Abstract
Chemotherapy, targeted therapy, and immunotherapy are used against advanced non-small cell lung cancer. A clinically efficacious method for relieving the adverse events associated of such therapies is lacking. Fifty-eight adult patients were enrolled in our trial to relieve pulmonary symptoms or the adverse events of drugs. Twenty patients who refused drug treatment were assigned equally and randomly to a hydrogen (H2)-only group and a control group. According to the results of tumor-gene mutations and drug-sensitivity tests, 10, 18, and 10 patients were enrolled into chemotherapy, targeted therapy, and immunotherapy groups in which these therapies were combined with H2-therapy, respectively. Patients underwent H2 inhalation for 4–5 hours per day for 5 months or stopped when cancer recurrence. Before study initiation, the demographics (except for tumor-mutation genes) and pulmonary symptoms (except for moderate cough) of the five groups showed no significant difference. During the first 5 months of treatment, the prevalence of symptoms of the control group increased gradually, whereas that of the four treatment groups decreased gradually. After 16 months of follow-up, progression-free survival of the control group was lower than that of the H2-only group, and significantly lower than that of H2 + chemotherapy, H2 + targeted therapy, and H2 + immunotherapy groups. In the combined-therapy groups, most drug-associated adverse events decreased gradually or even disappeared. H2 inhalation was first discovered in the clinic that can be used to control tumor progression and alleviate the adverse events of medications for patients with advanced non-small cell lung cancer. This study was approved by the Ethics Committee of Fuda Cancer Hospital of Jinan University on December 7, 2018 (approval No. Fuda20181207), and was registered at ClinicalTrials.gov (Identifier: NCT03818347) on January 28, 2019.
Keywords: adverse event, chemotherapy, hydrogen, immunotherapy, non-small-cell lung cancer, NSCLC, PFS, progression-free survival, targeted drug
INTRODUCTION
Lung cancer is the most prevalent form of cancer-related disease worldwide, of which non-small-cell lung cancer (NSCLC) accounts for ~85%.1 Many patients are at an advanced stage of lung cancer when the diagnosis is made,2 and progression-free survival (PFS) is usually < 6 months.3 In these cases, the tumors cannot be resected; therefore, chemotherapy,4 targeted therapy5 and immunotherapy6 are common treatment options. Each treatment has different indications, and also produces different adverse events. Serious adverse events (e.g., severe granulocytopenia,7 thrombocytopenia,8 abnormal liver function9) often lead to significant changes in vital signs and patients are forced to stop or change medications.
Hydrogen (H2) is an anti-inflammatory, antioxidant, and antiapoptotic molecule. It can diffuse into mitochondria, neutralize reactive oxygen species selectively,10,11 and restore cell viability by regulating expression of various genes.12 In animal experiments, H2 has been demonstrated to alleviate the serious adverse events caused by chemotherapy,13,14 and targeted therapy.15 H2 has been used in clinical trials of multiple non-neoplastic diseases, indicating the safety of H2 gas inhalation.16,17
In the present study, H2 therapy was used to control cancer progression and alleviate the adverse events of multiple standard therapies in patients with advanced NSCLC.
SUBJECTS AND METHODS
The inclusion and exclusion criteria
This clinical trial was registered at ClinicalTrials.gov (Identifier: NCT03818347) on January 28, 2019. The enrolled patients were divided into five groups according to the precise medical test results of tumors. The inclusion criteria were patients with: stage-III or -IV NSCLC diagnosed by imaging and pathology with specialist doctor; tumor number 1–6; maximum tumor length < 2 cm; Karnofsky performance status (KPS) score ≥ 70; expected survival time > 6 months; platelet count ≥ 80 × 109/L; white blood cell count ≥ 3 × 109/L; neutrophil count ≥ 2 × 109/L; hemoglobin ≥ 80 g/L. The exclusion criteria were patients with: a cardiac pacemaker; brain metastasis; grade-3 hypertension or diabetic complications; severe cardiac and pulmonary dysfunction. This study protocol received ethical approval from the Ethics Committee of Fuda Cancer Hospital of Jinan University on December 7, 2018 (approval No. Fuda20181207). Written informed consent was obtained from each patient.
Subjects
Between June and September 2019, 58 patients with advanced NSCLC at Fuda Cancer Hospital of Jinan University met the inclusion criteria mentioned above and were enrolled in the study. Thirty-four patients had surgery before enrollment, and 24 patients were in advance stage when diagnosis.
