Abstract
Aim
Ferritin is a hepatic protein that plays vital roles in diagnosing and predicting diseases, but its potential in coronavirus disease 2019 (COVID-19) remains unknown.
Method
We collected clinical records from 79 COVID-19 patients at Wuhan Union hospital (China). Spearman’s correlation analysis, receiver operating characteristic (ROC) curve and Kaplan–Meier survival curves were employed.
Results
Patients with elevated ferritin levels had a higher incidence of severity illness (50.0 vs 2.9%) and liver injury (52.3 vs 20.0%) when compared with patients with normal ferritin levels (p < 0.05). Ferritin could effectively identify the severity of illness (ROC area 0.873) and liver injury (ROC area 0.752). The elevated ferritin group showed longer viral clearance time (median 16 vs 6 days, p < 0.001) and in-hospital length (median 18 vs 10 days, p < 0.001).
Conclusions
It suggests that ferritin could act as an easy-to-use tool to identify liver injury and severity illness and predict the prognosis of COVID-19 patients. Intensive surveillance is necessary for patients with abnormal ferritin levels.
Keywords: Ferritin, liver injury, coronavirus, COVID-19, risk factor
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has spread to the whole world and the number of diagnosed cases is accelerating every day (Cao et al. 2020, Wu F et al. 2020). Medical resources in every country are in shortage, so there is an urgent need of seeking indicators for disease severity and prognosis at an early stage, which is believed to significantly reduce the medical stress and mortality rate.
As an acute-phase protein that can be induced in the setting of systemic inflammation, ferritin has been identified as a discriminative, predictive, or prognostic marker in patients with liver diseases (Kowdley et al. 2012, Jung et al. 2019, Shah and Kowdley 2019). In this fight against the COVID-19 pandemic, ferritin has been drawing increasing attention as a risk factor in the stratification or prognosis of COVID-19 (Kappert et al. 2020, Mehta et al. 2020, Sun et al. 2020, Wu C et al. 2020). However, its value still remains to be explored even though clinical observation has discovered the abnormal ferritin levels in severe COVID-19 patients (Chen G et al. 2020, Zhou et al. 2020). Herein, we aimed to assess the ability of ferritin in discriminating liver injury and severe illness on admission, as well as the potential of predicting outcome, to provide guidance for subsequent clinical practice.
Clinical significance
Ferritin could act as a simple and efficacious complementary tool to identify severe COVID-19 patients at early stage and predict their outcome.
This indicator would provide guidance for subsequent clinical practice, alleviate the medical stress and reduce the mortality.
Methods
Participants
This study had been approved by the Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Review date: March 24, 2020) and was exempted from the need for informed consent from patients because it was a retrospective assessment. All procedures followed the instructions of the Local Ethics Committee (approval 2020 NO. 0134). From 12 January 2020 to 21 February 2020, a total of 147 consecutive patients were initially enrolled from the department of infectious diseases of Wuhan Union Hospital, all of which were confirmed cases of COVID-19 after examination of COVID-19 RNA by RT-PCR (upper respiratory throat swab samples) and also chest computerized tomography (CT) scanning. One patient who died of traumatic brain injury with viral pneumonia was excluded. Patients without ferritin detection on admission were excluded. Therefore, 79 inpatients were enrolled in this study.
According to ‘diagnosis and treatment of novel coronavirus pneumonia’, severe illness on admission was defined when any of the following criteria is met: (1) shortness of breath (respire rate ≥30 times/min); (2) the oxygen saturation is less than 93% in resting-state; (3) arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa). As described previously, patients with elevated levels of alanine transaminase (ALT) or aspartate aminotransferase (AST) or bilirubin were defined as liver injury (Xie et al. 2020).
