Abstract
Chitosan is a deacetylated polycationic polysaccharide derived from chitin. It is structurally constituted of N-acetyl-D-glucosamine and β-(1-4)-linked D-glucosamine where acetyl groups are randomly distributed across the polymer. The parameters of deacetylation and depolymerization process greatly influence various physico-chemical properties of chitosan and thus, offer a great degree of manipulation to synthesize chitosan of interest for various industrial and biomedical applications. Chitosan and its various derivatives have been a potential molecule of investigation in the area of anti-microbials especially anti-fungal, anti-bacterial and antiviral. The current review predominantly highlights and discusses about the antiviral activities of chitosan and its various substituted derivatives against a wide spectrum of human, animal, plants and bacteriophage viruses. The extrinsic and intrinsic factors that affect antiviral efficacy of chitosan have also been talked about. With the rapid unfolding of COVID-19 pandemic across the globe, we look for chitosan as a plausible potent antiviral molecule for fighting this disease. Through this review, we present enough literature data supporting role of chitosan against different strains of SARS viruses and also chitosan targeting CD147 receptors, a novel route for invasion of SARS-CoV-2 into host cells. We speculate the possibility of using chitosan as potential molecule against SARS-CoV-2 virus.
Abbreviations: ACE2, Angiotensin Converting Enzyme 2; COS, Chitosan oligomers/oligosaccharides; COVID-19, Coronavirus Disease of 2019; DP, degree of polymerization; DD, degree of deacetylation; FCV, feline calicivirus; GCH, Guanidinylated Chitosan Hydrochloride; HIV, Human Immunodeficiency Virus; HPV, human papillomavirus; HTCC, N-2-hydroxypropyl)-3-trimethylammonium chitosan chloride; MERS-CoV, Middle East respiratory syndrome-Coronavirus; RBD, Receptor Binding Domain; SARS, Severe acute respiratory syndrome; TMV, Tobacco Mosaic Virus
Keywords: Chitosan, Sulfonated chitosan, Quaternary amino group, Systemic resistance elicitation, SARS-CoV-2, CD147 receptor
1. Introduction
As antibiotic resistance and zoonotic disease outbreaks are getting pervasive as documented over the past 30 years, these diminutive pathogens are accounting for almost 70% of the infectious diseases with a scale of public health [1,2]. According to the WHO 2014 global report on antimicrobial resistance, it has been highlighted how tuberculosis and gonorrhea treatment with current antibiotics have been an absolute failure due to anti-microbial resistance and is becoming a great concern worldwide [3]. As a countermeasure, current drug development needs to regularly focus on discovery and research for other alternative antimicrobial agents. Marine polysaccharides are one of the most explored molecules for various therapeutics and biomedical applications. They have been in studies and are in use for decades now because they exhibit strong antimicrobial activities against abroad spectrum of bacteria, viruses and fungi [4] e.g. fucoidans extracted from brown algae found in the marine environment has shown anti- Human Immunodeficiency Virus-1 (anti-HIV-1) activity [5].
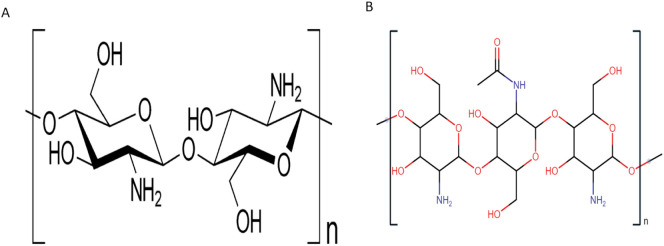
Out of numerous marine studied polysaccharides, chitosan has gained significant attention as a potent anti-microbial agent. Chitosan is a randomly deacetylated copolymer of β-(1-4)-linked d-glucosamine and N-acetyl-d-glucosamine (Fig. 1 ), derived from chitin which is the second most ubiquitous polysaccharide after cellulose and is present in the exoskeleton of various crustaceans, insects, and microfibrils of fungi [6]. Chitosan shows potent antifungal activities against deadly fungal strains like candida strains which contribute to a multitude of infections in humans, animals and bacterium species. Additionally, chitosan exhibits antibacterial action against E-coli(gram-negative) and Staphylococcus aureus (gram-positive) [7], and numerous other pathogenic bacterial strains [8] like Bacillus megaterium, Salmonella typhimurium, Pseudomonas fluorescens, Listeria monocytogenes, B. cereus, Lactobacillus plantarum, L. bulgaricus, L. brevis and Vibrio parahaemolyticus [9].
Fig. 1.
Chemical illustration of chitosan.
Chitosan and its derivatives are well-acknowledged as antiviral materials in the past. Previously S.N Chirkov has cited several research findings to support counter virus worth of chitosan and its various derivatives [10]. He spotlighted a peculiar characteristic of chitosan in terms of eliciting the molecular markers of Systemic Acquired Resistance (SAR) in plants and the same was later verified by Iriti et al [11]. Chitosan has shown to facilitate the expression of RNAse which was related to the induction of resistance to Potato virus X(PVX) infection, affecting viral replication [11]. Chitosan has been reported to elicit chemotaxis of macrophage and neutrophils along with stimulation of nitric oxide production in dog peripheral blood in-vitro suggesting stimulation of immune response [12,13]. Inhibition of Chlamydia trachomatis bacteria by chitosan in-vitro in HeLa cells line was reported [10]. In line with applications for therapeutics and biomedical uses, chitosan possesses properties of low allergenicity, biodegradability [14], non-toxicity [6], is economical to synthesize, and flexible to chemical modifications as has been documented over the years [8,[15], [16], [17]].
In concurrent to SARS-CoV-2 outbreak, this review explores and highlights similarity between the binding affinity of spike glycoprotein to Angiotensin-converting enzyme receptor-2(ACE 2) as a primary cell entry receptor for both Severe acute respiratory syndrome Coronavirus (SARS-CoV) and Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) [18,19]. Although studies are underway to completely comprehend the pathophysiology of SARS-CoV-2, yet inhibition of spike protein - ACE 2 receptors binding and cell fusion has been looked upon as potential approaches to treat SARS-CoV-2 infections directed by various computational modeling and molecular dynamic studies [[20], [21], [22]], and for this intervention, chitosan and its derivatives stand as a promising candidates as they are reported to bind with the spike protein subunits in in-vitro studies done on LLC-MK2 cell line and also in molecular modeling studies as discussed later in this review. Also, chitosan targeting a potential receptor molecule, CD147 having a crucial role in SARS-CoV-2 invasion has been discussed in this review. With a dearth of drug candidates to fight this new variant of coronavirus, we believe that this review will provide some new insights in this field and will underscore the antiviral potential of compounds derived from the marine ecosystem.
2. The Physico-chemical properties of Chitosan affect its antiviral activity
A gamut of factors may account for the anti-microbial efficacy of chitosan against abroad spectrum of microbes i.e. synthesis process (enzymatic, hydrolysis, etc.), conditions such as temperature, pH, degree of deacetylation (DD), degree of substitution (DS), molecular weight, deproteinization, concentration, and source of native chitin from which it is derived [9,15,23,24]. DD directly affecting solubility of chitosan in aqueous medium and its untimely effects on antiviral activity will be elaborated and discussed later in this review [25,26]. Extensive studies have been conducted to determine a correlation between antimicrobial activity and overall charge on chitosan. Chitosan as a native molecule has limited activity as an antiviral agent over a wide range of viruses, but substitution at the amino group and hydroxyl group can significantly alter its efficacy against various viral strains. Likewise, reports of sulfated chitosan have been shown to possess better antiviral property against HIV-1 and have provided better results in comparison to other derivatives due to similarity with heparin structure discussed in detail later in this review. Chitosan oligomers which are derived by hydrolysis of chitosan have also been reported for the wide-ranging antimicrobial actions by cell death or receptor binding inhibition. Moreover, the mechanism of action along with physico-chemical properties of chitosan derivatives differs, and is impacted by side-chain substitutions in concordance to chitosan itself [27] as mentioned in the review.
