ABSTRACT
Introduction: This study aimed to assess the correlation between nucleic acid amplification test (real-time reverse transcription-polymerase chain reaction, RT-PCR) positivity of patients presenting with suspected COVID-19 and pneumonic infiltration consistent with COVID-19-specific pneumonia diagnosis on thoracic computed tomography (CT), with symptoms, laboratory findings, and clinical progression.
Methods: The study included 286 patients (female:male 131:155; mean age, 53.3 ± 17.9 years) who were divided into two groups according to their RT-PCR test results. The symptoms, laboratory examinations, clinical findings, and thoracic CT imaging of the patients were evaluated.
Results: While the physical examination, comorbidities, and total CT scores were similar between the groups, taste/smell abnormalities were observed more frequently in the PCR-positive group. The use of moxifloxacin, lopinavir/ritonavir, and tocilizumab was higher in the PCR-positive group (p = 0.016, p < 0.001, and p = 0.002, respectively). The duration of hospitalization, intensive care requirement, and mortality rate of the studied groups did not differ between the groups.
Conclusions: Among patients presenting with suspected COVID-19 and pneumonic infiltration consistent with COVID-19 on thoracic CT, the symptoms, physical examination, total CT scores, duration of hospitalization, intensive care requirement, and mortality rate were similar between RT-PCR-positive and RT-PCR-negative patients. However, PCR-positive patients appeared to require more specific treatments.
KEYWORDS: COVID-19, SARS CoV-2, COVID-19 RT-PCR, pneumonia
1. Introduction
A novel type of coronavirus that is the seventh member of the coronavirus family, which causes unexplained pneumonia cases, was discovered in Wuhan state in China at the end of December 2019 [1]. The World Health Organization (WHO) initially defined this novel Coronavirus as a severe acute respiratory syndrome (SARS)-CoV-2 due to its similarities to the virus that caused the SARS epidemic in 2003. According to the latest data in December 2020, there have been nearly 80 million cases in more than 200 countries around the world since the WHO declared the disease a COVID-19 pandemic on 11 March 2020 [2]. It led to nearly 20,000 deaths in Turkey and 1.8 million total deaths worldwide by December 2020 Figure 1.
Figure 1.
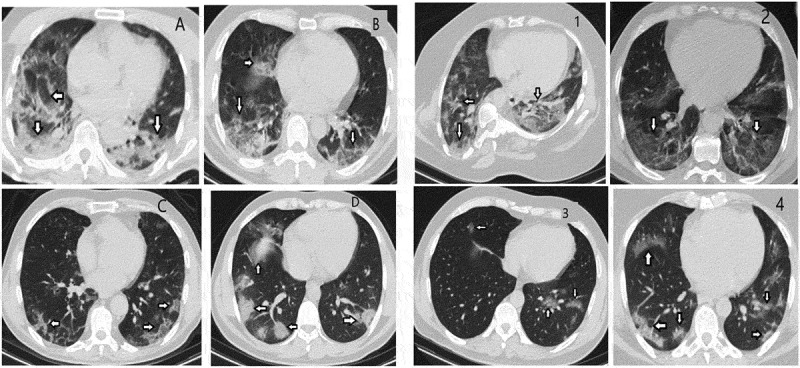
Thoracic computed tomography image samples of patients in PCR positive and negative groups
As the disease may be asymptomatic, associated with the clinical status of COVID-19, various symptoms are observed from upper and lower respiratory tract symptoms, such as fever, dry cough, complicated dyspnea, muscle pain, or fatigue to less commonly reported symptoms, such as taste and smell disorders [3]. The gold standard for the diagnosis of COVID-19 in our country, as well as the rest of the world, is real-time reverse transcription-polymerase chain reaction (RT-PCR) of nasopharyngeal swab sample test with an announced positivity rate of about 30–60% at the beginning. According to the Ministry of Health data in our country, nearly 22 million nucleic acid amplification tests have been performed, and nearly 10% of these tests identified positivity [4]. In addition to these tests, unenhanced chest computed tomography (CT) may also be considered as a reference for viral diagnosis with especially high sensitivity at initial presentation [5,6]. The actual COVID-19 diagnostic accuracy increases when the sensitivity and specificity of both RT-PCR and chest CT are taken into account together [7,8]. After all, it can be said that there is also no gold standard for the diagnosis of COVID-19, as well as for the therapy of disease.
