Abstract
Introduction
The QuitNic pilot trial aimed to test the feasibility of providing a nicotine vaping product (NVP) compared with combination nicotine replacement therapy (NRT) to smokers upon discharge from a smoke-free residential substance use disorder (SUD) treatment service.
Methods
QuitNic was a pragmatic two-arm randomized controlled trial. At discharge from residential withdrawal, 100 clients received telephone Quitline behavioral support and either 12-week supply of NRT or an NVP. Treatment adherence and acceptability, self-reported abstinence, cigarettes smoked per day (CPD), frequency of cravings, and severity of withdrawal symptoms were assessed at 6 and 12 weeks. Results are reported for complete cases and for abstinence outcomes, penalized imputation results are reported where missing is assumed smoking.
Results
Retention on was 63% at 6 weeks and 50% at 12 weeks. At 12 weeks, 68% of the NRT group reported using combination NRT while 96% of the NVP group used the device. Acceptability ratings for the products were high in both groups. At 12 weeks, 14% of the NVP group and 18% of the NRT group reported not smoking at all in the last 7 days. Mean CPD among continued smokers decreased significantly between baseline to 12 weeks in both groups; from 19.91 to 4.72 for the NVP group (p < .001) and from 20.88 to 5.52 in the NRT group (p < .001). Cravings and withdrawal symptoms significantly decreased for both groups.
Conclusions
Clients completing residential withdrawal readily engaged with smoking cessation post-treatment when given the opportunity. Further research is required to identify the most effective treatments postwithdrawal for this population at elevated risk of tobacco-related harm.
Trial registration number
ACTRN12617000849392
Implications
This pilot study showed that smoking cessation support involving options for nicotine replacement and Quitline-delivered cognitive behavioral counseling is attractive to people after they have been discharged from SUD treatment. Both nicotine vaping products and nicotine replacement therapies were highly acceptable and used by participants who reported reductions in cravings for cigarettes and perceptions of withdrawal symptoms and reductions in number of cigarettes smoked. Some participants self-reported abstinence from cigarettes—around one in five reported having quit smoking cigarettes at 12 weeks postdischarge. The results have significant public health implications for providing quit support following discharge from SUD treatment.
Introduction
Globally, rates of tobacco smoking amongst people with a substance use disorder are upwards of 80%,1 making smoking particularly harmful for this group and is a leading cause of death despite their other substance use.2–6 An 11-year cohort study of 845 persons previously in substance use disorder (SUD) treatment found that 51% of deaths were due to tobacco-related causes, twice that expected in the general population.2 Another study of people in SUD treatment found that cigarette smoking contributed to mortality above and beyond deaths due to other drug use, with death rates of smokers four times that of nonsmokers.3
Most people with SUDs smoke heavily and are more heavily nicotine dependent,1 experiencing nicotine withdrawal symptoms intensely when attempting to quit smoking.7,8 Although smoking cigarettes is socially acceptable amongst people with SUD,8 they report high levels of interest in quitting smoking and numerous quit attempts.9 However, most individuals in SUD treatment do not receive support to stop smoking tobacco,9,10 meaning that their quit attempts rarely convert to long-term sustained abstinence.11 Studies have found a number of reasons for low provision of smoking cessation support in SUD treatment settings including low staff confidence, and lack of supportive organizational leadership and systems.12
Most people who are discharged from settings enforcing abstinence (e.g., residential withdrawal or prison) return to smoking.13–15 Smoking cessation trials in SUD treatment settings using behavioral counseling and pharmacotherapies have shown that intervention effects are lost at 6 months follow-up or longer.16–18 One recent trial in an inpatient setting found that intolerance to withdrawal symptoms is a predictor of failed abstinence18,19 which means that use of nicotine replacement therapy (NRT) will likely help. Existing data on the acceptability of NRT, however, suggests that it is not well tolerated by people with SUD and this may be associated with low adherence rates.20
A new method of helping people quit that remains largely untested in people with SUD is the use of nicotine vaping products (NVPs). Otherwise known as electronic cigarettes (or e-cigarettes), NVPs may be a method of nicotine replacement that delivers nicotine more efficiently than conventional NRT, providing better relief from cravings and withdrawal symptoms.21 The use of NVPs involves inhaling vapor produced by heating nicotine liquid and is more similar behaviorally to cigarette smoking than other smoking cessation medicines, which may make them more attractive and acceptable to smokers wanting to quit.22
There remain some concerns that NVPs may constitute a gateway to nicotine dependence and eventual cigarette smoking by youth.23 Recent longitudinal studies show mixed results with some studies showing falls in smoking prevalence in youth with increased vaping and others the opposite effect.24–26 Also of concern has been the outbreak in the United States of lung damage cases called EVALI; however, the CDC have now reported that the damage was likely due to vitamin e acetate used by cannabis vapers and not nicotine vapes.27 Nonetheless, given they are a recent development with no long-term safety data available, concerns for the safety of vaping nicotine remain, and NVPs should be tested prior to use as smoking cessation support.