3. Chitosan and its derivatives combating plant viruses
3.1. Chitosan and its derivatives elicit resistance against plant viruses and their mechanism of action
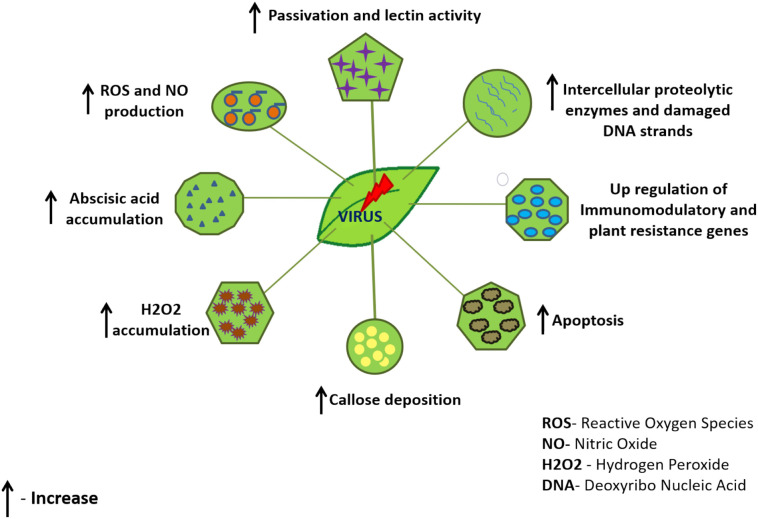
The direct inhibition mechanism of chitosan against plant virus replication has been rolled out throughout various reports and findings. Also, support for chitosan inducing systemic resistance has been eminent [11]. Various intrinsic factors like the impacted plant variety and sometimes, virus type determine the impact of chitosan to promote viral resistance in plants. Lectin proteins in plants serve as a protective barrier by showing their enhanced activity during biotic stress to develop host plant resistance through a complex signaling pathway. Chitosan as a response inducer of changes in lectin activity was studied in tobacco plant leaf discs and wild type potato tuber. It was noticed that pre-treatment with chitosan was able to slightly augment lectin activity and developed resistance in tobacco plant against Tobacco Mosaic Virus (TMV) (Fig. 2 ) [28]. The efficacy of chitosan to induce resistance in cucumber plant against Cucumber mosaic virus was evaluated. Chitosan was used as astandalone target molecule and in combination with glycine betaine. The results of the study indicated significant upregulation of defense genes, as demonstrated by gene expression analysis along with enhanced phytohormones, enzymatic and non-enzymatic antioxidants, and osmoprotectant protective mechanisms (Fig. 2). Conversely, the amount of oxidative stress indicator, malondialdehyde, and stress hormones, abscisic acid significantly declined. Furthermore, treatment with a combination of Chitosan and glycine betaine showed a superior effect than individual chitosan in terms of decreasing the infection by 90% and 84.21% respectively vs. the control group which was 95% affected. Also, a significant decline of disease severity to 2.88% and 6.22% respectively vs. the control group at 70% was observed along with uplifting of protective mechanisms of the host plant [29]. Chitosan caused an increase in intercellular proteolytic enzymes like RNases and proteases in pre-treated tobacco leaves leading to the fragmentation of the virions (Fig. 2). The study also underscored that the thinning of virions is caused by partial untwining of viral capsid subunits lowering the proteolysis of viral proteins exposed on the surface obstructing the binding capacity of virions and there was no evident toxicity recorded in this study [30]. Chitosan binding to plant cell surface receptors and stimulation of systemic resistance was also suggested to act as a cause and effect relationship. A research group concluded that chitosan binds to plant cell surface receptors and stimulates a defense response leading to the elicitation of systemic resistance. The mechanism of action was suggested to be through nitric oxide signaling via the production of a second messenger, phosphatidic acid-induced through phospholipase C in a coordinated effort with diacylglycerol kinase [31,32]. To underline the mechanism of chitosan induced viral infection resistance against Tobacco Necrosis Virus (TNV), it was suggested that chitosan induced apoptosis by a calcium-dependent mechanism which was directly time-dependent, triggering host defense system by acting as pathogen-associated molecular pattern molecule (PAMP) and activating the phospholipase-lipoxygenase pathway responsible for Reactive Oxygen Species (ROS) production. Calcium-dependent pathway, cause activation of callose synthase (leading to callose deposition which constraints cell to cell virus transport), Mitogen-activated protein kinases activation (MAPK), increase ROS and Nitric Oxide (NO) production which leads to a hypersensitive reaction at areas of attempted pathogen infection and activates hormonal signaling cascades (Fig. 2). Although many of these aforementioned mechanisms garnered support from high-impact studies yet some of them still face the fire of controversy [11,33]. Abscisic acid has also been noted to attribute as a major factor of plant-pathogen resistance mechanism as it was documented to elicit the accumulation of callose sites in palisade mesophyll of tobacco leaves. Since callose is part of systemic permeability in plasmodesmata and abscisic acid is essential for callose accumulation, the presence of chitosan not only help in eliciting the concentration of abscisic acid but also helps in forming a uniform network of callose sites which decreases the virus lesions sites in chitosan treated leaves affecting the viral spread from cell to cell (Fig. 2) [34]. Studies were also done to underscore the host immunomodulatory response at gene level induced by chitosan-N after inoculating papaya leaf with papaya leaf-distortion mosaic virus. The gene ontology analysis of differentially expressed genes demonstrated the potential of chitosan in upregulating plant disease resistance genes linked with pathways like starch and sucrose metabolism, phenylpropane biosynthesis and plant hormone signal transduction [35]. Altogether, these studies reflect that Chitosan promotes the induction of systemic resistance in plants by altering various signaling pathways.
Fig. 2.
Summarizing major protective mechanisms that plants adopt against plant viruses.
3.2. Factors affecting antiviralactivity of chitosan
The physico-chemical properties of chitosan such as 1) molecular structure, molecular mass, DD, 2) Degree of polymerization (DP), achieved by depolymerization methods such as enzymatic or chemical hydrolysis concentration, 3) plant variety, and in some cases virus type as discussed above for mechanism of action [11], significantly impacts viral resistance, as the receptor structure and function differ in different plant varieties with varying affinity to chitosan discussed above. Studies have elaborated that relatively small molecular mass (17 to 2 kDa) derivatives of chitosan obtained after chemical hydrolysis with hydrogen peroxide showed high antiviral activity as reflected by the reduction in the number of local viral necroses on tobacco leaves by 50–90% [36]. A study by Kulikov et al. demonstrated that antiviral activity on bean mosaic virus was impacted by the average molecular weight of chitosan fragments and method of hydrolysis used to deacetylate chitosan. There was significant inhibition of the viral spread on the inoculated bean plant with low molecular weight chitosan [37]. Furthermore, a related study on TMV infection reduction by a chitosan derivative Guanidinylated Chitosan Hydrochloride (GCH) showed that when half of the leave of N. glutinosa was pre-treated with GCH, the average local viral lesions count significantly declined by 84% in comparison to the use of chitosan alone where only 36% count decrease was observed suggesting initiation of viral resistance by GCH derivative. In the same study, Chitosan has also been shown to damage viral DNA strands in in-vitro studies because of its cationic property and high affinity to the nucleus(Fig. 2) [38,39]. Also, there was a correlation observed between the duration of exposure and the presence of viral count to a certain extent. The shorter span of GCH exposure and concentration of 2 mg/l has shown higher efficacy for infected leaves in producing systemic resistance against TMV infection. Also, the time exposure and antiviral efficacy were correlated to viral count present to a certain extent. GCH has also shown passivation activity against TMV which was also dependent on the time of exposure in-vitro (Fig. 2). Moreover, GCH showed direct killing activity by damaging the viral structure which was observed as flocculation in electron microscopic photographs [40]. Along similar lines, a study conducted by Noiket et al., treated a Thai variety of tomato plant Sridathip 3 which grows mostly in the Thailand peninsula and infected by Tomato yellow leaf curl virus diseases (TYLCV) with chitosan. During and after 25 days of the plant growth, it was grafted to an infected plant and viral aggregation was estimated by Enzyme-Linked Immunosorbent Assay (ELISA) monoclonal antibody test. A significant reduction of TYLCV irrespective of the concentration was observed [41]. Likewise, to get rid of the viruses like Papaya ringspot virus-W and Tomato chlorotic spot virus affecting the vegetable yield of cucurbits and tomatoes, chitosan was combined with different strains of Rhizobacteria, a plant growth promoter. The combination showed phenomenal infection inhibiting activity against both the viruses at the high dose of chitosan in a time-dependent manner. However, high chitosan concentration showed phytotoxicity in plants [42]. To recapitulate, chitosan alone or in combination has shown potent antiviral activity against many different plant viruses and its physico-chemical properties greatly influences this activity.
4. Chitosan and its derivatives in the battleground, defeating bacteriophage viruses
4.1. Chitosan and its physico-chemical properties affecting its antiviral efficacy against phage infections
The impact of physico-chemical properties of Chitosan on coliphages T2, T4, and T7 inactivation was studied by synthesizing its various derivatives differing in terms of the DP, the net charge, and the number of amino groups. Different phage strains showed different inactivation abilities in the presence of chitosan fragments, but it varied in a dose-dependent fashion except for the T2 page. Virulence of T2 Phage relied on the degree of polymerization, but no specific pattern was observed. With the same degree of polymerization, unmodified chitosan showed more activity than deaminated derivatives. Also, a model based on a decrease in anti-phage activity with a decrease in amino groups was observed. Conversely, anionic derivatives with a net negative charge showed a reduction of activity. Electronic microscopy pictures of T2 phage after treatment with chitosan fragments reflected distinct structure alterations in terms of the absence of long-tail fibers, contracted tail sheaths, and deformed basal plate [43]. Inhibition of phage virulence was reported at low DP due to absorption of chitosan in the bacterial cell wall whereas, in contrast, inhibition of phage infection at higher DP was due to decrease of phage bacteria replication. It was also concluded that inhibition of phage reproduction and inhibition of phage infection remained independent of the degree of deamination but were dependent on the concentration of chitosan [44]. An inverse relationship between the DP and concentration was noted for inhibition of phage infection i.e. lower DP and higher concentration had the best results whereas, in contrast, degree of deamination was not related to the inhibition property. Overall, low DP, high concentration, and deamination provided the best yield for Phage infection inhibitions, but it was speculated that inhibition was caused due to the antibacterial property of chitosan [45]. For phage viruses like MS2 and phi X 174, Davis et al., demonstrated the remarkable decrease in phage infection at different concentrations and molecular weights of chitosan for each of the phages in support of previous studies done before to emphasize the fact that overall charge on viral surface glycoproteins and charge on chitosan polymer affect the antiviral efficacy of chitosan. A notable decrease in MS2 virus by 5.2 logs and 2.6 logs (pfu)/mL) at a concentrations of (1% and 0.7% w/v respectively) at 222 kDa were recorded vs. 2.8 logs and 2.6 logs (pfu)/mL) at 53 kDa [46,47]. Although more considerable attention is given to pH and molecular weight, a study done by Chatain et al., suggested that different bacteriophages capsid arrangement and other physico-chemical factors can cause a greater difference in the antiviral activity of chitosan on different phage viruses. It was observed that the effect of concentration and pH was not significantly distinguishable for c2 bacteriophage in contrast to MS2 [48]. Similar studies done on MS2 bacteriophage by Wilson et al., for viral reduction and removal showed evidence of the role of overall charge, DD, substitution on the side chain, and molecular weight of chitosan macromolecule affecting its antiviral activity. Spot- plate method showed evidence of no viral lysis and these results were consistent with previous studies done. Also, the coagulation property of chitosan enables the overall antimicrobial property of modified chitosan in line including its surface charge and available surface area [49].
4.2. Suggested and proposed mechanism of action for antiviral activity
A comprehensive study enlisting antiviral activities by chitosan on various types of viruses cited some interesting reports to decipher the possible mechanism of chitosan antiviral activity. A decrease in the yield of such infectious gene containing phages was observed and chitosan binding to smaller peptide fragments in anagar medium was suggested. Oligomer reported better inhibition for phage infections unlike anionic sulfate substituted groups which were ineffective against phage infection in contradiction with other viruses [50]. Positive charge on chitosan amino groups was cited to be a major contributor for the overall cationic property of chitosan and was suggested to be responsible for inactivation of phage infection and it was valid for plant viruses too [51,52]. Three major mechanisms were cited to be responsible for chitosan antiviral efficiency against phage infections. 1) Cell death of phage bacteria 2) decrease in the virulence of daughter bacteriophages 3) inhibition of reproduction of virulent phage viruses. It was concluded that the bactericidal effect was not a contributor to the inhibition of the phage infection mechanism. It was cited that chitosan has transpired the morphological damage in virions in support of the second suggested mechanism, but it is still not ostensible which stage of replication chitosan affects in phage infection [10].