Many scientists all over the world are trying to find effective therapies for COVID-19 to either eradicate or reduce the severity of the disease. From the beginning of the disease, many therapies consisting of antiviral drugs, such as favipiravir, lopinavir, ritonavir [9], and remdesivir [10], antibiotics like macrolides [11], anti-inflammatory drugs like corticosteroids and colchicine [12], a recombinant humanized anti-human IL-6 receptor monoclonal antibody like tocilizumab, and vaccines, including four types such as whole virus, protein subunit, viral vector, and nucleic acid (RNA and DNA), have been investigated. Until now, 18 of 63 vaccine trials on humans have reached the final stage; however, only two of them have obtained emergency approval from the European Medicines Agency (EMA) and the US Food and Drug Administration. In addition to these community immunization efforts completing this pandemic period in the most harmless way, all countries continue widespread quarantine conditions and restrictions, which can have severe negative social and economic consequences. Many studies have already shown that lifestyle changes after COVID-19 pandemic restrictions, such as a decrease in physical activity, deterioration of eating habits and sleep patterns, depression, and anxiety disorders, may cause serious health problems in the future [13–15]. The importance of increasing physical activity for mental and physical health should be emphasized in addition to pharmacological treatments during the COVID-19 pandemic [16].
In our clinical practice, as well as in other countries, we see that the majority of the patients who were admitted to hospital with typical manifestations of COVID-19 underwent both diagnostic tests. It is known from the literature that a substantial proportion of COVID-19 patients might have negative initial RT-PCR tests and positive signs of chest CT or vice versa [17]. However, we have no clear knowledge yet about in which groups of patients, those with or without initial RT-PCR positivity and chest CT positivity, have worse clinical progression or other characteristics of the disease. Therefore, this study aimed to assess the correlation of RT-PCR positivity on nucleic acid amplification tests for patients presenting with suspicion of COVID-19 and with pneumonic infiltration consistent with COVID-19 diagnosis on thoracic CT with symptoms, laboratory findings, and clinical progression.
2. Methods
The study protocol was approved by the Biruni University Faculty of Medicine Ethics Committee and the Ministry of Health. The study was completed according to the principles of the Helsinki Declaration. All patients were provided full information about the study procedures before obtaining their written consent.
The study included 286 patients (female:male 131:155, mean age 53.3 ± 17.9 years) aged 18 years and older with COVID-19 suspicion attending the Biruni University Hospital Internal Medicine, Chest Diseases and Infectious Diseases clinics from March 18 to May 18. All the patients had infiltrative lung involvement consistent with COVID-19-specific viral pneumonia in the lower respiratory tract on chest CT examination at the beginning or follow-up period of the disease, and laboratory and clinical findings were consistent with COVID-19. Patients who had pulmonary findings of COVID-19 pneumonia at the initial presentation and a follow-up period of disease were formed according to initial RT-PCR test results as negative or positive groups. The patients whose chest CT findings were positive despite initial RT-PCR negativity was included in the PCR-negative group. Patients with positive nasal smears in terms of influenza, which could be another cause of viral pneumonia, were excluded from the study. All patients included in the study had oropharyngeal and nasal swab samples taken for the COVID-19 RT-PCR test developed with the virus sequence stated in the Ministry of Health guidelines for admission. We used Bio-Rad CFX96 Touch instruments and considered ct < 38 (200 RFU for threshold levels) sigmoidal curves as positive. In addition, blood samples were collected for laboratory tests during admission (full blood count, creatinine, sodium, potassium, alanine aminotransferase [ALT], aspartate aminotransferase [AST], lactate dehydrogenase [LDH], D-dimer, ferritin, troponin, and C-reactive protein (CRP)). Patient age, sex, symptoms, physical examination findings, the treatment used, duration of hospitalization, intensive care requirements, and mortality status were recorded.
