Abstract
CD19-targeted chimeric antigen receptor T (CAR T) cell therapy is a promising option to treat relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). However, the majority of CAR T-treated patients will eventually progress and require salvage treatment, for which there is no current standard. In this study, we analyzed data from 6 patients with R/R DLBCL who experienced progression following CD19-CAR T therapy, and then received CD19-specific CAR T cells that express a PD-1/CD28 chimeric switch-receptor (CD19-PD-1/CD28-CAR T) as salvage therapy at our institution. After the second infusion of CAR T cells, 3 of 6 patients achieved complete remissions and the duration of the response of responsive patients ranged from 8 to 25 months. One patient showed a stable disease. In contrast, 2/6 patients died on 60 days because of progression disease. Importantly, no severe neurologic toxicity or cytokine release syndrome was observed. These data suggest that CD19-PD-1/CD28-CAR-T cells, a novel anti-CD19 CAR-T cell therapy, elicit a potent and durable anticancer response, and can be used in the post-CD19-CAR T failure setting.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-021-01044-y.
Keywords: DLBCL, CAR T cell therapy, PD-1/CD28 chimeric switch receptor, Salvage therapy
To the Editor:
CD19-specific CAR T cell therapy has significantly improved the outcome of patients with R/R DLBCL, resulting in durable remissions in approximately 40% of heavily pretreated patients. Despite these encouraging results, nearly half of the patients could not achieve durable response after CD19-CAR T therapy and a significant proportion of patients will eventually relapse and develop treatment-refractory, fatal disease [1–4]. Recently, Spiegel et al. [5] reported on outcomes of large B-cell lymphoma patients who experienced progression following CD19-CAR T (Axicabtagene ciloleucel, axi-cel). The results showed that median overall survival (OS) from date of progression disease was 180 days (95% CI 105–242). Until now, there is no recommended therapeutic schedule for this fatal disease.
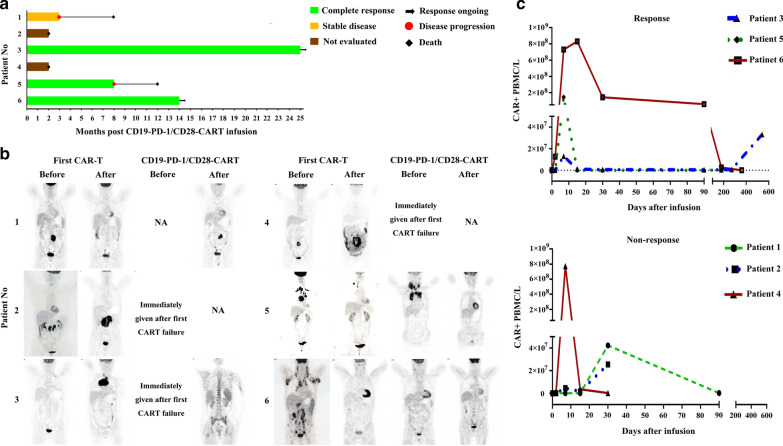
Six patients who relapsed or were refractory to CD19-CAR T therapy have been treated with CD19-PD-1/CD28-CAR-T, a novel anti-CD19 CAR-T cell therapy [6], on compassionate-use basis at our institution between January 2018 and August 2019. This retrospective study was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University (Hangzhou, China) and conducted in accordance with the principles of the Declaration of Helsinki. As of October 1, 2020, the median follow-up time was 14 months. Six consecutive patients were enrolled. Patients ranged from 47 to 63 years of age and had received prior CD19-CAR T therapy with CD28-based or 4-1BB-based CAR T cells. Three of the six patients were refractory to the first CAR T treatment, and two patients had partial remission (PR) with response duration of 3 and 4 months, respectively. One patient had a CR duration of 30 months before relapse (Table 1 and Fig. 1a). CD19-PD-1/CD28-CAR-T cells manufactured from the leukapheresed or cryopreservated autologous peripheral blood mononuclear cells were successful for all 6 patients. After failure of first CAR T therapy, patients received conditioning chemotherapy containing cyclophosphamide 500 mg/m2 and fludarabine 30 mg/m2 daily on days -5 to -3, and followed by a single