Abstract
Objective
This study aimed to investigate the efficacy and safety of convalescent plasma (CP) transfusion in a group of high-risk COVID-19 patients.
Methods
This prospective study included 204 patients from a single tertiary-care hospital, hospitalized with COVID-19, of whom 102 were treated with CP administration and standard care (PG) and 102 others who received standard care only (CG). The CG was selected from 336 hospitalized patients using the propensity-score matching (PSM) technique using age, MEWS score, and comorbidities. The primary outcome was mortality rate; secondary outcomes were the requirement of a ventilator, length of ventilator need, length of intensive care unit (ICU) stay, and length of overall hospital confinement. Additionally, parameters predicting death in COVID-19 patients were identified.
Results
Findings confirmed a significantly lower mortality rate in the PG versus the CG (13.7% vs. 34.3 %, p = 0.001) and a significant difference in the cumulative incidence of death between the two groups (p < 0.001). CP treatment was associated with lower risk of death (OR = 0.25 CI95 [0.06; 0.91], p = 0.041). There were no significant differences in ICU stay, ventilator time, and hospitalization time between the two groups.
Conclusions
A significantly lower mortality rate was observed in the group of patients treated with CP. Age, presence of cardiac insufficiency, active cancer, a ventilator requirement, and length of hospitalization significantly increased the risk of death in both groups. Our study shows that CP affords better outcomes when administrated in the earlier stage of high-risk COVID-19 disease.
Keywords: SARS-CoV-2, COVID-19, convalescent plasma
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-strand ribonucleic acid (RNA) virus similar to severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) which caused the 2002-2004 SARS outbreak (van Doremalen et al., 2020). During earlier MERS-CoV and SARS-CoV-1 outbreaks, many therapeutic possibilities were explored. Among them, convalescent plasma (CP) was proven to be one of the treatments that reduces mortality (Mair-Jenkins et al., 2015; Cheng et al., 2005). Nearly 18 years later, a similar challenge is being faced on an incomparably larger scale. Many effective treatments used during the SARS-CoV-1, MERS-CoV, and Ebola outbreaks produce no significant effect on SARS-CoV-2 infection, including lopinavir-ritonavir (Cao et al., 2020), tocilizumab (Radbel et al., 2020), and chloroquine (Cortegiani et al., 2020). Therefore, the need to investigate other treatment potentialities remains (Zhang and Liu, 2020, Depfenhart et al., 2020). Convalescent plasma, a passive immunization strategy, has been used effectively to prevent and treat epidemic infections for more than 100 years (Mair-Jenkins et al., 2015, Marson et al., 2020). Studies based on previous outbreaks proved good efficacy and no significant side effects (Van den Berg et al., 2020, Wooding and Bach, 2020). It has been described that the use of CP in SARS-CoV and avian influenza, an H5N1 virus infection, decreased viral load in the respiratory tract (Hung et al., 2011, Kong and Zhou, 2006). Convalescent plasma contains antiviral neutralizing antibodies, which are crucial in virus clearance. Other putative mechanisms underpinning the possible therapeutic effect of convalescent plasma include an overly active immune system (cytokine storm, Th1/Th17 ratio, complement activation) (Tobaiqy et al., 2020). As possibly shown in this study, viremia peaks in the first week of infection; therefore, it may be critical to administrate CP in the early stage of the disease. Convalescent plasma obtained from COVID-19 patients contains high titers of SARS-CoV-2 neutralizing antibodies. Antibodies play an essential role in communicating the presence of a pathogen to immune effector cells. Neutralizing antibodies interrupt viral entry into the human cell, attacking one of the functional subunits of S glycoprotein, and play a crucial role in the direct neutralization of the virus (Lindholm et al., 2020). Moreover, neutralizing antibodies can activate immune cells, such as dendritic cells, T-cells, B-cells, and other mechanisms of the immune system. High titers of virus-neutralizing antibodies present in plasma after COVID-19 may offer therapeutic value (Rojas et al., 2020, Rajendran et al., 2020).
Objectives
This study's main aim was to investigate the efficacy and safety of treatment with convalescent plasma transfusion in a group of severe COVID-19 patients, hospitalized at a tertiary center.
Study design and setting
This single-center prospective observational cohort study of 204 patients took place in the Central Clinical Hospital of the Ministry of Internal Affairs in Warsaw, Poland, from April 24 to September 28, 2020.
Participants
Donors
Eligibility criteria for 49 plasma donors were in accordance with the Minister of Health Regulations in force in Poland regarding the collection of blood from blood donor candidates and blood donors.