Data collection
The data of demographics, laboratory examinations, CT scan, clinical characteristics, treatments and outcomes were acquired by the hospitalization management system. All the laboratory examinations were completed in the same laboratory department with the same standard in Union hospital. All the data were collected by well-trained researchers with a double-blind method. Data collection of laboratory results were defined using the first-time examination at admission, except that ferritin levels of Figure 1(B,D) were monitored at different time points during the whole hospitalization of patients. The date of symptom onset, admission, negative detection of COVID-19 RNA, discharge and death were recorded accurately. The date of admission was used as the starting point of the virus clearance process, and the date of the negative detection of COVID-19 RNA was calculated as the ending point of viral clearance.
Figure 1.
(A) The levels of ferritin in severe and non-severe patients on admission; (B) The variation of ferritin in severe and non-severe patients (days were calculated as the interval between the time point of symptom onset and ferritin examination); n = 11, 15 and 9 for days 1–10, 11–20 and 21–30 of severe group while n = 22, 36 and 16 for days 1–10, 11–20 and 21–30 of non-severe group); (C) The levels of ferritin in liver injury and normal liver patients on admission; (D) The variation of ferritin in liver injury and normal liver patients (days were calculated as the interval between the time point of symptom onset and ferritin examination; n = 13, 18 and 8 for days 1–10, 11–20 and 21–30 of liver injury group while n = 20, 33 and 20 for days 1–10, 11–20 and 21–30 of normal liver group); (E) CT manifestation of COVID-19 patients (the corresponding ferritin levels and time points are displayed on the top of each image). The data are shown as median (IQR); **p < 0.001; *p < 0.01. The classification of severity illness or liver injury were based on the symptoms or laboratory examinations on admission.
Statistical analysis
Spearman’s correlation analysis was used to explore the coefficients of ferritin with liver damage/function biomarkers. The performance of the discriminant model was characterized by estimating the area under the receiver operating characteristic (ROC) curve (AUC). The cut-off value of ROC was calculated based on the maximum Youden index, which was used to assess global diagnostic effectiveness. Cumulative survival curves of hospitalisation span and viral clearance time were estimated using the Kaplan–Meier estimation method for two groups with normal and elevated ferritin levels (ferritin ≤ 200 ng/mL and ferritin >200 ng/mL) by log-rank test. All of the analyses were performed with the R software version 3.4.3 (http://www.R-project.org, The R Foundation) and EmpowerStats version 2.20 (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A two-sided p-value <0.05 was determined as with statistically significant differences.
Results
Baseline data
All participants were divided into two groups according to ferritin levels of the clinical reference range. As shown in Supplementary Table 1, there were significant differences of sex between the two groups, with male patients accounting for 68.2% in the elevation ferritin group but only 17.1% in the normal ferritin group (p < 0.001). The average age of the elevation ferritin group was also significantly larger than the normal ferritin group (49.9 vs 36.8-years-old). The other characteristics including systolic blood pressure (SBP), diastolic blood pressure (DBP), respire, pulse, smoking, comorbidities and symptoms of both groups were recorded but no statistical difference was found. Of 79 patients enrolled, 2 died in hospital, 8 were transferred to other hospitals, and 69 were discharged.
Laboratory examinations
Table 1 shows the clinical characteristics of COVID-19 patients, it is obvious that the incidence of severe illness in the elevation ferritin group was significantly higher than that of the normal group (50.0 vs 2.9%, p < 0.001). Likewise, liver injury was more frequently found in patients with higher ferritin levels, compared to those with normal ferritin levels (52.3 vs 20.0%, p = 0.003), as demonstrated by abnormal levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP) and albumin (p < 0.05). A spearman’s correlation analysis was also employed to demonstrate the relationship between ferritin and hepatic biomarkers (Supplementary Figure 1). Ferritin was highly positively correlated to AST, ALT and GGT (r > 0.57 and p < 0.001), but weakly correlated with albumin, ALP and total bilirubin (TBIL) (r = −0.389, 0.248 and 0.274 for albumin, ALP and TBIL, respectively, p < 0.05).
Table 1.
