Abstract
Background
Breast cancer (BC) is the leading malignant tumor among women worldwide. Although attending regular BC screening effectively reduces cancer-related mortality, surveys testify that screening knowledge is critically low among women. We aimed to conduct a comparative cross-sectional survey to assess BC and BC screening-related knowledge in Hungary.
Methods
Women between 25 and 65 years of age without a previous history of malignant tumors were included with non-probability sampling in 2017. Respondents were recruited either from primary care (laywomen) or from the waiting rooms of mammography (screening attendees). A self-completion questionnaire was constructed with questions about BC (risk factors, signs and symptoms, curability, and mortality), BC screening (mammography and breast self-examination), and BC-related information sources to assess knowledge among laywomen and screening attendees. In addition to descriptive statistics, odds ratios with 95% confidence intervals were calculated in univariate analysis and logistic regression was used in multivariate analysis.
Results
Altogether, 480 women completed the questionnaire, of which 429 (227 laywomen and 202 screening attendees) were eligible for inclusion. Laywomen and screening attendees knew the recommended age at first mammography in 35.2% and 86.6%, the recommended frequency of screening in 33.9% and 12.9%, the recommended age at first breast-self examination in 38.8% and 51.2%, had sufficient knowledge of the risk factors of BC in 7.0% and 5.9%, and that of signs and symptoms of BC in 16.7% and 28.9%, respectively. A higher proportion of screening attendees correctly identified the recommended age of first BC screening correctly than that of laywomen (86.6% vs. 35.2%; p < 0.001). The most popular information sources were television among laywomen and general practitioners or specialists among screening attendees. In multivariate analysis, older age, higher education, and place of residency were significant predictors of the right answers.
Conclusions
Although knowledge was insufficient in almost all fields of the questionnaire, the most prominent gap was observed concerning risk factors and signs and symptoms of BC both in laywomen and, unexpectedly, screening attendees. Most laywomen were lacking knowledge of screening protocol. These results urge breast health and BC knowledge interventions in Hungary.
Supplementary information
The online version contains supplementary material available at 10.1186/s12905-021-01204-9.
Keywords: Breast cancer screening, Knowledge, Mammography
Background
Breast cancer (BC) was a leading malignant tumor regarding incidence, prevalence, and mortality among women worldwide as well as in Europe in 2018. Worldwide incidence of female BC was 2 million while BC was responsible for more than 0.6 million fatalities in 2018. Within Europe, BC incidence was 522,513 with more than 137,707 BC-related fatalities in 2018 [1]. In Hungary, annual BC incidence has been exceeding 8000 cases since 2015 and reached 8215 with a mortality of 2212 in 2018 [2]. Data testified that Hungary is considerably behind the EU average concerning standardized mortality ratio of BC in 2018 [1].
BC is a multifactorial disease with complex pathomechanisms. Risk factors of BC include but are not limited to positive medical history for conditions or disorders in obstetrics, gynecology, reproduction, and endocrinology. Age is deemed to be an independent risk factor of BC. Besides, the cumulative number of periods seems to be important, which supports the central role of endogenous estrogen in the pathogenesis. Risk of BC doubles in cases where the first delivery is later than 30 years of age [3]. Additional modifiable risk factors involve the exposure to certain exogenous hormones (e.g., postmenopausal hormone therapy applied frequently to relieve the undesired changes of menopause) [4], smoking [5], high-fat diet [6], being overweight or obese, sedentary lifestyle, alcohol consumption (more than 1–2 drinks daily), high socioeconomic status, oral contraceptives containing estrogen, progestagen, or their derivatives; and vitamin D deficiency [4]. Chest irradiation carries a delayed, 7- to 17-fold risk of BC [7–9]. Protective factors include suckling: 12-month breastfeeding reduces the risk of pre-menopausal BC by 4% [10]. Genetics may account for 5–10% of BC cases, of which even 30% may have BRCA 1 or BRCA 2 mutations [11]. These mutations increase the risk of BC tenfold, although several other genes (the so-called ‛BC genes’) have been implicated as contributors to the pathogenesis [12] Hereditary syndromes, e.g., Li-Fraumeni [13] and Cowden syndromes carry a high risk of BC [14].