5. Chitosan and its derivatives giving a tough fight against coronaviruses
5.1. Correlation between spike glycoprotein of coronaviruses and role of ACE 2 receptor and spike glycoprotein in cell entry
The virulence of the SARS-CoV virus is primarily attributed to the ability of its spike protein to bind the ACE-2 receptors present in the human respiratory tract and initiate infection [21,22,53]. Cryo-electron microscopy images have revealed the structure of spike protein as a homotrimeric peptide with theS1 and theS2 subunits in each monomer. Cryo-electron microscopy performed for spike protein in its prefusion state at 3 Å resolution put forward some insightful observations. It was noted that one of the three Receptor Binding Domains (RBDs) of S1 subunit of spike protein stays in up-conformation (state of instability and receptor accessible position) which is coveted for receptor binding. As soon as the receptor binds to it, the entire receptor binding domain undergoes hinge like movement leading to augmented instability and turning all the three RBDs in to up-conformation. Following this, the whole S1 subunit out of sheer instability sheds apart leading to refolding of S2 subunit [54]. This stimulates the S2 subunit to acquire a stable state required for membrane fusion which subsequently results in virus introduction into the host cell [21,22,55]. The Receptor Binding Domain (RBD) of theS1 subunit comprises a core and a receptor-binding motif, the latter one being highly desired for specific recognition and strong binding to ACE2 receptor (Fig. 3A) [56]. It has been observed that SARS-CoV and SARS-CoV-2 both use human ACE2 receptor as a port of entry to gain access to host cells (Fig. 3A). However, the binding affinity of the spike protein's RBD of latter virus to hACE2 is more but the overall interaction of its spike protein with hACE2 is low compared to the former virus type. These results were generated from a protein pull-down assay, using cell surface-expressed SARS-CoV-2 and SARS-CoV spike proteins as the targets and recombinant hACE2 as the bait. The paradox here pertains to the dynamic state of RBD and also, emphasizes the role of human proteases in dismantling the spike protein and exposing the receptor-binding domain. This study also points towards the deliberate evading of immune surveillance by the hidden receptor binding domain of SARS-CoV-2 [57]. Upon analyzing the crucial residues of receptor binding motif (the highly variable region of RBD of SARS-CoV-2) using sequence alignment approach and high resolution crystal structure analysis, it was found that nine single amino acid substitutions at Q498, S459, P499, Q474, N501, E484, K452, T470 and R439caused a loss of binding affinity of RBD for human ACE2 receptors. Six of them are already validated [55,56,58] and three new mutations (S459, P499, Q474) were observed in this study. Conversely, it was noteworthy that five single amino acid substitutions at Q493, P499, A475, F486 and L455 favored the binding. All these highlighted residues play direct or indirect role in virus binding to the receptor and subsequent stabilization. By leveraging single amino acid substitution mutagenesis scanning, some stark differences were concluded in the antigenic potential of RBD of SARS-CoV and SARS-CoV-2 viruses owing to different amino acid composition [59]. Analysis of Bioinformatics data also showed evidence of similarity between the spike glycoprotein gene sequences of SARS-CoV and SARS-CoV-2 [60]. The use of similar receptors for cell entry by SARS-CoV and SARS-CoV-2 was confirmed by Hoffmann at the cellular level [61]. A comparative study on the cellular tropism of NL63-spike protein-bearing pseudovirions with SARS-CoV-spike protein-bearing pseudotypes concluded that both use ACE2 for cell entry and infection. However, the binding affinity to ACE2 varied wildly because the alignment of the ACE2-binding site of SARS-CoV with the most closely related sequence in NL63-S had only 14% amino acid similarity, which makes it harder in getting one definite answer to CoV family infections. These variations in the amino acid residues involved in binding interactions may reflect different strategies evolved by difference strains of corona viruses to penetrate human cells, sometimes by binding to different regions of the receptor or using different amino acid residues in receptor recognition [62].
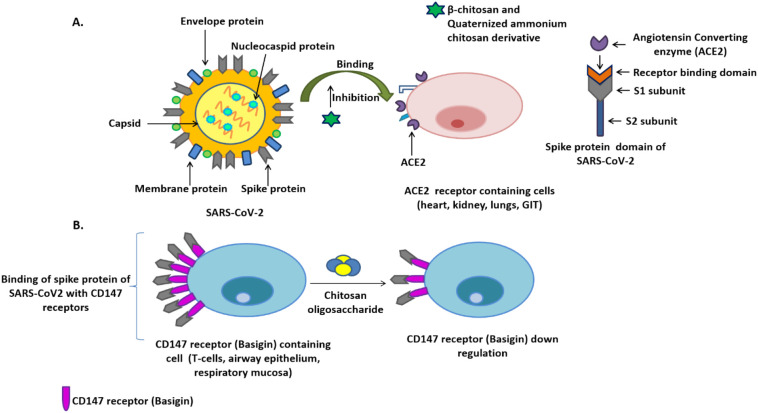
Fig. 3.
A) Proposed β-chitosan and quaternized ammonium chitosan derivatives preventing the SARS-CoV-2 infection by inhibiting the binding of spike protein to ACE2 receptors (recognized as primary receptor for SARS-CoV-2 binding). Alongside, interaction of ACE2 to receptor binding domain of SARS-CoV-2 has been displayed, B) Spike protein binds to the CD147 receptors, evolving as new route for virus invasion into the host cell. Chitosan oligosaccharide has been reported to downregulate the CD147 expression level which is witnessed as potential strategy for reducing the viral infection curve
5.2. Present antiviral derivatives of chitosan against different strains of coronaviruses, mechanism of action and future scope
As discussed above, molecular classifications of Human Coronavirus have suggested some structural similarity in spike protein alongside ACE2 being the choice of receptor to attach to the host cell and causing respiratory illnesses being a trait common among the corona viridae genus including SARS and SARS-CoV-2 [63]. Milewska et al., studied the inhibition factor of N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC) polymer, a quaternized chitosan, and its hydrophobic derivative. It was observed that these polymers had a significant inhibition factor for human coronavirus, HCoV-NL63 and human murine virus. Extensive study to fathom the mechanism of inhibition reflected that the polymer made a complex with the ectodomain of the spike protein of HCoV-NL63 without causing significant levels of cytotoxicity. It was also suggested that inhibition is only caused due to site-specific binding [64]. Later, a study based on the effect on viral entry inhibition by HTCC polymer in LLC-MK2 cell line delivered some interesting insights of the polymer inhibiting the interaction between cell receptors and spike protein by aggregation of spike proteins as one of the mechanisms of action for blocking its cell entry in support of his previous studies. Also, it demolishes the co-localization of ACE-2 receptors by virions. The range of the human coronavirus (HCoV-OC43, HCoV-229E,HCoV-NL63and HCoV-HKU1) infections being impeded was varied for different degrees of substitution. Another noteworthy result was inferred that closely related viruses required various degrees of substitution which was a peculiar trait observed concerning this moiety activity [65]. Later, a subsequent study was done on the antiviral efficacy of HTCC was determined in pathogenic coronaviruses, Middle East respiratory syndrome-Coronavirus(MERS-CoV), and SARS-CoV-2 through in-vitro studies using Vero and Vero E6 cell lines and ex-vivo studies by deploying upon human airway epithelium (HAE) model. The results of the survey reflected the potential of HTCC in hampering virus replication with more pronounced effects in SARS-CoV-2. It also significantly inhibited the internalization of viruses into vulnerable cells by reacting with spike protein through electrostatic interactions [66].
A recent molecular dynamics study by Kalathiya et al., underscored the tremendous potential of chitosan in having a strong binding affinity for homotrimer pocket and RBD of spike protein which can be further exploited to design chitosan based drugs and vaccines for attenuation of spike protein and disrupting virus binding to host receptors [67]. However, they could not validate their results by comparing them with those coming from a cryo-electron microscopy or crystallized structure of other structurally related proteins. Chitosan and its oligomers were cited to be able to inhibit ACE-1 activity too [68]. In a study to comprehend the role of β-chitosan in inhibiting the binding of SARS-CoV-2 spike protein RBD with ACE2 along with underlying mechanisms, some interesting insights were drawn. The native-PAGE analysis demonstrated that β-chitosan strongly binds to the ACE2 and spike-RBD under normal physiological conditions and further addition of β-chitosan silenced the binding of spike-RBD and ACE2. In-vitro analysis indicated downregulation of ACE2 levels by β-chitosan in Vero E6 cells, further diminishing the probability of spike-RBD binding to ACE2 and a significant reduction in the colocalization of RBD and ACE2 as was witnessed by dwindling immunofluorescence intensity of 6.91% against 33.6% when ACE2 and the spike-RBD were colocalized in β-chitosan's absence. Furthermore, addition of β-chitosan also exerted an anti-inflammatory effect in cells by reducing the expression of proteins surrounding Spike-RBD binding induced cell inflammation. In the in-vivo model when β-chitosan was intranasally administered, β-chitosan prevented lung morphological changes produced as an after-effect of SARS-CoV-2 binding with ACE2 receptors, downregulated ACE2 expression and inflammation, and blocked the binding of spike-RBD to lung cells harboring ACE2 receptors. The downregulation of ACE2 expression was attributed to the activation of ADAM17 metalloproteinase (Fig. 3A) [69].
