Abstract
With the commencement of the COVID19 pandemic, following its 1st case reported in Wuhan in China, the knowledge about the virus as well as the symptoms produced by the disease have drastically increased to this day. The manifestations of COVID19 is now known to affect multiple organ systems of the body, which have shown to have acute as well as chronic complications. Histopathological analysis of the biopsies from the affected organs have implied a direct cytopathic effect of the virus but at the same time not ruling out other causes like hypoxia metabolic changes etc., occurring during the course of the disease. In this review article, we have highlighted the histopathological changes in various organs as reported by various studies throughout the world for a better understanding of the etiopathogenesis of COVID19.
Keywords: COVID19, Multi-systemic, Lungs, Diffuse alveolar damage
1. Introduction
COVID19 has now, since its 1st case in December has been seen to show widespread and multisystemic manifestations [1].
. The disease is not just confined to the lungs, but with the spillage of the virus into the blood stream has lead to the involvement of almost all the organs of the body [2], including heart, liver, brain, kidney, skin, intestines and eyes. The multisystemic involvement can mainly be attributed to the widespread localization of the ACE2 receptors throughout the body, which acts as the major entry point for the virus [3]. The histo-pathological findings in various organs apart from the clinical symptoms and biochemical markers can provide major clue towards the pathogenesis of the disease.
2. Material and methods
We carried out systemic online search from databases with ‘COVID19’ and ‘Coronavirus’ as the keywords. The articles were taken mainly from PubMed, PMC, Embase, Cochrane library, Google Scholar, MEDLINE, UpToDate, and Web of Science databases. Articles were selected highlighting the systemic manifestations of the disease. The publication ranged from a period of 01 Jan 2020 to 20 July 2020.
All the publications screened and selected were carefully reviewed and analysed by two independent investigators. All the articles were studied in full text reviews. All studies not reporting original date and irrelevant data were excluded from this review.
3. The site of most action, lungs
The lungs by far, had been considered as the hotspot and are known to act as a niche for the proliferation of the virus. The residence of the virus in the lungs also is the main reason for the rapid transmission of the disease [4]. According to various studies published, the novel coronavirus enters by binding to ACE2 receptors present on the target cells through the Receptor Binding Domain (RBD) which is located on the viral particles [5].
In case of pulmonary involvement, the histological patterns vary from interstitial inflammation, diffuse alveolar damage (DAD), and necrotizing bronchitis/ bronchiolitis. By far the most common pattern observed is bilateral DAD [6]. In a case study by H. Zhang et al. (2020), the pulmonary biopsy of a 72-year-old diabetic male showed all the characteristic features of diffuse alveolar damage, which included presence of intra-alveolar fibrinous exudates, along with loose interstitial fibrosis. Chronic inflammatory infiltrates in the form of macrophages and lymphocytes (mainly CD4‑positive T cells) were also predominantly observed. Denuded alveolar lining cells with reactive type II pneumocyte hyperplasia, intra-alveolar loose fibrous plugs of organizing pneumonia were also present. The same study also showed immunostaining by rabbit polyclonal antibody, Rp3 NP on the lung sections, highlighting the alveolar epithelial cells. Positive immunostaining could also be appreciated in the damaged and desquamated cells found within the alveolar space [7] (Table 1 ).
Table 1.
Table enlisting various studies on COVID19, highlighting the radiological and pathological aspects of the disease in multiple systems of the body.
| Authors, Country | Aim of study | No. of cases studied | Clinical features & Radiological details | Biomarkers & Haematological findings | Histopathology & Cytomorphology | IHC/Immuno-floroscence | Electron microscopy |
|---|---|---|---|---|---|---|---|
| Zhang H et al, China | Histo-pathological findings and IHC in COVID19 infected lung | 01 | C/F: 72-year-old diabetic & hypertensive presented with fever & cough. | – | DAD, intra-alveolar fibrinous exudates & loose interstitial fibrosis. | Rp3 NP protein of SARS–CoV-2: prominent expression on alveolar epithelial cells & on damaged desquamated cells within alveolar space. | – |
| CT scan: Patchy ground glass–like opacifications with pleural thickening and mediastinal lymphadenopathy. | |||||||
| Copin MC et al, France | Histological patterns of lung injury | 06 | – | – | -01 case: Lymphocytic viral pneumonia | – | – |
| -05 cases: AFOP with intra-alveolar “fibrin balls” | |||||||
| Xu Z et al, China | Pathological findings of COVID-19 associated with acute respiratory distress syndrome | 01 | Chest X-ray: Multiple patchy shadows in B/L lungs | Peripheral blood flow cytometry: |
LUNGS: -B/L DAD, cellular fibro-myxoid exudate. |
– | – |
| - Reduced CD4 & CD8 T cells | -Viral cytopathic effect: multinucleated syncytial cells with atypical enlarged pneumocytes in intra-alveolar spaces. | ||||||
| - High proportions of HLA-DR (CD4 3·47 %) & CD38 (CD8 39·4%) double-positive fractions |
HEART: -Interstitial mononuclear inflammatory infiltrates |
||||||
| - Increased concentration of highly proinflammatory CCR6+ Th17 in CD4 T cells |
LIVER: -Moderate microvesicular steatosis with mild lobular & portal activity |
||||||
| Fox SE et al, USA | Autopsy series studying pulmonary and cardiac Pathology in Covid19. | 04 | C/F: Aged 44−78 years with hypertension -Mild cough & fever with sudden respiratory decompensation | -Elevated ferritin & fibrinogen levels | -LUNGS: (04 cases) -Bilateral DAD, mild-to-moderate lymphocytic infiltrate, megakaryocytes within small vessels & alveolar capillaries. |
– | – |
| -Increased AST | -Viral cytopathic effect: cytomegaly enlarged nuclei & bright, eosinophilic nucleoli within alveolar spaces. | ||||||
| -Increased d-dimer levels in all patients near death. |
HEART: (04 cases) -Scattered individual cell myocyte necrosis. |
||||||
| Schaller T et al, Germany | Postmortem Examination of Patients With COVID-19 | 10 | C/F: Fever, cough & dyspnea. | – |
LUNGS: (10 cases) -Disseminated DAD at different stages more pronounced in middle & lower lobes. |
– | – |
| X ray: (09 cases) ground-glass opacity predominantly in middle & lower lung fields. | -Fully established fibrosis in 01 case. | ||||||
| -Minor neutrophilic infiltration in 5 cases | |||||||
|
HEART: -Mild lymphocytic myocarditis (04 cases) & epicarditis (02 cases) |
|||||||
|
LIVER: -Non-specific Periportal liver lymphocyte infiltration & fibrosis |
|||||||
|
BRAIN: -No change |
|||||||
| Giani M et al, Italy | BAL fluid analysis | 01 | C/F: Fatigue & fever progressing to respiratory distress & hypoxia | – | CYTOMORPHOLOGY: | – | – |
| -Fibrino-hematic material with scattered alveolar macrophages & predominance of activated plasma cells (CD138+), admixed with T & scattered B lymphocytes. | |||||||
| -Alveolar macrophages: showed nuclear clearing or intranuclear cytopathic inclusions | |||||||
| Zhang Y et al, China | Assess liver impairment in COVID19 patients | 115 | C/F: Respiratory distress | Elevated levels of: |
LIVER: (01 case) -Non-specific findings. |
– | – |
| -ALT: 11/115 | -Mild sinusoidal dilatation with minimal lymphocytic infiltration | ||||||
| -AST: 17/115 | |||||||
| -S.biliubin: 08/115 cases. | |||||||
| Tian S et al, China | Pathological assessment of postmortem core biopsies, | 04 | C/F: Aged 59−81 years with each patient having at least one underlying disease, including immunocompromised status. | Case 1: -elevated pro-BNP & hypertensive cardiac troponin (d/t history of previous MI) | LUNGS: | – | – |
| CT scan (03 cases): Multiple patchy ground glass opacities | - DAD (in all 04 cases) | ||||||
