Abstract
The current scale of public and private testing cannot be expected to meet the emerging need for higher levels of community-level and repeated screening of asymptomatic Canadians for SARS-CoV-2. Rapid point-of-care techniques are increasingly being offered to fill the gap in screening levels required to identify undiagnosed individuals with high viral loads. However, rapid, point-of-care tests often have lower sensitivity in practice. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) for SARS-CoV-2 has proven sensitive and specific and provides visual results in minutes. Using a commercially available kit for RT-LAMP and primer set targetting nucleocapsid (N), we tested a blinded set of 101 archived nasopharyngeal (NP) swab samples with known RT-PCR results. RT-LAMP reactions were incubated at 65 °C for 30 min, using heat-inactivated nasopharyngeal swab sample in viral transport medium, diluted tenfold in water, as input. RT-LAMP agreed with all RT-PCR defined negatives (N = 51), and all positives with cycle threshold (Ct) less than 20 (N = 24), 65% of positives with Ct between 20−30 (N = 17), and no positives with Ct greater than 30 (N = 9). RT-LAMP requires fewer and different core components, so may not compete directly with the mainline testing workflow, preserving precious central laboratory resources for those with the greatest need. Careful messaging must be provided when using less-sensitive tests, so that people are not falsely reassured by negative results, but this caveat must be weighed against the clear benefits of reliably identifying those with high levels of virus in prioritized samples at the point of care.
Abbreviations: RT-LAMP, Reverse transcription loop-mediated isothermal amplification; RT-PCR, Reverse transcription polymerase chain reaction; Ct, cycle threshold, RPA : Recombinase polymerase amplification
Keywords: SARS-CoV-2, Point-of-care testing, Isothermal amplification, RT-LAMP, Extraction-free, Instrument-free
1. Introduction
In an unprecedented scientific feat, nucleic acid amplification tests for SARS-CoV-2 were published 21 days after the Chinese communicable disease control team arrived in Wuhan on 31 December 2019 [1], based on complete genome sequences published 10 days earlier [2]. Hundreds of molecular diagnostic tests for SARS-CoV-2 have been introduced since then [[3], [4], [5]]. The main diagnostic targets rely on specific host antibodies, viral proteins and viral RNA, each with its own specific benefits and limitations in accurately detecting SARS-CoV-2 during its infectious period, in order to more efficiently prevent its spread [6]. The best, most sensitive, tests require high levels of laboratory expertise and specialized facilities that understandably increase expense and turnaround time. This is especially true when challenged with recent, unprecedented testing volumes.
Advice on whether to test people “without symptoms” (which represents a range of pre- symptomatic, peri-symptomatic, sub-symptomatic and truly asymptomatic phenotypes) wavers as much over time as actual demand for testing by people “without symptoms” [7,8]. “Asymptomatic” testing has been characterized as wasteful, since only a small percentage come back positive, especially when investing the most precise, most expensive and most time-consuming test available. Testing programs that incorporate parallel strategies for detecting infection in those without symptoms have been demonstrated at large scale in Iceland [9] and more recently country-wide in Slovakia [10].
Several jurisdictions have approved and applied point-of-care assays that can rapidly indicate infection outside the laboratory. These include rapid antibody-detecting cassettes that use serum or a few drops of peripheral blood, rapid antigen tests on nasopharyngeal samples, and all-in-one RT-PCR platforms such as the Xpert® Xpress SARS-CoV-2 (Cepheid®), which provides highly sensitive and specific results relative to other RT-PCR-based workflows in a fraction of the time [11,12]. However, a key limitation is lack of scalability, with each (very expensive) machine only able to run one or a few samples at a time. Fluctuating demand for cartridges across all jurisdictions makes overall access unpredictable.
More recently, a number of nucleic acid isothermal amplification techniques for viral detection have been described, including loop-mediated isothermal amplification (LAMP) [[13], [14], [15]], using a 4- or 6-primer set and incubated at 65 °C for 30−60 min, recombinase-polymerase amplification (RPA) [16], using multiple enzymes and incubated at 42 °C for 15−20 min, and many others [[17], [18], [19]]. Isothermal assays have been shown to detect SARS-CoV-2 with very high sensitivity and specificity, and underlie several tests recently authorized for emergency use, including the ID NOW from Abbott, and CRISPR-based detection by SHERLOCK [20] (currently not approved for sale outside the United States).