Three large randomized controlled trials have found that NVPs alone or in combination with nicotine patches are safe and at least as effective as NRT28 or up to twice more effective than NRT at helping people quit smoking.29,30 Two small studies31,32 of NVPs in SUD treatment settings have been reported suggesting improvements in reductions in cigarettes smoked, high adherence rates, and self-reported abstinence. However, the trials are only single group studies with short follow-ups and very small sample sizes (n = 2531 and n = 1232). There is almost no evidence on the potential utility of NVPs with this population.
SUD treatment services that offer a period of residential admission in a smoke-free facility are an ideal opportunity to introduce smoking cessation interventions. Many services provide some NRT or behavioral support during the smoke-free stay to manage withdrawal symptoms during the forced abstinence.10 However, typically no follow-up smoking cessation support is given and most clients return to smoking immediately following discharge.10 The aim of this pilot trial (QuitNic) was to explore the feasibility, acceptability, and effectiveness of providing a NVP with a 12-week supply of liquid nicotine and telephone Quitline support compared with a 12-week supply of combination NRT and Quitline support to smokers upon discharge from a smoke-free SUD residential withdrawal service.
Methods
Design
QuitNic was a pragmatic, open-label, single-center, two-arm randomized controlled trial, with an active control. Clients of an SUD residential withdrawal service were recruited and randomized while in treatment to one of two groups to receive either 12-week supply of combination NRT (active control), or a NVP with 12-week supply of nicotine e-liquid upon discharge from the service. Both groups also received telephone Quitline behavioral support. Participants were followed up at 6 and 12 weeks following discharge from the unit. Ethics approval from the Eastern Health (E16–2016) and University of Newcastle (H-2017-0249) Human Research Ethics Committees was gained. The detailed protocol of the study is published33 and the trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617000849392).
Setting
Participants were recruited from a 12-bed residential withdrawal service in Melbourne, Australia, where the average length of stay is 8 days. The site is smoke-free and as part of standard care, all clients are offered NRT.
Participants
Eligible clients were those aged 18 years and over who were tobacco smokers at admission and with capacity to provide informed consent. Clients were not eligible to participate if they reported pregnancy or breast feeding, NVP use in the past month, participation in another research study at the site, or who were scheduled to be transferred to another residential treatment service following discharge.
Recruitment and Randomization
During the intake assessment, withdrawal unit staff screened clients for eligibility and interested clients were referred to a research assistant (RA) on site for more information, who sought written consent. After enrollment into the trial, participants completed an online baseline survey using a tablet device. Upon completing the baseline survey, participants were randomized 1:1 to an intervention via a computer-sequenced 4–6 block randomization embedded in the tablet device software.
Intervention Groups
Participants were informed of their intervention group by the RA and provided with a training session of up to 1 h. The training session provided participants with an overview of their discharge pack and how to best use its contents (either combination NRT or NVP) (see Supplementary Fig 3). All participants were told that they would receive proactive referral to telephone Quitline support for their quit attempt, during their inpatient withdrawal, and on days 1, 3, 7, 14, and 28 post-discharge. Participants were sent a text message prior to being called. The Quitline counselors were provided with a full-day training session with a clinical psychologist prior to the start of the trial, focusing on the correct use of NVPs and NRT and how to assist people who may have multiple addictions. On discharge, participants were provided with the packs containing the intervention products to which they were randomized. Interventions were provided upon discharge because inpatients are not permitted to vape on site.