intravenous infusion of CD19-PD-1/CD28-CAR-T as a salvage treatment for refractory or relapsed disease. The therapeutic doses of CAR T range from 0.32 × 106 to 4 × 106/kg of body weight. A total of 90 related adverse events occurring within 30 days of CAR-T infusion were recorded between grade 1 and 4 (Additional file 1: Table S1). Overall, 3/6 patients experienced grade 1 CRS, Patient 2 experienced grade 2 CRS, and Patient 4 and Patient 6 had both grade 2 CRS and grade 3 ICANS (immune effector cell‐associated neurotoxicity syndrome). Serum cytokine levels were detected in all patients during the first month following second CAR-T therapy (Additional file 2: Figure S1). IL-6, IL-4, IL-2, and TNFα were elevated in Patient 1. Patient 4 who experienced both grade 2 CRS and grade 3 ICANS exhibited increased levels of IL-6, IL-4, IL-2, IL-17A, and IFNγ, but such increased levels were not observed in Patient 6. Four cases of CRS resolved fully by supportive treatment while the 2 patients suffered from both CRS and ICANS resolved completely after treatment with supportive care, tocilizumab and glucocorticoids. The response was evaluated with FDG-PET-CT at 3 months after infusion (Fig. 1b), according to the International Working Group Response Criteria for Malignant Lymphoma. As shown in Table 1 and Fig. 1, three of six patients achieved a CR, and one patient showed stable disease. In contrast, 2/6 patients died on 60 days because of progression disease. Two of three patients achieving CR maintained ongoing response on the date for the last visit. But, another one relapsed within 8 months and eventually died 12 months after CAR T treatment. The presence of CAR-T cells in patients' blood was monitored by qPCR. The number of blood CAR+ cells peaked within 2 weeks after infusion. However, peak blood CAR+ cell numbers did not differ significantly between patients with response and those without response. Interestingly, CAR+ cell numbers dropped rapidly after peaking but increased significantly by day 540 after CAR T treatment in Patient 3 who achieved durable remission with long, treatment-free interval (Fig. 1c).
Table 1.
Clinical characteristics and post-CAR T outcomes
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (years) | 51 | 51 | 53 | 47 | 56 | 63 |
| Sex | Male | Male | Female | Female | Female | Male |
| Diagnosis/subtype | TFL | DLBCL/GCB | DLBCL/non-GCB | HGBL (Triple hit) | DLBCL/non-GCB | DLBCL/non-GCB |
| ECOG PS1 | 1 | 3 | 1 | 3 | 3 | 1 |
| Prior therapy | R-CHOP; 2-HyperCVAD-A | R-CHOP; R-BEAM + ASCT | R-CHOP; R-GDP; R2-COP + mitoxantrone; BEAM + ASCT | CHOP; R- EPOCH; R-DA-EPOCH; DHAP | R- EPOCH; R-GeMox; ICE2 R-COP2; Ibrutinib2 | R-CHOP; R-GDP; R2-MINE; R-DHAP |
| Disease Stage1 | 4 | 3 | 4 | 4 | 4 | 3 |
| B Symptom1 | A | A | B | A | A | A |
| PD-L11 | 20% | 25% | Negative | Negative | Negative | Negative |
| Ki-671 | 80% | 50% | 80% | 100% | 40% | 80% |
| IPI Score1 | 2 | 3 | 3 | 3 | 4 | 2 |
| First CAR-T | ||||||
| Costimulatory domain | 4-1BB | 4-1BB | CD28 | CD28 | 4-1BB | 4-1BB |
| Dose (106/kg) | 0.50 | 1.00 | 0.88 | 1.78 | 4.00 | 2.00 |
| Response | PR | PD | PD | PD | PR | CR |
| DOR (Month) | 4 | NA | NA | NA | 3 | 30 |
| Second CAR-T | ||||||
| ECOG PS | 2 | 4 | 1 | 4 | 3 | 1 |
| Dose (106/kg) | 0.32 | 0.63 | 0.50 | 2.90 | 4.00 | 2.00 |
| Response | PD | ED | CR | ED | CR | CR |
| DOR (Month) | NA | NA | 25 | NA | 8 | 14 |