Additional eligibility criteria are as follows:
- male sex,
- positive test for SARS-CoV-2 (Real-Time PCR method for SARS-CoV-2 RNA detection), and
- a double-negative SARS-CoV-2 test with a minimum 24 -h interval between the two tests
(Real-Time PCR method for detecting SARS-CoV-2 RNA).
Recipients
Inclusion criteria:
-
-
Detection of SARS-CoV-2 RNA in the reverse transcription-polymerase chain reaction (gene targets RdRp, E, and N) from nasopharyngeal swabs,
-
-
Modified Early Warning Score (Smid et al., 2020) (MEWS) ≥ 2,
-
-
Clinical manifestation compatible with COVID-19, and
-
-
Findings compatible with COVID-19 in either chest X-ray or CT.
From April 24 to September 28, 2020, CP was administered to 102 patients hospitalized in the Central Clinical Hospital of the Ministry of Internal Affairs in Warsaw, Poland, which had been converted into a single-purpose hospital customized for COVID-19 patients only. Every patient was subjected to a physical examination before CP administration.
Methods
As soon as ten days after a study participant tested negative two times, the participant's plasma was collected. Convalescent plasma to be used for transfusion was tested to confirm compatibility with the AB0 blood type. Each plasma donation produced one to three units (200 milliliters [ml] each). If the titer of IgG antibodies indicated the need for subsequent plasma collection, it occurred after a minimum of 14 days. The TRIMA Accel separator, using automatic apheresis, was used to obtain the plasma. Plasma was treated to inactivate biological pathogens (system Mirasol) (Kwon, 2014).
The level of neutralizing antibodies was measured using a Liaison SARS-CoV-2 S1/S2 IgG test (Diasorin) for quantitative determination of IgG anti-S1 and IgG anti-S2 specific antibodies to SARS-CoV-2 - an indirect chemiluminescence immunoassay (CLIA), normal values < 15 AU/ ml, that had been proven effective in a comparison of six SARS–CoV-2 immunoassays with microneutralization (specificity/sensitivity 96%/90.3%) (Strömer et al., 2020, Bonelli et al., 2020).
Patients in the CP group received their first 200-ml infusion of CP no later than the fourteenth day after a COVID-19 diagnosis. Some patients received more than one infusion: 56 patients (54.9%) received one (200 ml) infusion, 38 (33.95%) received two (400 ml), and eight patients (7.1%) received three (600 ml) infusions. All subsequent infusions were administered with a 24 -h interval in between. The decision regarding administered plasma volume (more than 200 ml) was made depending on the patient's general condition. The control group (102 propensity-matched patients) was selected from a cohort of 336 COVID-19 patients at a single hospital.
Convalescent plasma was administrated in parallel with a standard regimen of care. The Bioethics Committee granted approval for this study (agreement number 40/2020 of 03/04/2020). Before administering CP, patients had to sign an informed consent form to participate in the clinical trial. The consent form included information on the study's protocol and possible side effects of CP. Copies of signed forms are stored together with patients’ medical records in the hospital.
Variables
Our study's primary endpoint was mortality rate, and the secondary outcomes were the requirement of a ventilator, length of ventilator needs, length of intensive care unit (ICU) stay, overall hospitalization duration, and SARS-CoV-2 elimination time.
Levels of SARS-CoV-2 neutralizing antibodies were assessed in the donor blood.
Additionally, factors associated with mortality in SARS-CoV-2 patients were identified using logistic regression analysis.
Statistical analysis
Propensity-score matching (PSM) analysis was used to balance baseline confounders between plasma and non-plasma groups. Data from previous COVID-19 CP studies led to the assumption of a 28% death rate, indicating a sample size of 95 patients per each of two groups (190 total study participants) would be needed. This number was determined to be large enough to achieve a power of 90% to detect a 65% difference in death rates between the two study groups (28% - 65% = 18pp) with a two-sided p-value of 0.05. The control group was chosen from a cohort of 336 COVID-19 patients in one hospital. This method maintains the comparability of the two exposure groups in terms of baseline characteristics. For each patient, we used logistic regression to predict the probability (propensity score) of plasma. Variables in propensity-score estimation included sex, age, hypertension, arrhythmia, cardiac insufficiency, diabetes, chronic dialysis, diagnosed cancer, and/or recent stroke. The nearest-neighbor approach was used to match propensity scores. There were no issues with missing data for variables used in PSM. There was no missing data for sex and age; one out of 438 patients had missing data for the remaining variables. A patient from the plasma group was excluded from the final analysis before the start of patient matching and data analysis. Since the issue of missing data was marginal, no missing data imputation method was applied.
