Abstract
Early in the SARS-CoV-2 pandemic, convalescent plasma (CP) therapy was proposed as a treatment for severely ill patients. We conducted a CP treatment protocol under the Mayo Clinic Extended Access Program at University Hospital Brooklyn (UHB). Potential donors were screened with a lateral flow assay (LFA) for IgM and IgG antibodies against the SARS-CoV-2 S1 receptor-binding domain (RBD). Volunteers that were LFA positive were tested with an ELISA to measure IgG titers against the RBD. Subjects with titers of at least 1:1024 were selected to donate. Most donors with positive LFA had acceptable titers and were eligible to donate. Out of 171 volunteers, only 65 tested positive in the LFA (38.0%), and 55 (32.2%) had titers of at least 1:1024. Before our donation program started, 31 CP units were procured from the New York Blood Center (NYBC). Among the 31 CP units that were obtained from the NYBC, 25 units (80.6%) were positive in the LFA but only 12 units (38.7%) had titers of at least 1:1024. CP was administered to 28 hospitalized COVID-19 patients. Patients who received low titer CP, high titer CP and patients who did not receive CP were followed for 45 days after presentation. Severe adverse events were not associated with CP transfusion. Death was a less frequent outcome for patients that received high titer CP (>1:1024) 38.6% mortality, than patients that received low titer CP (≤1:1024) 77.8% mortality.
Keywords: SARS-CoV-2, COVID-19, Convalescent plasma donation and treatment, ELISA, Lateral flow antibody assay, ICU patients
Abbreviations: AABB, American Association of Blood Banks; CI, confidence interval; COVID-19, coronavirus disease of 2019; CP, convalescent plasma; ELISA, enzyme-linked immunosorbent assay; H1N1, influenza A virus subtype H1N1; H5N1, highly pathogenic Asian avian influenza A subtype H5N1; HIV, human immunodeficiency virus; HR, hazard ratio; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; LFA, lateral flow assay; MERS, Middle East respiratory syndrome; NYBC, New York Blood Center; RBD, receptor binding domain; RNA, ribonucleic acid; RT PCR, reverse transcriptase polymerase chain reaction; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SUNY, State University of New York; UHB, University Hospital of Brooklyn
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 has posed a treatment challenge. The disease emerged in Wuhan, China in December 2019 and has since arrived in major cities in the United States. Early in the pandemic, New York City was particularly affected [1]. In the USA, there had been a disproportionate burden of illness and death affecting racial and ethnic minorities [1].
Plasma from individuals who recovered from illness has been used as treatment for infectious diseases since the 1890′s [2]. During the 1918 Spanish flu pandemic, convalescent plasma was used to treat those with pneumonia [3], [4]. More recently, convalescent plasma was utilized with reported success in the management of MERS [5], Ebola [6], H5N1 [7], H1N1 [8], and SARS infections [9]. COVID-19 convalescent plasma (CP) is procured by plasmapheresis from donors who have recently recovered from COVID-19. CP contains specific antibodies that are expected to provide passive immunization to neutralize the pathogen [2]. Challenges to understanding the utility of this procedure include donor procurement, assessing an adequate antibody titer for infusion and selection of CP recipients.
Several recent studies have shown a beneficial effect of administering CP to COVID-19 patients [10], [11], [12], [13], though some studies have not [14], [15]. Differences in when in the course of the disease CP was administered, and the strength and specificity of CP could account for different outcomes. We hypothesized, therefore, that timely administration of potent CP would provide a survival benefit for hospitalized COVID-19 patients.
University Hospital of Brooklyn (UHB) was designated a COVID-19 only hospital on March 28, 2020 by the New York State Governor. Between March 13 and June 30, 765 COVID-19 positive patients were admitted and managed in the institution for moderate to severe symptoms. We describe our experience below in obtaining high titer CP from volunteer donors who had recovered from COVID-19 and the clinical course of 28 severely ill COVID-19 patients who were treated with high titer or low titer CP.
2. Methods
2.1. Study design
This is a retrospective descriptive study on the experience of a CP donation and treatment program. One group, composed of 17 COVID−19 patients who did not receive CP transfusion as part of their management, was observed and compared with two CP recipient groups, one with high titer and one with low titer anti-SARS-CoV-2 IgG antibody as described below. The non-CP treated group had similar severity of illness as the CP treated patients. A cutoff for CP donation was selected based on the Mayo Clinic recommendation of 1:160 titer or greater where inhibition of neutralization decreased by 50%. The Mayo program did not give guidance for an ELISA titer. In preliminary studies, we found that 50% reduction in OD in ELISA assays typically was 1:256 (close to neutralizing titer of 1:160) when the end point titration was 1:1024.