In our study, thoracic CT images were taken with a Siemens Somatom Scope 16-slice CT device with a section thickness of 1.5 mm, obtaining images without gaps between slices (gapless) using low-dose radiation (mAs: 50, Kvp: 120) were assessed. CT positivity was defined by findings assessed as consistent with COVID-19 pneumonia (peripheral, bilateral ground-glass appearance, multifocal rounded ground-glass areas, reverse halo) and findings assessed as consistent with viral pneumonia, including COVID-19 (peripheral and non-rounded multifocal, diffuse, perihilar or unilateral ground-glass opacity, low numbers, and very small peripheral and non-rounded ground-glass areas)(Figure-1). The severity of pulmonary involvement was obtained by dividing both lungs into three sections: upper, middle, and lower zones for a total of six regions. The volume involvement in each region was graded with 1 point for 0–25%, 2 points for 25–50%, 3 points for 50–75%, and 4 points for 75–100% [18,19].
2.1. Statistical analysis
Our study was a retrospective cross-sectional study. The fit to a normal distribution of all data was analyzed using the Kolmogorov–Smirnov test. Categorical variables are presented as percentages, while continuous variables are presented as mean ± standard deviation. Categorical variables were analyzed using the chi-square test, while continuous variables in two-way groups were analyzed using the t-test. All data were tested using the SPSS 20.0 (SPSS, Chicago, IL, USA) software, and values of p < 0.05 were considered statistically significant.
3. Results
The demographic characteristics, comorbidities, and symptoms in the studied groups are shown in Table 1. Age, sex, and smoking habits did not differ significantly among the groups. There was no significant difference in comorbidities between the study groups, but the frequency of coronary artery disease was higher in the PCR-negative group (p = 0.04). The symptoms were similar between the study groups, but the frequency of taste/smell abnormalities was higher in the PCR-positive group (p = 0.018).
Table 1.
Demographic characteristics, comorbidities, and symptoms of the study population
| All patients (n = 286) | PCR positive (n = 157) | PCR negative (n = 129) | p | |
|---|---|---|---|---|
| Age (years) | 53.3 ± 17.9 | 51.8 ± 17.4 | 55.1 ± 18.5 | 0.119 |
| Gender (female) | 131 (45.8) | 76 (48.4) | 55 (42.6) | 0.584 |
| Smoking | 82 (28.7) | 47 (29.9) | 35 (27.1) | 0.679 |
| Comorbidities | ||||
| Hypertension | 129 (45.1) | 69 (43.9) | 60 (46.5) | 0.890 |
| Diabetes mellitus | 54 (18.9) | 27 (17.2) | 27 (20.9) | 0.450 |
| COPD/asthma | 33 (11.5) | 17 (10.8) | 16 (12.4) | 0.713 |
| Coronary artery disease | 32 (11.2) | 12 (7.6) | 20 (15.5) | 0.04 |
| Hyperlipidemia | 30 (10.5) | 16 (10.2) | 14 (10.9) | 0.849 |
| Atrial fibrilation | 12 (4.2) | 7 (4.5) | 5 (3.9) | 0.807 |
| Chronic renal failure | 10 (3.5) | 4 (2.5) | 6 (4.7) | 0.355 |
| Malignancy | 10 (3.5) | 6 (3.8) | 4 (3.1) | 0.741 |
| Symptoms | ||||
| Fever | 243 (85) | 137 (87.3) | 106 (82.2) | 0.248 |
| Cough | 185 (64.7) | 107 (68.2) | 78 (60.5) | 0.214 |
| Fatigue | 150 (52.4) | 88 (56.1) | 62 (48.1) | 0.192 |
| Dyspnea | 75 (26.2) | 40 (25.5) | 35 (27.1) | 0.788 |
| Myalgia | 51 (17.8) | 30 (19.1) | 21 (16.3) | 0.482 |
| Taste/smell abnormalities | 29 (10.1) | 22 (14) | 7 (5.4) | 0.018 |
| Anorexia | 28 (9.7) | 18 (11.5) | 10 (7.8) | 0.413 |
| Headache | 21 (7.3) | 11 (7) | 10 (7.8) | 0.889 |
| Pharyngalgia | 19 (6.6) | 10 (6.4) | 9 (6.9) | 0.851 |
| Diarrhea | 14 (4.9) | 8 (5.1) | 6 (4.7) | 0.862 |
| Nausea/vomiting | 12 (4.2) | 6 (3.8) | 6 (4.7) | 0.952 |
| Chest pain | 11 (3.8) | 6 (3.8) | 5 (3.9) | 0.901 |
| Palpitation | 9 (3.1) | 6 (3.8) | 3 (2.3) | 0.745 |
| Abdominal pain | 6 (2.3) | 2 (1.3) | 4 (3.1) | 0.813 |
The medication and physical examination findings and chest CT findings (at initial and follow-up period) in the study groups are shown in Table 2. The use of moxifloxacin, lopinavir/ritonavir, and tocilizumab was higher in the PCR-positive group (p = 0.016, p < 0.001, and p = 0.002, respectively). The physical examination and total score of thoracic CT findings did not differ among the groups.