A potential approach to coping with high BC-related mortality is the introduction of BC screening programs. Studies proved that BC public health interventions promote early detection. Screening uptake of at least 70% of the target population reduces BC-related mortality significantly [15]. Although mammography reduces BC-related mortality by a remarkable 25% among women between 50 and 69 years of age, the risk reduction is less prominent in younger women between 40 and 49 years of age [16]. On the contrary, studies recorded a humble 15% decrease in BC-related mortality with frequent overdiagnosis (30%) and, consequently, frequent overtreatment [17]. The success of BC screening can be attributed to early detection because the immediate removal of tumors with a diameter < 10 mm results in an average survival of 20 years in approximately 90% of the cases [18].
Mass screening for BC had been incorporated into public health services in Hungary in 2001 [15]. The target population of BC screening involves asymptomatic women between 45 and 65 years of age who are regularly invited to participate in screening with X-ray mammography once every two years [19, 20]. Hungarian data from 2015 revealed that invitation for BC screening reaches 78.5% of the target population. Digital mammography accounted for 60% of cases. These quality indicators are far behind the 2015 EU average [20].
There was not a comprehensive report which assessed women’s knowledge of the field in Hungary, while insufficient knowledge may contribute to the suboptimal screening attendance rate. This inspired us to assess the knowledge of BC and BC screening among Hungarian women. In addition, we aimed to explore which channels are used by women to gather relevant information.
Methods
The study is reported following the STROBE Statement.
Study design and settings
This study is a cross-sectional survey. Subjects were recruited from 12 general practitioner clinics and the outpatient Department of Radiology, University of Pécs, Medical School, Hungary. Recruitment period lasted from March 2017 to June 2017.
Sample
We recruited a total of 480 women aged between 25 and 65 years with non-probability sampling (this age interval was the inclusion criteria). Missing data resulted in the exclusion of 52 women. Finally, data of 428 women (89.1%) with complete dataset were eligible for the analysis. The study population was divided into two groups by site of recruitment: 227 women were recruited from primary care (laywomen) and another 201 from the waiting rooms of mammography of the Department of Radiology (screening attendees). The exculsion critera was the history of malignant tumors.
Questionnaire development and validation
We constructed a self-completion questionnaire. As the first step of production, our team of epidemiologists indicated the main areas of interest and phrased index questions accordingly. Then, the set of questions was revised by an oncologist to validate the medical content. Laypeople appraised the wording of questions. The English-language version of the questionnaire is provided in Appendix as Additional file.
The first set included 7 questions concerning sociodemography (sex, age, place of residence, marital status, education, financial situation, and religion). Followed by a set of 16 questions regarding knowledge of BC and BC screening, including multiple-choice questions about information sources. There were 2 multiple and 14 single choice questions given. In terms of signs and symptoms of BC, respondents indicating correctly at least 5 options of the 8 given (all options were true) were considered to have sufficient knowledge. In terms of risk factors of BC, respondents indicating correctly at least 2 options and incorrectly maximum 1 option of 21 given (13 and 12 were true in the groups of screening attendees and lay women, respectively) were considered to have sufficient knowledge.
Medical assistants distributed the questionnaire and obtained signed informed consent from participants. We secured anonymity of participants by linking informed consents to the corresponding questionnaires with a numeric code. Documents were kept in locked cabinets separately until processing.
Statistical analysis
We performed descriptive statistics including the calculation of central tendencies (means or medians) with the measure of dispersion and relative frequencies. In univariate analysis, we used the Mann–Whitney U-test to examine the association between the level of education and knowledge of the risk factors of BC. We used the χ2 test with Z test to reveal the association between participation in screening and knowledge of timing of BC screening. In multivariate analysis, we used logistic regression with a probability of 95% with explanatory variables including age, education, and place of residency to examine the association between the sociodemographic characteristics and the dichotomous outcomes. Data were analyzed using SPSS 20.0 statistical software.
Results
Baseline sociodemographic characteristics of the study population are summarized in Table 1. Main findings are summarized in Table 2.
Table 1.