2.2. Donation of CP
Announcements for donation of CP were accomplished by advertisement on the UHB website and e-mail. This led to word-of-mouth spread to external donors, increasing the interest of many. Volunteer donors were screened at UHB based on the following criteria: (1) self-reported recovery from a documented COVID-19 infection, (2) asymptomatic for at least 14 days, (3) negative SARS-CoV-2 RNA RT PCR assay (only for those asymptomatic for ≤28 days), (4) positive lateral flow test (POS++ or greater), (5) high ELISA IgG titer (titer of at least 1:1024) and (6) no detectable anti-HLA antibody (Immucor, Delux Screen, Atlanta, GA). While the donation program was ramping up, the UHB blood bank reached out to New York Blood Center (NYBC) for COVID-19 CP. Plasmapheresis was performed by the Brooklyn Blood Donation Center at Maimonides Medical Center. These units were delivered frozen to the SUNY Downstate Blood Bank.
Twelve of the 28 patients in this analysis received CP that was donated at UHB and 16 patients received CP collected by the NYBC. Six of the 16 CP units from NYBC had an IgG titer of less than 1:1024.
2.3. IgM/IgG lateral flow test
All the plasma/serum samples from NYBC units, potential UHB donors and recipients were tested with a lateral flow test kit (Singuway, Shenzhen City, China) that detects IgM and IgG antibodies against the SARS-CoV-2 S1 spike protein receptor binding domain (RBD), following manufacturer’s protocol. In brief, one drop of plasma/serum was placed into the designated sample well followed by two drops of the kit’s diluent. The result was observed after exactly 10 min and recorded photographically (Fig. 1 ). The presence of at a red line along the marked area (IgM and/or IgG) indicated a positive test. A line must be present in “Control” for the test to be valid. The intensity of the red line was used to stratify the strength of the antibody: a faintly visible red line signifies +/- and red lines of increasing intensity compared to the control line (POS+++) were scored as POS+, POS++ or POS+++.
Fig. 1.

An example of a positive lateral flow assay. The intensity of the control line (indicated by “A”) was considered as POS+++. The IgG line (indicated by “B”) in this example had a POS++ intensity (weaker intensity than the control). The IgM line (indicated by “C”) had a POS+++ intensity (same intensity as the control).
2.4. ELISA-based plasma IgG
All recipients and donor plasma samples that tested positive by the lateral flow test were tested for levels of IgG antibody against the SARS-CoV-2 S1 spike protein RBD. Depending on the lateral flow result, assay controls and serum samples were diluted from 1:2 to 1:64 followed by 2-fold dilution out to 1:131072 and added to a 96-wells microtiter plate (Thermo Scientific Immulon, Waltham, MA, USA) that was coated with SARS-CoV-2 recombinant RBD (BEI Resources, Manassas, VA, USA). A secondary anti-human IgG (Fab specific) antibody labeled with horse radish peroxidase (Sigma-Aldrich, St. Louis, MO, USA), was added to each well to form a specific complex of antigen–antibody bound to the plate surface. The binding reaction was then enhanced visually with SIGMAFASTTM OPD (Sigma-Aldrich, St. Louis, MO, USA), and after application of the stop solution (3 M Hydrochloric acid), the optical density was immediately measured at 490 nm. When the absorbance value was greater than the cut-off value (OD490 = 0.15), the specimens were reported as a positive result and the resulting titer was reported.
2.5. SARS-CoV-2 viral RNA detection by RT-PCR
SARS-CoV-2 detection in recipients and potential donors was performed in-house using the Cepheid Xpert® Xpress SARS-CoV-2 RT-PCR assay (Cepheid, Sunnyvale, CA) following manufacturer’s protocol. Nasopharyngeal swab samples were collected from patients and volunteers, and the swab was placed into a tube containing 3 ml of viral transport medium. Using the pipette included in the Cepheid kit, 300 µl of the nasopharyngeal swab sample was transferred into the Cepheid cartridge tube, which contains the reacting reagents. The cartridge was then closed with the lid and placed into the GeneXpert® System for 45 min. Results were interpreted as positive if at least one of the three targets (N2 gene, E gene, SPC gene) were detected, and negative if none of the targets was present.
2.6. CP recipients
This treatment was performed at UHB between April 4, 2020 to June 26, 2020. The program ended because patients with severe symptoms were no longer seen in the hospital past this date. The Mayo Clinic protocol for Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19 (unique protocol identification number: 20–003312) was utilized. The program received approval from the local institutional review board.