Along similar lines, cross-linking of chitosan with genipin, a plant originated compound was done to obtain nano/micro spheres of N-(2-hydroxypropyl)-3-trimethyl chitosan. These spheres were evaluated for their antiviral efficiency in two strains of coronavirus namely human coronavirus NL63 (HCoV-NL63) and human coronavirus HCoV-OC43. The nano/micro spheres perfectly adsorbed former strain with high selectivity which led to plummeting virus infective potential by (7.2 ± 0.8) × 106 copies/ml, which accounts for 92% of virions but failed to show same activity with the later. The mechanism of action was elucidated to be electrostatic interaction between viral nucleic acid and the sphere complex. The ability of virus desorption from the adsorbent surface was found to be dependent on the cationic strength of the polymer complex [70]. A very recent opinion on the probable use of positively charged chitosan nanofibers in Personal Protective Equipments (PPE) against rising SARS-CoV-2 infection in healthcare providers bolsters the ambit of using Chitosan in this direction. This study centers on exploiting the electrostatic repulsive forces of Chitosan to reduce the viral load. It also touches upon a few quaternized chitosan derivatives like N,N,N-trimethyl chitosan (TMC), the single N-quaternized (QCS) and the double N-diquaternized (DQCS) as efficient molecules in this regard due to the presence of abundant cationic amino groups which can provide better viral repulsion results [71].
5.3. Another potential receptor for SARS-CoV-2 invasion and the potential role of chitosan oligosaccharide in targeting the same
The exploration of other potential receptors for SARS-CoV-2 binding shows that the spread of COVID-19 virus is not limited to ACE-2 expressing cells and it quickly gains access to innate and adaptive immune cells of the lungs and in the periphery, which do not express ACE-2 receptor. An exciting study highlighted the role of a transmembrane receptor, CD147 (also called basigin) in mediating SARS-CoV-2 infection to T cells and cells of epithelial origin in-vitro. The same receptor has shown cardinal importance in facilitating infection in HIV-1 and measles virus [72]. A study based on Gene profiling analysis reflected the abundance of CD147 receptors in airway epithelium and respiratory mucosa, strongly supporting the involvement of a variety of receptor proteins in mediating SARS-CoV-2 initial infection (Fig. 3B) [73]. A research-oriented study by Wang et al., is imperative in understanding the novel route of attacking CD147 receptor adopted by SARS-CoV-2 for invasion. Usage of monoclonal antibody against CD147 for its blockage has shown responsive dropinCOVID-19 infection in-vitro in Vero cell lines. This study suggests the interaction between the receptor-binding domain of spike protein of SARS-CoV-2 and CD147 for invading host cell [74]. With the same foundation, the clinical trial was conducted to estimate the safety and efficacy of a specifically targeted monoclonal antibody, Meplazumab against CD147 receptor to treat COVID-19 infection in patients. The results were very promising with patients recovering from COVID-19 associated pneumonia [75]. This draws attention to exploring CD147 targeting as a potential treatment option in tackling COVID infection. However, more investigation in this direction is demanded before something concrete can be concluded. Inflammation caused by cytokine storm is a cornerstone in the development of COVID infection. A study based on the involvement of immune cells in aggravating rheumatoid arthritis (RA) condition spotlighted the indulgence of CD147 receptors in causing local inflammation and bestowing the chemotactic ability to CD4+ and CD161+ expressing T-cells. CD147 interacts with CD98 for regulating T cell activation and function. Patients with RA expressed high levels of CD147 receptors on T-cells and a blockade of these receptors not only significantly reduced the local inflammation but also led to a remarkable decrease in the chemotactic ability of T-cells to the inflamed area [76]. Study done by Varadarajan et al., underscored the potential of CD147 receptor in binding toSARS-CoV-2 virus and facilitating its entry into the host cell. The results showed that upregulation of CD147 receptor in oral squamous cell carcinoma patients made them highly susceptible to COVID-19infection. The binding of the spike protein of coronavirus to CD147 receptor leads to its exhaustion and subsequent reduction in carcinogenesis [77]. Chitosan oligosaccharide has been shown to downregulate the expression of CD147 receptors in gastric cancer patients and thereby, reducing metastatic potential [78]. This study underscores that chitosan and its derivatives can be potential molecules in confining the spread of COVID-19 viral particles to immune cells, expressing CD147 receptors and demands further studies (Fig. 3B).
6. Chitosan and its derivatives standing strong against several human viruses
6.1. Correlation between heparin sulfate like structure and CD4+ receptor for anti-HIV-1 activity of sulfate and sulfonated derivatives of chitosan
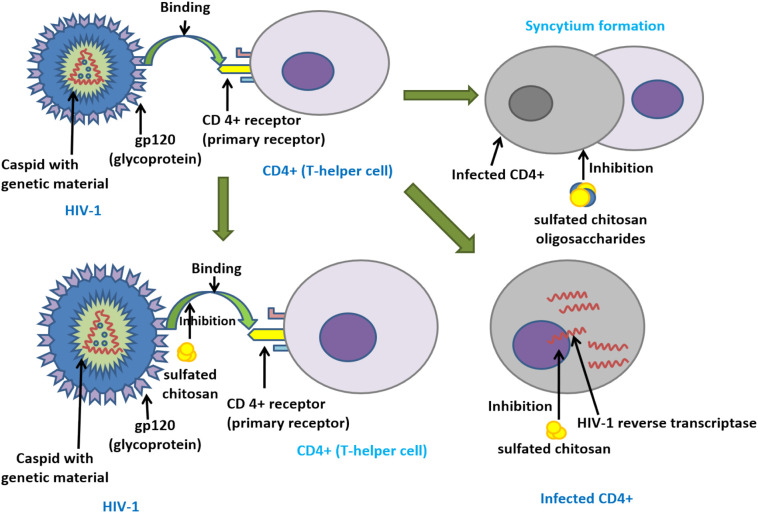
Several studies have suggested the Heparan sulfate binding receptor (GAGs)as the primary non-selective cell binding receptor for cellular entry and pathway mediator, by different viruses such asHIV-1, Sindbis virus, Herpes simplex virus, Japanese encephalitis virus, human cytomegalovirus, and several other retro, alpha, parvo, flavor, picorna, and papillomaviruses [[79], [80], [81], [82], [83], [84], [85]]. A study on Moloney murine leukemia virus having Heparan sulfate receptor inferred that sulfate substitution on succinic acid of succinyl Chitosan backbone showed the competitive inhibition effect in comparison to positive control dextran sulfate among other Chitosan derivatives, when studied on SC-1 and NIH-3T3 cell lines. The study also inferred that higher the molecular weight and degree of substitution, the greater will be the antiviral efficacy [86]. A study done by Artan et al., on inhibition of HIV-1 strains by sulfated chitosan oligosaccharides suggested that adding sulfation of amine group, and hydroxyl group can increase anti- HIV-1 activity at a lower molecular weight (MW 3–5 kDa being the most effective) and concentration. Sulfated chitosan oligosaccharide showed an EC [50] as 2.19 μg/ml, and lytic effect (EC [50] 1.43 μg/ml)) against HIV-1 virus. Also, data demonstrated that sulfated chitosan oligosaccharides inhibit the infected cell to cell fusion or syncytia formation and receptor interaction between HIV-1 strain and host cell receptor CD4+ (Fig. 4 ) [87]. Further studies indicated that sulfate substitution on the chitosan backbone. Nishimura et al., performed a comparative study with the addition of sulfonic groupsatan amine groupofcarbon-2 (C-2)&hydroxyl groupofcarbon-3 (C-3) versus Carbon-6 (C-6). The results derived with the addition of sulfonic group at C-2 and C-3 showed maximum inhibition compared to only atC-6, also it was observed that the addition of sulfonic groups at the hydroxyl groupofC-6 had no significant role in theanti-HIV-1 activity. They have suggested that theanti-HIV-1 activity of sulfonic groups at C-2 and C-3 is due to interaction with GP120 envelope glycoprotein as they have shown anticoagulant property whereas substitution atC-6 had non-specific ionic interaction with blood coagulating factors [88]. This argument of non-specific ionic interaction of sulfate substitution was supported by a study determining anticoagulation activity of sulfated chitosan, which was undertaken by Yang et al., by performing a coagulation assay of thrombin time (TT), prothrombin time (PT) and activated partial thromboplastin time (APTT),having sodium heparin as referral standard. In the APTT and TT assay, it was observed the anticoagulation activity of sulfated chitosan was dependent on concentration, molecular weight and degree of sulfation and it was inferred that sulfate substitution of chitosan only affected the intrinsic mechanism of coagulation. The ionic interaction was investigated as potential reason behind surface charges on sulfated chitosan and binding potential with cell receptors, in support of previous studies done on the same [89]. A sulfated derivative of Chitosan, N-carboxymethyl chitosan-N-O-sulfate was tested for its capacity to stop HIV-1 infection in CD4+ cells. It blocked virus binding to host cell receptors in CD4+ cells, virus adsorption on host cell and also participated in competitive inhibition of reverse transcriptase enzyme. The mechanism of action was attributed to its high and localized negative charge along with ampholytic nature. Dose-dependent inhibition was observed without any significant toxicity of chitosan anionic derivative to the host cell [90]. An article published by Jayakumar et al., comprehensively captured the anti-HIV-1 activity of sulfated polysaccharides like chitosan and its derivatives. They have mentioned the mechanism of action as inhibition of viral replication and blocking virus binding to host receptor, CD-4 (Fig. 4). It also touched upon the site selectivity by referring to the superior anti-HIV-1 activity of these polysaccharides with sulfation at Oxygen-2 and/or Oxygen-3 sites [91].
Fig. 4.
Underscoring the molecular mechanism behind antiviral activity of sulfated chitosan against HIV-1. Binding of GP120 receptors to the CD4+ receptors of T-cell is shown to be inhibited along with disruption of HIV-1 reverse transcriptase enzyme. Conversely, sulfated chitosan oligosaccharide has been shown to hamper the syncytium formation (merging of infected cell with neighboring healthy cells to give rise to multinucleate giant cell) of CD4+ cell infected with HIV-1 virus. This reduces virus replication and further infection.
Besides, Dimassi et al., have summarized various physico-chemical properties and substitution position of chitosan in a wide range of biomedical applications and new trends. Authors highlight that sulfonation of chitosan at various sites of free amino groups and/or at hydroxyl administers a better antimicrobial activity similar to heparin or other sulfated glycans which binds through different intra- and extracellular pathways inhibition mechanism. It also cites previous study done by Sosa et al. [90] based on using the process of random sulfonic substitution and determining inhibition potential of HIV-1 reverse transcriptase and CD-4 receptor binding along the line as discussed above. The paper also stipulates that the immune factor stimulation by sulfated chitosan through nitric oxide production in dose dependent manner has been comprised [92].