Group 1: Combination NRT
Participants randomized to this group received 12 weeks of NRT, with a 4-week supply of patches plus oral forms of NRT (gum, lozenges, and inhalators) in the discharge pack. Refills of NRT were provided after the initial 4-week period following phone contact with participants in weeks 3 and 7 (4-week supply mailed on each occasion). Thus, in total, participants received 3 × 4 weeks supply of NRT over the 12-week period. During the 3- and 7-week calls, they could specify their preferences for which types of NRT to be mailed to them. Written information on how to use NRT correctly and for how long, potential side effects (and when to notify a health-care provider), safe storage, and handling was also included in the discharge pack.
Group 2: NVP and Liquid Nicotine
Participants randomized to group 2 received a NVP starter kit which included the device (Innokin Endura T22; 1.5ohm atomizer, 4.5-mL tank) and 4-week supply of nicotine e-liquid. The unflavored e-liquid provided contained vegetable glycol, purified water, and nicotine. The dosing schedule of e-liquid provided to participants was dependent on their nicotine dependence score as measured by the Heaviness of Smoking Index.28,29 Participants scoring in the high nicotine dependence category were assigned an initial 4-week e-liquid supply (total 8- × 10-mL bottles) consisting of 2- × 10-mL bottles of 18 mg e-liquid and 6- × 10-mL bottles of 12-mg e-liquid. This allotment allows for a 1-week supply of 18-mg e-liquid while participants in this group familiarized themselves with the use of the device. The second and third batches of e-liquid (which were mailed to participants following calls at weeks 3 and 7) consisted of 8- × 10-mL bottles of 12-mg e-liquid only. Participants scoring in the moderate- and low-dependence categories received 3- × 4-week supplies of 8- × 10-mL bottles of 12-mg e-liquid. Written information on the risks and benefits of vaping, and instructions on how to use NVPs and safe storage and handling was included. A 1-week supply of 21-mg nicotine patches was also provided for use while learning how to use the NVP effectively.
Outcome Measures
Follow-up measures at 6 and 12 weeks were conducted via telephone surveys and included the following items.
Acceptability
Acceptability was measured using the question set: “Thinking about your use of and experience with [product], please indicate your agreement with the following statements: it was effective at reducing my cravings; it was easy to use; it was enjoyable to use.” Response options were as follows: (1) strongly disagree, (2) disagree, (3) undecided, (4) agree, or (5) strongly agree. Those in the NRT group were asked about each type of product individually.
Feasibility
Feasibility of conducting a NVP trial for smoking cessation in an SUD setting was examined by collecting consent and retention rates and comparing the demographic variables and outcomes between those retained and lost.
Treatment Adherence
Participants were asked if they had used and were currently using the products, frequency of use, and if they used a combination of products.
Abstinence From Tobacco Smoking
Abstinence from cigarette smoking was measured in two ways: continuous abstinence and 7-day point prevalence abstinence.34,35 Continuous abstinence was checked at each follow-up time point, i.e., by self-report at weeks 6 and 12, from the date of the previous interview: “Since [date] did you smoke at all, even part of a cigarette?” and among those who did: “In the past 6-weeks (that is, since [date]), have you smoked a cigarette, even a puff?” with the response options: (1) no, not a puff, (2) 1–5 cigarettes, or (3) more than five cigarettes. Continuous abstinence from tobacco smoking was defined as no more than five cigarettes since the date (i.e., each 6-week period). At 6 and 12 weeks, 7-day point prevalence smoking abstinence was assessed with: “Have you smoked at least part of a cigarette in the last 7 days?” The two measures are independent and thus some participants reporting continuous abstinence in the last 6 weeks (with up to 5 cigarettes smoked) may not be reporting abstinence at 7 days point prevalence.