Table 2.
Medication and physical examination findings of the study population
| All patients (n = 286) | PCR positive (n = 157) | PCR negative (n = 129) | p | |
|---|---|---|---|---|
| Medications | ||||
| Hydroxychloroquine | 257 (89.9) | 155 (98.7) | 128 (99.2) | 0.991 |
| Azithromycin | 183 (64) | 99 (64.7) | 84 (65.6) | 0.872 |
| Oseltamivir | 181 (63.3) | 102 (65) | 79 (61.2) | 0.539 |
| Moxifloxacin | 92 (32.2) | 60 (38.2) | 32 (24.8) | 0.016 |
| Favipiravir | 53 (18.5) | 33 (21.4) | 20 (15.6) | 0.225 |
| Lopinavir/ritonavir | 22 (7.7) | 21 (13.7) | 1 (0.8) | <0.001 |
| Tocilizumab | 15 (5.2) | 14 (9.1) | 1 (0.8) | 0.002 |
| Other antibiotics | 27 (9.4) | 20 (12.9) | 7 (5.5) | 0.042 |
| Physical examination findings | ||||
| Systolic blood pressure (mmHg) | 119.2 ± 20.1 | 117.1 ± 18.6 | 121.9 ± 21.6 | 0.094 |
| Diastolic blood pressure (mmHg) | 71.3 ± 9.4 | 70.9 ± 9 | 71.8 ± 9.9 | 0.508 |
| Heart rate (/min.) | 86.1 ± 12.5 | 87.3 ± 13.2 | 85.1 ± 11.2 | 0.232 |
| Oxygen saturation (%) | 94.1 ± 4.7 | 93.9 ± 4.8 | 94.1 ± 4.6 | 0.769 |
| Respiratory rate (/min.) | 18.4 ± 3.9 | 18.4 ± 3.7 | 18.3 ± 4.1 | 0.957 |
| Computed tomography findings | ||||
| Total score of computed tomography Initial | ||||
| 0 | 91 (31.8) | 39 (24.8) | 52 (40.3) | 0.084 |
| 1 | 131 (45.8) | 81 (51.6) | 50 (38.8) | |
| 2 | 46 (16.1) | 26 (16.6) | 20 (15.5) | |
| 3 | 8 (2.8) | 5 (3.2) | 3 (2.3) | |
| 4 | 10 (3.5) | 6 (3.8) | 4 (3.1) | |
| Follow-up | ||||
| 1 | 180 (62.9) | 102 (64.9) | 78 (60.5) | |
| 2 | 69 (24.1) | 38 (24.2) | 31 (24.1) | 0.185 |
| 3 | 18 (6.4) | 9 (5.7) | 9 (6.9) | |
| 4 | 19 (6.6) | 8 (5.2) | 11 (8.5) | |
The laboratory findings of the study groups are shown in Table 3. The leukocyte, neutrophil, and lymphocyte levels were significantly lower in the PCR-positive group (p < 0.001, each other). The hemoglobin, platelet, and D-dimer levels were significantly higher in the PCR-positive group (p = 0.023, p < 0.001, and p = 0.005, respectively). The duration of hospitalization, need for the intensive care unit, and mortality rate of the study population did not differ significantly among the studied groups (Table 4).
Table 3.