Clinical characteristics of COVID-19 patientsa.
| Total | Normal ferritin group (≤200 ng/mL) | Elevation ferritin group (>200 ng/mL) | p-Value | |
|---|---|---|---|---|
| n = 79 | n = 35 | n = 44 | ||
| Severity illness (%) | 23 (29.1%) | 1 (2.9%) | 22 (50.0%) | <0.001 |
| Liver injury (%) | 30 (38.0%) | 7 (20.0%) | 23 (52.3%) | 0.003 |
| Ferritin (ng/mL, 4.6–200) | 261.7 (95.3–674.9) | 82.6 (1.3–198.6) | 594.1 (200.5–2471.6) | <0.001 |
| Albumin (g/L, 35–55) | 40.2 (36.4–42.7) | 41.2 (26.4–47.7) | 37.7 (23.2–57.0) | 0.004 |
| ALP (U/L, 40–150) | 54.0 (46.0–66.0) | 51.0 (33.0–304.0) | 62.0 (31.0–199.0) | 0.028 |
| ALT (U/L, 5–40) | 23.0 (15.0–41.0) | 15.0 (5.0–179.0) | 36.5 (11.0–197.0) | <0.001 |
| AST (U/L, 8–40) | 25.0 (20.0–39.5) | 20.0 (9.0–208.0) | 33.0 (10.0–345.0) | <0.001 |
| GGT (U/L, 11–50) | 19.0 (13.0–36.5) | 13.0 (8.0–531.0) | 30.5 (12.0–214.0) | <0.001 |
| TBIL (μmol/L, 5.1–19.0) | 9.8 (8.0–11.9) | 8.7 (3.7–23.3) | 10.2 (4.7–422.8) | 0.077 |
| DBIL (μmol/L, 1.7–6.8) | 3.8 (2.7–5.6) | 3.8 (1.2–11.9) | 3.7 (1.9–248.9) | 0.566 |
| TBA (μmol/L, 0–10.0) | 2.0 (1.4–3.3) | 1.9 (0.5–5.5) | 2.0 (0.6–13.7) | 0.471 |
| FBG (mmol/L, 4.1–5.9) | 5.0 (4.6–5.7) | 4.8 (3.8–7.4) | 5.2 (3.6–13.4) | 0.017 |
| TG (mmol/L, <1.7) | 1.0 (0.8–1.4) | 0.8 (0.5–2.6) | 1.2 (0.6–4.5) | <0.001 |
| TC (mmol/L, <5.2) | 3.8 (3.2–4.4) | 3.6 (2.6–4.9) | 3.8 (2.6–6.0) | 0.222 |
| HDL-C (mmol/L, 1.16–1.42) | 1.0 (0.9–1.2) | 1.2 (0.6–1.8) | 0.9 (0.1–1.8) | 0.004 |
| LDL-C (mmol/L, 2.7–3.1) | 2.2 (1.8–2.7) | 2.1 (0.6–3.4) | 2.4 (1.1–4.0) | 0.128 |
| SAA (mg/L, <10.0) | 26.8 (5.6–287.5) | 6.6 (0.7–733.0) | 147.1 (5.4–739.8) | <0.001 |
| IL-6 (pg/mL, 0.12–2.9) | 6.3 (3.3–17.0) | 3.2 (2.8–6.9) | 9.5 (5.2–19.4) | <0.001 |
| Cre (μmol/L, 44.0–133.0) | 67.6 (58.5–79.0) | 61.0 (44.1–133.4) | 71.6 (51.3–158.0) | <0.001 |
| LDH (U/L, 109–245) | 66.0 (46.5–117.5) | 48.0 (26.0–165.0) | 73.0 (18.0–1447.0) | <0.001 |
| INR (0.80–1.31) | 1.0 (1.0–1.1) | 1.0 (0.9–42.9) | 1.0 (0.9–41.6) | 0.216 |
| D-dimer (mg/L, <0.5) | 0.5 (0.2–1.8) | 0.2 (0.2–13.9) | 0.7 (0.2–20.0) | 0.032 |
| Leucocytes (g/L, 3.5–9.5) | 5.0 (3.8–6.6) | 4.5 (2.3–12.7) | 5.7 (1.5–28.9) | 0.033 |
| Lymphocytes% (%, 20–50) | 27.4 (14.9–35.8) | 31.1 (7.6–52.1) | 22.4 (2.6–47.0) | 0.012 |
| Neutrophils (g/L, 1.8–6.3) | 2.8 (2.1–4.5) | 2.5 (1.1–8.8) | 3.1 (0.0–27.7) | 0.039 |
| NLR (%) | 2.2 (1.4–4.8) | 1.9 (0.7–11.6) | 3.0 (0.0–37.0) | 0.028 |