| X ray (01 case): Patchy high-density shadows with worsening in subsequent days. | All 04 cases: | - Case no. 02: also showed pneumocyte injury with focal sloughing & formation of syncytial giant cells | |||||
| -Normal AST/ALT/S.bilirubin levels | -Case no. 04: also showed fibrinoid necrosis in small vessels with abundant intra-alveolar neutrophilic infiltration, consistent with bronchopneumonia due to superimposed bacterial infection. | ||||||
| LIVER: | |||||||
| -Case 1: sinusoidal dilatation, nuclear glycogen in hepatocytes, focal macrovesicular steatosis and features of CLL | |||||||
| -Case 2: cirrhosis consistent with his history & mild zone 3 sinusoidal dilatation | |||||||
| -Case 3&4: mild lobular lymphocytic infiltration. | |||||||
|
HEART: -Non-specific findings with no epicardial/myocardial inflammation. |
|||||||
| Su H et al, China. | Histopathological analysis of kidney biopsy in 26 postmortem cases. | 26 | C/F: 26 cases died of respiratory failure due to COVID19 | - Leukocytois in 10 cases. | -Prominent ATI -Loss of brush border, vacuolar degeneration & dilatation of tubular lumen with cellular debris in almost all cases, |
DIF:Nonspecific IgM & C3 trapping in glomeruli. | Coronavirus-like particles: |
| −2 cases: multiple foci of bacteria & diffuse polymorphonuclear casts in tubular lumen. | Indirect IF: Positive granular nuclear and cytoplasmic staining with SARS-CoV nucleoprotein in tubular epithelium (in 03 out of 06 cases) | 65−136 nm, with distinctive spikes, 20 −25 nm in cytoplasm of renal proximal tubular epithelium/podocytes /less so in distal tubules. | |||||
| −3 cases: pigmented casts with high levels of CPK possibly due to rhabdomyolysis | |||||||
| −5 cases:Endothelial cell swelling with variable foamy degeneration in old/hypertensive/diabetic cases. | |||||||
| −3 cases: segmental fibrin thrombus in glomerular capillary loops with severe injury of the endothelium Occasional podocyte vacuolation & detachment from the glomerular basement membrane. | |||||||
| Christopher P. Larsen et al, USA | Collapsing Glomerulopathy in a Patient With COVID-19 | 01 | C/F: 63-year-old hypertensive male with fatigue, high-grade fever (39.7 | -Elevated CRP & D-dimer |
Kidney Biopsy: −14 glomeruli were globally sclerotic. Many of the intact glomeruli showed tuft collapse with overlying epithelial hypertrophy & hyperplasia in the Bowman space. |
DIF: Negative for IgA, IgG, IgM, C3, C1q, kappa & lambda in Glomeruli. | No definitive viral particles |
| -Lymphopenia | -Tubular epithelium injury: most prominent in the PCT | ||||||
| -Interstitial fibrosis, tubular atrophy, inflammatory infiltrate in interstitium consisting of lymphocytes, plasma cells with few scattered eosinophils | |||||||
| Buja LM et al, USA | Emerging spectrum of cardiopulmonary pathology of COVID-19: | 03 | C/F: Autopsy done on: Case 1: 62-year-old obese male history of respiratory illness died of COVID19 Case 2: 34-year-old obese, diabetic hypertensive with headache, shortness of breath & productive cough died of COVID19 Case 3: 48-year-old obese died of COVID19 | – | LUNGS: | – | Case 1: |
| CT scan: (Case 2) B/L upper & lower lobe ground-glass with early consolidative alveolar opacities of rounded morphology. | -Case 1: early DAD with multiple hyaline membranes & focal mild inflammation with lymphocytes & macrophages in some alveolar spaces. | -Strands of precipitated fibrin & entrapped neutrophils within alveolar capillaries -Larger deposits of fibrin in alveolar spaces. | |||||
| -Case 2:interstitial lymphocytic pneumonitis with lymphocytic infiltrates around small blood vessels & terminal bronchioles. Microthrombi in some pulmonary arterioles. | -No Viral particles in heart or lungs. | ||||||
| -Case 3: right pleura showed empyema. Right lung showed evidence of atelectasis & DAD. DAD was more pronounced in the expanded left lung. | |||||||
| LIVER: | |||||||
| -Case 1 & 2: Moderate macrovesicular steatosis | |||||||
| -Case 3: Moderate macrovesicular steatosis, lympho-plasmacytic triaditis with portal fibrosis and early portal-portal bridging fibrosis. | |||||||
| HEART: | |||||||
| -Case 1: Cardiomyocytes with moderately enlarged hyperchromatic nuclei with vacuolar degenerative change. No myocarditis. | |||||||
| -Case 2: Individual damaged cardiomyocytes | |||||||
| -Case 3: Multifocal lymphocytic infiltrates in epicardium. Myocytes: enlarged hyperchromatic nuclei, changes of acute injury. | |||||||
|
KIDNEY: -Case 1: Hyaline arteriolosclerosis with glomerulosclerosis |
|||||||
| -Case 2: Occasional fibrin-platelet thrombus in renal glomerular capillaries. | |||||||
| -Case 3: Mild hyaline arteriolosclerosis, periglomerular hyaline arteriolosclerosis with rare holo-sclerotic glomeruli. | |||||||
| Tavazzi G et al, Italy | Myocardial localization of coronavirus in COVID‐19 cardiogenic shock | 01 | C/F: 69-year-old patient with flu-like symptoms rapidly degenerating into respiratory distress, hypotension & shock. | -Lymphopenia |
HEART: -Cardiac myocytes showed non‐specific features consisting of focal myofibrillar lysis, and lipid droplets. |
Immune‐light microscopy: Large (>20 μm), vacuolated, CD68‐positive macrophages | Single / small groups of viral particles with electron‐dense spike‐like structures & size between 70–120 nm within the interstitial cells of myocardium. |
| -Raised CRP | |||||||
| - Increased hs‐TnI | |||||||
| Varga Z et al, Switzerland. | Endothelial cell infection and endotheliitis in COVID-19 | 03 | C/F: Autopsies done on: Hypertensive males with COVID19 disease developing multi-organ failure | – | -Case 1: Inflammatory cells associated with endothelium & apoptotic bodies in heart, small bowel & lungs | – | Case 1: In transplanted kidney- Viral inclusion structures (dense circular surface with lucid centre) in endothelial cells |
| -Case 2: Lymphocytic endotheliitis in lung, heart, kidney, and liver with liver cell necrosis. No lymphocytic myocarditis. | |||||||
| -Case 3: Small intestine resection showed prominent endotheliitis of the submucosal vessels with apoptotic bodies | |||||||
| Von Weyhern C.H., et al, Germany | Early evidence of pronounced brain involvement in fatal COVID-19 | 06 | C/F: Autopsies of 06 case aged 58−82 years who died from COVID-19 | -Elevated CRP & IL-6 in all the cases | BRAIN: | – | – |
| -Leukocytosis in 2 cases | All cases:-Lymphocytic pan-encephalitis & meningitis | ||||||
| -Patients with age <65yrs: Death due to Petechial bleeding and intracranial hemmorhage. | |||||||
| LUNGS: | |||||||
| −05 cases: DOD | |||||||
| =01 case: Organizing Pneumonia Pattern | |||||||
| Solomon IH et al, England | Neuropathological Features of Covid-19 | 18 | C/F: Myalgia headache & decreased taste | – | -All cases:Acute hypoxic injury in cerebrum & cerebellum with loss of neurons in the cerebral cortex, hippocampus & cerebellar Purkinje cell layer. No thrombi/vasculitis. | IHC to detect SARS-CoV: | – |
| Negative in neurons, glia, endothelium& immune cells. | |||||||
| −02 cases: perivascular lymphocytes | |||||||
| −01 case: focal leptomeningeal inflammation | |||||||
| Reichard RR et al, USA | A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology | 01 | C/F: 71-year-old male with fatigue and exertional dyspnea, | Elevated CRP, IL-6 & ferritin levels | -Perivascular acute disseminated encephalomyelitis (ADEM)-like pathology: Foci of intraparenchymal blood that disrupted the white matter, with macrophages at periphery of the lesions | – | – |
| CT Chest: Parenchymal consolidation and surrounding ground-glass opacities following a peri-broncho vascular distribution. | - Luxol fast blue: loss of myelin, | ||||||
| Poyiadji N et al, USA | COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy | 01 | C/F: 58-year-old female with 3-day history of cough, fever & altered mental status. | – | – | – | |
| NC-CT head: Symmetric hypoattenuation within the bilateral medial thalami. | |||||||
| MRI: Haemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes & subinsular regions | |||||||
| Galvan et al, Spain | Classification of the cutaneous manifestations of COVID ‐19 | 375 | C/F: | – | – | – | – |