Immunohistochemical assays of the ratio of programmed cell death-1: programmed cell death-1 ligands, tumor mutation burden, and microsatellite instability in tumor specimens were undertaken. Based on the results, 10 patients were administered a drug based on antibodies against programmed cell death-1 (Nivolumab [Opdivo®, Bristol-Myers Squibb, New York, NY, USA] or Pembrolizumab [Keytruda®, Merck, Kennyworth, NJ, USA]). Through detection of gene mutations in tumor specimens, 18 patients were selected to be given targeted therapy. Patients with a mutation in the epidermal growth factor receptor gene were administered Osimertinib (Tagrisso®, AstraZeneca, London, UK), Gefitinib (Iressa®, AstraZeneca) or Erlotinib (Tarceva®, Roche, Basel, Switzerland). Patients with a mutation in the anaplastic lymphoma kinase gene or receptor tyrosine kinase-1 gene were administered Crizotinib (Xalkori®, Pfizer, New York, NY, USA). Based on the data of drug-sensitivity tests, 10 patients were administered chemotherapy (Cisplatin or Carboplatin [both from Qilu Pharmaceutical, Haikou, Hainan Province, China]).
The remaining 20 patients who were not sensitive to common drugs or who failed to respond to treatment by common drugs were distributed evenly in the H2-monotherapy group or control group. Three groups of patients who had H2 treatment combined with another treatment (immunotherapy, targeted therapy or chemotherapy) started therapy before enrollment of our clinical trial. Most of those patients experienced significant effects on cancer-related lung symptoms after taking combination therapy, but new drug-related adverse events emerged. Lung symptoms or drug-related adverse events were compared before and after H2 treatment. The comparison of lung symptoms before hydrogen treatment and the changes of tumor or drug-related symptoms in each group after hydrogen treatment are shown in Figure 1. This study followed the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) Statement.
Figure 1.
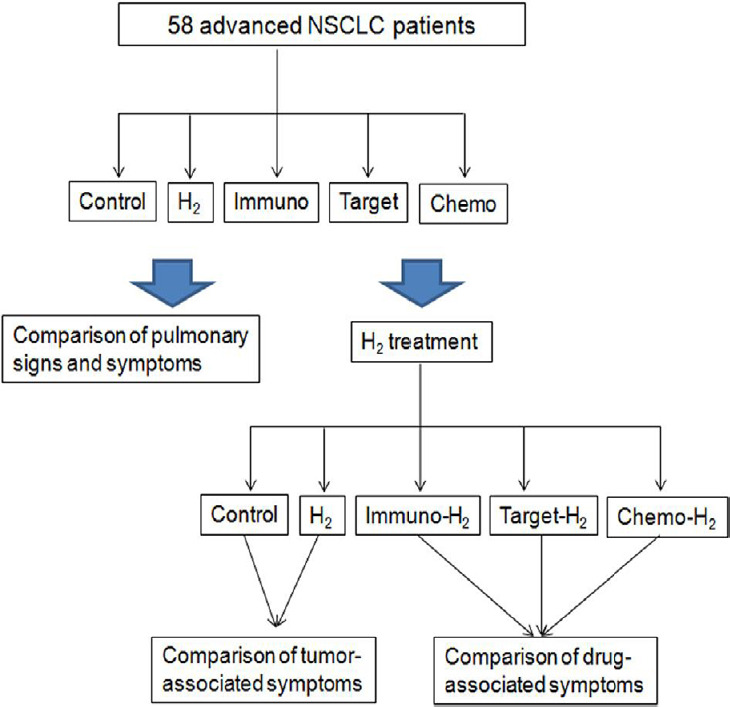
Symptom comparison of patients in each group before and after hydrogen treatment.
Note: Chemo: Chemotherapy; H2: hydrogen; Immuno: immunotherapy; NSCLC: non-small-cell lung cancer; Target: targeted therapy.
H2 inhalation
H2 was produced by a H2–oxygen nebulizer (H2 66.7% and oxygen 33.3%; maximum gas flow, 3 L/min; AMS-H-03, Shanghai Asclepius Meditec, Shanghai, China). The control group underwent a sham procedure (H2 0% and oxygen 33.3%; maximum gas flow 3 L/min; Shanghai Asclepius Meditec). Enrolled patients inhaled the gas mixture for 4–6 hours every day and underwent imaging examination every month until the existing lesions progressed significantly or new metastases appeared. PFS was calculated from the start of H2 inhalation to tumor progression. All patients received computed tomography or magnetic resonance examination every month. If the existing tumors grew up significantly or new metastases appeared, it is considered as tumor progression.
Pulmonary symptoms and drug-associated adverse events
The respiratory function of enrolled patients before H2 therapy was assessed by very experienced respiratory physicians using a pulmonary function tester (Autospiro AS-507; Minato Medical Science, Tokyo, Japan). Pulmonary tumor-related symptoms and the KPS score of all patients before H2 inhalation were evaluated. The adverse events of chemotherapy, targeted therapy or immunotherapy were assessed by the same respiratory physicians according to Common Terminology Criteria for Adverse Events 5.0.
Statistical analyses
Before H2 treatment, the demographics and tumor-associated symptoms of patients were compared using chi-squared and Fisher's exact tests; respiratory function and the KPS score were compared using one-way analysis of variance and Bonferroni's multiple comparison test. After H2 treatment, each tumor-associated symptom and drug-associated adverse event was compared using linear regression analysis; the PFS of each group was compared by one-way analysis of variance and Bonferroni's multiple comparison test. P < 0.05 was considered significant difference. Analyses were done using Prism 5.0 (GraphPad, San Diego, CA, USA).