| CRP (mg/L, <4.00) | 8.0 (3.1–40.0) | 3.1 (0.3–74.8) | 12.3 (0.6–163.0) | <0.001 |
| ESR (mm/h, 0.00–15.00) | 15.0 (8.0–33.0) | 10.0 (2.0–74.0) | 25.0 (3.0–101.0) | <0.001 |
Continuous variable was presented as median (IQR). p-Values were from t-test for normally distributed continuous data and from Mann–Whitney U test for abnormally distributed continuous data. Laboratory results were defined using the first-time examination at admission. The methods of chemiluminescence (ferritin, IL-6, and CRP), colorimetry (albumin and GGT), continuous monitoring assay (ALP and ALT), enzyme coupling (AST), diazonium salt (TBIL and DBIL), enzymatic cycling (TBA), glucose oxidase (FBG), lipoidase (TG), oxidase (TC), direct determination (HDL-c and LDL-c), immunoturbidimetry (SAA and D-dimer), picric acid (Cre), lactate dehydrogenase releasing (LDH), freezing (INR), flow cytometry (leucocytes, lymphocytes, neutrophils and NLR), and erythrocyte sediment rate analyser (ESR) were employed in the laboratory examinations, respectively.
Other biomarkers including fasting blood glucose (FBG), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), serum amyloid protein A (SAA), interleukin-6 (IL-6), creatinine (Cre), lactic dehydrogenase (LDH), D-dimer, leucocytes, lymphocytes%, neutrophils, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) also significantly differed between two groups (p < 0.05).
Determination of ferritin and corresponding CT manifestation
As mentioned above, there existed significantly more severe cases and liver injury patients in the elevation ferritin group than the normal group. In addition, we compared the ferritin levels between severe and non-severe groups or liver injury and normal liver groups, finding that the ferritin level of the severe group was significantly higher than that of the non-severe group (median 921.3 (IQR 440.0–1609.8) vs median 130.7 (IQR 58.8–320.4), p < 0.001) (Figure 1(A,B)). Similarly, the ferritin level of liver injury groups was also significantly higher than that of the normal liver group (median 499.4 (IQR 256.2–1456.7) vs median 130.8 (IQR 59.2–432.3), p < 0.001) (Figure 1(C,D)). In addition, we also collected CT images of three representative COVID-19 inpatients (the level of ferritin was also detected on the same day), it seemed that ferritin variations might be consistent with CT manifestation (Figure 1(E)). But this speculation still needs more supporting data.
