 2 years were 82.7 % while low NLR group was 94.6 % (P = 0.005). (Fig. 1).
Fig. 1.
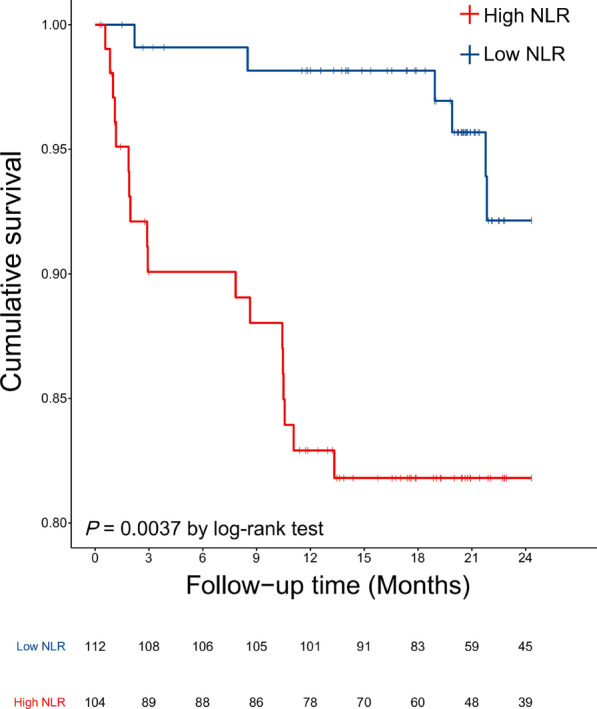
Kaplan–Meier survival analysis stratified by NLR value 4.8 (low NLR group, < 4.8; high NLR group, ≥ 4.8). The differences between the two groups was assessed with log-rank test. The freedom from early adverse events in the low NLR group was significantly higher than high NLR group (P = 0.004). NLR, neutrophil-to-lymphocyte ratio
Univariate and multivariable Cox-hazard regression analyses of early adverse events in the study population were shown in Table 4. Univariate analysis indicated that platelet count preoperatively, preoperative diastolic blood pressure and NLR preoperatively were the susceptive risk factors for early adverse events (P < 0.2). Multivariable regression analysis showed that the NLR preoperatively (HR per SD, 1.98; 1.14–3.44; P = 0.015), diastolic blood pressure on admission (HR, 0.86; 95 % CI, 0.78–0.95; P = 0.003) had independent influences on 2-year overall event-free survival.
Table 4.
Univariate and multivariable analysis of 2-year adverse events
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Age | 1.02 (1.00, 1.07) | 0.296 | ||
| Male gender | 0.40 (0.13, 1.22) | 0.105 | 0.49 (0.10, 2.31) | 0.363 |
| BMI | 0.94 (0.81, 1.11) | 0.479 | ||
| Smoking | 0.84 (0.23, 3.06) | 0.792 | ||
| Alcohol | 2.82 (0.92, 8.61) | 0.069 | 0.98 (0.23, 4.16) | 0.980 |
| Hypertension | 1.60 (0.44, 5.81) | 0.476 | ||
| False lumen patency | ||||
| Patent false lumen | Reference | 1 | ||
| Partial thrombosis | 0.67 (0.15, 3.10) | 0.608 | 0.50 (0.07, 3.28) | 0.466 |
| Complete thrombosis | 3.43 (0.74, 15.89) | 0.115 | 4.14 (0.51, 33.35) | 0.182 |
| Distance of LSCA ≤ 2 cm | 1.02 (0.28, 3.71) | 0.976 | ||
| Maximum diameter of abdominal aorta (cm) | 1.07 (0.97, 1.18) | 0.159 | 1.09 (0.92, 1.29) | 0.316 |
| SBP on admission (mmHg) | 1.00 (0.97, 1.03) | 0.907 | ||
| DBP on admission (mmHg) | 0.91 (0.83, 0.96) | 0.003 | 0.86 (0.78, 0.95) | 0.003 |
| Temperature preoperatively (℃) | 0.64 (0.14, 2.85) | 0.558 | ||
| Creatinine preoperatively (µmol/l) | 1.00 (0.98, 1.02) | 0.741 | ||
| Platelet count preoperatively (×109/l) | 0.99 (0.99, 1.00) | 0.107 | ||
| Preoperative NLR per SD | 1.58 (1.11, 2.26) | 0.011 | 1.98 (1.14, 3.44) | 0.015 |
| Alpha-blockers | 3.60 (0.47, 7.68) | 0.219 | ||
| Beta-blockers | 0.95 (0.31, 2.91) | 0.929 | ||
| ARBs | 1.06 (0.24, 4.79) | 0.939 | ||
| ACEIs | 1.12 (0.23, 4.81) | 0.939 | ||
| CCBs | 0.47 (0.16, 1.46) | 0.193 | 0.78 (0.14, 4.40) | 0.776 |
| Statins | 0.67 (0.21, 2.16) | 0.497 | ||
| Timing of procedure | ||||
| Acute (1–14 days) | Reference | |||
| Subacute (14–92 days) | 1.16 (0.39, 3.46) | 0.787 | ||
| ASA classification | ||||
| I | Reference | Reference | ||
| II | 0.54 (0.17, 1.77) | 0.309 | 0.51 (0.10, 2.73) | 0.433 |
| III | 2.96 (0.57, 15.36) | 0.195 | 3.19 (0.22, 46.42) | 0.395 |
| Adjunctive stents | 3.48 (1.07, 11.32) | 0.038 | 0.79 (0.13, 4.79) | 0.800 |
HR hazard ratio, CI confidence interval, BMI body mass index, LSCA left subclavian artery, NLR neutrophil-to-lymphocyte ratio, SBP systolic blood pressure, DBP diastolic blood pressure, ASA American Society of Anesthesiologists
Discussion