To define the control group, nominal variables were presented as n (% frequency of group), continuous variables as mean (SD - standard deviation), or median (range), depending on the normality of data distribution. Based on the visual assessment of histograms, skewness, and kurtosis values, the Shapiro-Wilk test verified data normality. Groups were compared with either the chi-square test or Fisher exact test for nominal variables and with the t-test or Mann-Whitney U test for continuous variables, as appropriate. Additionally, MD (mean or median difference) with a 95% confidence interval was calculated for continuous variables. Relative risk (RR) with a 95% confidence interval was calculated for nominal variables. Survival curves were prepared using the Kaplan-Meier approach, and a comparison of survival between groups with and without plasma treatment was made using the log-rank test. Additional analysis using logistic regression was conducted to identify parameters impacting death in COVID-19 patients. Initially, univariate models were calculated using all parameters. Subsequently, variables with p < 0.25 demonstrated in the univariate model were included as predictors into the final multivariate model, as recommended by Hosmer and Leweshow (Hosmer and Lemeshow, 2000). The stepwise method of adding predictors to the final model was used. All OR (odds ratios) mentioned in the text come from the multivariate model. Final model validation included the χ2 test, R2 Nagelkerke coefficient, and Hosmer and Lemeshow goodness of fit (GOF) test. Finally, ROC curves using Youden's criterium for a cut-off point were calculated for continuous parameters significant in logistic regression. All tests were two-sided, with α = 0.05. Statistical analysis was conducted using the R package, version 3.5.4 (Benedetto et al., 2018).
Results
Table 1 quantifies the propensity-score characteristics used to match CG and PG. As described in the statistical analysis section, the groups were comparable in terms of the baseline characteristics. Variables included in the propensity-score estimation included sex, age, hypertension, arrhythmia, cardiac insufficiency, diabetes, dialysis, active cancer, and active stroke. The nearest-neighbor approach was used in propensity-score matching. Hypertension was the most frequently observed comorbidity (54.9% and 52.9%), arrhythmia and cardiac insufficiency were second and third most frequent, and more than 13% of patients had an active neoplastic disease. Only less than 2% of patients had no other conditions.
Table 1.
Baseline characteristics of the unmatched and matched cohorts by plasma treatment.
| Unmatched |
Propensity-matched |
|||||
|---|---|---|---|---|---|---|
| Patients without plasma treatment | Plasma treated group | p | Patients without plasma treatment | Plasma treated group | p | |
| N | 336 | 103 | 102 | 102 | ||
| Sex, female, n (%) | 164 (48.8) | 46 (44.7) | 0.461 | 40 (39.2) | 45 (44.1) | 0.478 |
| Age, years, mean (SD) | 68.86 (17.56) | 62.80 (15.60) | 0.002 | 62.74 (20.55) | 63.04 (15.48) | 0.905 |
| No other conditions, n (%) | 7 (2.08) |
2 (1.94) |
2 (1.96) |
2 (1.96) |
NS | |
| Mean MEWS score |
2.66 | 2.53 | 0.004 | 2.59 | 2.54 | 0.384 |
| Hypertension, n (%) | 173 (51.5) | 54 (52.9) | 0.797 | 56 (54.9) | 54 (52.9) | 0.779 |
| Arrhythmia, n (%) | 90 (26.8) | 18 (17.6) | 0.061 | 16 (15.7) | 18 (17.6) | 0.707 |
| Heart failure, n (%) | 115 (34.2) | 15 (14.7) | < 0.001 | 15 (14.7) | 15 (14.7) | > 0.999 |
| Diabetes, n (%) | 82 (24.4) | 24 (23.5) | 0.857 | 26 (25.5) | 24 (23.5) | 0.745 |
| Dialysis, n (%) | 24 (7.1) | 3 (2.9) | 0.159 | 6 (5.9) | 3 (2.9) | 0.498 |
| Stroke active, n (%) | 11 (3.3) | 0 (0.0) | 0.075 | 0 (0.0) | 0 (0.0) | > 0.999 |
| Cancer active, n (%) | 60 (17.9) | 14 (13.7) | 0.324 | 14 (13.7) | 14 (13.7) | > 0.999 |
Groups were compared with the chi-square test or Fisher exact test for nominal variables or t-test for continuous variables (age).
As shown in Table 2 , the mortality rate in CG was 34.3%, while, in PG, the mortality rate was 13.7% (p = 0.001). However, hospitalization time was significantly longer in the PG group (13 vs. 20 days, p < 0.001). The requirement of respiratory treatment in CG was 21.6% compared to 11.8% in PG; however, this tendency did not reach statistical significance (p = 0.06).