Diagnostic ability of ferritin on severity illness
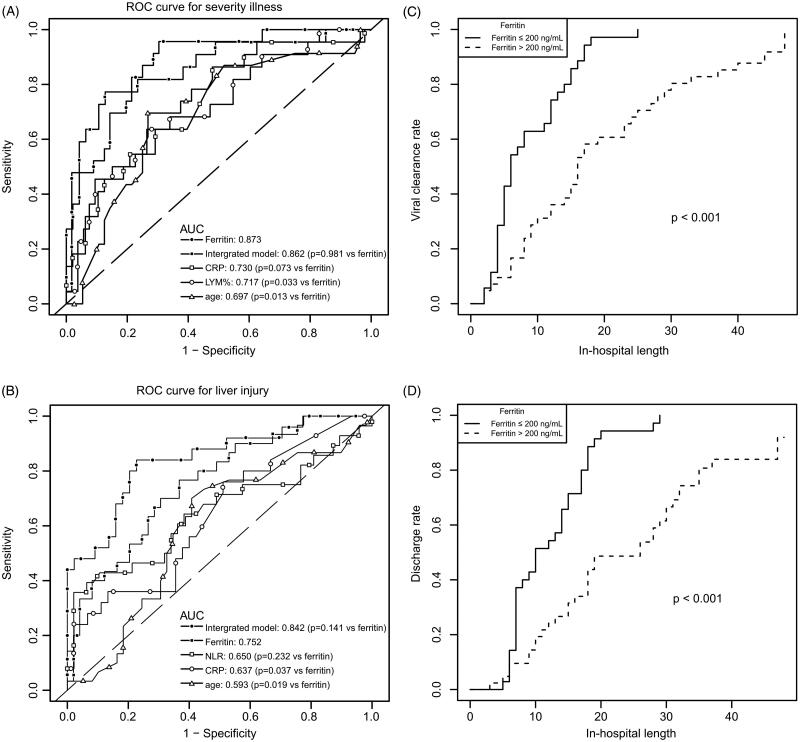
As shown in Figure 2(A), the diagnostic value of ferritin for the incidence of severe illness was compared to that of age, sex, CRP and LYM%, which had been recognised as the risk factors of COVID-19 (Huang et al. 2020, Jordan et al. 2020, Tan C et al. 2020, Tan L et al. 2020). AUC of ROC plot for ferritin was 0.873 (95% CI: 0.798–0.954), larger than that of age (0.697, 95% CI: 0.567–0.828), CRP (0.730, 95% CI: 0.602–0.859) and lymphocytes% (LYM%, 0.717, 95% CI: 0.586–0.847), indicating ferritin possesses a considerable diagnostic value for severity of patients on admission. The best cut-off value of ferritin was 272.5 ng/mL, with a sensitivity of 96% and specificity of 70%. Furthermore, we established a multivariable diagnostic model incorporating sex, age, ferritin, LYM% and CRP (n = 69, 0.862 (95% CI: 0.761–0.962), sensitivity 77%, specificity 87%). It is interesting to find that the diagnostic ability of the integrated model showed a similar value compared with ferritin alone (p = 0.981).
Figure 2.
(A) ROC curves of potential risk factors for severity illness (n = 79 for age and ferritin; n = 75 for LYM%; n = 70 for CRP; n = 69 for integrated model, integrating gender, age, ferritin, LYM% and CRP); (B) ROC curves of potential risk factors for liver injury (n = 79 for age and ferritin, n = 75 for NLR, n = 70 for CRP; n = 69 for integrated model, integrating gender, age, ferritin, NLR and CRP); Kaplan–Meier estimates of viral clearance rate (C) and inpatient discharge rate (D) over time stratified by serum ferritin levels (77 patients were included except 2 deceased ones).
Diagnostic ability of ferritin on liver injury
It was reported that NLR and CRP are associated with the liver function of COVID-19 patients (Zhang et al. 2020). Therefore, we compared the diagnostic ability of ferritin with age, NLR and CRP towards liver injury of COVID-19 patients (Figure 2(B)). The results showed that AUC of ROC plot for ferritin was 0.752 (95% CI: 0.644–0.861), larger than that of age (0.593, 95% CI: 0.462–0.724), CRP (0.637, 95% CI: 0.501–0.774) and NLR (0.650, 95% CI: 0.509–0.791), indicating ferritin possesses a considerable diagnostic value for liver injury of patients on admission. The best cut-off value of ferritin was 236.3 ng/mL, with a sensitivity of 77% and specificity of 63%. Furthermore, we established a multivariable diagnostic model incorporating sex, age, ferritin, NLR and CRP (n = 69, 0.842 (95% CI: 0.742–0.942), sensitivity 84%, specificity 77%). The AUC of this integrated model is larger than that of the ferritin independent model (n = 69, 0.770 (95% CI: 0.657–0.883), sensitivity 76%, specificity 68%), but no statistical significance was observed (p = 0.141).