The aim of this study was to evaluate the relationship between preoperative NLR and early outcomes of TEVAR. For every increase of one SD in preoperative NLR value, the risk of early adverse events increased by 98 % (95 % CI, 1.14–3.44) in the multivariate adjusted model. To the best knowledge of us, this is the first research to access the predictive role of NLR in the uTBAD patients receiving TEVAR with a cut-off value 4.8.
Although long-term safety and efficacy of TEVAR have been confirmed with multiple studies [3, 5, 17], early adverse events still attract controversy and impede the extensive application of TEVAR in uTBAD. In 2019, Professor Adams and his colleagues [6] suggested that high-risk subgroup of uTBAD patients may benefit from early intervention. However, most areas in China do not have a sound medical system, which would bring difficulties to follow-up and timely risk assessment of aortic dissection. It is therefore of great value to determine the optimal timing of TEVAR procedure to evade the early adverse events.
Previous studies focused on the association between NLR value and poor clinical outcomes of type A aortic dissection (TAAD) or severe aortic events after TEVAR or open surgery [18, 19]. Lafçi et al. [20] conducted a study with 123 patients and found that a high pre-NLR was associated with in-hospital mortality with a cut-off value 8. In another study, Bedel et al. [21] also observed an association between high levels of admission PLR and NLR and high mortality and organ dysfunction in acute TAAD patients. The studies above implied that combinations of inflammatory markers have a satisfied predictive capability for the in-hospital mortality. In contrast, in a large sample cohort study of 744 patients with TAAD, combinations of platelet, neutrophil and lymphocyte were unable to predict 30-day mortality [22]. Interestingly, when investigators combined all three biomarkers (platelet counts, lymphocyte-to-neutrophil ratio and lymphocyte monocyte ratio) in the predictive model, it provided the strongest predictive value of 30-day mortality. The reason behind this apparent contradiction might be unclear time of NLR measurement. In these studies, Azab et al. [23] used average NLR during the inpatient stay in the prediction model rather than admission, discharge, or maximum NLR. On the contrary, Park et al. [24] suggested that 24-hour NLR had a better predictive value of in-hospital mortality than the admission NLR. When compared with TAAD, TBAD showed less early postoperative mortality and complications such as renal insufficiency, liver insufficiency, and gastrointestinal hemorrhage. uTBAD Patients with strict blood pressure and heart rate control have a longer life expectancy and deserve an elective surgery instead of emergent one. King et al. [9] highlighted the importance of detection and treatment of high immune or inflammatory response in the endovascular aortic repair (EVAR) of elective abdominal aortic aneurysm (AAA). In this context, NLR 24 hours before operation is a better predictive index compared with the admission one. In our study, inflammatory index 24 hours before operation was utilized to predict the adverse events. We found significant difference of early outcomes between the high NLR group and low NLR group (adverse events, 17.3 % vs. 5.4 %, P = 0.005) despite that systemic inflammatory response of uTBAD is relatively lower than complicated aortic dissection or type A aortic dissection considering the lesion and comorbidities.