Table 2.
Plasma treatment (PG) and propensity-matched non-plasma treatment control group (CG) analysis.
| Characteristic | CG |
PG |
Relative risk or median (mean) difference (95% CI) | p | ||
|---|---|---|---|---|---|---|
| n | Level | n | Level | |||
| Deaths, n (%) | 102 | 35 (34.3) | 102 | 14 (13.7) | 2.50 (1.43, 4.36)2 | 0.001 |
| Ventilator, n (%) | 102 | 22 (21.6) | 102 | 12 (11.8) | 1.83 (0.96, 3.50)2 | 0.060 |
| Intensive care stay, n (%) | 102 | 25 (24.5) | 102 | 23 (22.5) | 1.09 (0.66, 1.78) 2 | 0.741 |
| Ventilator time, days, median (range) | 22 | 6.00 (1.00, 29.00) | 12 | 8.00 (1.00, 28.00) | −2.00 (-6.00, 4.00)1 | 0.701 |
| Hospitalization time, days, median (range) | 101 | 13.00 (0.00, 59.00) | 101 | 20.00 (0.00, 63.00) | −7.00 (-10.00, -5.00)1 | <0.001 |
| SARS-CoV-2 elimination time, days, mean (SD) | 64 | 18.63 (10.39) | 82 | 22.22 (11.50) | −3.59 (-7.23, 0.04)1 | 0.053 |
| Use of corticosteroids, n (%) | 102 | 11 (10.8) | 102 | 27 (26.5) | 0.41 (0.21;0.77) | 0.007 |
Groups were compared with a chi-square test for dichotomous variables and a t-test (elimination time) or Mann-Whitney U test (ventilator time, hospitalization time) for continuous variables, depending on distribution normality. Mean or median differences between groups were calculated as non-plasma group minus plasma group with 95% confidence interval for continuous variables, a relative risk with 95% confidence interval was calculated for nominal variables.
There were no significant differences in ICU stay, SARS-CoV-2 elimination time, and ventilator treatment time for both groups.
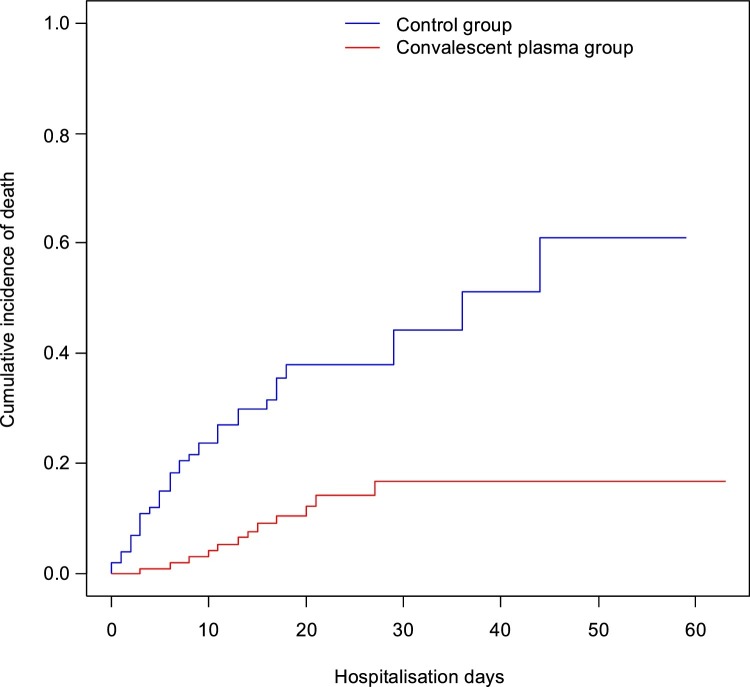
Cumulative death incidence for the entire study group (plasma + matched control group) at five days was 8.0%, CI95 (4.2%, 11.7%). By day ten, death rates had risen to 13.8% CI95 (8.8%, 18.5%). On day 20, the death rate was 24.4% CI95 (17.4%, 30.8%). The rate had risen to 36.8% CI95 (22.6%; 48.8%) by day 40 and, at the study's end (60 days), the death rate had risen to 76.3% CI95 (0.0%, 94.8%). The log-rank test confirmed statistically significant differences in the cumulative incidence of death between the plasma and no-plasma groups (p < 0.001) (Fig. 1 ).
Fig. 1.
Kaplan-Meier cumulative incidence of death in patients with SARS-CoV-2 with and without plasma treatment.