Prognostic ability of ferritin
The median duration of viral clearance was 6 days (IQR 2–25) in the normal ferritin group (≤200 ng/mL), while the clearance time in the elevation ferritin group (>200 ng/mL) was 16 days (IQR 2–47). The viral clearance events in the normal group and elevation ferritin group by using Kaplan–Meier survival analysis were displayed in Figure 2(C) (p < 0.001).
Besides, we recorded the length of hospitalization of all patients except 2 deceased ones and 8 patients that transferred to other hospitals. Our result showed that patients with higher ferritin levels on admission stayed in the hospital for a longer time (18 days, IQR 3–47) when compared with the ones with normal ferritin levels (10 days, IQR 5–29). The discharge events in the normal group and elevation ferritin group by using Kaplan–Meier survival analysis are displayed in Figure 2(D) (p < 0.001).
Discussion
Until now, there is no effective medicine available to treat COVID-19. Medical resources in every country are in shortage or even overdrawn, so there is an urgent need of seeking effective biomarkers that can identify the patients at risk upon their admission. Only in this way, can medical staffs make greater use of limited medical resources and reduce mortality. For the first time, the present study shows the ability of ferritin in identifying the liver injury and severe illness of COVID-19 patients.
Herein, the data of 79 patients with COVID-19 pneumonia were analyzed, the baseline characteristics of patients with elevated and normal ferritin levels were described and compared. The integrated models did not show better diagnostic ability on COVID-19 severity illness or liver injury significantly, compared with ferritin alone, demonstrating that ferritin might act as an independent easy-to-use indicator. In addition, we compared the viral clearance rate and in-hospital length between the elevation ferritin group and the normal group, finding that ferritin was also a discriminated indicator for the prognosis of COVID-19 patients. Viral clearance is the golden standard for defining the recovery of COVID-19 infections and predicting in-hospital length is extremely vital in COVID-19 treatment due to the lack of medical resources (Chen X et al. 2020). Thus, we suppose ferritin could act as effective discriminators for liver injury, severity illness, and prognosis.
Ferritin is an acute-phase protein that can be released from damaged hepatocytes (Koperdanova and Cullis 2015, Cao et al. 2019). Hyperferritinemia has been previously recognized in abnormal liver function conditions or metabolic syndrome (Trombini and Piperno 2007, Kowdley et al. 2012). In our study, COVID-19 patients with elevated ferritin levels possessed significantly more risk of liver injury and severity of illness. Previous observations also found that liver injury was prevalent in patients with COVID-19 (Mantovani et al. 2020, Xu et al. 2020, Zhang et al. 2020). Therefore, we recommend that patients should pay close attention to the risk of developing secondary liver diseases after they are discharged. Intensive surveillance and regular comprehensive medical examination are necessary, especially for those with abnormal ferritin levels.
However, there were some limitations in the study. First, this was a single-center retrospective study with limited size. Second, some cases had incomplete biochemistry determinations. Third, the present study failed to predict the mortality of COVID-19 patients due to the insufficient number of deaths.
In summary, these findings indicate that early examination of ferritin might effectively identify liver injury, severity illness and poor prognosis of COVID-19 patients. We suppose that ferritin could act as a simple complementary tool to help to guide clinical decisions and facilitating appropriate treatment. Patients with elevated admission ferritin level should be provided with strengthened attention and treatment. However, more studies are needed to confirm these findings and to explore exact pathological mechanisms.