The ID NOW compares favourably to GeneXpert [21], but also shares its disadvantages in terms of single sample per cartridge and single-source cartridges. Some kits, such as Twist Bioscience’s RPA-based assays [22] and the SHERLOCK LAMP/CRISPR assays, do not use special machines or cartridges, requiring only strip tubes or plates and p10 filter tips, hence can easily be scaled up to conduct hundreds or thousands of tests. New England Biolabs’ Colorimetric WarmStart LAMP kit can also be scaled and is available in Canada. In this study, we evaluated how a commercial colorimetric RT-LAMP kit combined with published SARS-CoV-2 primer sets and heat-treated, diluted sample compares with standard RT-PCR analysis.
2. Materials and methods
2.1. Archived sample set
A total of 101 leftover nasopharyngeal swab samples in viral transport medium, from both symptomatic and asymptomatic individuals attending for testing at walk-in or drive-through facilities in Winnipeg, Canada, tested for SARS-CoV-2 by RT-PCR using E gene primers and purified RNA template on the Cobas or Panther Fusion platforms, were used to validate a recently described RT-LAMP assay [23]. Comparisons were based on 50 RT-PCR positive samples, with fluorescence threshold values between 10 and 37 cycles, and 51 RT-PCR negative samples.
2.2. Sample processing and heat treatment
After assigning blinded sample identifiers to reduce potential bias in interpretation, samples were thawed and briefly spun down in a mini-centrifuge to collect cells and debris. An aliquot of 60 μl, drawn from the bottom of the tube near the pelleted material, was transferred to a 1.7 mL Eppendorf tube, labelled with the blinded code, then incubated in a heating block at 95 °C for 5 min. Serial tenfold dilutions of sample were prepared in nuclease-free water (New England Biolabs, Whitby, ON).
2.3. RT-LAMP assay and controls
RT-LAMP was carried out using the Colorimetric WarmStart LAMP Kit (New England Biolabs) and a published LAMP primer set targeting the N gene of SARS-CoV-2 (Table 1 ), previously shown to have high sensitivity [23,24] (Integrated DNA Technologies, Coralville, IA). A 10X mixture of the six primers in the set was prepared by first suspending all primers separately in nuclease-free water at a concentration of 100 μM, then combining 16 μl each of FIP/BIP, 4 μl each of LF/LB, 2 μl each of F3/B3, and 56 μl nuclease-free water (final volume 100 μl). Reactions were set up in a final volume of 20 μl (10 μl 2X Master Mix, 2 μl 10X primer mix, 6–7.5 μl nuclease-free water and 0.5–2 μl sample, either diluted or undiluted) and incubated in a heat block at 65 °C for 30 min. After sitting on ice for a few minutes to sharpen contrast, colour was assessed visually and photographed. Bright yellow colour indicates a positive result, while magenta indicates a negative result. Tests with orange or pink colour were considered failed reactions and re-tested.
Table 1.
Primers used in this study and positions in genome.
| Name | Sequence (5′-3′) | Start2 | Stop2 |
|---|---|---|---|
| FIP1 | TCTGGCCCAGTTCCTAGGTAGTCCAGACGAATTCGTGGTGG | 28,626 | 28,605 |
| TCTGGCCCAGTTCCTAGGTAGTCCAGACGAATTCGTGGTGG | 28,555 | 28,573 | |
| BIP1 | AGACGGCATCATATGGGTTGCACGGGTGCCAATGTGATCT | 28,654 | 28,675 |
| AGACGGCATCATATGGGTTGCACGGGTGCCAATGTGATCT | 28,719 | 28,702 | |
| LF | GGACTGAGATCTTTCATTTTACCGT | 28,589 | 28,575 |
| LB | ACTGAGGGAGCCTTGAATACA | 28,676 | 28,696 |
| F3 | TGGCTACTACCGAAGAGCT | 28,525 | 28,543 |
| B3 | TGCAGCATTGTTAGCAGGAT | 28,741 | 28,722 |
Primers shown twice to indicate each targets two distinct genome regions. Start and stop values are for the region shown in bold.