Laboratory findings of the study population
| All patients (n = 286) | PCR positive (n = 157) | PCR negative (n = 129) | p | |
|---|---|---|---|---|
| Glucose (mg/dL) | 112.8 ± 19.4 | 114.1 ± 21.1 | 111.3 ± 18.5 | 0.806 |
| Creatinine (mg/dL) | 1.1 ± 0.8 | 1.2 ± 0.9 | 1.1 ± 0.5 | 0.583 |
| Leukocyte (103/mL) | 7,6 ± 3.7 | 6.6 ± 3.3 | 8.9 ± 3.9 | <0.001 |
| Neutrophil (103/mL) | 5.4 ± 3.5 | 4.5 ± 3.1 | 6.4 ± 3.6 | <0.001 |
| Lymphocyte (103/mL) | 1.5 ± 0.8 | 1.3 ± 0.7 | 1.7 ± 0.9 | <0.001 |
| Hemoglobin (g/dL) | 13.1 ± 2.1 | 13.3 ± 1.8 | 12.7 ± 2.4 | 0.023 |
| Platelet (103/mL) | 228 ± 93 | 259 ± 108 | 204 ± 69 | <0.001 |
| CRP (mg/L) | 45.2 ± 63.1 | 46.7 ± 63.8 | 43.5 ± 62.6 | 0.687 |
| ALT (U/L) | 34.1 ± 53.8 | 36.1 ± 45.9 | 31.6 ± 62.1 | 0.492 |
| AST (U/L) | 31.8 ± 29.2 | 32.7 ± 19.7 | 30.7 ± 37.6 | 0.593 |
| LDH (U/L) | 285 ± 218 | 270 ± 127 | 305 ± 294 | 0.203 |
| Ferritin (ng/mL) | 266 ± 322 | 266 ± 275 | 266 ± 378 | 0.998 |
| D-Dimer (ng/mL) | 975 ± 1715 | 1370 ± 2330 | 679 ± 945 | 0.005 |
| Troponin I (pg/mL) | 229 ± 2467 | 310 ± 3256 | 131 ± 770 | 0.588 |
| Albumin (g/dL) | 3.6 ± 1 | 3.7 ± 1.1 | 3.5 ± 0.8 | 0.832 |
Table 4.
Duration of hospitalization, necessity of intensive care unit, and mortality rate of the study population
| All patients (n = 286) | PCR positive (n = 157) | PCR negative (n = 129) | p | |
|---|---|---|---|---|
| Duration of hospitalization | 4.4 ± 5.3 | 4.9 ± 5.4 | 3.9 ± 5.1 | 0.113 |
| Necessity of intensive care unit | 39 (13.6) | 24 (15.3) | 15 (11.6) | 0.392 |
| Mortality | 14 (4.9) | 10 (6.4) | 4 (3.1) | 0.274 |
The duration of hospitalization was significantly positively correlated with age (r = 0.299, p < 0.001), leukocyte count (r = 0,135, p = 0.028), CRP (r = 0.289, p = <0.001), ferritin (r = 0.274, p < 0.001), d-dimer (r = 0.207, p < 0.001), troponin I (r = 0.176, p = 0.008), and respiratory rate (r = 0.320, p < 0.001). A significant negative correlation between lymphocyte count (r = −0.231, p < 0.001) and oxygen saturation (r = −0.304, p < 0.001) was found. Oxygen saturation was significantly positively correlated with lymphocyte count (r = 0.247, p < 0.001) and significantly negatively correlated with age (r = −0.338, p < 0.001), leukocyte (r = −0,153, p = 0.028), CRP (r = −0.294, p = <0.001), ferritin (r = −0.252, p < 0.001), d-dimer (r = −0.231, p = 0.001), troponin I (r = −0.150, p = 0.042), and respiratory rate (r = −0.601, p < 0.001). Respiratory rate was significantly positively correlated with age (r = 0.427, p < 0.001), leukocyte (r = 0,173, p = 0.016), CRP (r = 0.269, p = <0.001), ferritin (r = 0.198, p = 0.007), d-dimer (r = 0.240, p = 0.001), and troponin I (r = 0.155, p = 0.043) and significantly negatively correlated with lymphocyte count (r = −0.227, p = 0.001).
4. Discussion
COVID-19 is a brutal disease that should be quickly diagnosed, controlled, and treated. From the beginning of COVID-19 history, the therapy is given to all patients with the slightest suspicion of the disease showing positive findings on RT-PCR test or chest CT. However, we did not know the clear differences between the patient groups with positive or negative RT-PCR tests at the beginning of the disease. In this study, the differences between RT-PCR-positive and RT-PCR-negative patients were researched for patients suspected of COVID-19 with pneumonic infiltration, which is consistent with COVID-19 on thoracic CT with similar age, sex, and comorbidities. The symptoms, physical examination findings, duration of hospitalization, intensive care requirement, and mortality rate were similar in both groups, but RT-PCR-positive patients were shown to require more specific treatments, such as moxifloxacin, lopinavir/ritonavir, and tocilizumab.