6.2. Other derivatives of chitosan showing anti –HIV-1 activity
Modification of low molecular weight chitosan oligomers (COS) with tripeptides like tryptophan (W), glutamine (Q) and methionine (M)in different combinations was done to determine their potential in reducing HIV-1 infectivity. The results highlighted a significant inhibition of syncytia formation, cytopathic effect, and viral replication along with downregulating levels of p24 antigenic protein visible on virus affected cells, envelope protein and viral infection factor proteins of virus and thus, reflecting a potent antiviral activity against various HIV-1 strains. QMW-COS effectively inhibited the host cell-virus interaction by altering the binding process. However, the effect was weaker for WMQ-COS [93]. Polyelectrolyte nano-complexes of chitosan and hyaluronan stabilized with Zinc (II) were evaluated for their synergistic effect when they were loaded with a very potent anti-HIV-1 drug, Tenofovir. When internalized inside human peripheral blood mononuclear cells infected with HIV-1, these nanocarriers caused considerable reduction in viral infection by limiting capsid p24 protein production [94].
6.3. Chitosan derivatives against other viruses and their mechanism of action
Studies reviewed by Ishihara et al., done on modified chitosan moiety for effectiveness against influenza virus A put out interesting insights [95]. One of them was a study done by Mori et al., centered around the use of chitosan silver nanoparticles composite nano-fiber sheet designed to trap virions and making them immobilized for better virucidal actions of silver nanoparticles against influenza virus A pertaining to the enhanced surface area provided by chitosan silver nanoparticles composite nano- fibersheet [96]. One similar review analysis was done on the antimicrobial activity of microbial polysaccharides by Smelcerovic et al., who cited the use of chitosan as an adjuvant that helped to boost the immuneresponse against influenza virus (Texas H1N1) and Panama virus when introduced with antigen vaccine in a mouse model [68].
Selectors modified Chitosan for inhibition and trapping of influenza viral particles was studied by Li et al., and derived some critical results. According to the study, the introduction of sialyllactose on chitosan moiety has shown a high affinity to bind with influenza viral surface resulting in blocking of host cell receptor interaction with viral surface in comparable levels to influenza virus blockers whereas, sialyllactose alone had no binding affinity to the viral surface. Degree of substitution has been a significant contributor to its efficacy as higher the replacement, higher was the inhibition potential [97]. Further, Cheng et al., synthesized chitosan sialyl oligosaccharides complex in support of the study done by Li et al., and found that this complex has a high potency of blocking cell receptor interaction with influenza viral glycoprotein for viral entry [98]. To explain the inhibition mechanism, it was suggested that glyco cluster formation disrupts viral adhesion to the cell surface as a previously studied phenomenon of glycoside cluster effect on glycan-protein interaction by Collin & Paulson et al., and Lee et al. [99,100].
Influenza virus infectivity inhibiting effect of chitosan was documented when intra nasal administration in mice model was studied against rapidly evolving strain of new Avian Influenza A virus, H7N9 virus which is one of the culprits behind virus-related various respiratory tract infections. The study reported a dose-dependent protective effect and survival rate directly proportional to the chitosan concentration (as survival rates started to dwindle with lower amounts of chitosan (30 μg or 10 μg)). They also observed a significant reduction in virus load and augmented inflammatory markers and leukocyte infiltration in lung/trachea tissue. Notably, the protective immune responses lasted for ten days. However, changing the route to intraperitoneal protected only 10% of animals even at the highest dose of chitosan (100 μg). Comparatively, a low protective effect was recorded against PR8 strain of influenza virus unlike H1N1 and H9N2 strains where full-fledged protection was seen [101].
Although metal ions have higher affinity binding with proteins, the study done by Lin et al., showed that type of protein residue, position, and pH affects their binding affinity. Also, it was observed that nickel ions had the best binding affinity to enterovirus-71 in the presence of chitosan as Ni2+ has six available coordination bonds. Chitosan chelating with nickel ions provided a greater chelation potential to Ni2+ with the exposed protein of enterovirus-71 and also provided more excellent stability of the bond formation between them concerningNi2+ and protein binding [102].
During a study conducted by Paul T as his scholarly work, antiviral action of chitosan on an adenovirus which causes respiratory ailments in younger children derived some exciting results. 0.1% chitosan treated infected NIH-3 T3 cell line of mouse embryo tissue with green fluorescent protein (GFP)-adenovirus, exhibited the best decline in fluorescence with respect to positive control group and to confirm the same, flow cytometry was done and the results were consistent. Also, 0.1% chitosan showed the best cell viability with respect to other concentration of 0.5 and 1 [103].
HPV has been one of the prominent causes of Sexually Transmitted Diseases (STD) and with vaccines being of high cost and lower economic background countries being susceptible groups at higher risk, alternative methods to prevent viral transmission are always looked into. As sulfonated chitosan has previously shown antiviral activity so, Gao et al., studied the substitution of H+ at 3 and 6 carbon of chitosan by sulfate groups and their study projected some interesting results. The modified chitosan, 3,6-O-sulfated chitosan (with58.3 kDa as average molecular weight and the sulfate content 45.8%) was able to inhibit a broad spectrum of HPV Pseudovirus (HPV PV). The inhibition was dose-dependent and chitosan binds with HPV PV capsid for the early stage of replication with a good selectivity index (CC50/IC50 = 1222.3), preferably at low pH conditions (pH = 5). Other observations on HeLa cell line showed a long incubation time required by sulfated chitosan to gain entry into the host cells while host cell surface binding happening immediately. While studying the mechanism, it was observed that the expression of L1 capsid protein was remarkably reduced which suggested cellular PI3K/Akt/mTOR pathway inhibition by sulfated chitosan in HPV infected cells, after western blot assay [104].
Sulfate substitution has added a new advantage over other derivatives. Ishihara et al., studied sulfate group substitution (with7.66% degree of sulfation) on carboxyl methyl chitin backbone chain. It showed an effective reduction of Friend murine leukemia virus foci studied in-vitro in dunni cells at high concentrations (100 and 300 μg/ml) and for herpes simplex type -1 virus. The decline showed dose-dependency which was comparable to dextran sulfate when treated during viral absorption. However, no activity was found before or after viral uptake, studied in-vitro on Vero & ATCC-VR-539 cell lines [105].
The antiviral efficacy of chitosan nanoparticles prepared from Luciliacuprina maggots was tested against three viruses; Herpes Simplex Virus (HSV-1- a DNA virus), Rift Valley Fever virus (RVFV) and Coxsackie viruses (RNA virus) on a Vero cell line. The outcomes of this study reflected a significant log reduction in virus infectivity titer and inhibition of cytopathic effects for all three infections with maximum inhibition for RVFV showing a 24.9% reduction in virus infection followed by Coxsackie virus at 26.1% and HSV-1 standing at 18.8% reduction post-treatment with chitosan nanoparticles compared to control [106].
In support of studies conducted using graphene as non-selective protein absorbent with N-2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC) for removal of virus in drinking water by Bai et al. [107], sulfate substitution have added new advantage over other derivatives. Later in line with the application of the quaternized derivative of chitosan, Mi et al., developed an electrospun mat with the use of polyvinyl alcohol (PVA) and cross-linker glutaraldehyde to decrease hydrophilicity of HTCC polymer for its use as virus absorbent in water treatment. They found that there was a higher log removal value than the recommendation by the Environmental Protection Agency (EPA) guidelines for enveloped Sindbis virus and non-enveloped Porcine parvovirus [108].
7. Highlighting potent antiviral activity of chitosan and its derivatives against animal viruses
Noroviruses present in unsafe food and environmental waters hold a strong threat to animals and humans' health by causing gastroenteritis and diarrheal diseases. Zhu et al. examined the antiviral property of chitosan microparticles against two human norovirus surrogates, murine norovirus, and bacteriophage MS2. They noticed a reduction in murine noroviral genome titer in a concentration and time-dependent manner of chitosan microparticles with 0.3% concentration and increased contact time of 24 h taking the viral titer to undetectable levels. Notably, chitosan microparticles showed immediate antiviral activity against both the surrogates at high concentrations. Plaque assay and Rt-qPCR studies based on the effect of chitosan microparticles on MS2 titer showed immediate antiviral activity at 0 h contact time at all chitosan microparticles concentrations. At 0.3% concentration of chitosan microparticle, MS2 viral titer fell to the limit of detection of 1.85 log PFU/ml. The binding study reflected that a majority of murine norovirus was present in the unbound state to chitosan microparticle in the supernatant, suggesting a different mechanism of action of chitosan microparticles for dismantling of virus [109]. Similarly, antiviral efficacy of different molecular weight Chitosan dissolved in two different solvents, water, and low pH acetic acid or aqueous Hydrochloric acid against human norovirus and four enteric virus surrogates including murine norovirus (MNV-1), feline calicivirus (FCV-F9), and bacteriophages, MS2 and phiX174 were determined. The study highlighted that the pH of the medium had a bigger say on virus infectivity than the type of solvent used to lower the pH of chitosan, except in the case with high molecular weight chitosan dissolved in hydrochloric and acetic acid and had activity against MS2 and FCV-F9 strains. Moreover, chitosans were found to be the most potent antiviral agents against MS2, followed by FCV-F9 and phi X174 viral strains, while no activity was observed against MNV-1. Decrease in MS2 infectivity by chitosan reflected a directly proportionate dependence on molecular weight but the sensitivity of FCV-F9 and phi X174viruses was found to be molecular weight independent [110].
Enterovirus is associated with wide-ranging human health syndromes. An in-vitro study based on treating Vero cells with chitosan‑nickel microcomposites was conducted for their effect on enterovirus 71 infection which showed blockage of viral cytopathic effects in cells, an inhibitory effect on virus infectivity by capsid binding with no virus residues observed at high concentration due to high osmolarity and virus replication [111].