Although previous clinical evidence has clearly shown that the increased NLR value is associated with poor outcomes after endovascular repair, the cut-off value varies among studies [8, 10, 19, 25, 26]. Octeau et al. [27] defined the median NLR 3.5 for all patients as the cut-off value in a retrospective study including 107 patients undergoing thoracic endovascular aneurysm repair. In another study, King et al. [9] used a ROC curve analysis to determine the cut-off value of NLR 4.0 with the highest specificity and sensitivity to correlate with mortality. In the present study, cut-off value 4.8 was obtained using a time-dependent ROC curve associated with 2-year poor outcomes. As NLR is a continuous dynamic value rather than a dichotomous variable, King et al. [9] used a tertile analysis, in which highest NLR group showed poorest outcomes compared with the lower two groups. In our study, effect of NLR per SD change was evaluated in the univariate and multivariable analysis (Table 4). It showed that the risk of early adverse outcomes increased by 98 % for every increase of one SD in preoperative NLR (multivariate HR, 1.98; 1.14–3.44; P = 0.015). Although there is a need to perform experiments with more subjects, this study shows potential risk of early adverse events for uTBAD patients undergoing TEVAR with a higher NLR than 4.8.
The effect of inflammatory markers in cardiovascular diseases has been studied routinely and a credible relationship between inflammatory markers and cardiovascular diseases has been confirmed [8, 28, 29]. Although the exact mechanisms of these markers are not well understood, it is thought that they reflect the complex interaction between the local immune response at the microenvironment of aortic dissection and the systemic inflammatory response [29–31]. The etiology of AD is complex, in which inflammation plays an important role [32, 33]. After onset, the systemic response to injury causes neutrophilia and massive neutrophil accumulation in the tunica adventitia of the dissected aorta [34]. The activation of neutrophils and endothelial cell adhesion to generate a large amount of reactive oxygen intermediate, in turn, increasing the vascular endothelial damage, and the excessive consumption of platelets after thrombosis may also increase the risk of aortic dissection rupture [35]. Clinical evidence has confirmed that systemic inflammatory markers like NLR is independently associated with mortality of AD patients [20, 25, 36, 37]. Taken together, inflammatory markers show promise in the diagnosis, timing of treatment and prognosis of AD. However, inflammatory markers are not factored into any risk stratification model of AAD until now [38]. It would be of interest to take the NLR value into consideration of predictive model of AAD for secondary prevention.
Limitations
Firstly, due to the retrospective study cohort from single center and small number, results may be biased. Secondly, details of adverse events could not be demonstrated via telephone. Thirdly, in clinical practice, however, there remains some controversy regarding the endovascular repair of uTBAD. Although routine TEVAR in uTBAD is standard practice at our institution, different TEVAR procedure manipulations and medical treatment would also affect the results. Fourthly, uTBAD patients who refused to endovascular repair and received optimal medical treatment alone were not included in the study, which may overstate the efficacy of endovascular repair of uncomplicated TBAD. Lastly, anatomic characteristics like oversizing were not included in the analysis, which demand a multi-center study in the future.
Conclusions
Current study revealed that high preoperative NLR value is an independent risk factor of early adverse events in uTBAD patients undergoing TEVAR. Further studies focused on predictive model combined with NLR value should be conducted to open new perspectives in the field of secondary prevention.
Acknowledgements
None.