Position in SARS-CoV-2 RefSeq genome (NC_045512).
2.4. Positive and negative controls
Serial tenfold dilutions in nuclease-free water of SARS-CoV-2-N Positive Control or MERS-CoV Negative Control DNA (Integrated DNA Technologies) at a starting concentration of 2 × 108 copies per ml were used as positive and negative controls, respectively, and included in every test batch (five dilutions from 108 to 104 copies/mL). Limit of detection of the assay was defined as the lowest concentration of control resulting in a positive result, adjusted for reaction volume (e.g. 106 copies/mL divided by 20-fold dilution in Master Mix = 5 × 104 copies/mL).
2.5. Data analysis
After categorization as either positive (yellow) or negative (magenta), samples were unblinded and compared. Sensitivity, specificity and positive/negative predictive values with confidence intervals were calculated using standard formulas with the help of MedCalc: https://www.medcalc.org/calc/diagnostic_test.php.
3. Results
3.1. Sensitivity, specificity and predictive values
Sensitivity of the RT-LAMP assay using raw, heat-inactivated sample was 77% compared to RT-PCR of purified RNA extracts (Table 2 ). Samples with the lowest Ct values (<22) were all bright yellow by RT-LAMP, even when diluted up to 1000-fold, indicating a similar range of detectable concentration to the positive control. All RT-PCR negatives were also RT-LAMP negative (Table 2). This indicates that RT-LAMP may be most useful to more quickly detect those with high viral loads.
Table 2.
Sensitivity, specificity and predictive values of RT-LAMP with raw sample input, compared to standard RT-PCR diagnosis.
| RT-LAMP | |||
|---|---|---|---|
| RT-PCR | Positive | Negative | N |
| Positive | 35 (70%) | 15 (30%) | 50 |
| < 20 cycles | 24 (100%) | 0 | 24 |
| 20−30 cycles | 11 (65%) | 6 (35%) | 17 |
| > 30 cycles | 0 | 9 (100%) | 9 |
| Negative | 0 | 51 (100%) | 51 |
| Result (95% CI) | |||
| Sensitivity | 77% (65−86%) | ||
| Specificity | 100% (90−100%) | ||
| NPV | 70% (60−78%) | ||
| PPV | 100 % | ||
| Accuracy | 85% (76−91%) |
3.2. Interpretation of colour change
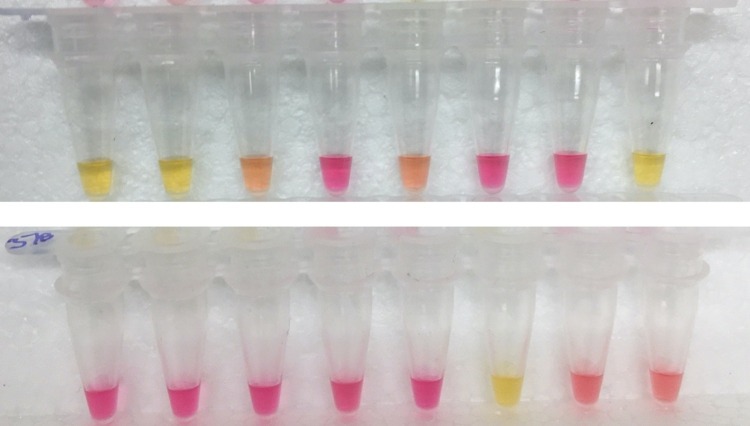
Only samples that were bright yellow after 30 min incubation were considered positive (Fig. 1 ). In some cases, a partial colour change resulted, from magenta to pink to orange, but did not become yellow within the period of observation (20−30 min, regardless of dilution factor down to 1000 copies). This partial colour change may indicate a weak positive (all samples do eventually turn yellow), a failed reaction (not indicating anything about the sample itself), or a true negative, depending on the reason for the weak change in pH. In this study, 9 samples initially resulted in an orange colour and all were negative when re-tested (8 were from PCR-negative samples, and the remaining sample was PCR-positive with a Ct value of 29).