RESULTS
Clinical data of advanced non-small cell lung cancer patients with H2 inhalation treatment
Patients underwent H2 inhalation per day for 5 months or stopped the inhalation when cancer relapsed. There was no significant difference in most patient characteristics (e.g., sex) in each group. However, the targeted therapy–H2-therapy group had a higher proportion of tumor-gene mutations than the other four groups (P = 0.005; Table 1).
Table 1.
Patient demographics of advanced non-small cell lung cancer patients in different groups
| Control (n = 10) | H2 (n = 10) | Immuno-H2 (n = 10) | Target-H2 (n = 18) | Chemo-H2 (n = 10) | P-value | |
|---|---|---|---|---|---|---|
| Sex | 0.5991 | |||||
| Female | 7 (70) | 5 (50) | 4 (40) | 12 (67) | 6 (60) | |
| Male | 3 (30) | 5 (50) | 6 (60) | 6 (33) | 4 (40) | |
| Age (yr) | 0.6239 | |||||
| 41–60 | 4 (40) | 4 (40) | 7 (70) | 8 (44) | 6 (60) | |
| 61–70 | 3 (30) | 5 (50) | 3 (30) | 6 (33) | 3 (30) | |
| 71–80 | 3 (30) | 1 (10) | 0 | 4 (22) | 1 (10) | |
| Pathology | 0.9999 | |||||
| Adenocarcinoma | 6 (60) | 7 (70) | 7 (70) | 12 (67) | 7 (70) | |
| Squamous cell carcinoma | 3 (30) | 2 (20) | 2 (20) | 4 (22) | 2 (20) | |
| Large cell cancer | 1 (10) | 1 (10) | 1 (10) | 2 (11) | 1 (10) | |
| TNM stage | 0.21 | |||||
| III | 2 (20) | 0 | 0 | 3 (17) | 3 (30) | |
| IV | 8 (80) | 10 (100) | 10 (100) | 15 (83) | 7 (70) | |
| Tumor number | 15 | 12 | 15 | 32 | 12 | 0.6716 |
| Lung, mediastinum and pleura | 6 (40) | 5 (42) | 5 (33) | 12 (38) | 6 (50) | |
| Brain | 3 (20) | 1 (8) | 1 (7) | 7 (22) | 4 (33) | |
| Bone | 4 (27) | 5 (42) | 5 (33) | 9 (28) | 2 (17) | |
| Others | 2 (13) | 1 (8) | 4 (27) | 4 (12) | 0 | |
| Tumor-gene mutation | 0.005 | |||||
| EGFR | 3 (30) | 1 (10) | 2 (20) | 14 (78) | 5 (50) | |
| ALK | 1 (10) | 2 (20) | 0 | 3 (17) | 0 | |
| ROS1 | 1 (10) | 0 | 0 | 1 (5) | 0 | |
| Not found | 5 (50) | 7 (70) | 8 (80) | 0 | 5 (50) |
Note: Data are expressed as number (percent), and analyzed by chi-squared and Fisher’s exact tests. H2: Hydrogen; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; ROS: receptor tyrosine kinase; TNM: tumor-node-metastasis.
Pulmonary signs and symptoms of advanced non-small cell lung cancer patients with H2 inhalation treatment
Before the start of H2 therapy, there was no significant difference in pulmonary function or the KPS score among the five groups of patients (Table 2). The prevalence of most pulmonary symptoms (e.g., mild dyspnea, non-cardiac chest pain, pleural effusion, and hemoptysis) was similar among groups. The prevalence of most pulmonary symptoms in the control group, H2-monotherapy group, and immunotherapy–H2-therapy group was higher than that in the target therapy–H2-therapy group and chemotherapy–H2-therapy group (P = 0.0137).
Table 2.
Pulmonary signs and symptoms before hydrogen therapy of advanced non-small cell lung cancer patients with H2 inhalation treatment
| Control (n = 10) | H2 only (n = 10) | Immuno-H2 (n = 10) | Target-H2 (n = 18) | Chemo-H2 (n = 10) | P-value | |
|---|---|---|---|---|---|---|
| Respiratory function | ||||||
| FEV1 (L) | 1.57±0.59 | 1.63±0.52 | 1.54±0.59 | 1.56±0.49 | 1.56±0.52 | 0.3897 |
| FVC (L) | 1.82±0.57 | 1.93±0.47 | 1.75±0.33 | 1.83±0.56 | 1.91±0.35 | 0.4623 |
| KPS score | 76±7 | 78±8 | 76±7 | 77±7 | 78±8 | 0.4007 |
| Tumor-associated symptoms | ||||||
| Moderate cough | 6 | 7 | 5 | 2 | 3 | 0.0137 |
| Mild dyspnea | 5 | 5 | 2 | 3 | 2 | 0.0757 |
| Non-cardiac chest pain | 4 | 4 | 2 | 4 | 2 | 0.6666 |
| Mild pleural effusion | 3 | 3 | 1 | 3 | 2 | 0.748 |
| Mild hemoptysis | 2 | 2 | 0 | 3 | 0 | 0.3683 |
Note: Data are expressed as mean ± SD or number. Respiratory function and the KPS score were compared using one-way analysis of variance and Bonferroni’s multiple comparison tests. Tumor-associated symptoms were compared using chi-squared and Fisher’s exact tests. H2: hydrogen; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; KPS: Karnofsky performance status.