The inclusion criteria for treatment with CP were: (1) at least 18 years of age, (2) had a diagnosis of SARS-CoV-2 infection laboratory confirmed by RT PCR assay, (3) admitted to UHB for the treatment of COVID-19 complications, (4) severe or life threatening COVID-19 or judged by the treating provider to be at high risk of progression to severe or life-threatening disease as judged by the need for supportive oxygen therapy, and (5) informed consent provided by the patient or healthcare proxy. There were no exclusion criteria. All patients were followed for 45 days after admission. Clinical chemistry and hematology parameters were monitored until either discharge or death.
CP was transfused within 24 h after thawing. Each CP unit, approximately 250 ml, was infused over one to two hours. Recipients were monitored every 15 min for signs of transfusion-related reactions and then followed post-transfusion for clinical course.
2.7. Non-CP treated group
A group of hospitalized critically ill patients who did not receive CP treatment was observed retrospectively and compared with CP recipients for outcomes. These patients, admitted from March 18 to May 1, 2020, were managed with similar COVID-19 therapeutic drugs as the CP recipients, except for the CP therapy itself.
2.8. Statistics
Statistical analyses were performed using IBM® SPSS Statistics Version 25 (IBM SPSS Japan, Tokyo, Japan) and GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Sample size calculations were not performed. The population analyzed included all volunteers and donors for the COVID-19 Convalescent Plasma Donation Program, all patients who received CP on or before June 26, 2020 at UHB and all patients in the non-CP group, unless otherwise indicated. Mortality in the CP and control cohorts was described with the use of Kaplan-Meier analysis. A chi-square analysis or Fisher’s exact test was used to assess for statistical difference between groups. Associations between cohort pretreatment characteristics, lab values, and mortality were evaluated with Cox proportional hazards regression. Mann-Whitney U test, and Kruskal-Wallis test where used to analyze continuous variables where appropriate. A two-sided p value of less than or equal to 0.05 was considered to indicate statistical significance and all values are shown without correction for multiple testing. The widths of the confidence intervals have not been adjusted for multiple comparisons; therefore, intervals should not be used to infer definite associations.
2.9. Study approval
The recruitment of patients for CP treatment was performed under registration with the Mayo Clinic Clinical Trial, Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04338360). Prior authorization to publish was secured from the Mayo Clinic PI. The trial protocol was approved by the SUNY Downstate institutional review board. Informed consent was obtained from each patient prior to transfusion. A separate IRB application was approved for retrospective review of electronic medical records of COVID-19 patients.
3. Results
3.1. Plasma donor Characteristics
Between April 23, 2020 to June 26, 2020, UHB volunteers (n = 171) were screened for possible donation. The process for evaluation of potential donors and initial screening results are shown in Fig. 2 . All volunteers were screened with a lateral flow assay that detects IgM and IgG antibodies against the RBD of S1 spike protein of SARS-CoV-2. Sixty-five (38.0%) tested positive for IgG and their anti-SARS-CoV-2 RBD IgG ELISA titers were determined. Six of the 65 (9%) tested positive for the viral RNA and were excluded from donation. Fifty-five subjects (32.2%) had titers of at least 1:1024. This level of antibody was chosen for donation pending final routine screening for blood product donation by the Brooklyn Blood Donation Center, Maimonides Medical Center.
Fig. 2.
Diagram of the screening process for CP donation. All UHB volunteer donors (N = 171) had mild to moderate COVID-19 related symptoms and were all asymptomatic for at least two weeks prior to presenting in the CP donation clinic. RT PCR – real-time reverse transcription polymerase chain reaction, ELISA – enzyme-linked immunosorbent assay.
Table 1 summarizes the characteristics and demographics of all screened volunteers at UHB. Ninety-two volunteers were female (53.8%). One hundred and twenty-nine (75.0%) reported mild symptoms and 42 (24.6%) had moderate symptoms. The median age was 44.2 years (IQR 33.5 to 54.5 years). Forty of the volunteers (23.4%) had a previous documented positive result for SARS-COV-2 RNA real-time reverse transcription polymerase chain reaction test (RT PCR), and 6 (3.5%) had a previous positive antibody test. All those who donated were at least 14 days without symptoms. The median period from date of last symptom to date of presentation at the donation clinic was 33 days (IQR 23 to 39.5 days).
Table 1.