| -Pseudo-chilbain: 71cases | |||||||
| -Vesicular: 34 | |||||||
| -Urticarial: 73 | |||||||
| -Maculo-papular: 176 | |||||||
| -Livedo/ necrosis: 21 | |||||||
| Recalcati et al, Spain | Cutaneous manifestations in COVID‐19: a first perspective | 18 | C/F: | – | – | – | – |
| out of 88 cases -Skin lesions | -Erythematous rash: 14 | ||||||
| -Urticaria: 03 -Chickenpox‐like vesicles :01 case | |||||||
| Marzano AV, Italy | Varicella-like exanthem as a specific COVID-19–associated skin manifestation | 22 | C/F: Fever, cough headache, weakness, coryza dyspnea, hyposmia, hypogeusia, pharyngodynia, myalgia with skin lesions | – | -Varicella-like papulovesicular exanthem showing:Basket-wave hyperkeratosis,slightly atrophic epidermis, vacuolar degeneration of the basal layer with multinucleate, hyperchromatic keratinocytes & dyskeratotic cells | – | – |
| Gianotti R et al, Italy | Clinical & histopathological study of skin dermatoses in patients with COVID-19 | 05 | C/F: Fever, sore throat & cough with development of skin lesions during hospital stay. | – | −02 cases: Grover & Kaposi’s varicelliform eruption- dyskeratotic cells, ballooning multinucleated cells, sparse necrotic keratinocytes with lymphocytic satellitosis. | – | – |
| −3rd case:Perivascular spongiotic dermatitis with exocytosis with a large nest of Langerhans cells, dense perivascular lymphocytic infiltration with eosinophils around the swollen blood vessels. | |||||||
| −4th case: Papular erythematous exanthema -edematous dermis with abundant eosinophils & lymphocytic vasculitis | |||||||
| −5th case: severe maular haemorrhagic rash d/t fintravascular microthrombi within the small dermal vessels | |||||||
| Xiao F et al, China | Evidence for Gastrointestinal Infection of SARS-CoV-2 | 73 | C/F: 73-year-old hospitalized patients infected with COVID19 | – |
Endoscopic biopsy: (01 case): -Lympho-plasmacytic infiltration along with interstitial edema in the lamina propria of stomach, duodenum, & rectum |
-ACE2: Rarely expressed on esophageal epithelium -abundantly distributed on cilia of glandular epithelia. | – |
| -Occasional lymphocytes in esophageal squamous epithelium | -Viral nucleocapsid protein: cytoplasm of gastric, duodenal & rectum glandular epithelial cell, but not in esophageal epithelium. | ||||||
| Carvalho A et al, USA | Gastrointestinal Infection Causing Hemorrhagic Colitis in COVID 19 | 01 | C/F: 71-year-old hypertensive female with nausea, vomiting, anorexia, diffuse abdominal pain & distention | Leukocytosis with neutrophilia | Edema in lamina propria with intact crypts with no colitis/ischemia/ or inflammatory bowel disease. | – | – |
| CE-CT abdomen: Severe colonic inflammation, most pronounced in ascending, transverse & descending colon | |||||||
| Yang M et al, China | Pathological Findings in the Testes of COVID-19 | 12 | C/F: Postmortem examination of the testes from 12 COVID-19 patients | – | -Sertoli cells showed swelling, vacuolation & cytoplasmic rarefaction, detachment from tubular basement membranes, loss and sloughing into lumens of the intratubular cell mass seen in all cases | ACE2: | No viral particles in all 03 cases tested |
| -Classified injury to seminiferous tubules (ST) as: | -Diffuse expression on Sertoli cells. | ||||||
| Mild – 02 cases | -Strongly expressed on Leydig cells. | ||||||
| Moderate – 05 | -No expression on spermatogonia. | ||||||
| Severe - 04 | |||||||
| Brancatella A et al, Italy | Subacute Thyroiditis After Sars-COV-2 Infection | 01 | C/F: 8-year-old female with fever, neck pain radiating to the jaw & palpitations | -Elevated free thyroxine & free triiodothyronine levels | – | – | – |
| USG neck: Bilateral and diffuse hypoechoic areas | -High inflammatory markers | ||||||
| -Leukocytosis on peripheral smear | |||||||
| Wei L et al, China | Pathology of the thyroid in severe acute respiratory syndrome | 05 | C/F: 05 autopsy cases who initially presented with fever and shortness of breath | Lymphopenia on peripheral smear | All cases: | Apoptosis with TUNEL assay: apoptosis was observed in both the follicular epithelium and the interfollicular region of all patients with SARS | – |
| -Destruction of the follicular epithelium and exfoliation of epithelial cells into the follicle. | |||||||
| -Follicles: dilated/ collapsed/distorted with an irregular outline/ microfollicle configuration. | |||||||
| −01 case: congested with severe damage in follicular epithelium. |
KEY:
DAD - Diffuse alveolar damage.
AFOP -Acute fibrinous and organizing pneumonia.
B/L - bilateral.
AKI - Acute Kidney injury.
C/F - clinical features.
CRP - C-reactive protein.
IL-6 - Interleukin 6.
hs‐TnI - Troponin I.
However, the other rare histological pattern which is being highlighted is acute fibrinous and organizing pneumonia (AFOP) in patients with COVID19 disease. This pattern was described in a short letter highlighting the autopsy findings in 06 French patients [8] and even earlier by Beasley et al. [9] in 2002, where it was first described. Out of these, 05 patients’ pulmonary histological examination showed deposition of extensive intra-alveolar “fibrin balls” (Table 1). They lacked the characteristic hyaline membranes and instead showed intra-luminal loose connective tissue within alveolar ducts and bronchioles (organizing pneumonia). Fibroblastic bodies and fibroblasts surrounding intra-alveolar fibrin along with evidence of vascular injury was also a prominent feature [8]. So, whether AFOP can be described as a histological pattern of COVID19 lung disease associated with a more aggressive course (due to its obliterative pattern), still needs to be studied further.
Xu Z et al., also described the presence of multi-nucleated syncytial cells with atypical enlarged pneumocyte. These cells showed a large nuclei, amphophilic granular cytoplasm, and prominent nucleoli which were suggestive of viral cytopathic changes [10]. Also observed were the presence of focal inflammatory infiltrate in the form of neutrophils, possibly due to secondary infection. The neutrophils, however, observed by Sharon E. Fox et al., in their autopsy findings were partially degenerated and were found to be entrapped in fibres, possibly depicting neutrophil extracellular traps (NETs) [11]. In a perspective, based on review of literature of autopsy results, suggested that NETs, although not very commonly seen in COVID19 disease may have a role in organ damage and increased mortality in COVID19 patients. This may be based on the association of COVID19 with excessive thrombus formation and cytokine storm [12]. However, this hypothesis needs to be proven further.
Tina Schaller et al. in 2020, performed post-mortem examination of 10 patients with other co-morbid conditions (including cardiovascular disease, obesity, pre-exiting lung damage, leukemias and thyroid conditions) who succumbed to COVID19, showed similar results of disseminated DAD as being the most common histological pattern. DAD was observed to be present in different stages of the disease, with heterogenous distribution and being more pronounced in the middle and lower lobes of the lung. They also postulated that the histological features observed in COVID19 infection has close resemblance to other Beta-coronavirus infections which includes Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome [13], which also showed DAD to be the main feature with varying degrees of edema, organization, hyaline membrane and fibrosis [14,15].
The broncho-alveolar lavage (BAL) sample analysed of a 66-year-old male, who presented with a short history of fever and fatigue, on cytomorphological examination showed the presence of scattered alveolar macrophages with fibrino-hematic material. It also showed presence of abundant CD138 positive plasma cells, with few showing plasmablastic features. Few T and B lymphocytes were also seen. The scattered macrophages, occasionally showed nuclear clearing and also intranuclear cytopathic inclusions attributable to SARS-CoV-2 infection. This case report highlighted the abundance of plasma cells in BAL specimens [16].