Tumor-associated symptoms of advanced non-small cell lung cancer patients with H2 inhalation treatment
At the beginning of H2 treatment, the prevalence of tumor-associated symptoms in the control group and H2-monotherapy group was similar (P = 0.9994). With prolongation of the treatment time, in the control group, the prevalence of moderate cough (P = 0.0023), mild dyspnea (P = 0.0019), mild non-cardiac chest pain (P = 0.0006), mild pleural effusion (P = 0.0023), and mild hemoptysis (P = 0.0028) increased significantly (Figure 2A). In the H2-monotherapy group, the prevalence of moderate cough (P = 0.0014), mild dyspnea (P = 0.0247), mild non-cardiac chest pain (P = 0.0136), mild pleural effusion (P = 0.0015), and mild hemoptysis (P = 0.0048) decreased significantly (Figure 2B).
Figure 2.
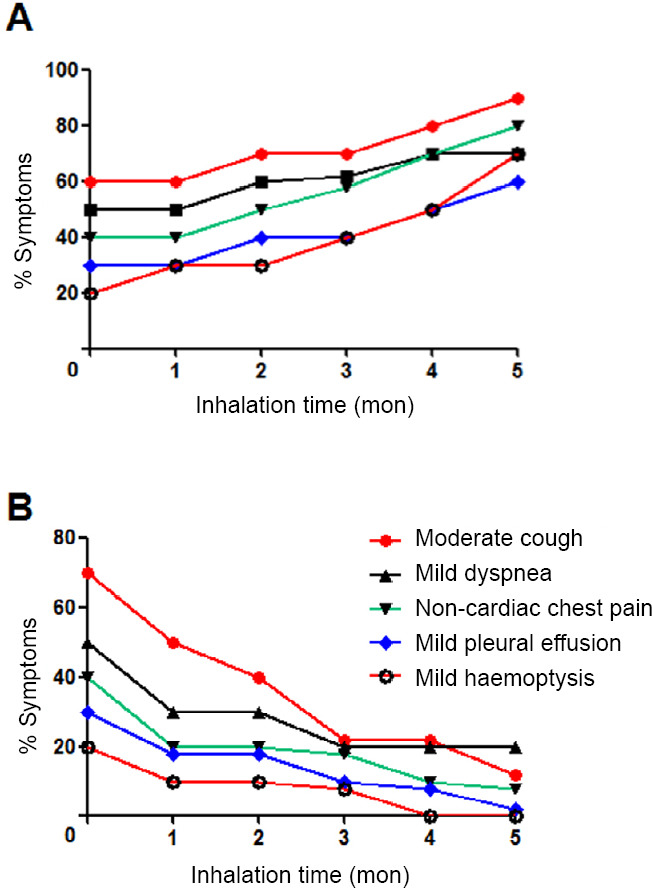
Tumor-associated symptoms varied with the inhalation time of hydrogen (H2).
Note: (A) Control group (inhalation of 33.3% oxygen gas and no H2 (n = 10). (B) H2 only group (inhalation of 66.7% H2 + 33.3% O2) (n = 10). Each tumor-associated symptom was compared using linear regression analysis.
Drug-associated adverse events of advanced non-small cell lung cancer patients with H2 inhalation treatment
At the beginning of H2 treatment, the prevalence of tumor-associated symptoms in the three groups was similar (P = 0.5120), but drug-associated symptoms in the three groups were quite different (Figure 3). With the prolongation of treatment time, the prevalence of cough and non-cardiac chest pain (P = 0.0013), maculopapular rash (P = 0.0021), hepatobiliary disease (P = 0.0064), and dizziness and headache (P = 0.0111) decreased significantly, but diarrhea did not (P = 0.4144) (Figure 3A). In the target therapy–H2-therapy group, the prevalence febrile granulocytopenia (P = 0.0026), nausea and vomiting (P = 0.0051), maculopapular rash (P < 0.0001), insomnia (P = 0.0144), and oral mucositis (P = 0.0007) decreased significantly (Figure 3B). In the chemotherapy–H2-therapy group, the prevalence of febrile granulocytopenia (P = 0.0086), anemia and thrombocytopenia (P = 0.0009), constipation and diarrhea (P = 0.0053) and anorexia (P = 0.0129) decreased significantly, but nausea and vomiting did not (P = 0.0720; Figure 3C).
Figure 3.