Cigarettes Smoked Per Day
A single-item from the two-item Heaviness of Smoking Index36 asked participants how many cigarettes they smoked per day.
Frequency of Cravings
Frequency of cravings was assessed by one item37: “Currently, how often do you get strong cravings to smoke tobacco?” with the response options: (1) hourly or more often, (2) several times per day, (3) at least once a day, or (4) less than daily.
Withdrawal Symptoms
Withdrawal was assessed using the eight-item Minnesota Nicotine Withdrawal Scale (MNWS),38 with symptoms rated on an ordinal scale, ranging from 0 (not present) to 4 (severe).
Psychological Distress
The 10-item Kessler Psychological Distress Scale (Kessler-10)39 of nonspecific psychological distress was administered. Scores under 20 suggest no psychological distress, 20–24 suggest mild mental distress, 25–29 suggest moderate mental distress, and over 30 likely to have severe mental distress.
Other Measures
A number of demographic and covariate measures were also taken at baseline. Quitting self-efficacy, motivation to quit, and the Heaviness of Smoking Index were assessed at baseline.40,41 The number of Quitline calls was obtained from the Quitline service and the number and type of adverse events were recorded.
Statistical Analysis
Participant characteristics at baseline were compared descriptively using proportions for categorical variables and means and standard deviation for continuous variables. The data were analyzed as a longitudinal data set, generalized linear mixed effects models were used to measure the difference in outcome responses between treatment groups at 6- and 12-week follow-up, and a random individual effect was included to account for repeated measures. The main comparison of interest was the differences in outcome measures between the treatment groups at 6 and 12 weeks. The distribution and conical link function for each model was assessed based on the outcome; binary outcomes (e.g., continuous and 7-day point prevalence abstinence) were modeled assuming a binomial distribution using a logit conical link function, continuous outcomes (e.g., Minnesota Nicotine Withdrawal Scale) were modeled assuming a normal distribution with an identity link function, ordinal outcomes (e.g., cravings) were modeled using a multinomial distribution with a cumulative logit conical link function, and count outcomes (e.g., number of days to relapse) were modeled using a log link function assuming a negative binomial distribution. For each model, the relevant diagnostics were checked to ensure sufficient fit and adherence to modeling assumptions. All analyses were undertaken in SAS Version 9.4.42
Sample Size
Following the recommendations of Lancaster et al.43 for pilot study sample size estimation, a convenience target of 100 participants was set for calculating response and attrition rates to aid larger trial design. QuitNic was not powered to detect differences in a primary outcome between groups at follow-up.