Abbreviations
- TEVAR
Thoracic aortic endovascular repair
- uTBAD
Uncomplicated type B aortic dissection
- IQR
Interquartile range
- NLR
Neutrophil-to-lymphocyte ratio
- LSCA
Left subclavian artery
- COPD
Chronic obstructive pulmonary disease
- CAD
Coronary artery disease
- TAAD
Type A aortic dissection
- EVAR
Endovascular aortic repair
- AAA
Abdominal aortic aneurysm
- PIS
Post-implantation syndrome
Authors’ contributions
HQZ: Investigation and Writing—original draft. LZ: Writing—original draft. TPL: Writing and Data curation. YML: Data curation. JZ: Writing—review and editing, Supervision. ZPJ: Conceptualization, Project administration.All authors read and approve the final version of the manuscript, and ensure it is the case.
Funding
The source of funding is the National Natural Science Foundation of China (81700430 and 9173910048) which supported the design of the study, and collection, analysis, interpretation of data, and preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This retrospective study was approved by the ethical review board of Changhai Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongqiao Zhu, Lei Zhang and Taiping Liang have contributed equally to this work
Contributor Information
Jian Zhou, Email: zhoujian1-2@163.com.
Zaiping Jing, Email: jingzaiping_vasc@163.com.
References
- 1.Tsai TT, Myrmel T, Smith DE, Llovet A, Distante A, Januzzi JL. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357(4):349–59. doi: 10.1056/NEJMoa063232. [DOI] [PubMed] [Google Scholar]
- 2.Durham CA, Aranson NJ, Ergul EA, Wang LJ, Patel VI, Cambria RP, Conrad MF. Aneurysmal degeneration of the thoracoabdominal aorta after medical management of type B aortic dissections. J Vasc Surg. 2015;62:900–6. doi: 10.1016/j.jvs.2015.04.423. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y-L, Wang F, Li T-X, Ding W, Deng G, Xie B, Teng G-J. Endovascular repair compared with medical management of patients with uncomplicated type B acute aortic dissection. J Am Coll Cardiol. 2016;67:2835–42. doi: 10.1016/j.jacc.2016.03.578. [DOI] [PubMed] [Google Scholar]
- 4.Heijmen R, Fattori R, Thompson M, Eggebrecht H, Degrieck I, Nienaber C, Cheshire N. Mid-term outcomes and aortic remodelling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE registry. Eur J Vasc Endovasc Surg. 2014;48:363–71. doi: 10.1016/j.ejvs.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Xiang D, Kan X, Liang H, Xiong B, Liang B, Wang L, Zheng C. Comparison of mid-term outcomes of endovascular repair and medical management in patients with acute uncomplicated type B aortic dissection. J Thorac Cardiovasc Surg. 2019;X:X. doi: 10.1016/j.jtcvs.2019.11.127. [DOI] [PubMed] [Google Scholar]
- 6.Tadros RO, Tang GHL, Barnes HJ, Mousavi I, Kovacic JC, Faries P, Olin JW, Marin ML, Adams DH. Optimal treatment of uncomplicated type B aortic dissection. J Am Coll Cardiol. 2019;74:11. doi: 10.1016/j.jacc.2019.07.063. [DOI] [PubMed] [Google Scholar]
- 7.Brizuela Sanz JA, González Fajardo JA, Taylor JH, Río Solá L, Muñoz Moreno MF, Vaquero Puerta C. Design of a new risk score in critical limb ischaemia: the ERICVA model. Eur J Vasc Endovasc Surg. 2016;51:90–9. doi: 10.1016/j.ejvs.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–7. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 9.King AH, Schmaier AH, Harth KC, Kumins NH, Wong VL, Zidar DA, Kashyap VS, Cho JS. Elevated neutrophil-lymphocyte ratio predicts mortality following elective endovascular aneurysm repair. J Vasc Surg. 2020;72:129–37. doi: 10.1016/j.jvs.2019.10.058. [DOI] [PubMed] [Google Scholar]
- 10.Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: a cross-sectional study. Vasc Endovasc Surg. 2011;45:227–31. doi: 10.1177/1538574410396590. [DOI] [PubMed] [Google Scholar]