The RT-PCR test is still the method accepted as the gold standard for screening and diagnosis of SARS-CoV-2 infection. The outbreak of the recently emerging SARS-CoV-2 poses a challenge for public health laboratories, especially for clinical laboratories in hospitals all over the world. The positivity on RT-PCR for the virus at initial presentation varies from 30% to 70% in different studies [8,20]. A study by Li et al. in China identified 64.3% RT-PCR-positivity for patients presenting with COVID-19 symptoms, while another study in Italy identified 39.4% RT-PCR-positivity in patients with findings consistent with COVID-19 on chest X-ray [21,22]. Similarly, in our study, RT-PCR positivity was identified in 54.9% of 286 patients with radiological findings consistent with COVID-19. These differences in the identification of SARS-CoV-2 with RT-PCR tests are thought to be due to many reasons, including inadequacy in obtaining and/or studying viral samples, false-negatives due to [23] variable accuracy rates in commercial tests, or unidentifiable positivity due to low viral load associated with the early period of disease [5].
Disease progression appears to affect individuals more severely if they are elderly, vulnerable, or with one or more comorbidities. The disease has more severe progression and may result in mortality, especially for men and those with cardiovascular disease [23–25]. This dramatic progression in the presence of hypertension leads to the consideration that injury to endothelial or alveolar epithelial cells expressing ACE2 receptors may form with hypertension in the lungs. However, the underlying mechanism for this connection is still not clearly known [26]. Nine months after the COVID-19 pandemic announcement, nearly 80 million cases were reported worldwide by December, with 2.2% mortality and, unfortunately, more than 1.5 million deaths. At the end of December, 2.13 million cases were observed in Turkey, and more than 19.000 citizens lost their lives (John Hopkins). A study in China found that 58% of patients were men, while the most commonly seen comorbidities were hypertension (26%), diabetes mellitus (10%), and cardiovascular disease (21). A study in the USA found that 59.6% of patients were men, with the most frequent comorbidities being hypertension (60%,) diabetes mellitus (37%), chronic lung diseases (22%), and coronary artery disease (13.1%) [27]. In our study, similar to all global data, the male rate was high (54.2%), with the most frequent comorbid conditions being hypertension, diabetes, chronic lung diseases, and coronary artery disease. The mortality rate was 4.8%, which is similar to the findings of other studies performed during the same period.
As COVID-19 is a viral infection affecting many systems, dominantly the respiratory tract, but including the gastrointestinal and cardiovascular systems, patients’ symptoms vary according to system involvement and include fever, cough, fatigue, dyspnea, myalgia, taste/smell abnormalities, anorexia, headache, pharyngalgia, diarrhea, nausea/vomiting, chest pain, palpitation, and abdominal pain. A study in China found that the most frequently observed symptoms were fever (86%), dry cough (53%), and fatigue (32%), while a study in the USA found cough (73%), fever (72%), and dyspnea (63%) [21,27]. Another study in Italy found that fever (86%), cough (56%), and dyspnea (40%) were the most common symptoms [22]. In our study, the most commonly observed symptom was fever (85%), followed by cough (64%), fatigue (52%), and dyspnea (36%). Apart from taste/smell abnormalities, there was no significant difference in the incidence of symptoms with PCR test positivity.
Since the SARS-CoV-2 isolation, there is still no effective medication to fully treat the virus. Several researchers around the world are working to find solutions to enhance the efficacy of drugs that will relieve COVID-19. Many drugs, including antivirals, remdesivir, antibacterial drugs, antiparasitic medication like ivermectin [28], antimalarial drugs with anti-inflammatory effects, and colchicine are being researched, and their efficacy is debated. In our study, similar medications were used (remdesivir and ivermectin are not available in Turkey, colchicine is not licensed or permitted for COVID-19), and PCR-positive patients were observed to require more specific treatments like lopinavir/ritonavir and tocilizumab. This situation suggests that after PCR-positive patients receive a higher viral load, it may lead to the administration of more intense specific treatments due to rapid progression in clinical disease. On the other hand, as the intensive care requirement and mortality rates of the patients are similar, this situation may be interpreted as the PCR-positivity at the beginning of the disease, which causes the doctors to keep the COVID-19 treatments given to the patient more broadly in the follow-up period with an observation of clinical progression.