Cumulative incidence of death in patients with eliminated SARS-CoV-2 was 7.4%, CI95 (0.0%, 16.4%).
Parameters predicting death in SARS-CoV-2 patients were identified using logistic regression analysis (Table 3 ). Age was found to significantly increase the risk of death, based on both univariate and multivariate models (OR = 1.14 CI95 [1.09; 1.22], p < 0.001). Presence of cardiac insufficiency or active cancer significantly increased the risk of death, based on both univariate and multivariate models, respectively OR = 12.00 CI95 (2.81; 62.88), p = 0.001 for cardiac insufficiency and OR = 29.29 CI95 (6.08; 199.06), p < 0.001 for active cancer. Ventilator use also increased the risk of death, based on both univariate and multivariate models, OR = 267.96 CI95 (40.82; 3222.89), p < 0.001. On the other hand, application of plasma and hospitalization time in days were decreasing the risk of death, both in univariate and multivariate models, respectively, OR = 0.25 CI95 (0.06; 0.91), p = 0.041 for plasma application and OR = 0.90 CI95 (0.85; 0.95), p < 0.001 for hospitalization time. The presence of arrhythmia, diabetes, stay in ICU, and ventilator use length significantly increased the risk of death in univariate models only. Other variables (sex, hypertension, dialysis, SARS-CoV-2 elimination time) were not significant in the model. Multivariate model evaluation using χ2 test confirmed that all variables jointly are significant (p < 0.001). The R2 Nagelkerke coefficient was at a high level (75%), indicating the model's good quality. Additional assessment with Hosmer and Lemeshow GOF test (p = 0.527) also confirmed a good fit of the model to the data.
Table 3.
Logistic regression for death in SARS-CoV-2 patients.
| Univariate models |
Multivariate model |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI for OR | p | OR | 95% CI for OR | p | |
| Sex, female | 0.95 | 0.49 to 1.83 | 0.890 | |||
| Age, years | 1.06 | 1.04 to 1.09 | < 0.001 | 1.14 | 1.09 to 1.22 | < 0.001 |
| Convalescent plasma treatment |
0.30 | 0.15 to 0.60 | 0.001 | 0.25 | 0.06 to 0.91 | 0.041 |
| Hypertension | 1.48 | 0.77 to 2.89 | 0.241 | |||
| Arrhythmia | 2.70 | 1.22 to 5.86 | 0.012 | |||
| Heart failure | 4.12 | 1.83 to 9.32 | 0.001 | 12.00 | 2.81 to 62.88 | 0.001 |
| Diabetes | 2.23 | 1.10 to 4.48 | 0.024 | |||
| Dialysis | 0.90 | 0.13 to 3.88 | 0.897 | |||
| Recent stroke | n/a | n/a | n/a | |||
| Cancer disease | 4.82 | 2.10 to 11.23 | < 0.001 | 29.29 | 6.08 to 199.06 | < 0.001 |
| Ventilator | 16.90 | 7.28 to 42.54 | < 0.001 | 267.96 | 40.82 to 3,222.89 | < 0.001 |
| Intensive care stay | 6.83 | 3.36 to 14.24 | < 0.001 | |||
| Ventilator time, days | 1.12 | 1.05 to 1.22 | 0.003 | |||
| Hospitalization time, days | 0.93 | 0.89 to 0.96 | < 0.001 | 0.90 | 0.85 to 0.95 | < 0.001 |
| SARS-CoV-2 elimination time, days | 1.01 | 0.89 to 1.10 | 0.913 | |||
OR – odds ratio with 95% confidence interval.
As shown in Table 4 , plasma application time from the beginning of hospitalization was correlated with respiratory treatment time, strongly indicating earlier administration of plasma resulted in a ventilator being needed for a shorter length of time (Spearman's correlation ratio was 0.41 [p < 0.001], which is a moderate correlation). No statistical correlation was found between hospitalization time, mortality, and ventilator need depending on the plasma dose.
Table 4.
Correlation between plasma application time with the length of hospitalization and requirement of ventilator time.
| Plasma application time from hospitalization start |
||
|---|---|---|
| rs | p | |
| Hospitalization time | 0.41 | < 0.001 |
| Ventilator time | −0.05 | 0.609 |
rs – Spearman’s correlation coefficient.