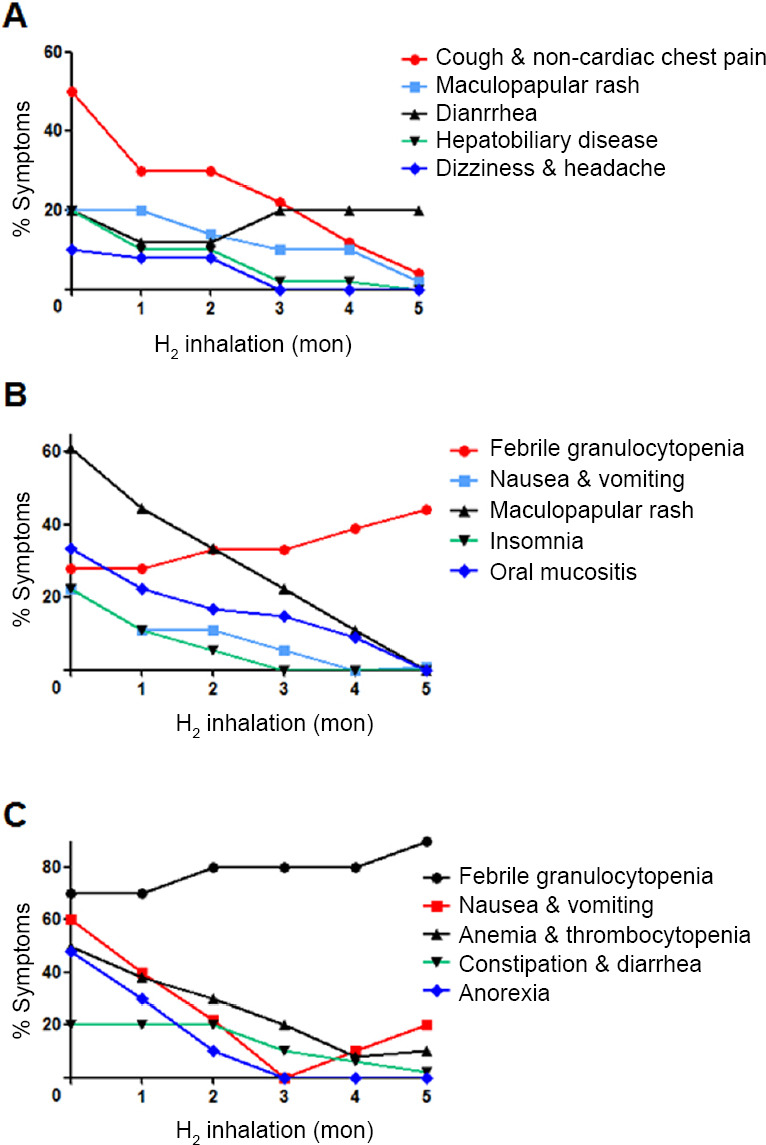
Drug-associated symptoms varied with the inhalation time of hydrogen (H2).
Note: (A) Immunotherapy-H2 group (n = 10). (B) Target-H2 therapy group (n = 18). (C) Chemotherapy-H2 therapy group (n = 10). Drug-associated adverse event was compared using linear regression analysis.
PFS of advanced non-small cell lung cancer patients with H2 inhalation treatment
After 16 months of follow-up, all 58 patients developed tumor progression. PFS for the control group was 4.4 ± 1.2 months, whereas that for the H2-only group was 7.9 ± 2.2 months, H2-immunotherapy group was 10.1 ± 2.6 months, H2-targeted therapy group was 9.4 ± 3.1 months, and H2-chemotherapy group was 8.5 ± 3.0 months. PFS of the four treatment groups was longer than that of the control group, and that of the three H2 therapy-combination groups was prolonged significantly (Figure 4).
Figure 4.
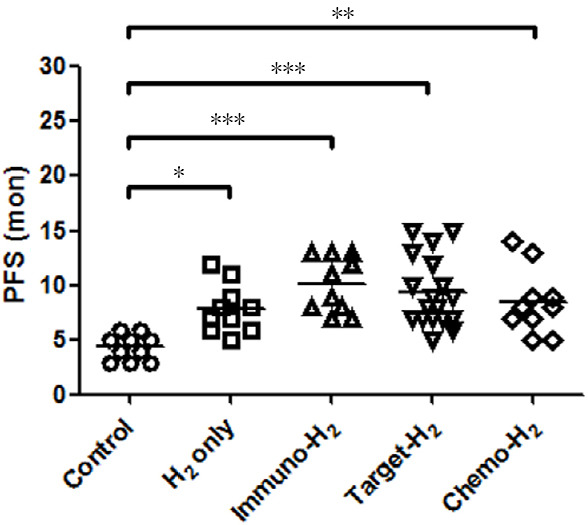
Comparison of progression-free survival (PFS) between groups after hydrogen (H2) treatment.