Results
Sample
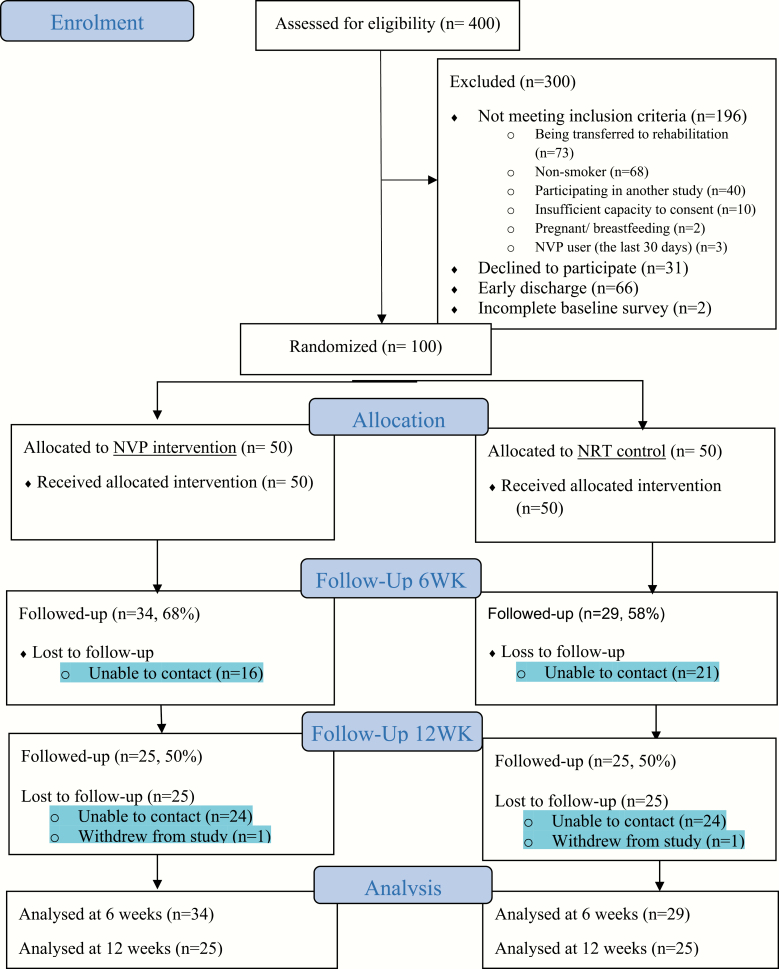
Figure 1 shows the flow diagram of participants through the study and retention rates. Of 400 clients approached to participate during the recruitment phase, 204 were eligible and 100 consented, completed the baseline survey, and were randomized to the two groups in equal numbers. At 6 and 12 weeks, 63 participants (63%) and 50 participants (50%) were followed up, respectively. Although slightly higher retention rates were evidence in the VNP group at 6 weeks (68% vs. 58% in NRT group; p = .300); there were no differences between groups at 12 weeks (25 recontacted in both arms; i.e., 50%). Comparisons were made for baseline demographic, smoking-related, and outcome variables between participants retained (at least one follow-up) and those lost to follow-up (no follow-up data) and no statistically significant differences were found for any variable (see Supplementary Table 1).
Figure 1.
Consort diagram of participant flow.
Table 1 shows the baseline demographic, smoking, and clinical characteristics of the sample, with very few noticeable differences between the groups with the exception of income, where a larger proportion of participants in the NVP group (82%) reported government benefits as their income source compared with the NRT group (64%), and a higher proportion of the NRT group reported being in paid employment (24%) than the NVP group (12%).
Table 1.
Numbers and Proportions of Participant Demographic Variables at Baseline, by Group and in Total
| VNP (n = 50) |
NRT (n = 50) |
Total (n = 100) |
||
|---|---|---|---|---|
| Age | Mean (SD) | 40.7 (10.4) | 41.0 (10.4) | 40.9 (10.4) |
| Gender | Male | 34 (68%) | 33 (66%) | 67 (67%) |
| Female | 16 (32%) | 16 (32%) | 32 (32%) | |
| Other | 1 (2.0%) | 1 (1.0%) | ||
| Aboriginal1 | Yes | 3 (6.0%) | 5 (10%) | 8 (8.0%) |
| Housing | Own House | 5 (10%) | 8 (16%) | 13 (13%) |
| Rental House | 16 (32%) | 16 (32%) | 32 (32%) | |
| With family or friends | 16 (32%) | 16 (32%) | 32 (32%) | |
| Supported / government housing/street living | 12 (24%) | 9 (18%) | 21 (21%) | |
| Other | 1 (2.0%) | 1 (2.0%) | 2 (2.0%) | |
| Education | Up to Year 9 | 8 (16%) | 9 (18%) | 17 (17%) |
| School Certificate/Year 10 | 9 (18%) | 11 (22%) | 20 (20%) | |
| HSC or Leaving Year 12 | 10 (20%) | 3 (6.0%) | 13 (13%) | |
| TAFE or other trade qualification | 18 (36%) | 21 (42%) | 39 (39%) | |
| University Degree | 5 (10%) | 6 (12%) | 11 (11%) | |
| Income | < US$100 per week | 5 (10%) | 4 (8.0%) | 9 (9.0%) |
| US$101–US$400 per week | 29 (58%) | 28 (56%) | 57 (57%) | |
| US$401–US$500 per week | 8 (16%) | 4 (8.0%) | 12 (12%) | |
| US$500 per week + | 4 (8.0%) | 8 (16%) | 12 (12%) | |
| Prefer not to answer | 4 (8.0%) | 6 (12%) | 10 (10%) | |
| Income source | Paid work (full/part time) | 6 (12%) | 12 (24%) | 18 (18%) |
| Government benefit | 41 (82%) | 32 (64%) | 73 (73%) | |
| Other | 3 (6%) | 6 (12%) | 9 (9%) | |
| Cigarettes per day | Mean (SD) | 20.0 (10.7) | 22 .4(16.4) | 21 (13.9) |