Fig. 1.
Interpretation of colour in tests on Samples 9-24 – Only bright yellow results after 30 min at 65 °C are considered positive. Magenta colour indicates a “true” negative (insufficient viral RNA), while orangey colour is considered a failed reaction and re-tested. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.3. Controls and limit of detection
Negative controls were reliably magenta throughout the experiments (Fig. 2 ) but did eventually get contaminated. Routine cleanup with DNAse and careful separation of pre-amplification and amplification/post-amplification areas are required (unless you use NEB’s more expensive dUTP kit) to prevent the massive DNA contamination that LAMP is prone to. The MERS-CoV DNA negative control at 50,000 and 500,000 copies/mL did not produce any yellow colour, however 5,000,000 copies/mL resulted in a positive reaction (not shown). SARS-CoV-2-N DNA positive control produced a bright yellow reaction within 30 min down to 50,000 copies/mL, but remained magenta at lower dilutions, indicating that this is the lower limit of detection of this test under current conditions (Fig. 2).
Fig. 2.
Positive and negative controls – Tubes 1-5: Tenfold dilutions of a new batch of SARS-CoV-2-N DNA (positive control), with final concentrations (copies per ml) of 5 × 106, 5 × 105, 5 × 104, 5 × 103, and 5 × 102. Tube 6: Previous batch positive control (5 × 105 per ml). Tube 7: MERS-CoV DNA (negative control), 5 × 105 per ml. Tube 8: No template control (NTC).
4. Discussion
In this study, we confirm that one of the fastest, simplest, cheapest and most scalable protocols available for detecting SARS-CoV-2 nucleic acid is so far reliable for strong positives only, but highly specific compared to RT-PCR detection. Other studies have shown similar reduced sensitivity but high specificity [23,[25], [26], [27], [28]]. One early study found that RT-LAMP was more sensitive than RT-PCR at detecting virus in an asymptomatic carrier monitored for several days [29]. RT-LAMP has been tested extensively at large scale in the UK and has recently been shown to have less-than-expected sensitivity (<50%) when rolled out to large populations as part of Operation Moonshot [30]. However, similarly to the current study, sensitivity was greatest in strong positives, indicating that only those with high viral loads may reliably be detected. Recent work has shown that SARS-CoV-2 could not be cultured from samples with Ct values greater than 24 and/or longer than 8 days past symptom onset [31]. This observation indicates that the RT-LAMP assay described here would detect all of those most likely to have culturable (and therefore infectious) virus, as has been shown when the two have been compared directly [25].
Many technologies have been introduced or adapted to rapid detection of SARS-CoV-2 at the point of care (Table 3 ). The pace of Canadian approvals for SARS-CoV-2 point-of-care diagnostic tests has been much faster than for other infections, while the FDA has provided Emergency Use Authorization for dozens of diagnostics, including the Sherlock Biosciences LAMP/CRISPR platform and the recent LAMP-based home test by Lucira [32]. Despite limitations in practice, all tests applied on a wider scale, with proper messaging about the limited significance of negative results, could be highly useful if strong positives are identified and acted upon more quickly [33].
Table 3.
Comparison of rapid tests to “gold standard” viral culture and RT-PCR.
| Method | Sample type1 | Cost | Complexity | Scalability | Sensitivity | Refs. |
|---|---|---|---|---|---|---|
| Viral culture | NP in VTM | Expensive | Complex | Not scalable | Culturable when Ct <24 | [31] |
| RT-PCR (central lab protocols) | Extracted NP |
Expensive | Complex | Kits, tubes and tips | Comparator | [45] |
| Cepheid GeneXpert | NP in VTM | Expensive | Simple | Platform and single-use cartridges | 95−100% | [11,21] |
| Abbott ID NOW | Direct NP | Expensive | Simple | Platform and single-use cartridges | 70−90% | [45,46] |
| Abbott Panbio Ag | Direct NP | Cheap | Simple | Single-use cartridges | 75−85% | [34,47,48] |
| NEB RT-LAMP | Extract-free NP | Cheap | Simple | Kit, tubes and tips | 75−95% | [23] |
NP: Nasopharyngeal, VTM: Viral transport medium.