Characteristics of volunteers screened for possible CP donation.
| Characteristics | N (%)/Median (IQR) (N = 171) |
|---|---|
| Age | 44.2 years (33.5–54.5 years) |
| Sex | |
| Male | 79 (46.2) |
| Female | 92 (53.8) |
| Symptom severity | |
| Mild | 129 (75.4) |
| Moderate | 42 (24.6) |
| Duration of Symptoms | 13.0 days (8.0–17.8 days) |
| Previous RT PCR | |
| Positive | 40 (23.4) |
| Negative | 15 (8.8) |
| No test | 116 (67.8) |
| Previous Antibody test | |
| Positive | 6 (3.5) |
| Negative | 1 (0.6) |
| No test | 164 (95.9) |
| Current RT PCR | |
| Positive | 16 (9.4) |
| Negative | 146 (85.4) |
| Not donea | 9 (5.3) |
| Current Antibody Test | |
| Positive | 65 (38.0) |
| Negative | 106 (62.0) |
| ELISA Titer (N = 59)b | |
| Negative | 1 (1.7) |
| 1:512 | 1 (1.7) |
| 1:1024 | 8 (13.6) |
| 1:2048 | 10 (16.9) |
| 1:4096 | 24 (40.7) |
| 1:8192 | 8 (13.6) |
| 1:16384 | 1 (1.7) |
| 1:32768 | 2 (3.4) |
| 1:65536 | 2 (3.4) |
| Lost to follow up | 2 (3.4) |
a. Volunteers were asymptomatic for >28 days.
b. Screened positive using the lateral flow assay and negative for viral RNA RT PCR.
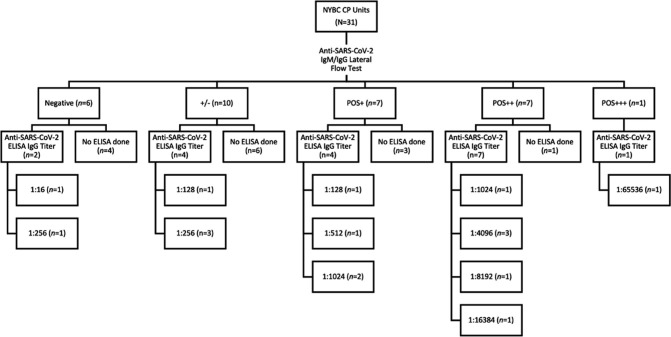
Thirty-one CP units were procured from NYBC. These units were collected from volunteers who were known to have SARS-CoV-2 infection (prior RT PCR positive test) but were not tested for antibody at NYBC. Subsequent lateral flow assays were performed at UHB on all these units and ELISA IgG titer was completed for 17 units (Fig. 3 ). Overall, 12 of 31 units (38.7%) had titers of at least 1:1024.
Fig. 3.
Diagram of the screening process for NYBC units. All units (N = 31) were tested with lateral flow assay and selected units were analyzed for antibody titer using ELISA. ELISA – enzyme-linked immunosorbent assay.
ELISA IgG titer determination was performed on 57 of the 59 potential UHB donors who screened positive on the lateral flow assay. Most (55/57, 96.5%) had titers of at least 1:1024 and were considered eligible to donate. The corresponding titers for each lateral flow assay positive result is shown in Fig. 4 . There was a significant correlation between IgG titer and lateral flow assay results for both NYBC units (Spearman r = 0.8904, 95% CI, 7087 to 0.9613; p = 0.005; Fig. 4A) and UHB volunteers (Spearman r = 0.7112, 95% CI, 0.5527 to 0.8200; p < 0.0001; Fig. 4B), where stronger intensity with lateral flow was observed to correspond with higher IgG titer.
Fig. 4.
Lateral flow test and ELISA IgG result. Panel A shows the lateral flow results and corresponding IgG titer for NYBC CP units. Panel B shows the lateral flow results and corresponding IgG titer for UHB potential donors. There was a significant correlation between IgG titer and lateral flow assay results for both NYBC units (Spearman r = 0.8904, 95% CI, 7087 to 0.9613; p = 0.005; Fig. 4A) and UHB volunteers (Spearman r = 0.7112, 95% CI, 0.5527 to 0.8200; p < 0.0001; Fig. 4B). Each data point denotes an individual result, the red line indicates median value. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
There is a significant difference in the number of potential donors with high titers when grouped according to severity of symptoms (p = 0.00015). Among the 129 volunteers with mild symptoms, only 28 (21.7%) had ELISA titers of >/= 1:2048 and most (91, 70.5%) had negative LFA. Among the 42 potential donors with moderate symptoms, most (22, 52.4%) had titers of >/=1:2048 and only 15 (35.7%) had negative LFA result.
In accordance with the American Association of Blood Banks (AABB) guideline, 8 of the 55 UHB volunteers were not allowed to donate due to height and/or weight restrictions. Other potential donors were unable to donate for a variety of reasons, including low hemoglobin, history of blood-borne infection, difficult venous access and a positive HLA antibody test. Seven of the 55 were lost to follow up, and 6 were placed on hold for donation as the program was suspended due to the low number of hospitalized COVID-19 cases. A total of 78 CP units were collected from 28 UHB donors.