New castle disease virus from the paramyxovirus group is known to cause avian viral infections and adding up tomortalities. Low molar weight sulfated chitosan, extracted from the cuttlebone of Sepia pharaonis was exploited for its antiviral activity through in-vitro studies. The data showed the exceptional abilities of this low molecular weight oligosaccharide to inhibit haemagglutination at a titer value of 64 against serially diluted virus-infected cultures and preventing viral proliferation inside the avian bloodstream and further host cell invasion by competing with the virus for Red Blood Cell (RBC) binding [112].
Previous studies have steered attention on the radical scavenging activity of amino groups in chitosan polymer, imparting the antimicrobial activity of chitosan. The introduction of amino groups to the chitosan polymer increased its antiviral activity against the Newcastle diseases virus (NDV). Inhibition effect studies at 1 g/L concentration along with bromine substitution at C6 of chitosan showed the best results by resulting in absolute inhibition with hemagglutination titer to a negligible level. The suspected reason was the electron density and 3-D structure of the chitosan polymer. However, the mechanism of action was not confirmed and was suspected to be an increase in immune response and decrease in genetic material as analyzed by Real Time-Polymerase Chain Reaction (RT-PCR) test [113]. Similar studies were done on NDV by Him et al., to understand how the 3-D structural arrangement affected the antiviral efficacy of chitosan. They had performed a hemagglutination titer test for ⍺- and β- chain arrangement of chitosan. And the results were eminent with respect to titer count reaching zero for direct inhibition of NDV by β- chitosan. Also, the better prevention effect was observed for β- chitosan than ⍺- chitosan at lower molecular weights. This provides evidence of a better opportunity for use of β- chitosan which is more abundant than the other two forms of chitosan [114]. In support of a previous study done by Suet al., where chitosan oligosaccharides and higher molecular weight water soluble chitosan were compared on inhibition capacity of FCV, higher concentration reflected high efficacy for inhibition in contrast to Phage infections where there was no evidence of the influence of concentration on infection inhibition [47]. The antiviral action of chitosan against foot and mouth diseases virus (FMDV) (which affect domestic livestock and some wild animals) was recorded in a study by Li et al., where it was noted that when suckling mice with FMDV was administered with chitosan, it helped in delaying the death of those mice [115], also it was inferred that chitosan accelerates the expression of the immune response to decreases the viral expression not only for FMDV but also for other viruses like H1N1 influenza virus and Panama virus when administered with respective viral antigens in mice [116]. Jan et al., study showed there was notable increase in NDV antibody titer, cytokines Interleukin-10 (IL-10), Interferon-ɣ(IFN- ɣ), and the cluster of differentiation 4(CD4) T-cells within 30 days in broiler chicken when the feed was mixed with diluted chitosan at a concentration of 25 mg/l. Lower mortality and diseases progression was observed with respect tithe control group [117].
Chitosan in combination with other antiviral drugs has demonstrated a synergistic effect with respect to augmenting efficacy and absorption of its partners. Curcumin has long been treated as a potent antiviral compound. However, its activity is limited by less solubility and bioavailability. To circumvent these pharmacokinetic issues, curcumin loaded chitosan nanocomposites were synthesized and their antiviral activity was evaluated against hepatitis C virus infection in the human hepatoma cell line. Nanocomposite and chitosan nanoparticles alone showed a100 percent reduction in viral titer (an indication of virus entry to the cells) compared to that of curcumin alone, underscoring synergism between chitosan nanoparticles and curcumin and also supporting antiviral efficiency of chitosan nanoparticles. Furthermore, a significant reduction in Hepatitis C Virus (HCV) core protein indicated that virus replication was observed for nanocomposites post treatment [118]. Caffeic acid abundant in Traditional Chinese medicine (TCM) herbs like Los Lonicerae Japonicae -Fructus Forsythiais a potent counter-virus agent but its use is limited by low bioavailability issues. In an in-vitro and in-vivo study, chitosan oligosaccharides were employed in combination separately with the four TCM herb preparations and they significantly improved integral absorption of caffeic acid by liberating tight junctions and acting as absorption enhancer which further strengthened its antiviral effect against influenza A virus subtype, H1N1 virus [119].
8. Conclusion
This review brings under light the immense potential hold by Chitosan as anti-microbial therapeutic and adjuvant for existing drugs given the challenges in antibiotics drug discovery especially antivirals. Chitosan, a cationic polysaccharide is obtained from Chitin, found ubiquitously in the exoskeleton of crustaceans, insects, and fungi and is generated abundantly as waste product of marine animal processing industry. Technical advancement and better characterization processes are required for mass usage of this macromolecule. In the last few decades, Chitosan and its' various derivatives have proved its worth against a gamut of deadly disease-causing bacteria, fungi and viruses. They have shown strong antiviral property against a wide spectrum of plant, animal, bacteria and human viruses including HIV-1 and Influenza virus. Chitosan's ability to mediate a two-pronged attack on plant viruses in terms of indirectly eliciting systemic resistance and directly affecting virus proliferation has been duly focused upon. With the COVID-19 pandemic grappling the World today and taking away precious human lives, there exists an exigency to develop interventions against this minuscule deadly virus. This review spotlights the ability of chitosan and its' derivatives to bind to the virus essential protein and host receptors required for successful virus binding and invasion into the host cell and initiating infection. We see Chitosan as a hope to combat this COVID-19 virus and strongly believe in the further exploration of this molecule in various preclinical models against SARS-CoV-2 infection to develop chitosan-based therapeutics and vaccines.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was proposed by Nivya Sharma and Chandrima Modak and supported by National Institute of Pharmaceutical Education and Research (NIPER) – Hyderabad under manuscript no. NIPER-H/2020/R056 and the supervision of Dr. Shashi Bala Singh. The search engines used were Pubmed and Google scholar. We are grateful to Dr. Pankaj Kumar Singh, Dr. Rahul Kumar and Dr. Dharmender Khatri for their collaborated assessment and critical manuscript evaluation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijbiomac.2021.02.090.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Supplementary Table 1 Chitosan derivatives against various viruses.
Supplementary Table 2 Effect of molecular weight and degree of deacetylation on activity against various viruses.
References
- 1.Wang L.F., Crameri G. Emerging zoonotic viral diseases. Rev. Sci. Tech. 2014;33(2):569–581. doi: 10.20506/rst.33.2.2311. [DOI] [PubMed] [Google Scholar]
- 2.Rossolini G.M., Arena F., Pecile P., Pollini S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014;18:56–60. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 3.WHO | Antimicrobial Resistance: Global Report on Surveillance 2014 [Internet]. WHO. World Health Organization; [cited 2020 Oct 22]. Available from: http://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/.
- 4.Abad MJ, Bedoya LM, Bermejo P. Marine compounds and their antimicrobial activities. Sci Microb Pathog Commun Curr Res Technol Adv. 2011;51:1293–306.
- 5.TTT Thuy, Ly B.M., Van T.T.T., Van Quang N., Tu H.C., Zheng Y., et al. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 6.Kumar M.R., Muzzarelli R., Muzzarelli C., Sashiwa H., Domb A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004;104(12):6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 7.Yang T.-C., Chou C.-C., Li C.-F. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol. 2005;97(3):237–245. doi: 10.1016/S0168-1605(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y.-E., Kim H., Seo C., Park T., Lee K.B., Yoo S.-Y., et al. Marine polysaccharides: therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017;40(9):1006–1020. doi: 10.1007/s12272-017-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.No H.K., Park N.Y., Lee S.H., Meyers S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002;74(1–2):65–72. doi: 10.1016/s0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 10.Chirkov S.N. The antiviral activity of chitosan (review) Prikl. Biokhim. Mikrobiol. 2002;38(1):5–13. [PubMed] [Google Scholar]
- 11.Iriti M., Varoni E.M. Chitosan-induced antiviral activity and innate immunity in plants. Environ. Sci. Pollut. Res. 2015;22(4):2935–2944. doi: 10.1007/s11356-014-3571-7. [DOI] [PubMed] [Google Scholar]
- 12.Mori T., Okumura M., Matsuura M., Ueno K., Tokura S., Okamoto Y., et al. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials. 1997;18(13):947–951. doi: 10.1016/s0142-9612(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 13.Peluso G., Petillo O., Ranieri M., Santin M., Ambrosic L., Calabró D., et al. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15(15):1215–1220. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 14.Khan T.A., Peh K.K., Ch’ng H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci. 2000;3(3):303–311. [PubMed] [Google Scholar]
- 15.Hosseinnejad M., Jafari S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016;85:467–475. doi: 10.1016/j.ijbiomac.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Aranaz I., Mengíbar M., Harris R., Paños I., Miralles B., Acosta N., et al. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009;3(2):203–230. [Google Scholar]