Sociodemographic characteristics of the groups
| Variable | Laywomen | Screening attendees |
|---|---|---|
| (N0 = 227) | (N0 = 202) | |
| Age (mean) | 47.22 | 53.33 |
| (median) | 48.00 | 55.00 |
| (minimum) | 25.00 | 31.00 |
| (maximum) | 64.00 | 64.00 |
| Place of residence (N0, %) | ||
| City town | 117 (51.5%) | 58 (28.9%) |
| Town | 56 (24.7%) | 74 (36.8%) |
| Village | 54 (23.8%) | 69 (34.3%) |
| Marital status (N0, %) | ||
| Unmarried | 23 (10.1%) | 13 (6.5%) |
| Married/common-law marriage | 136 (59.9%) | 145 (72.1%) |
| Divorced/separated | 47 (20.7%) | 23 (11.4%) |
| Widowed | 21 (9.3%) | 20 (10%) |
| The highest level of education (N0, %) | ||
| Less than primary school | 2 (0.9%) | 1 (0.5%) |
| Primary school | 24 (10.6%) | 18 (9%) |
| Vocational or industrial school | 53 (23.3%) | 43 (21.4%) |
| Secondary school | 50 (22.0%) | 81 (40.3%) |
| College or university | 98 (43.2%) | 58 (28.9%) |
| Health education (N0, %) | ||
| Yes | 140 (61.7%) | 148 (73.6%) |
| No | 87 (38.3%) | 53 (26.4%) |
| Religiosity (N0, %) | ||
| Yes | 102 (44.9%) | 113 (56.2%) |
| No | 125 (55.1%) | 88 (43.8%) |
| Employment status (N0, %) | ||
| Employed | 145 (63.9%) | 119 (59.2%) |
| Unemployed | 7 (3.1%) | 4 (2.0%) |
| Inactive | 66 (29.0%) | 73 (36.3%) |
| Dependant | 9 (4.0%) | 5 (2.5%) |
| Financial situation (N0, %) | ||
| Very bad | 7 (3.1%) | 1 (0.5%) |
| Poor | 27 (11.9%) | 15 (7.5%) |
| Average | 108 (47.6%) | 107 (53.2%) |
| Good | 76 (33.5%) | 74 (36.8%) |
| Very good | 9 (3.9%) | 4 (2.0%) |
Table 2.
Significant associated factors of respondents’ knowledge of BC screening, BSE and BC
| Outcome | Laywomen | Screening attendees | ||
|---|---|---|---|---|
| Predictive factors | Effect | Predictive factors | Effect | |
| Knowing the recommended age of first BC screening and the frequency of BC screening | Age | (ß = 0.039; p = 0.026; OR = 1.040 95% CI 1.005–1.076) | NS | |
| Knowing the recommended age of first BSE and the frequency of BSE | Education | (ß = 0.386; p = 0.016; OR = 1.472 95% CI 1.076–2.013) | NS | |
| Knowing that BC is a common cause of death in Hungary | Age | (ß = 0.029; p = 0.034; OR = 1.030 95% CI 1.090–1.058) | NS | |
| Education | (ß = 0.279; p = 0.014; OR = 1.322 95% CI 1.059–1.651) | NS | ||
| Knowing that there is an asymptomatic period of early BC | Age | (ß = 0.033; p = 0.043; OR = 1.033 95% CI 1.001–1.067) | Age | (ß = 0.046; p = 0.032; OR = 1.047 95% CI 1.004–1.093) |
| Education | (ß = 1.057; p = 0.028; OR = 1.985 95% CI 1.090–3.615) | Education | (ß = 0.395; p = 0.015; OR = 1.485 95% CI 1.080–2.041) | |
| Place of residency (county town vs. village) | (ß = 0.577; p < 0.001; OR = 1.780 95% CI 1.342–2.360) | NS | ||
| Being well-informed about symptoms | Education | (ß = 0.493; p = 0.001; OR = 1.638 95% CI 1.213–2.211) | Education | (ß = 0.353; p = 0.012; OR = 1.424 95% CI 1.081–1.876) |
All outcomes are adjusted for age, education and residency
Timing of BC screening
A higher proportion of screening attendees correctly identified the recommended age of first BC screening correctly (that is, 45 years of age in Hungary) than that of laywomen (35.2% vs. 86.6%; p < 0.001).
The recommended frequency of BC screening in average-risk women (that is, once every 2 years in Hungary) was identified correctly in 33.9% and 12.9% by laywomen and screening attendees, respectively. Comparing screening attendees to laywomen of screening age not attending BC screening in the past 2 years did not reveal a significant difference between groups. Respondents who knew both the recommended age of first BC screening and the recommended frequency of BC screening accounted for 16.7% and 10.0% of laywomen and screening attendees, respectively. Associated factors of knowing the right answers are listed in Table 2.