3.2. Plasma recipients characteristics
From April 4, 2020 to June 26, 2020, 28 severely ill COVID-19 patients (19 males and 9 females) received CP transfusion at UHB under the Mayo Clinic-led Expanded Access Program. The individual profiles of the 28 patients who received CP are shown in Supplementary Table 1. Table 2 provides data on demographics and outcomes for the CP recipients and the non-CP group. Most recipients were African American (85.7%), 3.6% were Asian, 3.6% were Hispanic-white. Two patients declined to identify their ethnicity. Most recipients were male (18/28, 64.3%) and between 50 and 70 years of age (13/28, 46.4%). The most common symptoms at disease onset were shortness of breath (71.4%), fever (50%) and cough (50%). Co-morbidities present in these patients were hypertension (60.7%), diabetes mellitus (37.0%), renal disease (28.6%), heart disease (21.4%), neurological disease (14.3%), lung disease (2.5%), cancer (3.6%) and other (32.1%; e.g. HIV, hyperlipidemia, bipolar disorder, glaucoma). Concurrent therapies received by the patients were azithromycin (60.7%), tocilizumab (46.4%), ceftriaxone (50.0%), corticosteroids (39.3%), vancomycin (28.6%), hydroxychloroquine (25.0%) and Remdesivir (7.1%).
Table 2.
Characteristics of patients given CP and those not given CP.
| Characteristic | All CP Recipients (n = 28) | Low titer (</=1:1024) (n = 9) | High titer (>1:1024) (n = 19) | No CP (n = 17) |
|---|---|---|---|---|
| Median age (IQR) – yr | 67.5 (53.3–75.0) | 68.0 (62.0–70.0) | 67.0 (53.5–76.5) | 62 (56.5–70.0) |
| Age Category – no. (%) | ||||
| < 50 yr | 4 (14.3) | 1 (11.1) | 3 (15.8) | 1 (5.9) |
| 50 to <70 yr | 13 (46.4) | 5 (55.6) | 8 (42.1) | 12 (70.6) |
| >= 70 yr | 11 (39.3) | 3 (33.3) | 8 (42.1) | 4 (23.5) |
| Sex – no. (%) | ||||
| Male | 19 (67.9) | 5 (55.6) | 14 (73.7) | 10 (58.8) |
| Female | 9 (32.1) | 4 (44.4) | 5 (26.3) | 7 (41.2) |
| Race – no. (%) | ||||
| Black | 24 (85.7) | 6 (66.7) | 18 (94.7) | 12 (70.6) |
| Non-Black | 2 (7.1) | 2 (22.2) | 0 (0) | 2 (11.8) |
| Undisclosed | 2 (7.1) | 1 (11.1) | 1 (5.3) | 3 (17.6) |
| Median days ill before transfusion (IQR) - days | 9 (7–16.5) | 12 (9.0–17.0) | 8 (5.0–14.5) | NA |
| Received mechanical ventilation (%) | 13 (46.4) | 6 (66.6) | 7 (36.8) | 17 (100.0) |
| Mortality (%) | 14 (50.0) | 7 (77.8) | 7 (36.8) | 9 (52.9) |
| Coexisting conditions - no. (%) | ||||
| Any condition | 22 (78.6) | 8 (88.9) | 15 (78.9) | 16 (94.1) |
| Hypertension | 17 (60.7) | 6 (66.6) | 11 (57.9) | 11 (64.7) |
| Renal disease | 8 (28.6) | 3 (33.3) | 5 (26.3) | 1 (5.9) |
| Diabetes | 7 (25.0) | 4 (44.4) | 6 (31.6) | 11 (64.7) |
| Respiratory (e.g., asthma, COPD) | 7 (25.0) | 1 (11.1) | 4 (21.1) | 2 (11.8) |
| Hyperlipidemia | 4 (14.3) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
Keys: COPD – chronic obstructive pulmonary disease; NA – not applicable.
All patients received supportive oxygen therapy at the start of their hospital admission. Thirteen patients were eventually placed on invasive mechanical ventilation (46.4%). The median duration of invasive mechanical ventilation was 10 days (IQR, 8 to 14 days). Ten of the 13 (76.9%) mechanically ventilated patients subsequently died of COVID-19 complications, while only 4 (26.7%) died among the 15 patients that did not require invasive oxygenation. Twelve of 15 recipients (80.0%) who did not require intubation for oxygen support were transfused with high IgG titer plasma. The risk of death was significantly greater in CP patients who were female and required mechanical ventilation support (Fig. 5 ). The median days of illness prior to CP transfusion was 9 days (IQR 7 to 16 days). This interval was shorter for the high titer group (median = 8 days; IQR 5 to 14.5 days) as compared to the low titer group (median = 12 days; IQR, 9 to 17 days). The survival of patients who received CP within 14 days of illness did not significantly differ from those who transfused after 14 days of illness (HR = 3.13, 95% CI = 0.73 to 13.39; p = 0.199).