4. Liver, features being comparatively non-specific
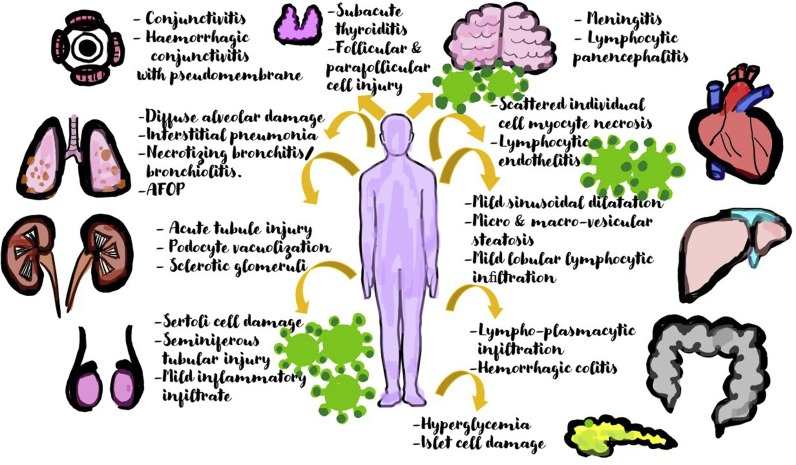
COVID 19, although having its nidus in the lungs has now been seen to show widespread manifestations. (Fig. 1 ) Many studies have been conducted to study the extent of liver involvement by COVID19, owing to the presence of ACE2 receptors not only on the hepatocytes but also on the intra-hepatic bile duct [17].
Fig. 1.
Diagrammatic picture showing the multisystemic involvement of COVID19 and enumerating the commonly observed histo-pathological changes in each affected organ.
The liver, being involved and getting affected can mainly be depicted by the deranged liver biochemical profile, as shown by various studies throughout the world. The most commonly affected levels include, abnormal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and also mildly raised total bilirubin (TB) levels. Yafei Zhang et al., in their study compared various liver function indices of patients with COVID19 with patients suffering from community acquired pneumonia (CAP). However, they found that the liver enzymes levels were not significantly different between the two groups. It was found that 9.57 % and 14.78 % of the 115 cases with COVID19 showed elevated ALT and AST respectively, which was almost comparable to the 11.4 %(ALT) and 21.92 %(AST) of 114 cases with CAP. In contrast, it was observed in their study that there was a significant elevation in the liver enzymes in severe cases compared to the mild ones [18]. On the other hand, another study when compared patients with anormal liver function to patients with normal liver function, found that the elevated ALT levels may be attributable to drinking history. However, they found that C- reactive protein (CRP) ≥20 mg/L and lymphocyte count< 1.1 × 109/L were independently related to the ALT elevation, indicative of hepatic injury suggesting an inflammatory cytokine storm [19].
On the other hand, the histological evaluation in most of the liver biopsies has mainly shown non-specific findings. Yafei Zhang et al., evaluated the liver biopsy of one of the autopsy cases and found mild sinusoidal dilatation with minimal lymphocytic infiltration in the liver tissue [18]. Similarly, another evaluation of liver biopsies in Beijing showed moderate micro-vesicular steatosis and active inflammation in the hepatic lobule portal area. But whether these findings were drug induced (since the patients received lopinavir/ritonavir treatment) or due to COVID19 infection, could not be concluded [10].
Another study which evaluated the microscopic histological changes in 04 cases, observed sinusoidal dilatation with nuclear glycogen accumulation in hepatocytes, focal macro-vesicular steatosis along with dense atypical small lymphocytes infiltrating the portal tracts in case 1 (patient being a known case of CLL). The 2nd case showed features of cirrhosis consistent with his history and, mild zone 3 sinusoidal dilatation along with only mild lobular lymphocytic infiltration in case 3 and 4 [20].
So, owing to the small sample size in most of the studies, whether the changes in the liver depict direct hepatic injury by the infection or drug induced changes [10] (owing to antivirals, antibiotics, traditional Chinese medicine, antipyretics and analgesics) or inflammatory cytokine storm or merely due to hypoxemia [18,19], still lies in its preliminary stage. However, at the same time, it can also be postulated that maybe all these mechanisms might be working hand in hand to bring about these changes.
5. Kidney: the newest organ of interest
Acute kidney injury (AKI) is now well established in patients with COVID19. The spread of the virus from the site of lung involvement, into the circulation with its further spread into the kidney seems to be the likely route [21]. The ACE2 receptor being widely distributed throughout the body is also seen to be present on the proximal tubules of the kidney at the brush border apical membrane. Studies have also shown their presence on the podocytes, which may act as the site of invasion by the viral particles [22].
Various studies have shown renal involvement by COVID19 infection, of which Yachun Chang et al., observed high prevalence of kidney disease in patients hospitalized with COVID19 in the form of raised serum creatinine in approximately 40 % of the cases. They tried to associate kidney involvement of these cases with the patient outcome, and observed that patients with increased serum creatinine levels were more likely to have ICU admissions. They highlighted the role of kidney function tests and advocated its use for prognostication even in patients presenting with mild form of respiratory symptoms [21].
Although slightly lacking, the histopathological features of the kidney with COVID19 has been observed to show renal involvement. On tissue examination, a diverse spectrum of features were observed by Hua su et al., in the very first evaluation of renal tissue. They studied 26 renal post-mortem cases and observed prominent proximal acute tubular injury (ATI) in the form of loss of brush border, vacuolar degeneration and dilatation of the tubular lumen with cellular debris. Occasionally areas of frank necrosis with detachment of epithelium with bare tubular basement membrane were also observed in few cases. The podocytes also showed vacuolization and detachment from the glomerular basement membrane. The peritubular and glomerular capillary loops showed diffuse erythrocyte aggregation (confirmed by CD235a) with obstruction of the lumen along with endothelial damage. Diabetic changes were frequently observed in many cases in the form of nodular mesangial expansion with hyalinosis of arterioles. Also, arteriosclerosis of medium-size arteries with ischemic glomeruli was seen in 11 of the patients with hypertension [23] (Table 1).
Another study, observed sclerotic glomeruli in a 44-year-old COVID19 positive woman who presented with fever, vomiting, worsening cough, and flank pain. She developed AKI superimposed on known chronic kidney disease, type II diabetes and hypertension. They analysed 24 glomeruli, 14 showed sclerotic changes and rest of the intact glomeruli showed features of tuft collapse with overlying epithelial hypertrophy and hyperplasia in the Bowman space. Similar to Hua Su et al., they also reported significant injury to the tubular epithelium, prominently in the proximal tubules in the form of reactive nuclei with mitotic figures, along with denudation of the brush borders [24]. However, they observed no definite viral particles on electron microscopy. Their direct immunofluorescence findings were negative for immune reactants in glomeruli, including IgA, IgG, IgM, C3, C1q, kappa, and lambda [24], which was in contrast to the other study which showed non-specific IgM and C3 trapping [23] (Table 1). But indirect immunofluorescence (by Hua su et al.) with SARS-CoV nucleoprotein when done on the same 06 cases, showed positive granular staining in a nuclear or cytoplasm pattern in tubular epithelium in 03 of the cases [23].
On the other hand, electron microscopic findings showed the presence of coronavirus-like particles in the cytoplasm of renal proximal tubular epithelium, also in the podocytes and comparatively lesser in the distal tubules. The size of the particles ranged from 65−136 nm in diameter, with characteristic spikes, around 20−25 nm, in a solar “corona” like appearance. Other features seen in coronavirus including adjacent double membrane with surface projections, nucleocapsid apposing to the viral envelope, and the interior electron-lucence of the particles had also been distinctively observed. Furthermore, the weak immunostaining with ACE2 on the proximal tubules in close correspondence with the location of the virus particles observed on electron microscopy, further supports the involvement of renal tubules as the major site of involvement by COVID19 infection [23]. Also, the detection of the presence of viral particles in the blood and urine of patients with COVID19 infection by polymerase chain reaction too, supports strong renal involvement [24].
Summarizing, the renal involvement of proximal tubules in the form of ATI, endothelial damage and podocyte vacuolization depict direct cytopathic effect of the virus. Whether collapsing glomerulopathy, which is a fulminant form of kidney disease, as observed in few cases, is associated with COVID19 related cytokine storm or is related to other underlying conditions still needs further evaluation and studies.
6. Cardiac disease in COVID19, involvement analysed
Myocarditis has been known to occur due to multiple causes. Viral induced myocarditis is also relatively common, mostly associated with Coxsackie virus, Epstein-Barr virus, Cytomegalovirus, Echovirus, Hepatitis B virus, Human Herpes virus 6, HIV -1 and Influenza virus B, amongst a few [25]. The abundance of expression of ACE2 receptors on the cardiac myocytes also poses easy feasibility of direct attack by the virus [26]. So the question arises, “Do COVID19 infection too commonly causes myocarditis?”, is still under study and is being evaluated worldwide.