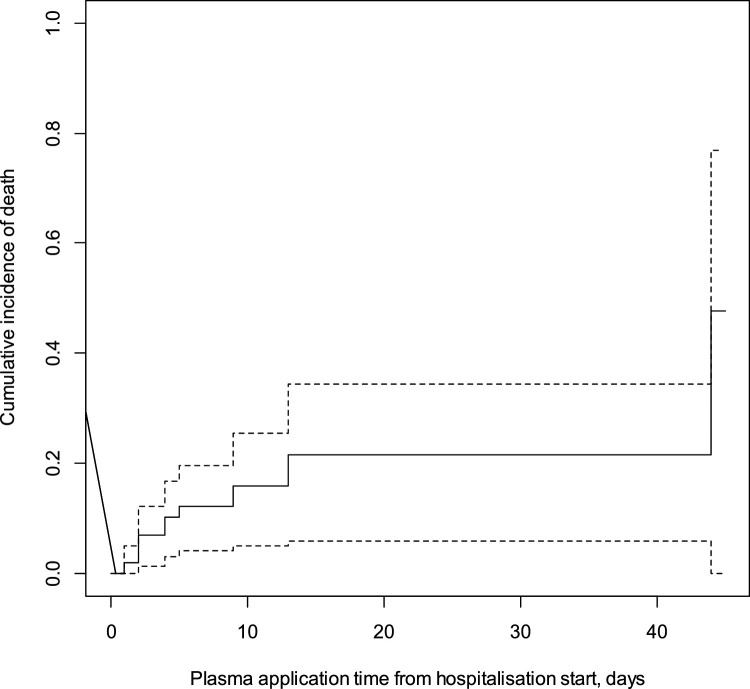
As shown in Fig. 2 , the sooner plasma is administered, the lower the cumulative incidence of death. At five days, cumulative incidence of death was 12.2%, CI95 (4.2%; 19.6%); at ten days 21.5%, CI95 (5.9%; 44.5%). During the study in total, the cumulative incidence of death was found to be 47.7%, CI95 (0.0%; 77.0%). Fig. 2 includes only patients from the plasma group. Its objective was to present survival depending on the timing of the 1st administration of plasma (Fig. 3 ).
Fig. 2.
Kaplan-Meier survival curve in patients with SARS-CoV-2 related to plasma application time from hospitalization start.
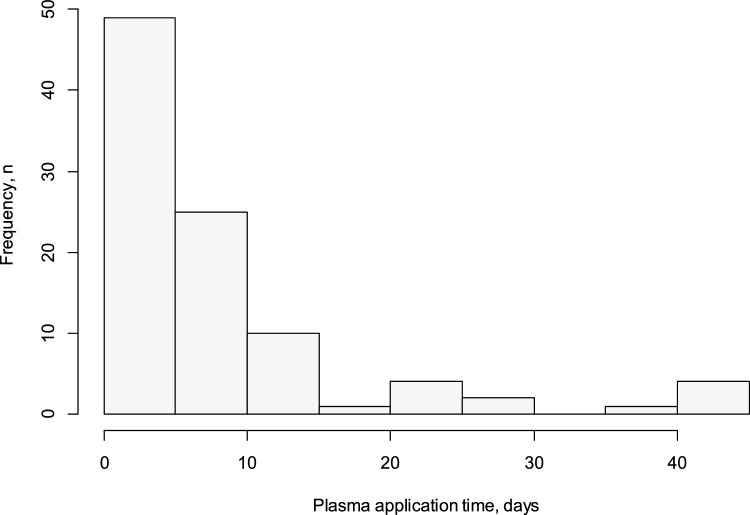
Fig. 3.
Histogram for plasma administration time (from positive COVID test to 1st plasma administration in days).
Donors
The mean level of NBas in the donor group was 142.75 (SEM ± 12.0057). Two of 44 donors had no NBas detected.
Discussion
This study compares mortality and other endpoints between patients treated with convalescent plasma (PG), and a propensity-score matched control group (CG) of patients hospitalized in the same medical facility and demonstrates a significantly lower mortality rate in the PG (13.7% vs. 34.3% in CG, p = 0.001). A recent randomized PlasmAr study (Simonovich et al., 2020) suggest no significant differences in mortality rate between patients treated with convalescent plasma and those who received placebo; however, baseline mortality in the untreated group in that study was 11.43% in comparison to 34.3 % in a similar group in our hospital.
The high mortality rate in our study is probably dependent on the high number of comorbidities among our patients in contrast to the PlasmAr study (35.2 % of patients with no coexisting conditions in the PlasmAr placebo group vs. 1.96 % in our study). In the tertiary center where our study was performed, patients at the highest risk of complications were also treated during a pandemic.
Our results suggest the benefits of convalescent plasma treatment in high-risk COVID-19 patients.