Supplementary Material
Acknowledgements
We acknowledge all the front-line medical staffs fighting against COVID-19. We show our respect to those who have sacrificed in this war.
Funding Statement
This study was supported by the National Key R&D Program of China [2017YFC0909900] and National Natural Science Foundation of China [81903901].
Author contributions
Peng Cao: data collection, writing-original draft preparation, conceptualization; Yuanjue Wu: data analysis, manuscript revision; Sanlan Wu: data collection; Tingting Wu: data collection; Rui Zhang: data collection; Qilin Zhang: data collection; Zhao Wang: data collection; Yu Zhang: supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cao, P., et al. , 2019. The preventative effects of procyanidin on binge ethanol-induced lipid accumulation and ros overproduction via the promotion of hepatic autophagy. Molecular nutrition & food research, 63 (18), e1801255. [DOI] [PubMed] [Google Scholar]
- Cao, P., et al. , 2020. The important role of polysaccharides from a traditional Chinese medicine-lung cleansing and detoxifying decoction against the COVID-19 pandemic. Carbohydrate polymers, 240, 116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G., et al. , 2020. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. Journal of clinical investigation, 130 (5), 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., et al. , 2020. Hypertension and diabetes delay the viral clearance in COVID-19 patients. medRxiv, 2020.03.22.20040774 [Google Scholar]
- Huang, C., et al. , 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 395 (10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, R.E., Adab, P., and Cheng, K.K., 2020. Covid-19: risk factors for severe disease and death. BMJ, 368, m1198. [DOI] [PubMed] [Google Scholar]
- Jung, J.Y., et al. , 2019. Serum ferritin level is associated with liver steatosis and fibrosis in Korean general population. Hepatology international, 13 (2), 222–233. [DOI] [PubMed] [Google Scholar]
- Kappert, K., Jahic, A., and Tauber, R., 2020. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers, 25 (8), 616–625. [DOI] [PubMed] [Google Scholar]
- Koperdanova, M. and Cullis, J.O., 2015. Interpreting raised serum ferritin levels. BMJ, 351, h3692. [DOI] [PubMed] [Google Scholar]
- Kowdley, K.V., et al. , 2012. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology, 55 (1), 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani, A., Beatrice, G., and Dalbeni, A., 2020. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver international, 40 (6), 1316–1320. [DOI] [PubMed] [Google Scholar]
- Mehta, P., et al. , 2020. Hlh across speciality collaboration, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 395 (10229), 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, R.A. and Kowdley, K.V., 2019. Serum ferritin as a biomarker for NAFLD: ready for prime time? Hepatology international, 13 (2), 110–112. [DOI] [PubMed] [Google Scholar]
- Sun, Y., et al. , 2020. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. Journal of autoimmunity, 112, 102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, C., et al. , 2020. C-reactive protein correlates with CT findings and predicts severe COVID-19 early. Journal of medical virology, 92 (7), 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, L., et al. , 2020. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal transduction and targeted therapy, 5 (1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombini, P. and Piperno, A., 2007. Ferritin, metabolic syndrome and NAFLD: elective attractions and dangerous liaisons. Journal of hepatology, 46 (4), 549–552. [DOI] [PubMed] [Google Scholar]
- Wu, C., et al. , 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine, 180 (7), 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, F., et al. , 2020. A new coronavirus associated with human respiratory disease in China. Nature, 579 (7798), 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, H., et al. , 2020. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver international, 40 (6), 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L., et al. , 2020. Liver injury during highly pathogenic human coronavirus infections. Liver international, 40 (5), 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., Shi, L., and Wang, F.S., 2020. Liver injury in COVID-19: management and challenges. The lancet. Gastroenterology & hepatology, 5 (5):428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., et al. , 2020. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver international, 40 (9), 2095–2103. [DOI] [PubMed] [Google Scholar]
- Zhou, F., et al. , 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 395 (10229), 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.