Like in the kidney and liver, the cardiac involvement can be depicted by various elevated bio-markers, which increase in case of myocardial injury. Troponin I and Troponin T appear to be the biomarkers of interest. Various studies have evaluated that increased Troponin I and Troponin T levels are associated with higher mortality among COVID19 patients and are mostly seen in older comorbid patients [27,28]. It was observed by Yishay Szekely et al., that higher troponin levels were associated with higher left atrial pressure and also decreased left ventricular diastolic function [29]. Other markers which have shown increased mortality include Interleukin-6, d-dimer, Lactate dehydrogenase (LDH), Ferritin, which in contrary to Troponins suggest inflammatory cytokine storm, rather than direct myocardial damage as a mechanism for cardiac injury [30].
The autopsy histo-pathological examination of the heart in most of the studies have shown minimal to negligent direct viral cytopathic changes on the myocytes. An autopsy series performed in the United States, highlighted the same and observed only scattered individual myocyte necrosis with no evidence of extensive necrotic areas. They found no evidence of massive lymphocytic infiltration, which is normally seen in viral myocarditis. At the same time most of the cases, grossly only showed cardiomegaly with right ventricular dilatation, mostly owing to pulmonary involvement by the virus [31]. Another study performed on 03 autopsy cardiac specimens, also revealed only scattered individual cell myocyte necrosis rarely with lymphocytes adjacent to but not seen to be surrounding degenerating myocytes, suggesting the possibility of an early viral cytopathic effect. Grossly, most of the specimens showed cardiomegaly and right ventricular dilatation [11].
On the other hand, Tavazzi et al. in 2020, demonstrated the presence of viral particles (electron‐dense spike‐like structures and size between 70–120 nm) in the interstitial cells of the myocardium, with the absence of these particles in endothelial cells or cardiomyocytes, thereby suggesting direct myocardial involvement during the viraemic stage of the disease [32] (Table 1). Zsuzsanna Varga et al., however observed lymphocytic endothelitis in various organs of the body including the cardiac vessels, thereby suggesting impaired microcirculation within the vascular channels [33]. Moreover, it has also been demonstrated in a few cases that the development of fulminant viral myocarditis is mostly associated with a higher viral load [34].
So, as demonstrated by most of the studies the myocardial involvement by COVID19 may not be as severe as compared to other viral infections. Also, the elevated Troponin levels may not truly anticipate the level of myocardial involvement [35,36]. Cardiac involvement seems to be more of a response to the inflammatory storm created by the infection as well as the extensive microvascular involvement owing to massive endothelitis [33,37]. On the other hand, many studies have also proposed the role of various drugs that are being used for treatment of COVID19 as reasons for potential deterioration in cardiovascular conditions. As a common example choroquine, which is extensively being used to reduce the incidence of complications in COVID19 patients, is known to cause cardiac toxicity in the form of restrictive or dilated cardiomyopathy along with conduction abnormalities [38].
7. Central nervous system manifestations in COVID19: Symptoms suggest involvement
With the onset of various neurological symptoms, the common view holds and suggests central nervous system (CNS) involvement by COVID19. The major symptoms mostly presenting include headache, convulsions, change in mental status and symptoms of encephalitis [39]. Furthermore, the appearance of anosmia as a fairly common symptoms, also may suggest neurological involvement [40]. Jason Netland et al., in their experimental study conducted on transgenic mice, showed that the entry of SARS-CoV virus into the brain occurred by the olfactory bulb, further leading to trans-neuronal spread. They observed significant fraction of neurons to be expressing viral antigen by day 4 and suggested that the death of the mice occurred due to the death of infected neurons, particularly the neurons located in the cardiorespiratory centres of the medulla [41].
A histo-pathological examination of 06 patients who died of COVID19 in Germany, revealed meningitis, lymphocytic panencephalitis, localized perivascular inflammation and brainstem neuronal cell damage, in almost all the cases examined. Also, they observed that all the younger aged cases (<65yrs) exhibited diffuse petechial haemorrhages owing to massive intracranial haemorrhage due to COVID19 associated coagulopathy. Hence, in the younger cases, they attributed CNS haemorrhage to be a fatal complication. However, they attributed none of these changes to be due to COVID19 induced hypoxia [42]. On the other hand, Isaac H. Solomon et al., suggested only hypoxia induced injury in the cerebrum and cerebellum, with no evidence of meningitis, encephalitis or thrombi and vasculitis. They also found no cytoplasmic viral staining on immunohistochemical examination [43].
The post-mortem brain examination of a 71-year-old male who died of COVID19 revealed widespread haemorrhagic white matter lesions distributed throughout the cerebral hemisphere. Histo-pathology showed a perivascular acute disseminated encephalomyelitis (ADEM)-like appearance, along with features of axonal damage. Also, observed were clusters of macrophages at the periphery of the lesions. (Table 1) Only scant CD3 positive perivascular lymphocytes were observed. Hence, this case report highlighted that the postulated mechanism for this type of insult could be due to para-infectious post viral autoimmune response apart from primary vascular disease [44]. Another case study, reported a case of acute necrotizing encephalopathy in a patient with COVID19, which they postulated might be due to intracranial inflammatory cytokine storm like seen in patients with Influenza. In this case symmetrical hypoattenuation within the bilateral medial thalami was seen on non-contrast CT imaging. MRI showed haemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes and subinsular regions suggestive of acute necrotizing encephalopathy [45].
The neurological symptoms so commonly appearing in patients with COVID19 infection, could be due to various reasons. Some of the few explanations include, direct cytopathic effect by the virus, hypoxia induced changes, cerebrovascular insults due to coagulopathy and also metabolic changes [46].
8. Cutaneous involvement: a rare manifestation
With the establishment of COVID19 as having multisystemic manifestations, cutaneous involvement by the virus is also now being widely studied. Various presentations have been described ranging from maculopapular rash, vesicular rash, petechia/purpura, perniosis (chilblains), urticaria, livedo racemosa, and also distal ischemia [47]. I Hamming et al., studied the localization of ACE2 receptors on various tissues of the body and found ACE2 to be present in the basal cell layer of the epidermis (including basal layer of hair follicles) and smooth muscle cells surrounding the sebaceous glands with weak cytoplasmic staining in sebaceous gland cells. Also, strong granular staining was observed in eccrine gland cells [5].
C. Galvan Casaset al., in Spain described 05 cutaneous patterns found to be associated with COVID19 which included pseudo-chilblain, monomorphic vesicles, urticaria, maculo-papules and livedo/ necrosis. Monomorphic disseminated vesicular lesions, which contained haemorrhagic content along with pseudo-chilblain, they suspected might be useful indicators of the disease. On the other hand, urticarial and maculopapular lesions they postulated might be associated with various drug reactions and the occurrence of livedo or necrosis suggesting an occlusive vascular disease [47]. Zhang et al., similarly, in the meanwhile observed acral ischemic changes in the form of dry gangrene, cyanosis and skin bullae in 07 patients through which they highlighted the hypercoagulable states commonly seen in COVID19 patients [48].
Another study in Italy, evaluated skin manifestations in 18 patients, of which 08 showed skin involvement at the onset of the disease, and in 10 patients, the manifestations happened during the course of hospitalization. Erythematous rash mostly present on the trunk was the most commonly observed presentation in 14 patients followed by widespread urticaria seen in 03 patients and chickenpox like vesicles seen in only 01 patient [49]. Varicella-like papulovesicular exanthem has also been described as a rare yet specific cutaneous manifestation of COVID19, by Marzano AV et al. They described these lesions to be dominant on the trunk in a scattered distribution with a few lesions being associated with mild pruritis, in others pruritis being absent. These lesions mostly appeared 3 days after the onset of systemic symptoms and disappeared 8 days later. Histo-pathological evaluation of 04 cases revealed basket-wave hyperkeratosis with mildly atrophic epidermis. Also seen was vacuolar degeneration of the basal layer with hyperchromatic multinucleated keratinocytes and dyskeratotic cells. However, they observed significant absence of inflammatory infiltrate [50] (Table 1).