A recently published study from the Mayo Clinic showed that early administration of high-titer CP to infected adults reduced the mortality rate as well as the progression of COVID-19. Like our study, the benefits of CP treatment were associated with early administration (up to 72 hours from the onset of symptoms) (Ma et al., 2021).
The authors of the PLACID trial (Agarwal et al., 2020) did not establish a reduction in mortality or progression to severe COVID-19 in patients treated with convalescent plasma; however, most of the patients in this study experienced only mild or moderate symptoms (94.2% of all participants). In contrast, CP proved beneficial in a group of patients with severe or life-threatening disease in Mount Sinai Hospital, New York City (Liu et al., 2020a, Liu et al., 2020b); these patients experienced a significant reduction of oxygen requirement ratio and significantly lower mortality rate. It is worthwhile to mention that the sooner the plasma was administrated, the lower the cumulative incidence of death was observed in our study. On day five, for example, the cumulative incidence of death was 12.2% but, on day ten, the rate had escalated to 21.5%.
Hegerova et al. report a study of 20 patients treated by CP with severe or critical coronavirus disease that showed the best results when a transfusion was undertaken within the first seven days of hospitalization (Hegerova, 2020). Similar to our study, that study suggests that high-risk patients with comorbidities and severe COVID-19 symptoms receive more significant benefit from CP when administered in the early stage of hospitalization. We have shown that the mortality rate increases proportionally to hospitalization length, which further strengthens the argument for early treatment.
A trial of CP conducted in Wuhan, China (Li et al., 2020) showed no statistical significance of mortality rate (p = 0.3); however, interpretation of this finding is limited by early termination of the trial. It is worthwhile to mention that some studies of SARS-CoV-2's viral kinetics revealed that naturally reducing viral-antibody titers are seen seven to ten days after onset of symptoms in most patients (Liu et al., 2020a, Liu et al., 2020b, Zou et al., 2020). Nagoba et al. (2020) stressed that it is essential to administer CP before a humoral immune response for COVID-19. In the present study, no differences in ICU time and ventilator time were shown for either group. The tendency to minimize ventilator use in the PG was demonstrated; however, this value did not reach statistical significance. The small number of study participants in each group probably influenced this finding. For this study, a single unit of donated plasma measured between 200 ml and 300 ml. Recent trials in China increased the dose to a maximum of 2,400 ml (Ye et al., 2020). When outcomes of the two studies are compared, there is no significant difference between them. The maximum dose in this study was 600 ml, and we are suggesting this was sufficient since no statistical correlation was found between hospitalization time, mortality, and ventilator need in relation to the size of plasma dose.
In our study, contrary to various other publications (Abolghasemi et al., 2020), we were not able to demonstrate shortened hospitalization in the PG group. This finding is likely a result of significantly higher mortality rates in the control group when linked to shorter hospitalization time. Another influence could be that this study involved administering multiple doses of plasma; this dosing regimen started a median of 14 days after admittance to the hospital.
Early reporting from the United States (US) Food and Drug Administration suggests viral neutralizing antibody titers should be at least 1:160. A Chinese publication found that SARS-CoV-2 neutralizing antibody levels correlate with the severity of COVID-19 pneumonia (Chen et al., 2020). This finding could explain the high level of donor NBas in our study as all donors were recruited while hospitalized for COVID-19, therefore known to carry a moderate to severe viral load. Robiani et al. found 18% of donors with no neutralizing antibodies, whereas we found only 4.55%. With these studies in mind, it is noteworthy to consider routinely documenting the NBas level of all plasma donors; since the science of NBas kinetics is still in its infancy, it is perhaps imperative to document donor NBas levels at this time. Furthermore, donated plasma may be of enhanced value to plasma recipients when the plasma donor has personally experienced moderate or severe COVID disease.
This trial took place during the early days of the COVID-19 pandemic when there were no effective treatment options yet available. Now, enough time has elapsed that multiple studies on potential treatment options are in publication. Some studies involved comparing various potential treatments against each other, such as comparing CP outcomes to those of dexamethasone. In the Roback study, some patients received more than one therapeutic agent, for example, giving one patient both antiviral medications and steroids (Roback and Guarner, 2020). This approach makes it difficult to disentangle the specific clinical responses derived when multiple elements are compared in a study or to single out the beneficial properties of an individual treatment plan.
This study found CP administration to be safe; only one study participant experienced a side effect (mild and temporary skin rash). This finding is in keeping with a study done in the US that produced similar results (Joyner et al., 2020). Other adverse reactions reported in various studies include immunological reactions or increased risk of infection at the transfusion site; these reactions are typical responses to the transfusion of blood products, including plasma. As at the tertiary hospital, baseline mortality in patients during standard treatment was high, a finding that may be similar in other COVID-19 studies.