Several jurisdictions use the Panbio Ag Rapid Test Device to identify positives early, assuming that negative results should be confirmed [[34], [35], [36]]. One recent study showed that none of those who were test discordant (positive by RT-PCR but negative using Panbio) had culturable virus in their sample [34], indicating potentially reduced transmissibility by individuals defined as “false negatives” in current protocols. Other studies have indicated that sensitivity of the Panbio devices drops in people with no symptoms [36], or when using self-collected specimens such as nasal swabs and saliva [37].
The impact of mutations on our ability to accurately detect SARS-CoV-2 with primers based on older genome sequences is an area of growing concern [38], requiring constant vigilance and updating of primers being used. Mutations across the genome (including in the N gene targetted in this report) are regularly reported around the world [39], and can conveniently be monitored online (www.covidcg.org) [40]. Other strategies to mitigate the risk of false negative RT-LAMP tests due to mutations, for example by targetting more than one gene (standard in RT-PCR assays), have also recently been reported [41].
Molecular diagnostic techniques that use raw or minimally processed sample are fastest, but less sensitive, since the background chemistry of a raw sample may introduce uncontrolled variability [25,42]. Potential inhibitors or other contaminants may reduce the reliability of both positive and negative results. This concern is somewhat lessened in the context of the current report, which we have confirmed is highly specific and can amplify target molecules even in highly diluted samples. However, the current assay’s limit of detection (50,000 copies/mL, similar to other studies [25]) does decrease the likelihood of detecting a person with low viral load. In contrast, Sherlock Biosciences claims on its website (www.sherlock.bio) that its LAMP-based assay can detect 7000 copies per ml VTM or more than 7 times fewer than this report.
Is the risk of missing someone with a low viral load greater than making a person with a high viral load wait days to find out they are positive? In all cases, expectations about what a negative test result actually means must be properly managed. In this study, RT-LAMP with raw, heat-treated sample was ∼75% sensitive compared to nasopharyngeal RT-PCR, meaning that a false negative result can be expected 1 out of 4 times the test is performed. Therefore, careful explanation should be provided as part of pre-test and post-test counselling that a single negative test does not mean a person is free of SARS-CoV-2.
Another way to have more confidence in less sensitive tests is by repeated testing, increasing confidence that a negative test result is not just due to random error, transience of viral shedding or low test sensitivity [43]. Regular testing is facilitated when methods are fast, cheap, based on saliva, extract-free and easy to conduct in diverse settings, identifying the earliest timepoint at which a person is infectious, and increasing the immediacy and impact of contact tracing. Building an expanded repertoire of testing platforms that do not all depend on the same instruments, reagents and laboratory infrastructure is an essential strategy to improve surge capacity [33]. Ideally, new testing modalities should not compete with central laboratory tests for equipment, consumables or personnel, all of which are presently stretched very thin. Core costs and processing time of extraction-free RT-LAMP are similar to RT-PCR workflows (Table 4 ), but can be done without expensive machines or specialized labs, by trained non-specialists with oversight and support. This frees nurses and other front-line staff for less technical duties and preserves public health testing resources for those most in need.
Table 4.
Base cost (CAD$) of commercial kit and procedure time to conduct extract-free LAMP assay.