Similarly, another study in Northern Italy which examined 05 patients with skin lesions observed maculopapular lesions on the trunk of two patients with fever, cough and sore throat, suggestive of Grover disease. On histo-pathology, they observed dyskeratotic cells, ballooning multinucleation of cells along with presence of sparse necrotic keratinocytes with lymphocytic satellitosis, hence showing simultaneous features of both Grover and Kaposi’s varicella form eruption. The 3rd patients’ skin lesions were studied over a series of three punch biopsies at different stages of the disease. The lesion began with an exanthema located on the trunk and limbs, which morphologically revealed diffuse small telangiectatic blood vessels in the upper dermis, subsequently showing within the epidermis, nests of Langerhans cells and finally progressing to a purpuric maculo-papulo-vesicular rash. The rash revealed perivascular spongiotic dermatitis with exocytosis. Also, there were large nests of Langerhans cells along with eosinophil rich dense perivascular lymphocytic infiltration surrounding the swollen blood vessels with extravasated RBCs. The 4th patient developed papular erythematous exanthema which showed an edematous dermis with abundant eosinophils with features of lymphocytic vasculitis, with the 5th patient revealing a severe macular haemorrhagic rash attributed to the formation of intravascular microthrombi within the small dermal vessels. The development of the exanthema they attributed to the hematogenous spread of the virus along the cutaneous vascular system with subsequent activation of the immune system involving the recruitment of lymphocytes and Langerhans cells present along the skin-nodal pathway [51].
Hence, the skin involvement by the virus, presenting as a vast spectrum of skin manifestations although slightly uncommon, has emerged as a new area of interest for studying COVID19. Although, the exact mechanism is still not clear, postulated theories include hematogenous dermal spread of the virus, with further immune activation and cytokine release. There may also be the activation of Langerhans cells explaining the development of spongiosis and vasodilation. Few other symptoms like livedo and gangrene however, can be clearly explained by the propensity to form microthrombi during the course of the infection [47,48,51]. However, this calls for more studies to further establish the specificity of these skin lesions to COVID19 infection.
9. Gastrointestinal tract and COVID19: is there a link?
With the isolation of COVID 19 in stool samples, there is increased speculation about gastro-intestinal tract (GIT) involvement by the virus during the course of the disease. Many studies have been conducted throughout the world confirming the same, and also there have been cases demonstrating the isolation of COVID19 virus in the stool samples of patients presenting without any GIs symptoms, thus raising suspicion of an alternate route of infection through feco-oral transmission [52,53]. Yongjian Wu et al., also observed in their study that fecal samples showed positivity for SARS-CoV-2 RNA for a mean period of 11.2 days even after the respiratory tract samples tested negative for SARS-CoV-2 RNA, hence suggesting viral transmission through faeces as a potential risk in people residing in closed and contained premises [54].
The role of ACE2 receptors and transmembrane serine protease 2 (TMPRSS2) was emphasized by Hao Zhang et al., in the pathogenesis of COVID19 in the GIT. They found that ACE2 and TMPRSS2 were not only co-expressed in the alveolar type II cells of the lungs but also in the esophageal glandular and upper GI epithelial cells and the absorptive enterocytes of the ileum and colon, thereby, suggesting an entry route for COVID19 virus in the digestive tract [55]. Tong Meng et al., also stated that since the entry of COVID19 into host cells depends on the cell receptor recognition as well as proteases cleaving, the host cells should be co-expressing both ACE2 receptors and cell proteases TMPRSS2 [56]. Similarly, immunofluorescent data has also shown ACE2 protein to be expressed on the glandular cells of gastric, duodenal and rectal epithelium [57].
The most common GIT symptoms include diarrhoea, nausea, vomiting and anorexia [58,59]. In around 50.5 % (103 out of 204) of the patients admitted to a hospital in China were found to be showing one or more digestive symptoms. Out of these 103 patients, 97 presented with both respiratory as well as digestive symptoms and 06 patients only developed digestive symptoms without any respiratory symptoms [59]. Ren Mao et al., in their systemic review and meta-analysis postulated that patients presenting with gastrointestinal symptoms tended to have an increased risk of acute respiratory distress syndrome and liver injury. They also stated that patients with severe COVID-19 disease were more likely to present with abdominal pain in comparison to patients with non-severe COVID disease [60].
The histo-pathological data regarding the COVID19 changes in the GIT is very limited. The endoscopic biopsy of 01 patient done by Fie Xiao et al., revealed no significant damage of the esophageal, gastric, duodenal and rectal mucosal epithelium on histological examination. However, they reported lympho-plasmacytic infiltration along with interstitial edema in the lamina propria of the stomach, duodenum, and rectum along with occasional lymphocytes in esophageal squamous epithelium [61] (Table 1). Another autopsy report showed segmental dilatation and stenosis in the small intestine of an 85-year-old male positive with COVID19 infection [62]. A case of hemorrhagic colitis was reported in United States, in a 71-year-old COVID19 positive lady, who developed nausea, vomiting, anorexia, diffuse abdominal pain with distention and 10–20 bloody bowel movements per day. Her CT with intravenous contrast, revealed severe colonic inflammation (more pronounced in the ascending, transverse, and descending colon). The endoscopic evaluation showed patchy focal erythema without ulceration in the descending colon, sigmoid colon, and rectum and the histological examination of the colon and rectal biopsies revealed edema in the lamina propria with intact crypts with no evidence of infective colitis/ischemia/or inflammatory bowel disease [63].
In conclusion, GIT involvement by COVID19 is now well established with widespread GI manifestations of the disease [[58], [59], [60]]. Feco-oral transmission could be another mode of transmission by the virus [[52], [53], [54]] which needs to be further studied so that adequate precautions and measures can be instilled to curb further spread of the virus. However, information regarding the histo-pathological changes in the GIT caused by the virus, still remains relatively sparce.
10. Ocular involvement in COVID19: new symptoms emerge
COVID19 being a respiratory virus with, aerosol and droplet transmission being the major mode of transmission, the exposed conjunctival surfaces of the eyes also seem to be an important portal of entry for the virus, which also explains the new emerging ocular symptoms in infected patients [64]. Newer studies, like previously discussed, highlight the role of both ACE2 and TMPRSS2 in the entry of the virus [55,56]. Dia Ma et al. in 2004, in their study stated that the cornea as compared to the conjunctiva had higher propensity to be infected by COVID19 as mouse corneal tissue showed higher expression of ACE2 and TMPRSS2 [65].
The most common symptom being described in humans is conjunctivitis, [66] with pyogranulomatous anterior uveitis, choroiditis with retinal detachment and retinal vasculitis being described in felines [67]. A 65-year-old COVID19 positive woman admitted to a hospital in Italy presented with cough, sore throat, coryza, and bilateral conjunctivitis. An ocular swab when done detected the presence of viral RNA in the ocular sample collected [68]. Similarly, Sergio Zaccaria Scalinci et al., also studied 05 patients whose preliminary symptoms included features of acute conjunctivitis which showed conjunctival hyperemia, epiphora, discharge, and photophobia [69]. Similarly, in a case series of 38 patients in Hubei, China, 12 patients presented with ocular symptoms which included epiphora, conjunctival congestion and chemosis. Out of these 38 patients, 02 patients showed a positive COVID19 test, done on the conjunctival specimen. However, they also observed that patients who presented with ocular symptoms were more likely to have leukocytosis and neutrophilia along with higher procalcitonin, CRP, and LDH levels as compared to patients without ocular symptoms. This could possibly suggest that ocular symptoms appear more commonly in patients with severe pneumonia [70].
The presence of COVID19 was evaluated in the tears and conjunctival secretions, by Jianhua Xia MM et al. They reported that out of the 30 COVID19 positive patients that they studied (21 had common disease and 09 had severe disease), the only tear and conjunctival sample that came out to be positive was that of the only 01 patient who presented with symptoms of conjunctivitis. Hence, they reported detection of no viral RNA in the tear fluid or the conjunctival secretions of the severe or common-type patients without conjunctivitis [71].
The vast diversity of ocular symptoms of COVID19 along with its late sequelae, was also highlighted by Valentin Navel et al., who reported haemorrhagic conjunctivitis with pseudomembrane in a 63-year-old male who tested positive for COVID19. He initially presented with only respiratory symptoms but on day 17, viral conjunctivitis developed with subsequent formation of follicles, petechias, tarsal hemorrhages and chemosis. Also, observed was the development of a pseudomembrane which was thin yellowish-white translucid on the tarsal conjunctiva of lower lids which could be easily peeled off without bleeding. However, COVID19 viral RNA could not be demonstrated in the conjunctival secretions and tears of the patient [72].
Despite paucity of literature, the involvement of COVID19 in the occurrence of various ocular symptoms, with conjunctivitis being the commonest, seems very likely [66,68,69]. The detection of COVID19 RNA in the tear and conjunctival secretions, even though seen only in a few cases, confirms the same [[69], [70], [71],73]. One of the widely accepted theories include direct inoculation of the virus, through aerosol and droplet into the conjunctival surface with subsequent movement via the nasolacrimal duct or hematogenous spread [[74], [75], [76]].