Breast self-examination (BSE)
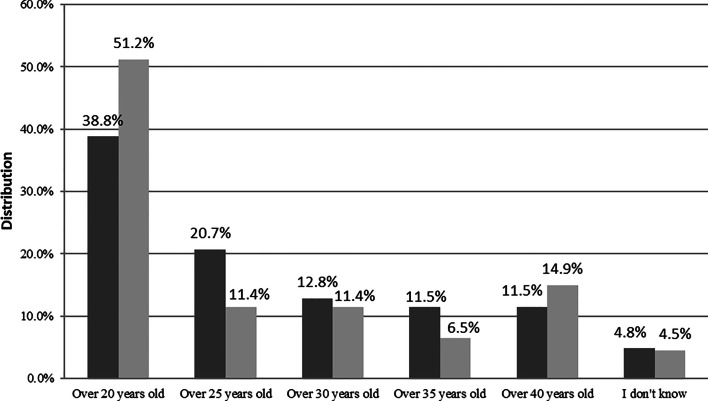
The recommended age of first BSE (that is, 20 years of age) was rightly recognized in 38.8% and 51.2% by laywomen and screening attendees, respectively (Fig. 1).
Fig. 1.
Age at first breast self-examination.
 Laywomen,
Laywomen,
 Screening attendees
Screening attendees
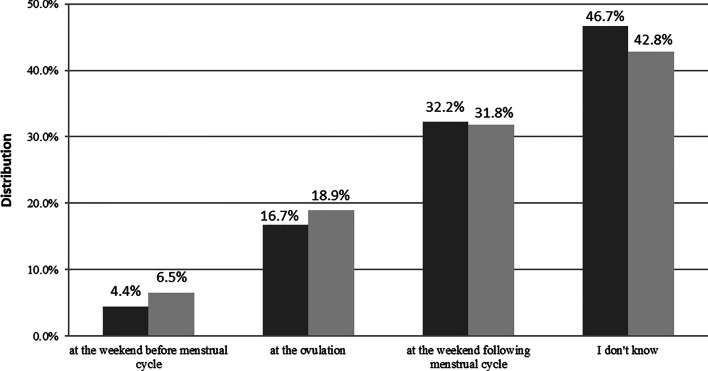
The recommended timing of BSE (that is, at the weekend following period) was identified correctly in 32.2% and 31.8% by laywomen and screening attendees, respectively; without a significant difference between screening attendees and laywomen of screening age without attending for BC screening in the past 2 years (Fig. 2). Respondents who knew both the recommended age of first BSE and the recommended timing of BSE accounted for 13.7% and 18.4% of laywomen and screening attendees, respectively. Associated factors of knowing the right answers are listed in Table 2.
Fig. 2.
Timing of breast self-examination.
 Laywomen,
Laywomen,
 Screening attendees
Screening attendees
Curability and mortality of BC
The majority of laywomen believed that early BC can be curable (92.5%), the ratio was similar among screening attendees (96.5%).
Laywomen and screening attendees stated that BC is a common cause of death in Hungary in 68.8% and 76.6% respectively. Associated factors of knowing the right answers are listed in Table 2.
Signs and symptoms of BC
An early BC can be asymptomatic according to 80.6% and 78.1% of laywomen and screening attendees, respectively.
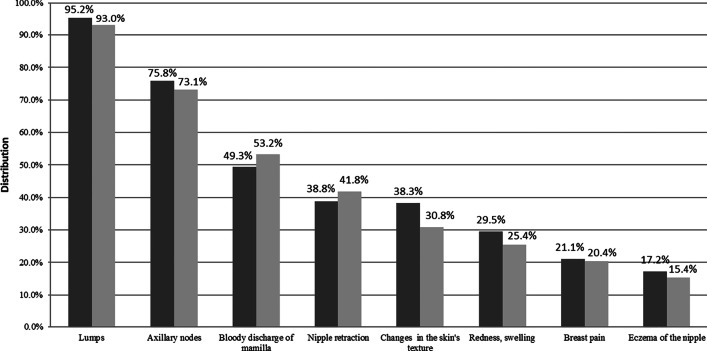
16.7% and 28.9% of laywomen and screening attendees had sufficient knowledge of symptoms, respectively. Agreement on the 3 most common symptoms was good between groups: lumps (95.2% and 93.0%), axillary nodes (75.8% and 73.1%), and bloody discharge of mamilla (49.3% and 53.2%) were indicated by laywomen and screening attendees, respectively (Fig. 3). Associated factors of having sufficient knowledge are listed in Table 2.