Fig. 5.
Kaplan-Meier curves displaying the 45-day survival of CP recipients by sex (A) and by necessity for mechanical ventilation therapy (B). Panel A shows that female patients who received convalescent plasma had lower survival (HR as compared with male patients, 3.03; 95% CI, 1.04 to 8.78, p = 0.041; panel A). This result is in contrast with previously published findings. Racial differences and other susceptibility parameters (such as specific co-morbidities) should be investigated and controlled to further draw conclusions on the effect of gender on the severity of COVID-19. Panel B demonstrates that the risk of death was significantly greater in CP patients who required mechanical ventilation support (compared to patients who received non-mechanical respiratory support, HR = 3.72; 95% CI, 1.15 to 12.02, p = 0.028; panel B). The extent of lung injury in COVID-19 remains an important predictor of clinical outcome in our patient population.
A group of 17 hospitalized critically ill COVID-19 patients who did not receive CP as part of their management was compared to the CP treated groups. These patients, admitted from March 18 to May 2, 2020, were selected based on similarity to the CP treatment groups on age and severity from about 600 admitted patients. We note that CP infusions occurred from April 2 to June 24, 2020. “Severe” COVID-19 respiratory symptoms was defined by deterioration of respiratory function requiring supplemental oxygen greater than 4 L/minute to maintain oxygen saturation at >90%. The non-CP treated patients received similar COVID-19-related management as the treatment groups except for CP. The major reasons for not receiving CP include: (1) CP was not yet available for treatment, (2) consent was not given by the patient or their health proxy, and (3) the patient recovered or died before CP could be administered.
Fourteen of the 28 CP (50.0%) recipients died within the 45-day follow up period. The median 45-day survival in this group was 21 days (IQR 14.85 to 21.75 days). Among the non-CP group, 9 of 17 (52.9%) patients died during the 45 day follow up period with a median survival of 24 days (IQR, 19.62 d to 30.71 d). The mortality of the high titer group was lower than that of the non-CP cohort (36.8% and 52.9%, respectively), while the mortality of the low titer group (77.8%) was higher than that of the non-CP and the high titer cohorts. Fig. 6 shows a Kaplan-Meier 45-day survival plot for the three cohorts. Patients transfused with high titer CP (>1:1024) had better survival than the cohort the received low titer CP (</=1:1024) (logrank p = 0.079, NS). Factors other than CP treatment may have also affected the observed outcome. For example, the period in which low titer and high titer CP was administered differed by 5 weeks. The low titer recipients received CP earlier in the pandemic as shown in Fig. 7 . Additionally, the interval between admission to the hospital and CP treatment decreased so that many patients received high titer CP sooner in the course of their disease (Fig. 8 ).
Fig. 6.

Kaplan-Meier analysis of surviving fraction versus days of hospitalization for patients that received low titer (≤1:1024) or high titer (>1:1024) CP and no CP. Patients transfused with high titer (>1:1024) CP trended to have better survival (logrank p = 0.079).
Fig. 7.
Survival of recipients who received low and high titer CP units based on date of admission. Most patients who received low titer CP were admitted on or before 4/29/2020, and most of them eventually died. Most patients who were given high titer CP were admitted on or after 4/19/2020, and most of them survived.
Fig. 8.
The interval between admission and CP transfusion according to date of admission. Most patients who received high-titer CP units also received the plasma soon after admission. High titer plasma and earlier management with CP were apparent in patients admitted after 4/29/2020.
The concurrent laboratory values of recipients (before and after the CP transfusion) and non-CP group (days 1 and 7 after admission) are shown in Supplementary Table 2 and Supplementary Fig. S1. The SARS-COV-2 antibody titers of the recipients were determined pre-transfusion (as baseline) and serially during the post-transfusion period (Supplementary Fig. S2). The median pre-transfusion antibody titer was 1:4096. Serial IgG titers did not differ significantly between those who received high and low IgG titer CP. A small increase in IgG titers was observed between day three and day seven of treatment.
Three patients had reported adverse events after transfusion with CP (11%), which were all deemed unrelated to the transfusion. The adverse events noted were atrial fibrillation with rapid ventricular response one day after transfusion, progression of lung disease requiring intubation one day after transfusion and arterial oxygen desaturation to 85% six hours after transfusion.