11. Endocrine system and COVID19: understanding the intricate changes
The presence of ACE2 receptors on endocrine organs like pancreas and testis [77,78] along with multiple case studies reporting COVID19 related endocrinal abnormalities, raises the question of a possible link between the two.
11.1. Genital system
The effect of COVID19 on the testis is still debated, however, the abundance of ACE2 receptors on the testis including spermatogonia, Leydig and Sertoli cells, raises the need for more research on the topic [78]. Ling Ma et al., in order to study the effects of COVID19 on male sex hormones, compared the levels of sex-related hormones between COVID19 positive reproductive-aged men and age-matched healthy men. They found the levels of serum luteinizing hormone (LH) to be significantly increased in COVID19 positive men but observed the ratio of testosterone to LH and the ratio of follicle stimulating hormone to LH were dramatically decreased in males with COVID19 [79]. An autopsy series conducted on the testicular tissue of 11 COVID19 men in China, revealed Sertoli cells to be most prominently affected showing cellular swelling, vacuolation and cytoplasmic rarefaction along with detachment from the tubular basement membrane. They also observed sloughing and loss of the intratubular cell mass and graded the injury to the seminiferous tubular based on its severity into mild (02), moderate (05), or severe (04) (Table 1). Also, interstitial edema with mild inflammatory infiltrates comprising of CD3-positive T lymphocytes and CD68-positive histiocytes was observed and 08 out of 11 cases showed variable degrees of spermatogenic alteration, thereby highlighting evidence of testicular injury in COVID19 positive men. However, the presence of viral RNA was detected in the testis of only 01 case [80]. Scrotal discomfort was reported by 19 % of COVID19 positive cases under evaluation in China, suggestive of viral orchitis, however, no comprehensive genitourinary examination was performed to confirm the same and none of the semen samples collected from these patients detected COVID19 RNA [81]. With the lack of enough evidence of the presence of viral RNA in testicular tissue and seminal fluid raises various doubts, but the development of viral orchitis and Sertoli cell changes during the course of COVID19 infections, calls for the need to evaluate and study more data.
There is paucity of literature regarding the female genital tract and COVID19, however, a recent study suggested a possible link between polycystic ovarian syndrome (PCOS) and COVID19, stating that females with PCOS might be at a higher risk of developing adverse COVID19 related outcomes [82]. But, the effect of COVID19 infection on the female reproductive health still remains unexplored.
11.2. Thyroid
Thyroid involvement, especially the development of subacute thyroiditis (SAT) post-viral infection is quite common [83]. An isolated case report of an 18-year-old female, was reported by Alessandro Brancatella et al., in Italy who developed fever, neck pain radiating to the jaw along with palpitations, 15 days post a positive COVID19 report. Her laboratory tests showed increased free thyroxine and free triiodothyronine levels, and elevated inflammatory markers and leukocytosis. The neck ultrasound revealed bilateral and diffuse hypoechoic areas and was diagnosed with SAT [84]. An autopsy series of 05 patients in 2007, reported thyroid dysfunction in SARS-CoV patients, and observed parafollicular and follicular cell injury along with evidence cellular necrosis. They found few of the thyroid follicles to be dilated, others collapsed and distorted with irregular outline, also with others showing a microfolliclar configuration [85] (Table 1). But with just few studies in this area the knowledge of the effect of COVID19 infection on the thyroid gland is still scare.
11.3. Pancreas
Exocrinal involvement in the form of acute pancreatitis was reported in a 35-year-old COVID19 positive obese female [86] and studies also state that the high and aberrantly glycosylated ACE2 in uncontrolled hyperglycemia, could aid the cellular entry of COVID19 thereby causing a much more severe disease [87]. Increased blood glucose levels and abnormal variability of glucose can be seen in diabetic patients with COVID19 disease possibly due to stressful conditions triggering elevated release of hyperglycemic hormones [88]. Jin-Kui Yang et al., compared 39 SARS patients who had no previous diabetes and had received no steroid treatment with 39 matched healthy siblings in a 3-year follow-up period. They found that 20 patients of the 39 developed diabetes during hospitalization however after 3 years, only two of these patients continued to be diabetic. Hence, they suggested that the localization of ACE2 receptors in the endocrine part of the pancreas lead to increased uptake of the SARS coronavirus, subsequently leading to uncontrolled hyperglycemia and development of acute diabetes [89].
Thereby, literature suggests that the presence of diabetes not only could act as a risk factor for the development of more severe COVID19 disease, but also COVID19 infection in a previously euglycemic patient could cause a sustained inflammatory state causing hyperglycemia and subsequently leading to the development of diabetes.
11.4. Does COVID19 adversely affect outcome in pregnancy?
Maternal to fetal transmission of infections are well known especially for the TORCH group of infections, as well as with the newer viruses including Zika and Ebola [90]. The prospect of whether there is vertical transmission of COVID19 from mother to infant still mostly remains unexplored. Various reports exist of miscarriages, intrauterine growth restriction and preterm birth in women infected with SARS-CoV, however no vertical transmission of infection was noted by Schwartz DA, of SARS-CoV in these women [91].
A recent study in China, analysed 10 neonates born to COVID-19 positive mothers, out of which nasopharyngeal swabs were collected from 09 of these neonates who tested negative for COVID19. They clinically assessed all the neonates and concluded that the presence of perinatal COVID19 infection may cause adverse effects like: premature labor, fetal distress, neonatal respiratory distress, thrombocytopenia with abnormal liver function, and even death [92]. On the contrary, Xiaotong Wang et al. in 2020, reported the case of a 30-week COVID19 positive pregnant woman with atypical pneumonia who delivered a preterm healthy baby with no evidence of COVID-19 [93]. Similarly, L Zhang et al., compared the pregnancy outcomes of 16 COVID19 positive women with 45 women without COVID19, they too reported no significant difference between the two groups in terms of blood loss, neonatal birth weight, fetal distress, meconium-stained amniotic fluid and neonatal asphyxia. Also, none of the neonates tested positive for COVID19 on RT-PCR [94]. Huijun Chen et al., also when retrospectively analysed 09 pregnant woman with COVID 19 they found no significant difference in the clinical characteristics of COVID19 pneumonia in pregnant women as compared to non-pregnant adult patients with COVID19 pneumonia, and reported no fetal infection due to intrauterine vertical transmission [95].
Reviewing the current available literature, none of the studies so far provided evidence of maternal to fetal transmission of COVID19 infection, however due to the scantiness of the studies available so far, the conclusion of no vertical transmission is still premature.
12. Conclusion
COVID19 with its myriad of symptoms is now being well established as a pan-systemic disease. The lungs, however still seem to be the most commonly affected organ, with multiple bilateral patchy ground glass opacities being the most frequently observed radiological findings. DAD with intra-alveolar fibrinous exudates and loose interstitial fibrosis can safely be described as the most common histological pattern observed in pulmonary biopsies in patients with COVID19. GI involvement of the disease, presenting in the form of diarrhoea raises the speculation of feco-oral transmission of COVID19, although the histopathological findings of the gastro-intestinal tract and the liver still appear quite non-specific. Information regarding COVID19 as a renal disease, is sparse, however, few studies have established the presence of Corona-virus like particles in the cytoplasm of renal proximal tubular epithelium, podocytes and less so in the distal tubules.
The histological examination of the heart appears non-specific findings with nil to minimal epicardial and myocardial inflammation, ruling out the occurrence of viral myocarditis, which is commonly seen in other viral infections. Evidence of meningitis and lymphocytic panencephalitis in patients with COVID19 has also been observed by few studies, but, whether to describe these changes as due to direct viral cytopathic changes or due to hypoxia and other metabolic changes still remains unclear. Conjunctivitis is a new symptom, now increasing being seen in cases with COVID19, and the expression of ACE2 and TMPRSS2 on the corneal surface can also act as a new portal of entry for the COVID19 virus. Cutaneous involvement, although quite rare, has also been observed in the form of livedo and gangrene due to microthrombi formation owing to a hypercoagulable state.
The presence of ACE2 receptors on the testis, and the evidence of Sertoli cell injury with varying damage to the seminiferous tubules, raises the question of prolonged long term effect on the male reproductive health. The development of a hyperglycaemic state in patients with COVID19 also poses the threat of developing acute diabetes, as observed in some studies. However, there remains no evidence of maternal to fetal transmission of COVID19 infection till date, to the best of our knowledge.