Fig. 3.
Signs and symptoms of breast cancer.
 Laywomen,
Laywomen,
 Screening attendees
Screening attendees
Risk factors of BC
The majority of both laywomen and screening attendees had sufficient knowledge of risk factors of BC (7.0% vs. 6.0%, respectively). Laywomen believed that genetic predisposition (81.1%), physical trauma (55.5%), and smoking (47.1%) are the three most common risk factors, whereas screening attendees favored to choose genetic predisposition (85.6%), physical trauma (49.3%), and irradiation (45.8%). The data did not satisfy the conditions of logistic regression model because only a small proportion of the respondents had sufficient knowledge of risk factors so that we analysed this outcome with univariate statistics exclusively. Among screening attendees, women who had sufficient knowledge were better educated (p = 0.01).
Source of information
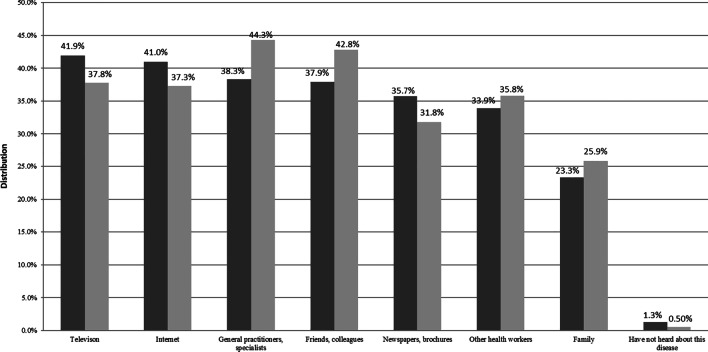
Television (41.9%), the internet (41.0%), and general practitioners or specialists (38.3%) were the three most common information sources among laywomen. On the contrary, screening attendees favored to choose general practitioners or specialists (44.3%), friends and colleagues (42.8%), and television (37.8%) to be the three most common sources (Fig. 4).
Fig. 4.
Information sources among laywomen and screening attendees.
 Laywomen,
Laywomen,
 Screening attendees
Screening attendees
Discussion
This study aimed to assess the knowledge of women (laywomen vs. screening attendees) of BC and BC screening. Since screening uptake is suboptimal in Hungary, an improvement would be desirable to reduce BC-related mortality. One potential tool for increasing awareness would be the initiation of public health interventions.
As we expected, there was a prominent difference between groups in knowing the recommended age at first BC screening: the ratio of correct answers favored screening attendees over laywomen of screening age not attending for BC screening in the past 2 years (86.6% vs. 35.2%, respectively). This might be attributed to the invitation letters for BC screening when reaching the appropriate age of screening (i.e., 45 years in Hungary) [15]. The difference between groups highlights the efficacy of population-based invitation letters for mass screening, even if the entire target population is not covered (around 80%). However, we failed to detect a similar difference regarding the recommended frequency of BC screening; here, the ratio of correct answers was extremely low in both groups. The importance of screening is highlighted by the fact that the 5-year relative survival rate of stage III BC is about 72%, women in this stage can often be treated successfully. On the contrary, stage IV (metastatic) BC has a 5-year relative survival rate of about 22% [21]. Knowledge of BC screening protocol, particularly for those reaching the recommended age at first screening, should be improved. Among laywomen, respondents who had sufficient knowledge were older, which highlights the importance of education of the younger generation. The required information may be transmitted via the internet and the television for the younger women, and by family doctors and specialists or by distribution of flyers for the older women because our findings highlighted these as the most frequently chosen information sources.
Although BSE should be started more than two decades earlier than mammography (20 years vs. 45 years, respectively), most respondents were unaware of this. These results corroborate the findings of the study of Do Thi Thanh Toan et al. from North-Vietnam, in which only 19.3% of the respondents knew when to perform the first BSE [22]. Knowledge of screening attendees was not better significantly compared to that of laywomen of screening age not attending for BC screening in the past two years; this is true concerning the timing of regular BSE (i.e., at the weekend following period) as well. Although physical examination has a low sensitivity (54%), its specificity is high (94%) [23] and seems more relevant for women aged 40–49 years than those aged above 50 years [24]. Laywomen with better education were more likely to know the recommended age of first BSE and the recommended timing of BSE. Since our findings were disappointing regarding BSE, it would be important to include the most important features of this screening modality (i.e., technique, timing in the period, and from what age) in the sexual education program of primary schools. This preventive activity may facilitate the early recognition of BC.