The primary limitation of this study was a lack of randomization, which was replaced by PSM analysis. The decision not to randomize in our study was based on ethical considerations at the beginning of the pandemic; however, this analysis's results were based on population groups of sufficient size and advanced statistics, as described in the methodology.
In summary, convalescent plasma transfusion seems to be a safe and effective therapy for SARS-CoV-2 infection in a group of high-risk patients. Moreover, our study's results suggest that early administration may be crucial for an optimal outcome. This study demonstrated that age, presence of cardiac insufficiency, active cancer, the requirement of a ventilator, and/or the length of hospitalization significantly increased the death risk in the studied group. Randomized prospective trials in the subgroup of high-risk patients are still needed.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding Source
The study was supported by The National Centre for Research and Development in Poland.
Ethical approval
Approved (agreement number 40/2020 of 03/04/2020).
References
- Abolghasemi H., Eshghi P., Cheraghali A.M., Imani Fooladi A.A., Bolouki Moghaddam F., Imanizadeh S. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., PLACID Trial Collaborators Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicenter randomized controlled trial (PLACID trial) BMJ (Clin Res Ed) 2020;371 doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto U., Head S.J., Angelini G.D., Blackstone E.H. Statistical primer: propensity score matching and its alternatives. Eur J Cardio-Thoracic Surg. 2018;53:1112–1117. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- Bonelli F., Sarasini A., Zierold C., Calleri M., Bonetti A., Vismara C. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Eng J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zhang J., Qin X., Wang W., Xu M., Wang SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depfenhart M., de Villiers D., Lemperle G., Meyer M., Di Somma S. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Internal Emerg Med. 2020;15:801–812. doi: 10.1007/s11739-020-02383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerova L. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136:759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer D., Lemeshow S. Second Edition. John Wiley & Sons, Inc.; New York: 2000. Applied Logistic Regression. page 95. [Google Scholar]
- Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Investig. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.K., Zhou B.P. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J. 2006;12:489. [PubMed] [Google Scholar]
- Kwon S.Y. Pathogen inactivation efficacy of mirasol PRT system and intercept blood system for non-leucoreduced platelet-rich plasma-derived platelets suspended in plasma. Vox Sanguinis. 2014:254–260. doi: 10.1111/vox.12158. Web. [DOI] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm Paul F., Ramsey Glenn, Kwaan Hau C. Passive immunity for coronavirus disease 2019: a commentary on therapeutic aspects including convalescent plasma. Semin Thromb Hemost. 2020;46(7):796–803. doi: 10.1055/s-0040-1712157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Tengfei, Wiggins C.C., Kornatowski B.M., Hailat R.S., Clayburn A., Guo W. The role of disease severity and demographics in the clinical course of COVID-19 patients treated with convalescent plasma. medRxiv. 2021 doi: 10.3389/fmed.2021.707895. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson P., Cozza A., De Silvestro G. The true historical origin of convalescent plasma therapy. Transfus Apher Sci. 2020;59(5):102847. doi: 10.1016/j.transci.2020.102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoba B., Gavkare A., Jamadar N., Mumbre S., Selkar S. Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID-19. J Infect Public Health. 2020;13:1818–1822. doi: 10.1016/j.jiph.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158:e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran K., Krishnasamy N., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020;(September):1475–1483. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;09:1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid L., Osman L., Arend S.M., de Groot S., de Boer M.G.J. Evaluation of a modified early warning score (MEWS) adjusted for COVID-19 patients (CEWS) to identify risk of ICU admission or death. medRxiv. 2020 Web, October 28. [Google Scholar]
- Strömer A., Grobe O., Rose R., Fickenscher H., Lorentz T., Krumbholz Diagnostic accuracy of six commercial SARS-CoV-2 IgG/total antibody assays and identification of SARS-CoV-2 neutralizing antibodies in convalescent sera. medRxiv. 2020;(June) Web. [Google Scholar]
- Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A. Therapeutic management of patients with COVID-19: a systematic review. Infect Preven Pract. 2020;2:10006. doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg K., Vermeulen M., Glatt T.N., Wasserman S., Barrett C.L., Peter J., Brittain D. COVID-19: convalescent plasma as a potential therapy. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2020;110:562–563. [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Eng J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Eng J Med. 2020;7:619–629. doi: 10.1056/NEJMoa2031304. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding D.J., Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26:1436–1446. doi: 10.1016/j.cmi.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92:1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Eng J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Viroly. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]