- 11.Authors/Task Force members. Erbel R, Aboyans V, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult the task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–926. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 12.Lancellotti P, Marechal P, Donis N, Oury C. Inflammation, cardiovascular disease, and cancer: a common link with far-reaching implications. Eur Heart J. 2019;40:3910–2. doi: 10.1093/eurheartj/ehz645. [DOI] [PubMed] [Google Scholar]
- 13.Tse LW, MacKenzie KS, Montreuil B, Obrand DI, Steinmetz OK. The proximal landing zone in endovascular repair of the thoracic aorta. Ann Vasc Surg. 2004;18:178–85. doi: 10.1007/s10016-004-0008-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Lu Q, Zhou J, Jing Z, Zhao Z, Bao J. Alternative management of the left subclavian artery in thoracic endovascular aortic repair for aortic dissection: a single-center experience. Eur J Med Res. 2015;20:57. doi: 10.1186/s40001-015-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trimarchi S, Eagle KA, Nienaber CA, Pyeritz RE, Jonker FHW, Suzuki T, O’Gara PT, Hutchinson SJ, Rampoldi V, Grassi V, Bossone E, Muhs BE, Evangelista A, Tsai TT, Froehlich JB, Cooper JV, Montgomery D, Meinhardt G, Myrmel T, Upchurch GR, Sundt TM, Isselbacher EM. on behalf of the international registry of acute aortic dissection (IRAD) investigators. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the international registry of acute aortic dissection (IRAD) Circulation. 2010;122:1283–9. doi: 10.1161/CIRCULATIONAHA.109.929422. [DOI] [PubMed] [Google Scholar]
- 16.Gorla R, Erbel R, Kahlert P, Tsagakis K, Jakob H, Mahabadi A-A, Schlosser T, Eagle K, Bossone E, Jánosi RA. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur J Cardiothorac Surg. 2016;49:1239–47. doi: 10.1093/ejcts/ezv355. [DOI] [PubMed] [Google Scholar]
- 17.Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Fattori R, Ince H. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–16. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Luo S, Ding H, Liu Y, Huang W, Xie N, Li J, Xue L, Luo J. Predictors associated with an increased prevalence of postimplantation syndrome after thoracic endovascular aortic repair for type B aortic dissection†. Eur J Cardiothorac Surg. 2019;55:998–1005. doi: 10.1093/ejcts/ezy379. [DOI] [PubMed] [Google Scholar]
- 19.Kordzadeh A, Malietzis G, Browne T, Prionidis I, Panayiotopoulos YP. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): a retrospective cohort study. Int J Surg. 2015;15:45–8. doi: 10.1016/j.ijsu.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Lafçi G, Çi̇Çek ÖF, Uzun HA, Yalçinkaya A, Di̇Ken A, Turak O, Çağli K, Taşoğlu İ, Gedi̇K HS, Korkmaz K, Günertem OE, Çağli K. Relationship of admission neutrophil-to-lymphocyte ratio with in-hospital mortality in patients with acute type I aortic dissection. Turk J Med Sci. 2014;44:186–92. doi: 10.3906/sag-1301-136. [DOI] [PubMed] [Google Scholar]
- 21.Bedel C, Selvi F. Association of platelet to lymphocyte and neutrophil to lymphocyte ratios with in-hospital mortality in patients with type A acute aortic dissection. Braz J Cardiovasc Surg. 2019;34(6):694–8. doi: 10.21470/1678-9741-2018-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Lin Y, Zhang H, Peng Y, Li S, Huang X. Relationship of platelet counts and inflammatory markers to 30-day mortality risk in patients with acute type A aortic dissection. BioMed Res Int. 2020;2020. [DOI] [PMC free article] [PubMed]
- 23.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D, Lafferty J. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–6. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 24.Park JJ, Jang H-J, Oh I-Y, Yoon C-H, Suh J-W, Cho Y-S, Youn T-J, Cho G-Y, Chae I-H, Choi D-J. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–42. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Appleton ND, Bailey DM, Morris-Stiff G, Lewis MH. Neutrophil to lymphocyte ratio predicts perioperative mortality following open elective repair of abdominal aortic aneurysms. Vasc Endovasc Surg. 2014;48:311–6. doi: 10.1177/1538574413519713. [DOI] [PubMed] [Google Scholar]