| per reaction | per result1 | per 24 | per 96 | |
|---|---|---|---|---|
| WarmStart Colorimetric Kits2 | ∼$2−3 | ∼$10−15 | ∼$150-$230 | ∼$600-$900 |
| Master Mix/Primers | $1.76−2.82 | $8.45−13.53 | $135−216 | $540−860 |
| LAMP Primer Mixes | $0.02−0.06 | $0.10−0.30 | $2.40−7.20 | $10-$30 |
| Procedure time3 | – | ∼1−1.5 hours | 2−3 hours | 3−5 hours |
| Master mix setup (96) | – | 4 min. | 15 min. | 30 min. |
| Sample processing/dilution | – | 15 min. | 60 min. | 90 min. |
| Transfer to Master Mix | – | 5 min. | 15 min. | 30 min. |
| Isothermal amplification | – | 35 min. | 35 min. | 35 min. |
| Evaluate and record result | – | 6 min. | 15 min. | 45 min. |
| Repeat failed reactions | – | 45 min. | 65 min. | 110 min. |
Assuming an average of four reactions per screen (multiple dilutions and/or primer sets), plus 20% for cost of positive and negative controls (4 per 48 reactions) and re-tests (6 per 48 reactions).
Cost variance due to different sizes of kits and formulations available and whether 20 or 25 μl test volume is used.
Does not include sample collection or transport time.
In conclusion, scalable rapid tests, such as the one evaluated in this study, may efficiently detect individuals with high viral loads at the point of care. Our findings suggest RT-LAMP could be useful as a screening mechanism for prioritized samples within the existing test chain, reliably identifying those with highest virus concentrations ahead of the standard RT-PCR workflow, and able to be scaled to any required number of tests per day. Further work must focus on improving sensitivity, incorporating saliva or other self-collected samples to facilitate repeated testing, and triangulating evidence from different testing modalities [44] to better ascertain an individual’s infectious period.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to Dr. Kerry Dust, Dr. Paul van Caeseele, Dr. Jared Bullard, Dr. Derek Stein, Adam Hedley and all the staff and scientists at Cadham Provincial Laboratory who originally processed the samples for SARS-CoV-2 testing. Thanks to Dr. Keith Fowke, Dr. Grant McClarty, Dr. Pam Orr, Sheila Ang and Steve Wayne from the Department of Medical Microbiology and Infectious Diseases, Rady Faculty of Health Sciences, University of Manitoba, for space, advice, encouragement and practical support.
References
- 1.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan Jf, Kok Kh, Zhu Z., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Health Security, Bloomberg School of Public Health; 2020. Serology-based and Molecular-based Tests Tracker. URL: https://www.centerforhealthsecurity.org/resources/COVID-19/COVID-19-SerologyandMolecular-basedTests.html. Accessed Nov. 20, 2020. [Google Scholar]
- 4.2020. COVID-19 In Vitro Diagnostic Devices and Test Methods Database. URL: https://covid-19-diagnostics.jrc.ec.europa.eu/devices. Accessed Nov. 20, 2020. [Google Scholar]
- 5.2020. Coronavirus Test Tracker: Commercially Available COVID-19 Diagnostic Tests. 360Dx.com. URL: https://www.360dx.com/coronavirus-test-tracker-launched-covid-19-tests. Accessed Nov. 20, 2020. [Google Scholar]
- 6.Kumar R., Nagpal S., Kaushik S., Mendiratta S. COVID-19 diagnostic approaches: different roads to the same destination. Virus Dis. 2020;31(2):97–105. doi: 10.1007/s13337-020-00599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chagla Z., Mertz D. Put an end to asymptomatic testing, before the system gets overwhelmed. National Post. 2020;(September) URL: https://nationalpost.com/opinion/opinion-put-an-end-to-asymptomatic-testing-before-the-system-gets-overwhelmed, Accessed Oct. 1, 2020. [Google Scholar]
- 8.Padula W.V. Why only test symptomatic patients? Consider random screening for COVID-19. Appl. Health Econ. Health Policy. 2020;18(3):333–334. doi: 10.1007/s40258-020-00579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudbjartsson D.F., Helgason A., Jonsson H., et al. Spread of SARS-CoV-2 in the icelandic population. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavelka M., Van-Zandvoort K., Abbott S., et al. The effectiveness of population-wide, rapid antigen test based screening in reducing SARS-CoV-2 infection prevalence in Slovakia. medRxiv. 2020 doi: 10.1101/2020.12.02.20240648. [DOI] [Google Scholar]