| PBMC Source | Leukapheresis | Cryopreservation | Cryopreservation | Cryopreservation | Leukapheresis | Leukapheresis |
| Time for CAR-T culture (Day) | 15 | 14 | 14 | 13 | 14 | 12 |
| Naïve T (%) | 1.89 | 29.85 | 25.35 | 51.9 | 31.25 | 28.64 |
| TCM T (%) | 60.48 | 26.84 | 40.49 | 42.89 | 47.97 | 13.66 |
| TEM T (%) | 1.14 | 21.35 | 23.26 | 3.81 | 13.61 | 13.16 |
| TEMRA T (%) | 36.49 | 21.96 | 10.90 | 1.40 | 7.17 | 44.55 |
ASCT, autologous stem cell transplant; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; COP, cyclophosphamide, vincristine, prednisone; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; BEAM, carmustine, etoposide, cytarabine, melphalan; GDP, gemcitabine, dexamethasone, cisplatin; GEMOX, gemcitabine, oxaliplatin; ICE, ifosfamide, carboplatin, etoposide; HyperCVAD, cyclophosphamide, vincristine, doxorubicin, dexamethasone; DHAP, dexamethasone, high dose cytarabine, cisplatin; ICE, ifosfamide, carboplatin, etoposide; MINE, mesna, ifosfamide, mitoxantrone, etoposide; R, rituximab; R2, rituximab combined with lenalidomide; DLBCL, diffuse large B cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; GCB, germinal center B cell; TFL, transformed follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; HGBL, high grade B-cell lymphoma; Triple hit, MYC, BCL2 and BCL6 rearrangements. DOR, Duration of Response; TCM, T cells with central memory; TEM, T cells with effector memory; TEMRA, terminally differentiated effector T cells
1These data were collected before first CAR-T
2The chemotherapy regimens were conducted after first CAR-T
Fig. 1.
CD19-PD-1/CD28-CAR T induced durable clinical responses in R/R large B-cell lymphoma patients after failure of CD19-CAR T therapy. a Treatment response of each patient and the duration of response post-infusion with CD19-PD-1/CD28-CART. b Representative PET-CT scans at baseline and 90 days post first CAR-T infusion and salvage CAR T therapy. c The absolute number of CAR + peripheral blood mononuclear cells in patients who achieved clinical responses or non-responses were quantified by PCR
Treatment options are limited for DLBCL patients when disease fails to respond to or relapses after CD19 CAR-T cell therapy. Novel therapies with different mechanisms of actions are critical to improve unmet clinical needs and the outcome of these patients. Alternative CD19-specific CAR-T product may be an active salvage therapy although no clinical trials have defined the optimal approach. A recent study evaluated the efficacy of axi-cel, a CD28 costimulatory-based CD19-CAR T, as salvage therapy after failure of tisagenlecleucel or investigational CD19 CAR-T with 4-1BB costimulation in patients with DLBCL. One of three patients achieved CR, but died 180 days after axi-cel treatment because of progressive disease while two of three patients were refractory [7]. In another clinical study, second infusion of CD19-CAR T was used as salvage treatment after failure of first CD19 CAR T-cell therapy. Of 21 Non-Hodgkin lymphoma (NHL) patients, the overall response rate after the second CAR T was 52% (CR, n = 4; PR, n = 7) [8]. In the present study, 3 of 6 patients achieved CRs, 2 of 3 CRs are ongoing, suggesting that CD19-PD-1/CD28-CAR-T elicit a potent and durable anticancer response, and can be used in the post-CD19 CAR T failure setting. However, we did not find an association between the costimulatory domain of CAR T and disease control (Table 1).