Note: PFS of each group was compared by one-way analysis of variance and Bonferroni's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Molecular H2 has been used to treat pulmonary symptoms in animal models of acute lung injury,18,19,20,21 asthma22 and chronic obstructive pulmonary disease.23,24,25 The principle of H2 therapy includes inhibition of secretion of cytokines such as interleukin-4, interleukin-13,22 interleukin-6 and tumor necrosis factor-α.15 H2 therapy can alleviate pulmonary inflammation without impairing anti-tumor effects.14,26 Therefore, H2 gas can be adopted as adjuvant therapy to suppress these symptoms.
Chemotherapy, targeted therapy, and immunotherapy are first-line treatments against advanced NSCLC.27,28,29 The vastly increased generation of reactive oxygen species during treatment is believed to contribute to adverse events, resulting in oxidative stress, inflammation and apoptosis.30,31 In the present study, H2 therapy was shown to alleviate drug-related adverse events, most of which have been reported in animal models, including lung injury caused by various factors,18,19,20,21 hepatobiliary diseases,32,33,34 maculopapular rash,35 diarrhea and constipation,36,37,38 nausea and vomiting,39,40 oral mucositis,41,42 anemia,43 thrombocytopenia,44 and anorexia.45 We found that the prevalence of insomnia, dizziness and headache could be reduced significantly after H2 inhalation, which could be related to relief of diseases of the central nervous system, such as cerebral hemorrhage,46,47,48 Parkinson's disease49,50 and Alzheimer's disease,51,52 observed in animal experiments. The mechanism of action observed in animal experiments could be used as a reference for clinical research. Surprisingly, H2 therapy, which is non-toxic and can alleviate adverse events in multiple organs simultaneously, has been used rarely.
We found that H2 monotherapy could prolong the PFS of patients with advanced NSCLC from 4.4 ± 1.2 months to 7.9 ± 2.2 months, suggesting that H2 could inhibit the growth of lung cancer cells independently. This hypothesis has been bolstered by data from an in vitro and in vivo study, which confirmed that H2 can inhibit the proliferation, migration, and invasion of the lung-cancer cell lines and tumor growth in mouse model.53 These data suggested that H2 could serve as new therapy against lung cancer. However, for patients eligible for first-line treatment, drugs will produce more pronounced effects upon tumor control. Whether a combination of drug therapy and H2 therapy can elicit better tumor control must be studied further, but gradual reduction of most drug-associated adverse events after H2 inhalation is clear.
Several delivery methods of molecular H2 are available and convenient: inhalation, drinking H2-dissolved water, injection with H2-saturated saline, and taking a “H2 bath.”54 H2 is non-toxic, inexpensive, can be administered readily, can diffuse into tissues and cells55 and cross the blood–brain barrier.56 Hence, H2 could be used to treat tumors of the head, neck and chest. Because of the risk of explosion of H2 and oxygen mixed in air, often such gas mixtures are inhaled using catheters and masks whereas, in animal experiments, drinking or injection of H2-dissolved water is employed. Use of a machine with a sufficiently high flow rate (3 L/min) and inhalation duration every day (4–6 hours) of H2 in this trial may enable control of tumor growth and reduce the prevalence of adverse events of drugs.
In general, H2 inhalation was first discovered in the clinic that can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced NSCLC. The main limitation of this study is that the number of patients enrolled is relatively small, and more accurate patient benefits are still awaiting the results of large samples. Whether this therapeutic effect can be improved further, as well as determination of the synergistic effect of drugs and H2 therapy, must be explored further.
Footnotes
Conflicts of interest
None declared.
Financial support
None.
Institutional review board statement
This study protocol received ethical approval from the Ethics Committee of Fuda Cancer Hospital of Jinan University on December 7, 2018 (approval No. Fuda20181207) and conformed to the specifications of the World Medical Association's Declaration of Helsinki.