- 11.Tibbetts R., Callahan K., Rofoo K., Zarbo R.J., Samuel L. Comparison of the NeuMoDX, Diasorin Simplexa, Cepheid and Roche CDC SARS-CoV EUA assays using nasopharyngeal/nasal swabs in universal transport media (UTM) and sputum and tracheal aspirates. bioRxiv. 2020 doi: 10.1101/2020.05.26.118190. [DOI] [Google Scholar]
- 12.Zhen W., Smith E., Manji R., Schron D., Berry G.J. Clinical evaluation of three sample-to-Answer platforms for the detection of SARS-CoV-2. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/JCM.00783-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alekseenko A., Barrett D., Pareja-Sanchez Y., et al. Direct detection of SARS-CoV-2 using non-commercial RT-LAMP reagents on heat-inactivated samples. Sci. Rep. 2021;11(1820) doi: 10.1038/s41598-020-80352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augustine R., Hasan A., Das S., et al. Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-Care test for coronaviruses in the context of COVID-19 pandemic. Biology. 2020;9(8) doi: 10.3390/biology9080182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma V., Chaudhry D., Kaushik S. Evaluation of clinical applicability of reverse transcription-loop-mediated isothermal amplification assay for detection and subtyping of Influenza A viruses. J. Virol. Method. 2018;253:18–25. doi: 10.1016/j.jviromet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobato I.M., O’Sullivan C.K. Recombinase polymerase amplification: basics, applications and recent advances. Trends Anal. Chem. 2018;98:19–35. doi: 10.1016/j.trac.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdolahzadeh A., Dolgosheina E.V., Unrau P.J. RNA detection with high specificity and sensitivity using nested fluorogenic Mango NASBA. RNA. 2019;25(12):1806–1813. doi: 10.1261/rna.072629.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham J., Meyer S., Nguyen C., et al. Performance characteristics of a high throughput automated transcription mediated amplification test for SARS-CoV-2 detection. J. Clin. Microbiol. 2020;58(10) doi: 10.1128/JCM.01669-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W., Hsu H., Su J., Clapper J., Hsu J. Room temperature isothermal colorimetric padlock probe rolling circle amplification for viral RNA detection. bioRxiv. 2020 doi: 10.1101/2020.06.12.128876. [DOI] [Google Scholar]
- 20.Joung J., Ladha A., Saito M., et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. medRxiv. 2020 doi: 10.1101/2020.05.04.20091231. [DOI] [Google Scholar]
- 21.Smithgall M.C., Scherberkova I., Whittier S., Green D.A. Comparison of cepheid xpert xpress and abbott ID now to Roche cobas for the rapid detection of SARS-CoV-2. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian J., Boswell S.A., Chidley C., et al. An enhanced isothermal amplification assay for viral detection. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-19258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loan Dao Thi V., Herbst K., Boerner K., et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Trans Med. 2020;12(eabc7075) doi: 10.1126/scitranslmed.abc7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Odiwuor N., Xiong J., et al. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. medRxiv. 2020 doi: 10.1101/2020.02.26.20028373. [DOI] [Google Scholar]
- 25.Dudley Dm, Newman Cm, Weiler Am, et al. Optimizing direct RT-LAMP to detect transmissible SARS-CoV-2 from primary patient samples. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow F.W., Chan T.T., Tam A.R., et al. A rapid, simple, inexpensive, and mobile colorimetric assay COVID-19-LAMP for mass on-site screening of COVID-19. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L., Wu S., Hao X., et al. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin. Chem. 2020;66(7):975–977. doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Österdahl M.F., Lee K.A., Lochlainn M.N., et al. Detecting SARS-CoV-2 at point of care: preliminary data comparing loop-mediated isothermal amplification (LAMP) to polymerase chain reaction (PCR) BMC Infect. Dis. 2020;20(1):783. doi: 10.1186/s12879-020-05484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X., Deng Q., Li J., et al. Development and clinical application of a rapid and sensitive loop-mediated isothermal amplification test for SARS-CoV-2 infection. mSphere. 2020;5(4) doi: 10.1128/mSphere.00808-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iacobucci G. Rapid test missed over 50% of positive cases in Manchester pilot. BMJ. 2020;371:m4323. doi: 10.1136/bmj.m4323. [DOI] [PubMed] [Google Scholar]