| Quit attempts—ever | Yes | 39 (78%) | 38 (76%) | 77 (77%) |
| Quit attempts—12 months | Yes | 22 (44%) | 15 (30%) | 37 (37%) |
| Motivation to quit | Median (SD) | 7.3 (2.4) | 7.7 (2.1) | 7.5 (2.4) |
| Quit self-efficacy | Not at all sure | 7 (14%) | 3 (6%) | 10 (10%) |
| Slightly sure | 10 (20%) | 5 (10%) | 15 (15%) | |
| Moderately sure | 15 (30%) | 24 (48%) | 39 (39%) | |
| Very sure | 14 (28%) | 14 (28%) | 28 (28%) | |
| Extremely sure | 4 (8%) | 4 (8%) | 8 (8%) | |
| Heaviness of smoking | Low | 13 (26%) | 9 (18%) | 22 (22%) |
| Medium | 25 (50%) | 28 (56%) | 53 (53%) | |
| High | 12 (24%) | 13 (26%) | 25 (25%) | |
| Psychological distress | Low (< 20) | 3 (6%) | 1 (2%) | 4 (4%) |
| Moderate (21–29) | 13 (24%) | 16 (28%) | 26 (26%) | |
| High (> 30) | 35 (70%) | 35 (70%) | 70 (70%) | |
| Primary drug of concern | Alcohol | 31 (62%) | 27 (54%) | 58 (58%) |
| Methamphetamine | 7 (14%) | 10 (20%) | 17 (17%) | |
| Cannabis | 3 (6.0%) | 7 (14%) | 10 (10%) | |
| Other | 9 (18%) | 6 (12% | 15 (15%) |
1Self-identify as Aboriginal and/or Torres Strait Islander.
Treatment Adherence
Of the 34 participants randomized to the NVP group who were contactable at 6 weeks, 32 (97%) reported using the device during that period. Assuming all drop outs were no longer using the device, this figure is reduced to 64% use. Only 15 (45%) reported using the 1-week supply of patches provided to the NVP group at discharge. Of the 25 participants contacted at 12 weeks, 24 reported use of the device in the past 6 weeks (96% or 48% assuming drop outs were no longer using the device).
Treatment adherence in the NRT arm varied by product type (see Supplementary Table 2). Overall, 20 of the 29 (69%) participants contactable at 6 weeks in the NRT arm reported use of more than one type of NRT (or 40% assuming drop outs were not using) and 17 of the 25 (68%) contactable at 12 weeks reported doing so (or 34% if lost to follow-up assumed no longer using).
Acceptability
Acceptability ratings were generally high for both groups, with some variation in the NRT group based on type of NRT used. In the NRT group, of the participants that adhered to treatment at 12-week follow-up (complete case analysis), agreement (strongly agree and agree) that the NRT was (1) “effective at reducing my cravings” ranged from 61.5% (for lozenge users) to 93.3% (for inhalator users); (2) “easy to use” ranged from 91.7% (for nicotine gum) to 100% (for patches, lozenge, inhalator, and mouth spray); and (3) “enjoyable to use” ranged from 16.7% (for nicotine gum) to 93.3% (for inhalators), with other forms of NRT moderate (40% of mouth spray users, 42.1% of patch users, and 46.2% of lozenge users agreed). The equivalent outcomes for the NVP group were (1) 91.7% agreed that the NVP was “effective at reducing their cravings”; (2) 91.7% agreed that the NVP was “easy to use,” and (3) 75% agreed that the NVP was “enjoyable to use,” at 12-week follow-up (see Supplementary Table 3).