4. Discussion
Hospitalized COVID-19 patients who received high ELISA IgG titer CP (>1:1024) had better survival than patients receiving low titer CP (≤1:1024). Although this finding did not reach statistical significance, a clear trend was observed. Failure to achieve statistical significance may be due to small sample size. Indeed, two larger studies concluded that there was a survival benefit for CP recipients [11], [13]. A recent study found significantly lower mortality rates among recipients that received high titer IgG CP compared to low antibody level plasma units [12], [13]. Our results support the importance of transfusing COVID-19 patients with high titer anti-SARS-CoV-2 antibody in order to provide a survival benefit.
However, in our experience such survival benefit may not be solely attributed to CP. At the beginning of the pandemic in March 2020, the medical and nursing staff at UHB were unfamiliar with the disease and most patients arrived in the hospital already in critical condition. Early patients were treated with hydroxychloroquine and azithromycin; both have been linked to poorer outcomes or without benefit [16]. During this period, the only CP units available to our patients were those coming from NYBC, which were later found to have low antibody titers. From the second half of April onwards, COVID-19 patients were given high antibody titer CP units from our screened donors. During this period, the treatment of COVID-19 was also improving as evidence became available on the benefit of dexamethasone, Remdesivir and CP as well. Furthermore, the interval between admission to the hospital and CP treatment decreased so that patients received CP sooner in the course of their disease. An additional factor is that the general public’s overall awareness of COVID-19 improved, and patients began to seek medical attention earlier in the disease process. All these circumstances may have contributed to the improved survival of the high titer COVID-19 recipients. Indeed, multi-center randomized controlled trials found no benefit with the use of convalescent plasma in severe COVID-19 pneumonia when some of these factors were controlled [14], [15]. However, in the latter studies, most patients were treated with CP late in the course of their disease as compared to our study (>14 days vs 9 days, respectively).
Screening and subsequent antibody titer determination of CP potency against the SARS-CoV-2 spike RBD is an essential step to ensure the efficacy of this treatment. Preliminary evidence with COVID-19 suggests that patients with mild symptoms may develop very low titer antibodies [17], [18], [19]. One study observed that 18% of convalescent COVID-19 donors had undetectable neutralizing titers in their plasma samples collected an average of 30 days after the onset of symptoms [20]. A larger study performed by NYBC showed that more than half of CP donors had low neutralizing titers and that there was a large variation in antibody titers among donors [21]. In our experience, using ELISA binding as a surrogate for neutralizing antibodies, among the 171 volunteers, only 55 (32.2%) had high levels of antibody. Out of 31 NYBC CP units, only 12 (38.7%) had titers of at least 1:1024. If CP is considered a valid therapeutic option, there is an existing challenge to screen CP donors for antibody titers, as evidence shows that most mild COVID-19 patients may not develop an adequate level of anti-SARS- CoV-2 antibodies to provide a therapeutic benefit to recipients.
The lateral flow assay permitted us to quickly screen volunteers who potentially had high titers. Donors at UHB who tested POS++ with the lateral flow assay had antibody ELISA titers of > 1:1024. Automated systems for screening are now available for larger testing capacity.
It is important to note that the mortality of our patient population was greater than in previously published cohorts. This circumstance may be why a CP effect was seen despite the small numbers of patients. Joyner et al (2020) reported a seven-day mortality of only 15% among their CP recipients [13]. Liu et al (2020) reported mortality rates of 12.8% among plasma recipients and 24.4% among their matched control patients with a median follow up of 11 days for the plasma group and 9 days for the control group [11]. Our data shows a 44% death rate after a 45-day follow-up. The prolonged follow-up period in our study may have revealed a higher mortality rate. However, baseline health status differences, as well as racial and genetic factors, need to be further investigated in a larger cohort. In contrast to previous studies, the patients included in our treatment program were predominantly Black. COVID-19 case surveillance reports showed higher prevalence among racial and ethnic minority group [22]. In a large cohort study in a health institution in Louisiana [23], 76.9% of COVID-19 patients were Black, whereas Blacks comprise only 31% of the institution’s population, and most deaths were also in Black patients (70.6%). CDC has emphasized that inequities in the social determinants of health, such as poverty and healthcare access, affecting minority groups are interrelated and influence a wide range of health and quality-of-life issues and outcomes.
Greater mortality rate was also observed at UHB among female patients, with a hazard ratio three times higher than male patients. This result is in contrast with findings published in one Chinese study where it was concluded that male gender is a risk factor for worse outcome in 43 COVID-19 patients independent of age and susceptibility [24]. Racial differences and other susceptibility parameters (such as specific co-morbidities) should be investigated and controlled to further draw conclusions on the effect of gender on the severity of COVID-19.