- 31.Bullard J., Dust K., Funk D., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa638. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucira health’s At-Home covid test, explained. Bloomberg. 2020;(November) URL: https://www.bloomberg.com/opinion/articles/2020-11-18/lucira-health-s-at-home-covid-test-explained, Accessed Nov. 18, 2020. [Google Scholar]
- 33.Heidt A. COVID-19 diagnostics: how do saliva tests compare to swabs? Scientist. 2020;(October) URL: https://www.the-scientist.com/news-opinion/covid-19-diagnostics-how-do-saliva-tests-compare-to-swabs--68035, Accessed October 9, 2020. [Google Scholar]
- 34.Torres I., Poujois S., Albert E., et al. Evaluation of a rapid antigen test (PanbioTM COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.12.022. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulilete O., Lorente P., Leiva A., et al. Evaluation of the PanbioTM rapid antigen test for SARS-CoV-2 in primary health care centers and test sites. medRxiv. 2020 doi: 10.1101/2020.11.13.20231316. [DOI] [Google Scholar]
- 36.Linares M., Perez-Tanoira R., Carrero A., et al. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020;133 doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agullo V., Fernandez-Gonzalez M., Ortiz de la Tabla V., et al. Evaluation of the rapid antigen test Panbio COVID-19 in saliva and nasal swabs in a population-based point-of-care study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison, A. FDA Issues Alert Regarding SARS-CoV-2 Viral Mutation to Health Care Providers and Clinical Laboratory Staff (Press release, Jan. 8, 2021). URL: https://www.fda.gov/news-events/press-announcements/fda-issues-alert-regarding-sars-cov-2-viral-mutation-health-care-providers-and-clinical-laboratory, Accessed Oct. 15, 2020.
- 39.Islam M.R., Hoque M.N., Rahman M.S., et al. Genome-wide analysis of SARS-CoV-2 virus strains circulating worldwide implicates heterogeneity. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-70812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen A.T., Altschuler K., Zhan S.H., Chan Y.A., Deverman B.E. COVID-19 CG: tracking SARS-CoV-2 mutations by locations and dates of interest. bioRxiv. 2020 doi: 10.2144/btn-2020-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Ren G., Buss J., Barry A.J., Patton G.C., Tanner N.A. Enhancing colorimetric loop-mediated isothermal amplification speed and sensitivity with guanidine chloride. BioTechniques. 2020;69(3):179–185. doi: 10.2144/btn-2020-0078. [DOI] [PubMed] [Google Scholar]
- 42.Schermer B., Fabretti F., Damagnez M., et al. Rapid SARS-CoV-2 testing in primary material based on a novel multiplex LAMP assay. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0238612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramdas K., Darzi A., Jain S. ’Test, re-test, re-test’: using inaccurate tests to greatly increase the accuracy of COVID-19 testing. Nat. Med. 2020;26(6):810–811. doi: 10.1038/s41591-020-0891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mlcochova P., Collier D., Ritchie A., et al. Combined point-of-Care nucleic acid and antibody testing for SARS-CoV-2 following emergence of D614G spike variant. Cell Rep. Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu Y.-P., Iqbal J.Z., O’Leary T.J. Evaluation of ID NOW and RT-PCR for detection of SARS-CoV-2 in an ambulatory population. medRxiv. 2020 doi: 10.1101/2020.12.07.20245225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Comer S., Fisk D. An extended laboratory validation study and comparative performance evaluation of the Abbott ID NOW TM COVID-19 assay in a coastal California tertiary care medical center. medRxiv. 2020 doi: 10.1101/2020.06.14.20130518. [DOI] [Google Scholar]
- 47.Fenollar F., Bouam A., Ballouche M., et al. Evaluation of the Panbio Covid-19 rapid antigen detection test device for the screening of patients with Covid-19. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.02589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gremmels H., Winkel B.M.F., Schuurman R., et al. Real-life validation of the Panbio COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]