- 26.Erturk M, Cakmak HA, Surgit O, et al. The predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol. 2014;64(5):371–6. doi: 10.1016/j.jjcc.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Octeau D, Barnes HJ, Faries CM, Nakazawa KR, Ting W, Marin ML, Faries PL, Tadros RO. Association of preoperative neutrophil-to-lymphocyte ratio with rates of adverse events after thoracic endovascular aneurysm repair. J Vasc Surg. 2020;72:e186. doi: 10.1016/j.jvs.2020.04.500. [DOI] [PubMed] [Google Scholar]
- 28.Ueland T, Kjekshus J, Frøland SS, Omland T, Squire IB, Gullestad L, Dickstein K, Aukrust P. Plasma levels of soluble tumor necrosis factor receptor type I during the acute phase following complicated myocardial infarction predicts survival in high-risk patients. J Am Coll Cardiol. 2005;46:2018–21. doi: 10.1016/j.jacc.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, Meghani M, Akhtar M, Costantino T. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–9. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 30.Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, Sano M. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res. 2015;116:612–23. doi: 10.1161/CIRCRESAHA.116.304918. [DOI] [PubMed] [Google Scholar]
- 31.Andreata F, Syvannarath V, Clement M, Delbosc S, Guedj K, Fornasa G, Khallou-Laschet J, Morvan M, Even G. Macrophage CD31 signaling in dissecting aortic aneurysm. J Am Coll Cardiol. 2018;72:45–57. doi: 10.1016/j.jacc.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, Tabacco F, De Santis V, Vecchione A, Mitterhofer AP, Nofroni I, Amodeo R, Trappolini M, Aliberti G. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42:622–9. doi: 10.3109/07853890.2010.518156. [DOI] [PubMed] [Google Scholar]
- 33.Kuehl H, Eggebrecht H, Boes T, Antoch G, Rosenbaum S, Ladd S, Bockisch A, Barkhausen J, Erbel R. Detection of inflammation in patients with acute aortic syndrome: comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart. 2008;94:1472–7. doi: 10.1136/hrt.2007.127282. [DOI] [PubMed] [Google Scholar]
- 34.Anzai A. Inflammatory mechanisms contributing to aortic expansion and rupture after acute aortic dissection. J Immunol Sci. 2018;2:22–6. doi: 10.29245/2578-3009/2018/4.1156. [DOI] [Google Scholar]
- 35.Tsai M-T, Wu H-Y, Roan J-N, Tsai Y-S, Hsieh PCH, Yang Y-J, Luo C-Y. Effect of false lumen partial thrombosis on repaired acute type A aortic dissection. J Thorac Cardiovasc Surg. 2014;148:2140–6.e3. doi: 10.1016/j.jtcvs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Karakoyun S, Gürsoy MO, Akgün T, Öcal L, Kalçık M, Yesin M, Erdoğan E, Külahçıoğlu S, Bakal RB, Köksal C, Yıldız M, Özkan M. Neutrophil–lymphocyte ratio may predict in-hospital mortality in patients with acute type A aortic dissection. Herz. 2015;40:716–21. doi: 10.1007/s00059-014-4121-2. [DOI] [PubMed] [Google Scholar]
- 37.Kalkan ME, Kalkan AK, Gündeş A, Yanartaş M, Oztürk S, Gurbuz AS, Ozturk D, Iyigun T, Akcakoyun M, Emiroglu MY, Tuncer MA, Koksal C. Neutrophil to lymphocyte ratio: a novel marker for predicting hospital mortality of patients with acute type A aortic dissection. Perfusion. 2017;32:321–7. doi: 10.1177/0267659115590625. [DOI] [PubMed] [Google Scholar]
- 38.Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, Myrmel T, Sangiorgi GM, De Vincentiis C, Cooper JV, Fang J, Smith D, Tsai T, Raghupathy A, Fattori R, Sechtem U, Deeb MG, Sundt TM, Isselbacher EM. Simple risk models to predict surgical mortality in acute type A aortic dissection: the international registry of acute aortic dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