More than 90% of respondents had sufficient knowledge of the curability of early BC. In a study from Beirut, respondents had severe knowledge gaps regarding curability but were well informed about signs and symptoms [25]. A Mongolian study resulted in results comparable to ours: 91.1% of respondents knew that early recognition of BC could improve survival [26]. On the contrary, only about 70% of the respondents knew that BC is a common cause of death. In Hungary, BC is the most common malignant tumor and the third most common cause of tumor-related mortality among women [1, 2]. Higher education and older age seem to be associated with better knowledge in laywomen.
About 80% of respondents knew that BC can be asymptomatic. However, both laywomen and screening attendees had insufficient knowledge of the typical clinical presentation: less than one-fourth of the respondents proved to have sufficient knowledge of signs and symptoms of BC. It would be important to extend women’s knowledge of this aspect because recognizing signs and symptoms is a key moment in the detection and effective therapy of early BC. Literature states that the common signs and symptoms are lumps, mastitis, breast pain, and mamillar discharge [27]. The answers of respondents from the groups partly overlap: lumps were indicated by almost all respondents, whereas only half of them indicated mamillar discharge. Moreover, more than 70% indicated that axillary nodes belong to common symptoms while lymphatic metastases are rather characteristic of advanced BC. It is important to highlight that the third most frequently indicated symptom was the bloody nipple discharge, indicated approximately by half of the respondents in both groups. In the study of Linsell et al. from the UK, most women (85%) knew that lumps and axillary nodes could be the signs of BC, but less than half of respondents knew that BC could be associated with non-nodular signs as well [28]. The attendance rate of mammography can be increased only if women are aware of the warning signs and symptoms of BC; otherwise, they remain unrecognized and women will not see the doctor. Again, higher education, older age, living in county town were amenities, as demonstrated concerning signs and symptoms. According to a report, a positive family history for BC is associated with better knowledge (Western Turkey; 2011) [29]. According to our survey, BC among friends proved to be an associated factor of correctly knowing the frequency of BC.
A dramatically insufficient knowledge was explored regarding the risk factors of BC: less than 10% of respondents had sufficient knowledge. Opposing to the low knowledge in the Hungarian cohort of subjects, Trupe et al. demonstrated that about one-third (31.3%) of respondents had sufficient knowledge of risk factors of BC (in South African women aged 18–68 years; 2017) [30]. In our survey, both laywomen and screening attendees chose the same answers to be the two most common risk factors: genetic predisposition (81.1% vs. 85.6%, respectively) and physical trauma (55.5% vs. 49.3%, respectively). Discrepant answers were given regarding the third most common factor: laywomen favored smoking (47.1%), whereas screening attendees favored irradiation (47.1%). In an Indian study, respondents listed smoking and alcohol consumption as risk factors of BC, but many respondents highlighted the importance of positive family history for BC [31]. The role of genetics and irradiation was overestimated by the respondents: in fact, genetic predisposition is responsible only for around 5–10% of all BC cases, the most common association is with the mutations of BRCA genes [11]. Most respondents were misinformed about the role of physical trauma: it has not been proven without doubt that trauma increases the risk of BC [32]. Screening attendees who had sufficient knowledge were significantly better educated.
The most popular information sources were television and the internet among laywomen, whereas screening attendees favored general practitioners or other specialists, and colleagues or friends. Among adult Nigerian women (2014), the most common sources of information were the media and health care workers [33]. According to the survey of Maloney EK et al., laywomen preferred the internet to doctors to gather information about BC screening. Respondents frequently searched for information from the websites of cancer organizations about alternative therapies, adverse effects of therapies, conventional therapies, and traditional therapies (in Americans aged 27–79 years; 2015) [34]. Although the reliability and credibility of websites are questionable and often misleading, it is rare that patients, or at least their relatives, do not search for information about BC on the internet. The discrepant information sources between groups may explain the differences in the answers to the questions related to screening protocol and clinical phenotype of BC.