Informed consent statement
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for the images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study followed the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) Statement.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices). Study protocol and informed consent form will be available immediately following publication, without end date. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Reinke LF, Feemster LC, Backhus LM, Gylys-Colwell I, Au DH. Assessment and management of symptoms for outpatients newly diagnosed with lung cancer. Am J Hosp Palliat Care. 2016;33:178–183. doi: 10.1177/1049909114557635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot PM, Chung JH, Ackman JB, et al. ACR Appropriateness Criteria® noninvasive clinical staging of primary lung cancer. J Am Coll Radiol. 2019;16:S184–S195. doi: 10.1016/j.jacr.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16:653–660. doi: 10.1586/14737140.2016.1170596. [DOI] [PubMed] [Google Scholar]
- 5.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 6.Perrotta F, Rocco D, Vitiello F, et al. Immune checkpoint blockade for advanced NSCLC: a new landscape for elderly patients. Int J Mol Sci. 2019;20:2258. doi: 10.3390/ijms20092258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou JG, Tian X, Cheng L, et al. The risk of neutropenia and leukopenia in advanced non-small cell lung cancer patients treated with Erlotinib: a prisma-compliant systematic review and meta-analysis. Medicine. 2015;94:e1719. doi: 10.1097/MD.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Cai XJ, Chen LY, et al. Factors potentially associated with gemcitabine-based chemotherapy-induced thrombocytopenia in Chinese patients with nonsmall cell lung cancer. J Cancer Res Ther. 2018;14:S656–S660. doi: 10.4103/0973-1482.187338. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yuan Z, Wang Q, Fan W, Zhang G. Meta-analysis of overall incidence and risk of ALK inhibitors-induced liver toxicities in advanced non-small-cell lung cancer. Medicine (Baltimore) 2019;98:e13726. doi: 10.1097/MD.0000000000013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelso GF, Porteous CM, Coulter CV, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer MD, Sureda A, Tauler P, Palacín C, Tur JA, Pons A. Impaired lymphocyte mitochondrial antioxidant defences in variegate porphyria are accompanied by more inducible reactive oxygen species production and DNA damage. Br J Haematol. 2010;149:759–767. doi: 10.1111/j.1365-2141.2010.08149.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita T, Kusakabe Y, Kitamura A, Okada S, Murase K. Investigation of protective effect of hydrogen-rich water against cisplatin-induced nephrotoxicity in rats using blood oxygenation level-dependent magnetic resonance imaging. Jpn J Radiol. 2011;29:503–512. doi: 10.1007/s11604-011-0588-4. [DOI] [PubMed] [Google Scholar]
- 14.Meng X, Chen H, Wang G, Yu Y, Xie K. Hydrogen-rich saline attenuates chemotherapy-induced ovarian injury via regulation of oxidative stress. Exp Ther Med. 2015;10:2277–2282. doi: 10.3892/etm.2015.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terasaki Y, Suzuki T, Tonaki K, et al. Molecular hydrogen attenuates gefitinib-induced exacerbation of naphthalene-evoked acute lung injury through a reduction in oxidative stress and inflammation. Lab Invest. 2019;99:793–806. doi: 10.1038/s41374-019-0187-z. [DOI] [PubMed] [Google Scholar]
- 16.Ono H, Nishijima Y, Ohta S, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: a randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. 2017;26:2587–2594. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Asanuma H, Kitakaze M. Translational study of hydrogen gas inhalation as adjuncts to reperfusion therapy for acute myocardial infarction. Circ J. 2017;81:936–937. doi: 10.1253/circj.CJ-17-0520. [DOI] [PubMed] [Google Scholar]
- 18.Zhai Y, Zhou X, Dai Q, Fan Y, Huang X. Hydrogen-rich saline ameliorates lung injury associated with cecal ligation and puncture-induced sepsis in rats. Exp Mol Pathol. 2015;98:268–276. doi: 10.1016/j.yexmp.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Sun Q, Cai J, Liu S, et al. Hydrogen-rich saline provides protection against hyperoxic lung injury. J Surg Res. 2011;165:e43–49. doi: 10.1016/j.jss.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Liu K, Sun Q, et al. Consumption of hydrogen water reduces paraquat-induced acute lung injury in rats. J Biomed Biotechnol. 2011;2011:305086. doi: 10.1155/2011/305086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Fu XJ, Gu C, et al. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J Burn Care Res. 2011;32:e82–e91. doi: 10.1097/BCR.0b013e318217f84f. [DOI] [PubMed] [Google Scholar]
- 22.Xiao M, Zhu T, Wang T, Wen FQ. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-κB in a murine model of asthma. Eur Rev Med Pharmacol Sci. 2013;17:1033–1043. [PubMed] [Google Scholar]
- 23.Liu Z, Geng W, Jiang C, et al. Hydrogen-rich saline inhibits tobacco smoke-induced chronic obstructive pulmonary disease by alleviating airway inflammation and mucus hypersecretion in rats. Exp Biol Med (Maywood) 2017;242:1534–1541. doi: 10.1177/1535370217725249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Ma C, Wang X, et al. Hydrogen coadministration slows the development of COPD-like lung disease in a cigarette smoke-induced rat model. Int J Chron Obstruct Pulmon Dis. 2017;12:1309–1324. doi: 10.2147/COPD.S124547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki Y, Sato T, Sugimoto M, et al. Hydrogen-rich pure water prevents cigarette smoke-induced pulmonary emphysema in SMP30 knockout mice. Biochem Biophys Res Commun. 2017;492:74–81. doi: 10.1016/j.bbrc.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Yang H, Fan Y, Li L, Fang J, Yang W. Hydrogen-rich saline attenuates cardiac and hepatic injury in doxorubicin rat model by inhibiting inflammation and apoptosis. Mediators Inflamm. 