Declaration of Competing Interest
None.
Submission declaration and verification
Not been published previously.
Study registration
No registration was required as the article submitted is a review article.
Ethical clearance
No ethical clearance was required as the article submitted is a review article.
Author contributions
The authors mentioned in the article confirm being the sole contributors of this work and have approved it for publication.
References
- 1.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;39:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain A. COVID-19 and lung pathology. Indian J. Pathol. Microbiol. 2020;63(2):171–172. doi: 10.4103/IJPM.IJPM_280_20. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Zhou P., Wei Y., Zhang J., Huang L., Zhang C., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copin M.C., Parmentier E., Duburcq T., Poissy J., Lille Mathieu D. COVID-19 ICU and Anatomopathology Group, Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beasley M.B., Franks T.J., Galvin J.R., Gochuico B., Travis W.D. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch. Pathol. Lab. Med. 2002;126:1064–1070. doi: 10.1043/0003-9985(2002)126<1064:AFAOP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Marki B., et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanden Brand J.M., Smits S.L., Haagmans B.L. Pathogenesis of Middle East respiratory syndrome coronavirus. J. Pathol. 2015;235:175–184. doi: 10.1002/path.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giani M., Seminati D., Lucchini A., Foti G., Pagni F. Exuberant plasmocytosis in Bronchoalveolar Lavage specimen of the first patient requiring extracorporeal membrane oxygenation for SARS-CoV-2 in Europe. J. Thorac. Oncol. 2020;15:e65–e66. doi: 10.1016/j.jtho.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A., et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCov infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 18.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Li S., Xu M., Yu P., Zheng S., Duan Z., et al. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv. 2020 doi: 10.1101/2020.02.28.20028514. preprint. [DOI] [Google Scholar]
- 20.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye M., Wysocki J., William J., Soler M.J., Cokic I., Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 2006;17(11):3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 23.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kissling S., Rotman S., Gerber C., Halfon M., Lamoth F., Comte D., et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennert R., Crijns H.J., Heymans S. Acute viral myocarditis. Eur. Heart J. 2008;29(17):2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G., Hu R., Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens. Res. 2020;43(6):588–590. doi: 10.1038/s41440-020-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan H., Zhang L., Huang B., Zhu M., Zhou Y., et al. Cardiac injuries in patients with coronavirus disease 2019: not to be ignored. Int. J. Infect. Dis. 2020;96:294–297. doi: 10.1016/j.ijid.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Association of cardiovascular disease and myocardial injury with outcomes of patients hospitalized with 2019-corona virus disease (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020;63(3):390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman A.R., Bularga A., Mills N.L. High-sensitivity cardiac troponin can Be an ally in the fight against COVID-19. Circulation. 2020;141(22):1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 37.Inciardi Rm, Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aslam S., Mehra M.R. COVID-19: yet another coronavirus challenge in transplantation. J. Heart Lung Transpl. 2020;39(5):408–409. doi: 10.1016/j.healun.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kipnis J. Immune system: the “seventh sense”. J. Exp. Med. 2018;215(2):397–398. doi: 10.1084/jem.20172295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., et al. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)‑like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y., Xu X., Yang L., Liu C., Yang C. Nervous system damage after COVID-19 infection: Presence or absence? Brain Behav. Immun. 2020;87:55. doi: 10.1016/j.bbi.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández-Nieto D., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Cao W., Xiao M., Li Y.J., Yang Y., Zhao J., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(Mar (0)):E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. Chinese. [DOI] [PubMed] [Google Scholar]
- 49.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 50.Marzano A.V., Genovese G., Fabbrocini G., Pigatto P., Monfrecola G., Piraccini B.M., et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J. Am. Acad. Dermatol. 2020;83(1):280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gianotti R., Zerbi P., Dodiuk-Gad R.P. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J. Dermatol. Sci. 2020;98(2):141–143. doi: 10.1016/j.jdermsci.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of Sars-CoV-2 viral rna in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69(6):1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 56.Meng T., Cao H., Zhang H., Kang Z., Xu D., Gong H., et al. The insert sequence in SARS--CoV-2 enhances spike protein cleavage by TMPRSS. bioRxiv. 2020 doi: 10.1101/2020.02.08.926006. [DOI] [Google Scholar]
- 57.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6) doi: 10.1053/j.gastro.2020.02.055. 1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of novel coronavirus infection in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan L., Mu M., Ren H.G., Tan J.Y., Li X.H., Liang J., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J., et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6) doi: 10.1053/j.gastro.2020.02.055. 1831-1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z., et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. English, Chinese. [DOI] [PubMed] [Google Scholar]
- 63.Carvalho A., Alqusairi R., Adams A., Paul M., Kothary N., Peters S., et al. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am. J. Gastroenterol. 2020;115(6):942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Broff B. COVID-19: ocular manifestations, ocular secretions, and ocular portal of entry. Adv. Ophthalmol. Vis. Syst. 2020;10(2):48–49. doi: 10.15406/aovs.2020.10.00382. [DOI] [Google Scholar]
- 65.Ma D., Chen C., Jhanji V., Xu C., Yuan X.L., Liang J.J., et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye. 2020;34:1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., et al. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doherty M.J. Ocular manifestations of feline infectious peritonitis. J. Am. Vet. Med. Assoc. 1971;159:417–424. PMID: 5107089. [PubMed] [Google Scholar]
- 68.Colavita F., Lapa D., Carletti F., Lalle E., Bordi L., Marsella P., et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA. Ann. Intern. Med. 2020;173:242–243. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scalinci S.Z., Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Navel V., Chiambaretta F., Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am. J. Ophthalmol. Case Rep. 2020;19 doi: 10.1016/j.ajoc.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun X., Zhang X., Chen X., Chaohua D., Xiaojing Z., Weiyong L., et al. The infection evidence of SARS-COV-2 in ocular surface: a single-center cross-sectional study. medRxiv. 2020 doi: 10.1101/2020.02.26.20027938. [DOI] [Google Scholar]
- 75.Qing H., Li Z., Yang Z., Shi M., Huang Z., Song J., et al. The possibility of COVID-19 transmission from eye to nose. Acta Ophthalmol. (Copenh) 2020;98(3):e388. doi: 10.1111/aos.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seah I., Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul. Immunol. Inflamm. 2020;28(3):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020;18(9) doi: 10.1016/j.cgh.2020.04.040. 2128-2130.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan C., Li K., Ding Y., Lu W., Wang J., et al. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. medRxiv. 2020 doi: 10.1101/2020.02.12.20022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma L., Xie W., Li D., Shi L., Mao Y., Xiong Y., et al. Effect of SARS-CoV-2 infection upon maL.e gonadal function: a single center-based study. medRxiv. 2020 doi: 10.1101/2020.03.21.20037267. [DOI] [Google Scholar]
- 80.Yang M., Chen S., Huang B., Zhong J.M., Su H., Chen Y.J., et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur. Urol. Focus. 2020;6(5):1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan F., Xiao X., Guo J., Song Y., Li H., Patel D.P., et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil. Steril. 2020;113(6):1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kyrou I., Karteris E., Robbins T., Chatha K., Drenos F., Randeva Hs. Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked female patient population at potentially higher risk during the COVID-19 pandemic. BMC Med. 2020;18(1):220. doi: 10.1186/s12916-020-01697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Desailloud R., Hober D. Viruses and thyroiditis: an update. Virol. J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brancatella A., Ricci D., Viola N., Sgrò D., Santini F. Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J. Clin. Endocrinol. Metab. 2020;105(7):dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei L., Sun S., Xu C.H., Zhang J., Xu Y., Zhu H., et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum. Pathol. 2007;38(1):95–102. doi: 10.1016/j.humpath.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aloysius M.M., Thatti A., Gupta A., Sharma N., Bansal P., Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20(5):1026–1027. doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 epidemic. J. Med. Virol. 2020;92(7):770–775. doi: 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang A., Zhao W., Xu Z., Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwartz D.A. The origins and emergence of Zika virus, the newest TORCH infection: what’s old is new again. Arch. Pathol. Lab. Med. 2017;141(1):18–25. doi: 10.5858/arpa.2016-0429-ED. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from Coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin. Infect. Dis. 2020;71(15):844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L., Jiang Y., Wei M., Fu Z., Ke C., Zhi Z., et al. Zhonghua Fu Chan Ke Za Zhi Z. 2020;55(3):166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 95.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang P.W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]