Interpretation of the results of our study are limited due to the small sample size of the cohorts, the observational design, as well as the heterogeneity of pre-existing co-morbidities and pharmaco-therapeutic interventions. Even though these limitations prevent deriving broad conclusions regarding CP donation and therapy, the current information supports and extends previous studies on CP treatment for COVID-19. Our study provides guidance for procuring potent CP for treatment of hospitalized COVID-19 patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority under Contract No. 75A50120C00096 awarded to the Mayo Clinic.
Disclaimer: The views and opinions expressed in this publication are those of the authors and do not reflect the official policy or position of the US Department of Health and Human services and its agencies including the Biomedical Research and Development Authority and the Food and Drug Administration, as well as any agency of the U.S. government. Assumptions made within and interpretations from the analysis are not reflective of the position of any U.S. government entity.
The authors would like to thank the following for their technical contribution: residents of the Department of Pathology of SUNY Downstate Health Sciences University, staff of the Transplant Laboratory of SUNY Downstate Health Sciences University, staff of Mirimus, Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.humimm.2021.02.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Centers for Disease Control and Prevention, United States COVID-19 Cases and Deaths by State. https://COVID.cdc.gov/COVID-data-tracker/#cases_casesinlast7days. since January 21, 2020 (accessed 15 July 2020).
- 2.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfusion. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis F.D., Hall M.W., Gaines A.R. Early use of convalescent serum in influenza. Mil. Surg. 1920;47:177–179. [Google Scholar]
- 4.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann. Intern. Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization MERS-CoV Research Group State of knowledge and data gaps of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;12:5. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization, Experimental therapies: growing interest in the use of whole blood or plasma from recovered Ebola patients (convalescent therapies) Ebola situation assessment - 26 September 2014. http://www.who.int/mediacentre/news/ebola/26-september-2014/en/ (accessed 15 July 2020).
- 7.C.P. Simmons, N.L Bernasconi, A.L. Suguitan, K. Mills, J.M. Ward, N.V. Chau, T.T. Hien, F. Sallusto, Q. Ha do, J. Farrar, M.D. de Jong, A. Lanzavecchia, K. Subbarao, 2007. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza, PLoS Med. 4(5), e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed]
- 8.Kong L.K., Zhou B.P. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med. J. 2006;12:489. [PubMed] [Google Scholar]
- 9.Wong V.W., Dai D., Wu A.K., Sung J.J. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med. J. 2003;9:199–201. [PubMed] [Google Scholar]
- 10.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.S.T.H. Liu, H.M. Lin, I. Baine, A. Wajnberg, J.P. Gumprecht, F. Rahman, D. Rodriguez, et al., Convalescent plasma treatment of severe COVID-19: A matched control study, medRxiv preprint. https://doi.org/10.1101/2020.05.20.20102236. 22 May 2020.
- 12.Joyner M.J., Wright R.S., Fairweather D.L., Senefeld J.W., Bruno K.A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M.J. Joyner, J.W. Senefeld, S.A. Klasses, J.R. Mills, P.W. Johnson, et al., Effects of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience, medRxiv preprint. https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1. 12 August 2020.
- 14.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V. A randomized trial of convalescent plasma in COVID-10 Severe Pneumonia. NEJM. 2020 doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A.B. Cavalcanti F.G. Zampieri R.G. Rosa L.C.P. Azevedo et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate COVID-19 N Engl J Med 383 (2020) 2041–2052. doi: 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed]
- 17.E. Salazar, S.V. Kuchipudi, P.A. Christensen, T.N. Eagar, X. Yi, et al. Relationship between anti-Spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. bioRxiv preprint. https://www.biorxiv.org/content/10.1101/2020.06.08.138990v1. 9 June 2020.
- 18.Klein S., Pekosz A., Park H.S., Ursin R., Shapiro J., Benner S. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J .Clin. Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madariaga M.L.L., Guthmiller J.J., Schrantz S., Jansen M.O., Christensen C. Clinical predictors of donor antibody titer and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial. J. Intern. Med. 2020 doi: 10.1111/joim.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbiani D.F., Gaebler C., Muecksch F., Cetrulo Lorenzi J., Wang Z. Convergent antibody responses to SARS-CoV-2 infection in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luchsinger L.L., Ransegnola B.P., Jin D.K., Muecksch F., Weisblum Y., Bao W. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.02005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.[22] E.K. Stokes, L.D. Zambrano, K.N. Anderson, E.P. Marder, K.M. Raz, et al., Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 69 (2020) 759–765. http://dx.doi.org/10.15585/mmwr.mm6924e2external icon. [DOI] [PMC free article] [PubMed]
- 23.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N. Engl. J. Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J.M., Bai P., He W., Wu F., Liu X.F. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.