Abstinence From Cigarette Smoking
A summary of 7-day point prevalence abstinence and 6-week continuous abstinence in NVP and NRT groups at both follow-up times, by complete cases analysis and Penalized imputation where missing is assumed smoking analysis is provided in Table 2. No differences between groups were found to be statistically significantly different.
Table 2.
Self-reported 7-Day Point Prevalence Abstinence and 6-Week Continuous Abstinence Rates, by Group at 6- and 12-Week Follow-up Time Points
| 6-Week outcomes | |||||
|---|---|---|---|---|---|
| NVP | NRT | OR1 | 95% CI | p | |
| Self-reported 7-day point prevalence abstinence | |||||
| Complete cases Penalized imputation |
5/34 (15%) 5/50 (10%) |
7/29 (24%) 7/50 (14%) |
0.59 0.71 |
0.13–2.66 0.17–2.93 |
0.479 0.636 |
| Self-reported 6-week continuous abstinence | |||||
| Complete cases Penalized imputation |
8/34 (24%) 8/50 (16%) |
12/29 (41%) 12/50 (24%) |
0.43 0.59 |
0.11–1.66 0.18–1.97 |
0.212 0.388 |
| 12-Week outcomes | |||||
| NVP | NRT | OR1 | 95% CI | p | |
| Self-reported 7-day point prevalence abstinence | |||||
| Complete cases Penalized imputation |
7/25 (28%) 7/50 (14%) |
9/25 (36%) 9/50 (18%) |
0.75 0.76 |
0.17–3.27 0.21–2.73 |
0.696 0.676 |
| Self-reported 6-week continuous abstinence | |||||
| Complete cases Penalized imputation |
9/25 (36%) 9/50 (18%) |
10/25 (40%) 10/50 (20%) |
0.89 0.91 |
0.21–3.81 0.27–3.03 |
0.876 0.872 |
1Calculated from crude logistic mixed effect models (NRT = reference group).
Cigarettes Per Day
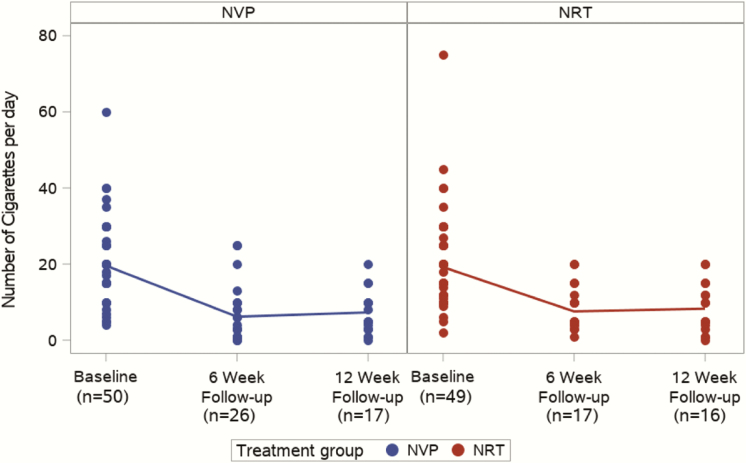
Figure 2 shows the number of CPD by group (for those not reporting quitting smoking) at baseline, 6 and 12 weeks. For the NVP group, the mean CPD were 19.91, 4.96, and 4.72 at each time point, representing significant reductions from baseline (p < .001 at both 6 and 12 weeks). Similarly, there were significant reductions in the mean CPD in the NRT group from baseline of 20.88, to 5.24 at 6 weeks (p < .001) and 5.52 at 12 weeks (p < .001). The differences between groups were not significant at 6 weeks (IRR: 0.79, 95% CI: 0.58–1.08, p = .142) and 12 weeks (IRR: 0.86, 95% CI: 0.61–1.20, p = 0.368) follow-up.
Figure 2.
Number of cigarettes per day by group and follow-up timepoint (baseline, 6weeks, and 12 weeks).