The current study has some limitations; first, its results cannot be generalized to the entire population because it was conducted in a single center and included only patients above 18 years of age; second, the long-term outcomes of our patients were not researched. We did not demonstrate the improvement of pathophysiological changes in the lung; for this, patients should undergo pulmonary function tests and CT examinations after 6–8 weeks. Finally, a viral panel was not examined in the PCR-negative group, and thus, other viral infections could not be excluded.
In conclusion, there was no significant difference in the symptoms, physical examination findings, CT findings, duration of hospitalization, intensive care requirement, and mortality rates between RT-PCR positive and negative patients presenting with the suspected COVID-19 and pneumonic infiltration consistent with COVID-19 on thoracic CT. It has been observed that PCR-positive patients require more specific treatments. Even though RT-PCR studies associated with the diagnosis and process of COVID-19 have given much more knowledge and clinical experience to the literature, chest CT screening is still a more sensitive method than RT-PCR test in the beginning and also follow-up period of the disease. In our study, the chest CT findings turned positive in the follow-up period of the disease, but clinical outcomes of COVID-19 patients with or without RT-PCR positivity were found to be similar. Therefore, the treatment selection for COVID-19 patients should be decided primarily according to their chest CT screening tests and clinical findings.
Funding Statement
This paper was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Coronavirus disease 2019 (COVID-19) situation report-83. WHO. 2020. [Google Scholar]
- 3.Carlos WG, Dela Cruz CS, Cao B, et al. Novel Wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201(4):P7–p8. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . COVID-19 situation report Turkey (ENG). 2020. October 16. Available from: https://covid19.saglik.gov.tr/TR-75241/covid-19-situation-report-turkey-eng.html.
- 5.Yang Y, Yang M, Shen C, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020. February 17;11. [Preprint]. DOI: 10.1101/2020.02.2020 [DOI] [Google Scholar]
- 6.He JL, Luo L, Luo ZD, et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168:105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Hou H, Wang W, et al. Combination of CT and RT-PCR in the screening or diagnosis of COVID-19. J Glob Health. 2020;10(1):010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020;382(24):2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohe M, Shida H, Jodo S, et al. Macrolide treatment for COVID-19: will this be the way forward? Biosci Trends. 2020;14(2):159–160. [DOI] [PubMed] [Google Scholar]
- 12.Deftereos SG, Siasos G, Giannopoulos G, et al. The Greek study in the effects of colchicine in COVID-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol. 2020;61(1):42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall G, Laddu DR, Phillips SA, et al. A tale of two pandemics: how will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog Cardiovasc Dis. 2020. DOI: 10.1016/j.pcad.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesser IA, Nienhuis CP.. The impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health. 2020;17(11):3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravalli S, Musumeci G. Coronavirus outbreak in Italy: physiological benefits of home-based exercise during pandemic. J Funct Morphol Kinesiol. 2020;5(2):31. DOI: 10.3390/jfmk5020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maugeri G, Castrogiovanni P, Battaglia G, et al. The impact of physical activity on psychological health during COVID-19 pandemic in Italy. Heliyon. 2020;6(6):e04315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z-H, Li Y-J, Wang X-J, et al. Chest CT of COVID-19 in patients with a negative first RT-PCR test: comparison with patients with a positive first RT-PCR test. Medicine (Baltimore). 2020;99(26):e20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA – secondary publication. J Thorac Imaging. 2020;35(4):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooi GC, Khong PL, Müller NL, et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. [DOI] [PubMed] [Google Scholar]
- 20.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296(2):E115–e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Liu W, Zheng Y, et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020;22(4–5):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ippolito D, Pecorelli A, Maino C, et al. Diagnostic impact of bedside chest X-ray features of 2019 novel coronavirus in the routine admission at the emergency department: case series from Lombardy region. Eur J Radiol. 2020;129:109092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh HJ, Kim DH, Heo EY, et al. Clinical characteristics of COVID-19: clinical dynamics of mild severe acute respiratory syndrome coronavirus 2 infection detected by early active surveillance. J Korean Med Sci. 2020;35(32):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrera FJ, Shekhar S, Wurth R, et al. Prevalence of diabetes and hypertension and their associated risks for poor outcomes in COVID-19 patients. J Endocr Soc. 2020;4(9):bvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez J, Albaiceta GM, García-Clemente M, et al. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene. 2020;762:145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]