2016;2016:1320365. doi: 10.1155/2016/1320365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu S, Chen Z, Hu C, et al. Nedaplatin plus Docetaxel versus Cisplatin plus Docetaxel as first-line chemotherapy for advanced squamous cell carcinoma of the lung - a multicenter, open-label, randomized, phase III trial. J Thorac Oncol. 2018;13:1743–1749. doi: 10.1016/j.jtho.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Choi YW, Jeon SY, Jeong GS, et al. EGFR exon 19 deletion is associated with favorable overall survival after first-line gefitinib therapy in advanced non-small cell lung cancer patients. Am J Clin Oncol. 2018;41:385–390. doi: 10.1097/COC.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 29.Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy. 2018;10:93–105. doi: 10.2217/imt-2017-0121. [DOI] [PubMed] [Google Scholar]
- 30.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radical Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui Q, Wang JQ, Assaraf YG, et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda KI, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 33.Tan YC, Xie F, Zhang HL, et al. Hydrogen-rich saline attenuates postoperative liver failure after major hepatectomy in rats. Clin Res Hepatol Gastroenterol. 2014;38:337–345. doi: 10.1016/j.clinre.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Yang L, Tao K, et al. Protective effects of hydrogen enriched saline on liver ischemia reperfusion injury by reducing oxidative stress and HMGB1 release. BMC Gastroenterol. 2014;14:12. doi: 10.1186/1471-230X-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou P, Lin B, Wang P, et al. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J Radiat Res. 2019;60:17–22. doi: 10.1093/jrr/rry074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X, Mao Y, Cai J, et al. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res. 2009;43:478–484. doi: 10.1080/10715760902870603. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Sun YP, Hu PF, et al. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J Surg Res. 2011;167:316–322. doi: 10.1016/j.jss.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Shigeta T, Sakamoto S, Li XK, et al. Luminal injection of hydrogen-rich solution attenuates intestinal ischemia-reperfusion injury in rats. Transplantation. 2015;99:500–507. doi: 10.1097/TP.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Chen Z, Mao N, Xie Y. The protective of hydrogen on stress-induced gastric ulceration. Int Immunopharmacol. 2012;13:197–203. doi: 10.1016/j.intimp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JY, Wu QF, Wan Y, et al. Protective role of hydrogen-rich water on aspirin-induced gastric mucosal damage in rats. World J Gastroenterol. 2014;20:1614–1622. doi: 10.3748/wjg.v20.i6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasuyama K, Tomofuji T, Ekuni D, et al. Hydrogen-rich water attenuates experimental periodontitis in a rat model. J Clin Periodontol. 2011;38:1085–1090. doi: 10.1111/j.1600-051X.2011.01801.x. [DOI] [PubMed] [Google Scholar]
- 42.Yoneda T, Tomofuji T, Kunitomo M, et al. Preventive effects of drinking hydrogen-rich water on gingival oxidative stress and alveolar bone resorption in rats fed a high-fat diet. Nutrients. 2017;9:64. doi: 10.3390/nu9010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao S, Mei K, Qian L, et al. Therapeutic effects of hydrogen-rich solution on aplastic anemia in vivo. Cell Physiol Biochem. 2013;32:549–560. doi: 10.1159/000354459. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi S, Wada K, Nagatani K, Osada H, Otani N, Nawashiro H. Hydrogen may inhibit collagen-induced platelet aggregation: an ex vivo and in vivo study. Intern Med. 2012;51:1309–1313. doi: 10.2169/internalmedicine.51.7161. [DOI] [PubMed] [Google Scholar]
- 45.McCarty MF. Potential ghrelin-mediated benefits and risks of hydrogen water. Med Hypotheses. 2015;84:350–355. doi: 10.1016/j.mehy.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Manaenko A, Lekic T, Ma Q, Zhang JH, Tang J. Hydrogen inhalation ameliorated mast cell-mediated brain injury after intracerebral hemorrhage in mice. Crit Care Med. 2013;41:1266–1275. doi: 10.1097/CCM.0b013e31827711c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao A, Wu H, Hong Y, et al. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-kappaB pathway and NLRP3 inflammasome. Mol Neurobiol. 2016;53:3462–3476. doi: 10.1007/s12035-015-9242-y. [DOI] [PubMed] [Google Scholar]
- 48.Zhuang Z, Sun XJ, Zhang X, et al. Nuclear factor-κB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res. 2013;91:1599–1608. doi: 10.1002/jnr.23281. [DOI] [PubMed] [Google Scholar]
- 49.Fu Y, Ito M, Fujita Y, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson's disease. Neurosci Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Fujita K, Seike T, Yutsudo N, et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. PLoS One. 2009;4:e7247. doi: 10.1371/journal.pone.0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer's disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Li J, Liu Q, et al. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer's disease. Neurosci Lett. 2011;491:127–132. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788–797. doi: 10.1016/j.biopha.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 54.Zhang JY, Liu C, Zhou L, et al. A review of hydrogen as a new medical therapy. Hepatogastroenterology. 2012;59:1026–1032. doi: 10.5754/hge11883. [DOI] [PubMed] [Google Scholar]
- 55.Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011;17:2241–2252. doi: 10.2174/138161211797052664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res. 2013;3:10. doi: 10.1186/2045-9912-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