Limitations
Cross-sectional surveys do not permit causal generalizations. Another limitation of the study is the nonprobability sampling used, raising concerns about self-selection bias.
Conclusions
Our results revealed that Hungarian women including laywomen and, unexpectedly, screening attendees are often mis- and underinformed about the risk factors as well as about the signs and symptoms of BC. Most laywomen are lacking knowledge of screening protocol. These findings urge for immediate BC screening and breast health knowledge intervention to increase knowledge among people, especially in the younger and less educated strata of society and villagers. Since electronic media (among laywomen) and healthcare workers (among screening attendees) are the major information sources, distribution of reliable and easily digestible information via these channels may improve knowledge, therefore improving awareness of BC screening. Our findings implicate that additional education may be recommended for BC screening attendees.
Supplementary Information
Acknowledgements
Authors would like to thank all general practitioners and medical assistants for their participation in the survey.
Abbreviations
- BC
Breast cancer
- OR
Odds ratios
- CI
Confidence intervals
- BSE
Breast self-examination
Authors’ contributions
The study was designed by DK, GN, and IK. DK, GN, and ZS took part in the acquisition of data and performed statistical analyses. DK prepared the draft of the paper. IK supervised the study, participated in the interpretation of the data, and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by “GINOP-2.3.2-15-2016-00048—STAY ALIVE” co-financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020, and by Human Resources Development Operational Programme Grant, Grant Number: EFOP 3.6.2‐16‐2017‐00006—LIVE LONGER which is co-financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on request.
Ethics approval and consent to participate
Ethical approval was granted by the Regional and Institutional Ethical Committee of University of Pecs, Hungary (6542). Written informed consent was obtained from all participants.
Consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The International Agency for Research on Cancer. GLOBOCAN 2018. France. http://globocan.iarc.fr. Accessed 9 Feb 2019.
- 2.National Institute of Oncology. http://www.onkol.hu/hu/rakregiszter-statisztika. Accessed 9 Feb 2019
- 3.Li C, Beaber E, Mei-Tzu C, et al. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137:579–587. doi: 10.1007/s10549-012-2365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. http://www.who.int/Accessed. 2 Dec 2018
- 5.Reynolds P. Smoking and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(1):15–23. doi: 10.1007/s10911-012-9269-x. [DOI] [PubMed] [Google Scholar]
- 6.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristin Rojas MD, Ashley Stuckey MD. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016;59(4):651–672. doi: 10.1097/GRF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 8.Aisenberg C, Finkelstein D, Doppke K, et al. High risk of breast carcinoma after irradiation of young woman with Hodgkin’s disease. Cancer. 1997;79:1203–1210. doi: 10.1002/(SICI)1097-0142(19970315)79:6<1203::AID-CNCR20>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow A, Barber J, Hudson G, et al. Risk of second malignancy after Hodgkins’s disease in a collaborative British cohort: the relation to age treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of invidual data from 47 epidemiological studies in 30 countries, including 50302 woman with breast cancer and 96973 woman without disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 11.Economopoulou P, Dimitriadis G, Psyrri A. Beyond BRCA: new hereditary breast cancer susceptibility genes. Cancer Treat Rev. 2015;41(1):1–8. doi: 10.1016/j.ctrv.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Silver D, Cantor S, et al. BRCA 1, BRCA2, and Rad51 operative in a common DNA damage response pathway. Cancer Res. 1999;59:1752–1756. [PubMed] [Google Scholar]
- 13.Garber JE, Goldstein AM, Kantor AF, Dreyfus MG, Fraumeni JF, Li FP. Follow up study of twenty-four families with Li-Fraumeni syndrome. Cancer Res. 1991;5:6094–6097. [PubMed] [Google Scholar]
- 14.Tan M-H, Mester JL, Ngeow J, Rybicki LA, Orloff MS. Charis Eng Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18(2):400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lajos D. Daganatok szűrése minőségbiztosítási kézikönyv és módszertani útmutatóOrszágos Tisztifőorvosi Hivatal. Budapest. 2013;213:141–228. [Google Scholar]
- 16.IARC . Handbooks of cancer prevention. Breast cancer screening. Lion: IARC; 2002. [Google Scholar]
- 17.https://www.cochrane.org/CD001877/BREASTCA_screening-for-breast-cancer-with-mammography. Accessed 2 Dec 2018.