- 17.Chandy T., Sharma C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs. 1990;18(1):1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 18.Satija N., Lal S.K. The molecular biology of SARS coronavirus. Ann. N. Y. Acad. Sci. 2007;1102(1):26. doi: 10.1196/annals.1408.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Zhou Y., Zhang M., Wang H., Zhao Q., Liu J. Updated approaches against SARS-CoV-2. Antimicrob. Agents Chemother. 2020;64(6) doi: 10.1128/AAC.00483-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercurio I., Tragni V., Busto F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell Mol Life Sci [Internet] 2020 Jul 4 doi: 10.1007/s00018-020-03580-1. [cited 2021 Jan 18]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turoňová B., Sikora M., Schürmann C., WJH Hagen, Welsch S., Blanc F.E.C., et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370(6513):203–208. doi: 10.1126/science.abd5223. Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahariah P., Masson M. Antimicrobial chitosan and chitosan derivatives: a review of the structure–activity relationship. Biomacromolecules. 2017;18(11):3846–3868. doi: 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- 24.Šim\uunek J., Brandysová V., Koppová I. The antimicrobial action of chitosan, low molar mass chitosan, and chitooligosaccharides on human colonic bacteria. Folia Microbiol (Praha) 2012;57(4):341–345. doi: 10.1007/s12223-012-0138-1. [DOI] [PubMed] [Google Scholar]
- 25.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RCF Cheung, Ng T.B., Wong J.H., Chan W.Y. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs. 2015;13(8):5156–5186. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muanprasat C., Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol. Ther. 2017;170:80–97. doi: 10.1016/j.pharmthera.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Babosha A.V. Changes in lectin activity in plants treated with resistance inducers. Biol Bull Russ Acad Sci. 2004;31(1):51–55. [PubMed] [Google Scholar]
- 29.Sofy A.R., Dawoud R.A., Sofy M.R., Mohamed H.I., Hmed A.A., El-Dougdoug N.K. Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in Cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules. 2020;25(10):2341. doi: 10.3390/molecules25102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagorskaya V., Reunov A., Lapshina L., Davydova V., Yermak I. Effect of chitosan on tobacco mosaic virus (TMV) accumulation, hydrolase activity, and morphological abnormalities of the viral particles in leaves of N. tabacum L. cv. Samsun. Virol Sin. 2014;29(4):250–256. doi: 10.1007/s12250-014-3452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewen Q., Yijie D., Yi Z., Shupeng L., Fachao S. Plant immunity inducer development and application. Mol. Plant-Microbe Interact. 2017;30(5):355–360. doi: 10.1094/MPMI-11-16-0231-CR. [DOI] [PubMed] [Google Scholar]
- 32.Raho N., Ramirez L., Lanteri M.L., Gonorazky G., Lamattina L., Ten Have A., et al. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J. Plant Physiol. 2011;168(6):534–539. doi: 10.1016/j.jplph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Iriti M, Sironi M, Gomarasca S, Casazza AP, Soave C, Faoro F. Cell death-mediated antiviral effect of chitosan in tobacco. Plant Physiol. Biochem.. 2006;44(11−12):893–900. [DOI] [PubMed]
- 34.Iriti M., Faoro F. Abscisic acid is involved in chitosan-induced resistance to tobacco necrosis virus (TNV) Plant Physiol. Biochem. 2008;46(12):1106–1111. doi: 10.1016/j.plaphy.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 35.An N., Lv J., Zhang A., Xiao C., Zhang R., Chen P. Gene expression profiling of papaya (Carica papaya L.) immune response induced by CTS-N after inoculating PLDMV. Gene. 2020 doi: 10.1016/j.gene.2020.144845. [DOI] [PubMed] [Google Scholar]
- 36.Davydova V.N., Nagorskaya V.P., Gorbach V.I., Kalitnik A.A., Reunov A.V., Solov’Eva T.F., et al. Chitosan antiviral activity: dependence on structure and depolymerization method. Appl. Biochem. Microbiol. 2011;47(1):103–108. [PubMed] [Google Scholar]
- 37.Kulikov S.N., Chirkov S.N., Il’ina A.V., Lopatin S.A., Varlamov V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. Microbiol. 2006;42(2):200–203. [PubMed] [Google Scholar]
- 38.Hadwiger L.A., Beckman J.M., Adams M.J. Localization of fungal components in the pea-Fusarium interaction detected immunochemically with anti-chitosan and anti-fungal cell wall antisera. Plant Physiol. 1981;67(1):170–175. doi: 10.1104/pp.67.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J.J., Klosterman S.J., Hadwiger L.A. A comparison of the effects of DNA-damaging agents and biotic elicitors on the induction of plant defense genes, nuclear distortion, and cell death. Plant Physiol. 2001;125(2):752–762. doi: 10.1104/pp.125.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y., Cai J., Du Y., Lin J., Wang C., Xiong K. Preparation and anti-TMV activity of guanidinylated chitosan hydrochloride. J. Appl. Polym. Sci. 2009;112(6):3522–3528. [Google Scholar]
- 41.Noiket N., Boonthip T., Riangwong K. Thai Society for Biotechnology, Electronic Proceeding of the 26th Annual Meeting of the Thai Society for Bio-technology and International Conference Mae Fah Luang University Chiang Rai Thailand, November 26th- 29th. 2014. Evaluation of potential for chitosan to control TYLCV disease and promote the growth of Sridathip 3 tomato; pp. 252–259. [Google Scholar]
- 42.Abdalla O.A., Bibi S., Zhang S. Integration of chitosan and plant growth-promoting rhizobacteria to control Papaya ringspot virus and Tomato chlorotic spot virus. Arch Phytopathol Plant Prot. 2017;50(19–20):997–1007. Dec 14. [Google Scholar]
- 43.Kochkina Z.M., Surgucheva N.A., Chirkov S.N. Coliphages inactivation using chitosan derivatives. Mikrobiologiia. 2000;69(2):261–265. [PubMed] [Google Scholar]
- 44.Kochkina Z.M., Chirkov S.N. Effect of chitosan derivatives on the development of phage infection in cultured Bacillus thuringiensis. Mikrobiologiia. 2000;69(2):266–269. [PubMed] [Google Scholar]
- 45.Kochkina Z.M., Chirkov S.N. Effect of chitosan derivatives on the reproduction of Coliphages T2 and T7. Mikrobiologiia. 2000;69(2):257–260. Apr. [PubMed] [Google Scholar]
- 46.Davis R, Zivanovic S, Davidson PM, D'Souza DH. Enteric viral surrogate reduction by chitosan. Food Environ Virol. 2015;7(4):359–65. [DOI] [PubMed]
- 47.Su X., Zivanovic S., D’SOUZA D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009;72(12):2623–2628. doi: 10.4315/0362-028x-72.12.2623. [DOI] [PubMed] [Google Scholar]
- 48.Chatain-Ly M.H., Moussaoui S., Rigobello V., Demarigny Y., Vera A. Antiviral effect of cationic compounds on bacteriophages. Front. Microbiol. 2013;4:46. doi: 10.3389/fmicb.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson J. 2015. The Efficacy of Utilizing Chitosan as an Antiviral Agent in Water Treatment. [Google Scholar]
- 50.Kochkina Z.M., Chirkov S.N. Influence of the chitosan oligomer on the phage particles and reproduction of phage 1-97A in the culture of bacillus thuringiensis. Microbiology. 2001;70(6):706–710. Nov 1. [PubMed] [Google Scholar]
- 51.Kochkina Z.M., Chirkov S.N. Influence of chitosan derivatives on the development of phage infection in theBacillus thuringiensis culture. Microbiology. 2000;69(2):217–219. [PubMed] [Google Scholar]
- 52.Polyakova A.M., Kravchenko A.V., Ernak I.M., Gorbach V.I., Astrina O.S., Luk’yanov P.A., et al. Effects of chitosan on the biological properties of gram-negative bacteria endotoxins. Bull. Exp. Biol. Med. 1995;120(2):814–817. [Google Scholar]
- 53.Wang S., Guo F., Liu K., Wang H., Rao S., Yang P., et al. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008;136(1–2):8–15. doi: 10.1016/j.virusres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 56.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi C., Sun X., Ye J., Ding L., Liu M., Yang Z., et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020:1–10. doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. 2020 Jan 31. The Novel Coronavirus 2019 (2019-nCoV) Uses the SARS-Coronavirus Receptor ACE2 and the Cellular Protease TMPRSS2 for Entry into Target Cells. bioRxiv. (2020.01.31.929042) [Google Scholar]
- 62.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. 2005;102(22):7988–7993. doi: 10.1073/pnas.0409465102. May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500(7461):227–231. doi: 10.1038/nature12328. Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milewska A., Ciejka J., Kaminski K., Karewicz A., Bielska D., Zeglen S., et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013;97(2):112–121. doi: 10.1016/j.antiviral.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milewska A., Kaminski K., Ciejka J., Kosowicz K., Zeglen S., Wojarski J., et al. HTCC: broad range inhibitor of coronavirus entry. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milewska A., Chi Y., Szczepanski A., Barreto-Duran E., Liu K., Liu D., et al. 2020. HTCC as a Highly Effective Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalathiya U., Padariya M., Mayordomo M., Lisowska M., Nicholson J., Singh A., et al. Highly conserved Homotrimer cavity formed by the SARS-CoV-2 spike glycoprotein: a novel binding site. J. Clin. Med. 2020;9(5):1473. doi: 10.3390/jcm9051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smelcerovic A., Knezevic-Jugovic Z., Petronijevic Z. Microbial polysaccharides and their derivatives as current and prospective pharmaceuticals. Curr. Pharm. Des. 2008;14(29):3168–3195. doi: 10.2174/138161208786404254. [DOI] [PubMed] [Google Scholar]
- 69.Alitongbieke G., Li X.-M., Wu Q.-C., Lin Z.-C., Huang J.-F., Xue Y., et al. 2020. Effect of β-Chitosan on the Binding Interaction between SARS-CoV-2 S-RBD and ACE2. bioRxiv. [Google Scholar]
- 70.Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubia\lka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater Sci Eng C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hathout R.M., Kassem D.H. Positively charged electroceutical spun chitosan nanofibers can protect health care providers from COVID-19 infection: an opinion. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., Wawrzyniak P., et al. Distribution of ACE2, CD147, CD26 and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020 doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aguiar JA, Tremblay BJ-M, Mansfield MJ, Woody O, Lobb B, Banerjee A, et al. Gene Expression and In Situ Protein Profiling of Candidate SARS-CoV-2 Receptors in Human Airway Epithelial Cells and Lung Tissue. BioRxiv. 2020;. [DOI] [PMC free article] [PubMed]
- 74.Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, et al. SARS-CoV-2 Invades Host Cells Via a Novel Route: CD147-spike Protein. BioRxiv. 2020;.
- 75.Bian H, Zheng Z-H, Wei D, Zhang Z, Kang W-Z, Hao C-Q, et al. Meplazumab Treats COVID-19 Pneumonia: An Open-labelled, Concurrent Controlled Add-on Clinical Trial. MedRxiv. 2020;.