CAR T cell efficacy can be enhanced by using engineering strategies to address the challenge relating to T cell exhaustion induced by PD-1/PD-L1 pathway [9–11]. Until now, few cases report rare lymphoma patients who could obtain better efficacy by a combination of CAR-T cell therapy and PD-1 blockade [10, 12]. Recently, we have reported that CD19-PD-1/CD28-CAR T cells exhibited a superior capability of killing PD-L1+ B-cell lymphoma cells in vitro and in vivo relative to the prototype, CD19-CAR T cells. We also demonstrated that this therapy had a favorable safety profile and induced durable clinical responses in the patients with PD-L1+ R/R DLBCL [6]. An interesting aspect of the current study was that CD19-PD-1/CD28-CAR T was generally well-tolerated and resulted in a high response rate that was durable in R/R large B-cell lymphoma after failure of CD19-CAR T therapy. In conclusion, our data demonstrate the ability to augment CAR T cells targeting CD19+ lymphoma by co-expressing a chimeric PD-1/CD28 switch-receptor, and that this therapy has potential as a salvage treatment when first CAR T proves ineffective or resistant.
Supplementary Information
Additional file 1: Table S1. Treatment-emergent adverse events.
Additional file 2: Figure S1. Kinetics of serum cytokines. A-H. Fold change of listed serum cytokines obtained from patients at the indicated times was calculated relative to the baseline.
Acknowledgements
Not applicable.
Abbreviations
- DLBCL
Diffuse large B cell lymphomas
- NHL
Non-Hodgkin lymphoma
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death 1 ligand
- CAR T
Chimeric antigen receptor T
- R/R DLBCL
Relapsed/refractory diffuse large B-cell lymphoma
- CRs
Complete remissions
- PR
Partial remission
- OS
Overall survival
- CRS
Cytokine release syndrome
- ICANS
Immune effector cell-associated neurotoxicity syndrome
Authors' contributions
W. Qian, A. Liang, K. H. Young, and Z. Lu were responsible for conception, design and manuscript writing. Y. Liang, H. Liu, W. Lei, C. Zhang and P. Li were responsible for collection, analysis, and interpretation. All authors were involved at each stage of manuscript preparation and approved the final version. All authors read and approved the final manuscript.
Funding
This study was supported by funds from Translational Research Grant of NCRCH (2020ZKZC01), and the National Natural Science Foundation of China (No. 81830006, 81830004).
Availability of data and materials
All data generated or analyzed during this study are included in this paper and its Supplementary files.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University (Hangzhou, China) and all patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yun Liang, Hui Liu and Zheming Lu are co-first author
Contributor Information
Aibin Liang, Email: lab7182@tongji.edu.cn.
Ken H. Young, Email: ken.young@duke.edu
Wenbin Qian, Email: qianwb@zju.edu.cn.
References
- 1.Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol. 2019;12(1):59. doi: 10.1186/s13045-019-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel JY, Dahiya S, Jain MD, Tamaresis JS, Nastoupil L, Jacobs MT, et al. Outcomes of patients with large B-cell lymphoma progressing after Axicabtagene Ciloleucel. Blood. 2020 doi: 10.1182/blood.2020006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Lei W, Zhang C, Yang C, Wei J, Guo Q, et al. CD19-specific CAR-T cells that express a PD-1/CD28 chimeric switch-receptor is effective in patients with PD-L1 positive B-cell lymphoma. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-20-1457. [DOI] [PubMed] [Google Scholar]
- 7.Chow VA, Gopal AK, Gauthier J, Tseng YD, Turtle CJ, Maloney DG, et al. Axicabtagene ciloleucel for relapsed or refractory lymphoma after prior treatment with a different CD19-directed CAR T-cell therapy. Blood Adv. 2020;4(19):4869–4872. doi: 10.1182/bloodadvances.2020002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauthier J, Bezerra ED, Hirayama AV, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B cell malignancies. Blood. 2020 doi: 10.1182/blood.2020006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, et al. Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res. 2017;23(22):6982–6992. doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 10.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, et al. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68(3):365–377. doi: 10.1007/s00262-018-2281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Deng Q, Jiang YY, Zhang R, Zhu HB, Meng JX, et al. CAR-T 19 combined with reduced-dose PD-1 blockade therapy for treatment of refractory follicular lymphoma: A case report. Oncol Lett. 2019;18(5):4415–4420. doi: 10.3892/ol.2019.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Treatment-emergent adverse events.
Additional file 2: Figure S1. Kinetics of serum cytokines. A-H. Fold change of listed serum cytokines obtained from patients at the indicated times was calculated relative to the baseline.
Data Availability Statement
All data generated or analyzed during this study are included in this paper and its Supplementary files.