- 18.Zunzunegui RG, Chung MA, Oruwari J, et al. Casting-type calcifications with invasion and high-grade ductal carcinoma in situ a more aggressive disease? Arch Surg. 2003;138(5):537–540. doi: 10.1001/archsurg.138.5.537. [DOI] [PubMed] [Google Scholar]
- 19.Giordano L, von Karsa L, Tomatis M, Majek O, de Wolf C, Lancucki L, et al. Mammographic screening programmes in Europe: organization, coverage and participation. J Med Screen. 2012;19:72–82. doi: 10.1258/jms.2012.012085. [DOI] [PubMed] [Google Scholar]
- 20.Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, et al. Status of implementation and organization of cancer screening in the European Union Member States—Summary results from the second European screening report. Int J Cancer. 2018;142:44–56. doi: 10.1002/ijc.31043. [DOI] [PubMed] [Google Scholar]
- 21.https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.htmlAccessed. 10 Dec 2018.
- 22.Toan DTT, Son DT, Hung LX, Minh LN, Mai DL, Hoat LN. Knowledge, attitude, and practice regarding breast cancer early detection among women in a mountainous area in Northern Vietnam. Cancer Control. 2019;26(1):1073274819863777. doi: 10.1177/1073274819863777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thistlethwaite J, Stewart RA. Clinical breast examination for asymptomatic women—exploring the evidence. Aust Fam Physician. 2007;36:145–150. [PubMed] [Google Scholar]
- 24.Hassan LM, Mahmoud N, Miller AB, Iraj H, Mohsen M, Majid J, Reza SM, Mojgan M. Evaluation of effect of self-examination and physical examination on breast cancer. Breast. 2015;24(4):487–490. doi: 10.1016/j.breast.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 25.El Asmar M, Bechnak A, Fares J, Al Oweini D, Alrazim A, El Achkar A, Tamim H. Knowledge, attitudes and practices regarding breast cancer amongst lebanese females in Beirut. Asian Pac J Cancer Prev. 2018;19(3):625–631. doi: 10.22034/APJCP.2018.19.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yerramilli P, Dugee O, Enkhtuya P, Knaul FM, Demaio AR. Exploring knowledge, attitudes, and practices related to breast and cervical cancers in Mongolia: a national population-based survey. Oncologist. 2015;20(11):1266–1273. doi: 10.1634/theoncologist.2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343–349. [PubMed] [Google Scholar]
- 28.Linsell L, Burgess CC, Ramirez AJ. Breast cancer awareness among older women. Br J Cancer. 2008;99(8):1221–1225. doi: 10.1038/sj.bjc.6604668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köşgeroğlu A, Ayrancı Ü, Özerdoğan N, et al. Knowledge of women on early diagnosis methods and risk factors for breast cancer in a province of Western Turkey: a descriptive study. Pak J Med Sci. 2011;27:646–650. [Google Scholar]
- 30.Trupe LA, Rositch A, Dickerson L, Lucas S, Harvey SC. Knowledge and attitudes about breast cancer in Limpopo, South Africa. J Glob Oncol. 2017;3(5):509–514. doi: 10.1200/JGO.2016.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prusty RK, Begum S, Patil A, Naik DD, Pimple S, Mishra G. Knowledge of symptoms and risk factors of breast cancer among women: a community based study in a low socio-economic area of Mumbai, India. BMC Womens Health. 2020;20(1):106. doi: 10.1186/s12905-020-00967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigby JE, Morris JA, Lavelle J, Stewart M, Gatrell AC. Can physical trauma cause breast cancer? Eur J Cancer Prev. 2002;11:307–311. doi: 10.1097/00008469-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Olajide TO, Ugburo AO, Habeebu MO, Lawal AO, Afolayan MO, Mofikoya MO. Awareness and practice of breast screening and its impact on early detection and presentation among breast cancer patients attending a clinic in Lagos, Nigeria. Nigerian J Clin Pract. 2014;17(6):802–807. doi: 10.4103/1119-3077.144404. [DOI] [PubMed] [Google Scholar]
- 34.Maloney EK, D'Agostino TA, Heerdt A, Dickler M, Li Y, Ostroff JS, Bylund CL. Sources and types of online information that breast cancer patients read and discuss with their doctors. Palliat Support Care. 2015;13(2):107–114. doi: 10.1017/S1478951513000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on request.