- 76.Lv M., Miao J., Zhao P., Luo X., Han Q., Wu Z., et al. CD147-mediated chemotaxis of CD4+ CD161+ T cells may contribute to local inflammation in rheumatoid arthritis. Clin. Rheumatol. 2018;37(1):59–66. doi: 10.1007/s10067-017-3800-9. [DOI] [PubMed] [Google Scholar]
- 77.Varadarajan S., Balaji T.M., Sarode S.C., Sarode G.S., Sharma N.K., Gondivkar S., et al. EMMPRIN/BASIGIN as a biological modulator of oral cancer and COVID-19 interaction: novel propositions. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo Z., Dong X., Ke Q., Duan Q., Shen L. Downregulation of CD147 by chitooligosaccharide inhibits MMP-2 expression and suppresses the metastatic potential of human gastric cancer. Oncol. Lett. 2014;8(1):361–366. doi: 10.3892/ol.2014.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WuDUNN D., Spear P.G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 1989;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Compton T., Nowlin D.M., Cooper N.R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193(2):834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 81.Su C.-M., Liao C.-L., Lee Y.-L., Lin Y.-L. Highly sulfated forms of heparin sulfate are involved in Japanese encephalitis virus infection. Virology. 2001;286(1):206–215. doi: 10.1006/viro.2001.0986. [DOI] [PubMed] [Google Scholar]
- 82.Klimstra W.B., Ryman K.D., Johnston R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998;72(9):7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Byrnes A.P., Griffin D.E. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 1998;72(9):7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y., Maguire T., Hileman R.E., Fromm J.R., Esko J.D., Linhardt R.J., et al. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997;3(8):866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 85.Kroschewski H., Allison S.L., Heinz F.X., Mandl C.W. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology. 2003;308(1):92–100. doi: 10.1016/s0042-6822(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 86.Stepanov O.A., Prokof’eva M.M., Stocking K., Varlamov V.P., Levov A.N., Vikhoreva G.A., et al. Replication-competent gamma-retrovirus Mo-MuLV expressing green fluorescent protein as efficient tool for screening of inhibitors of retroviruses that use heparan sulfate as primary cell receptor. Mol. Biol. 2012;46(3):457–466. [PubMed] [Google Scholar]
- 87.Artan M., Karadeniz F., Karagozlu M.Z., Kim M.-M., Kim S.-K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010;345(5):656–662. doi: 10.1016/j.carres.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 88.Nishimura S.-I., Kai H., Shinada K., Yoshida T., Tokura S., Kurita K., et al. Regioselective syntheses of sulfated polysaccharides: specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998;306(3):427–433. doi: 10.1016/s0008-6215(97)10081-7. [DOI] [PubMed] [Google Scholar]
- 89.Yang J., Luo K., Li D., Yu S., Cai J., Chen L., et al. Preparation, characterization and in vitroanticoagulant activity of highly sulfated chitosan. Int. J. Biol. Macromol. 2013;52:25–31. doi: 10.1016/j.ijbiomac.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 90.MAG Sosa, Fazely F., Koch J.A., Vercellotti S.V., Ruprecht R.M. N-Carboxymethylchitosan-N, O-sulfate as an anti-HIV-1 agent. Biochem. Biophys. Res. Commun. 1991;174(2):489–496. doi: 10.1016/0006-291x(91)91443-g. [DOI] [PubMed] [Google Scholar]
- 91.Jayakumar R., Nwe N., Tokura S., Tamura H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007;40(3):175–181. doi: 10.1016/j.ijbiomac.2006.06.021. Feb 20. [DOI] [PubMed] [Google Scholar]
- 92.Dimassi S., Tabary N., Chai F., Blanchemain N., Martel B. Sulfonated and sulfated chitosan derivatives for biomedical applications: a review. Carbohydr. Polym. 2018 Dec 15;202:382–396. doi: 10.1016/j.carbpol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 93.Karagozlu M.Z., Karadeniz F., Kim S.-K. Anti-HIV activities of novel synthetic peptide conjugated chitosan oligomers. Int. J. Biol. Macromol. 2014;66:260–266. doi: 10.1016/j.ijbiomac.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 94.Wu D., Ensinas A., Verrier B., Primard C., Cuvillier A., Champier G., et al. Zinc-stabilized colloidal polyelectrolyte complexes of chitosan/hyaluronan: a tool for the inhibition of HIV-1 infection. J. Mater. Chem. B. 2016;4(32):5455–5463. doi: 10.1039/c6tb00898d. [DOI] [PubMed] [Google Scholar]
- 95.Ishihara M., Nguyen V.Q., Mori Y., Nakamura S., Hattori H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int. J. Mol. Sci. 2015;16(6):13973–13988. doi: 10.3390/ijms160613973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mori Y., Ono T., Miyahira Y., Nguyen V.Q., Matsui T., Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013;8(1):93. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X., Wu P., Gao G.F., Cheng S. Carbohydrate-functionalized chitosan fiber for influenza virus capture. Biomacromolecules. 2011;12(11):3962–3969. doi: 10.1021/bm200970x. [DOI] [PubMed] [Google Scholar]
- 98.Cheng S., Zhao H., Xu Y., Yang Y., Lv X., Wu P., et al. Inhibition of influenza virus infection with chitosan–sialyloligosaccharides ionic complex. Carbohydr. Polym. 2014;107:132–137. doi: 10.1016/j.carbpol.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 99.Collins B.E., Paulson J.C. Cell surface biology mediated by low affinity multivalent protein–glycan interactions. Curr. Opin. Chem. Biol. 2004;8(6):617–625. doi: 10.1016/j.cbpa.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Lee Y.C., Lee R.T. Carbohydrate-protein interactions: basis of glycobiology. Acc. Chem. Res. 1995;28(8):321–327. [Google Scholar]
- 101.Zheng M., Qu D., Wang H., Sun Z., Liu X., Chen J., et al. Intranasal administration of chitosan against influenza A (H7N9) virus infection in a mouse model. Sci. Rep. 2016;6:28729. doi: 10.1038/srep28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin Y.-C., Lin S.-T., Chen C.-Y., Wu S.-C. Enterovirus 71 adsorption on metal ion-composite chitosan beads. Biotechnol. Prog. 2012;28(1):206–214. doi: 10.1002/btpr.699. [DOI] [PubMed] [Google Scholar]
- 103.Pauls T. 2016. Chitosan as an Antiviral. [Google Scholar]
- 104.Gao Y., Liu W., Wang W., Zhang X., Zhao X. The inhibitory effects and mechanisms of 3, 6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018;198:329–338. doi: 10.1016/j.carbpol.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 105.Ishihara C., Yoshimatsu K., Tsuji M., Arikawa J., Saiki I., Tokura S., et al. Anti-viral activity of sulfated chitin derivatives against Friend murine leukaemia and herpes simplex type-1 viruses. Vaccine. 1993;11(6):670–674. doi: 10.1016/0264-410x(93)90315-o. [DOI] [PubMed] [Google Scholar]
- 106.Hassan MI, Mohamed AF, Taher FA, Kamel MR. Antimicrobial activities of chitosan nanoparticles prepared from Lucilia cuprina maggots (Diptera: Calliphoridae). J. Egypt. Soc. Parasitol.. 2016;46(3):563–70. [PubMed]
- 107.Bai B., Mi X., Xiang X., Heiden P.A., Heldt C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013;380:137–142. doi: 10.1016/j.carres.2013.08.020. Oct 18. [DOI] [PubMed] [Google Scholar]
- 108.Mi X., Vijayaragavan K.S., Heldt C.L. Virus adsorption of water-stable quaternized chitosan nanofibers. Carbohydr. Res. 2014;387:24–29. doi: 10.1016/j.carres.2014.01.017. Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu S., Barnes C., Bhar S., Hoyeck P., Galbraith A.N., Devabhaktuni D., et al. Survival of human Norovirus surrogates in water upon exposure to thermal and non-thermal antiviral treatments. Viruses. 2020;12(4):461. doi: 10.3390/v12040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davis R, Zivanovic S, D'Souza DH, Davidson PM. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol.. 2012;32(1):57–62. [DOI] [PubMed]
- 111.Lin Y.-C., Chang C.-H. In vitro inhibition of enterovirus 71 infection with a nickel ion/chitosan microcomposite. Virus Res. 2014;190:17–24. doi: 10.1016/j.virusres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 112.Karthik R., Manigandan V., Saravanan R., Rajesh R.P., Chandrika B. Structural characterization and in vitro biomedical activities of sulfated chitosan from Sepia pharaonis. Int. J. Biol. Macromol. 2016;84:319–328. doi: 10.1016/j.ijbiomac.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 113.He X., Xing R., Liu S., Qin Y., Li K., Yu H., et al. The improved antiviral activities of amino-modified chitosan derivatives on Newcastle virus. Drug Chem. Toxicol. 2019:1–6. doi: 10.1080/01480545.2019.1620264. [DOI] [PubMed] [Google Scholar]
- 114.He X., Xing R., Li K., Qin Y., Zou P., Liu S., et al. Beta-chitosan extracted from Loligo Japonica for a potential use to inhibit Newcastle disease. Int. J. Biol. Macromol. 2016;82:614–620. doi: 10.1016/j.ijbiomac.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 115.Li D. Chitosan can stop or postpone the death of the suckling mice challenged with foot-and-mouth disease virus. Virol. J. 2010;7(1):1–4. doi: 10.1186/1743-422X-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bacon A., Makin J., Sizer P.J., Jabbal-Gill I., Hinchcliffe M., Illum L., et al. Carbohydrate biopolymers enhance antibody responses to mucosally delivered vaccine antigens. Infect. Immun. 2000;68(10):5764–5770. doi: 10.1128/iai.68.10.5764-5770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jan S.-S., Liu D.-C., Dong X.-Y., Hu Y.-M., Chen J. Effects of chitosan and its derivative added to water on immunological enhancement and disease control. Immunotherapy. 2012;4(7):697–701. doi: 10.2217/imt.12.68. [DOI] [PubMed] [Google Scholar]
- 118.Loutfy S.A., Elberry M.H., Farroh K.Y., Mohamed H.T., Mohamed A.A., Mohamed E.B., et al. Antiviral activity of chitosan nanoparticles encapsulating Curcumin against hepatitis C virus genotype 4a in human Hepatoma cell lines. Int. J. Nanomedicine. 2020;15:2699–2715. doi: 10.2147/IJN.S241702. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Zhou W., Yin A., Shan J., Wang S., Cai B., Di L. Study on the rationality for antiviral activity of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple preparations improved by Chito-oligosaccharide via integral pharmacokinetics. Mol J Synth Chem Nat Prod Chem. 2017;22(4) doi: 10.3390/molecules22040654. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6154603/ Internet. Apr 20 [cited 2020 Oct 23. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Chitosan derivatives against various viruses.
Supplementary Table 2 Effect of molecular weight and degree of deacetylation on activity against various viruses.