Frequency of Strong Cravings
Reports of “hourly to several times a day” cravings decreased in the NVP group from 74% of participants to 52% at 6 weeks and 48% at 12 weeks; and in the NRT group from 68% at baseline to 58% and 40% at 6 and 12 weeks. The differences between groups were not significant at either follow-up point (6 weeks: OR: 1.51, 95% CI: 0.51–4.45, p = .454; 12 weeks: OR: 0.89, 95% CI: 0.28–2.80, p = .844).
Withdrawal Symptoms
Total scores on the MNWS fell from baseline to 6 and 12 weeks for both groups (see Supplementary Figure 1), with differences between groups not significant (6-week estimate: 0.09, 95% CI: −0.24–0.42, p = .580; 12-week estimate: −0.10, 95% CI: −0.46–0.25, p = .570).
Psychological Distress
For the NVP group, the mean K10 scores were 31.96, 24.66, and 22.17 at each time point which were significant reductions from baseline (p < .001 for both 6 and 12 weeks). Similarly, there were significant reductions in the mean K10 scores in the NRT group from baseline of 32.88 to 21.42 at 6 weeks (p < .001) and 22.52 at 12 weeks (p < .001). The differences between groups were not significant at 6 (IRR: 2.12, 95% CI: −1.81–6.05, p = .288) or 12 weeks (IRR: −1.29, 95% CI: −5.73–3.16, p = .567) follow-up (see Supplementary Figure 2).
Adverse Events
In the NVP group, 15 participants reported 19 adverse events and one serious adverse event. In the NRT group, 10 participants reported 14 adverse events. No adverse events or serious adverse events were classified as probably or definitely caused by the study products.
Quitline Calls
There were no differences in the number of Quitline calls received by group: 43 participants in the NVP group received Quitline calls (mean: 2.58, SD: 2.6, range: 1–12) and 42 participants in the NRT group received Quitline calls (mean: 2.57, SD: 2.98, range: 1–13) over the 12-week study period.
Discussion
This pilot study showed that smoking cessation support involving options for nicotine replacement and Quitline-delivered cognitive behavioral counseling is attractive to people after they have been discharged from SUD treatment. Both NVPs and NRT were rated highly acceptable and well used by those participants who returned at 6- and 12-week follow-up. Those participants reported reductions in cravings for cigarettes and perceptions of withdrawal symptoms and reductions in number of cigarettes smoked. Some participants self-reported abstinence from cigarettes—around one in five reported having quit smoking cigarettes at 12-week postdischarge. This pilot study was not powered to detect differences between groups in cessation or acceptability outcomes, but rather suggests that powered trials of NVPs and NRT in this population are warranted.
Our findings suggest that clients will engage with smoking cessation support after discharge from SUD treatment if given the opportunity, and that many will report benefits such as reductions in cigarettes smoked, withdrawal symptoms, and cravings for cigarettes. Some clients even reported periods of abstinence from cigarettes. Implications of this finding are that providing smoking cessation support postdischarge from a smoke-free facility is an opportunistic time to intervene which may lead to longer term smoking cessation. Further examination of the optimal forms of smoking cessation support for people discharged from SUD treatment is warranted, as is longer term follow-up as some of those still using a product had not quit completely, and it will be of interest to see if further use of nicotine products eventually does lead to more cessation.
The Quitline support was designed to overcome previous reports from clients in SUD treatment that they could not relate to Quitline counselors and therefore rarely engaged with Quitline.20 The counselors involved in this trial were trained by clinical psychologists in provision of smoking cessation support to people with SUD. Overall, 84 of the 100 participants recruited into the trial engaged with Quitline with an average of 2.5 calls, which is an encouraging result for this population. Also encouraging was the evident reduction in psychological distress in both treatment groups. Scores on the psychological distress scale suggested that participants were likely to have been experiencing severe mental distress at baseline, which fell to mild levels across the duration of the study.
Very similar results were found for the two types of nicotine products (VNPs or NRT) with use and acceptability high, suggesting that it is important to continue examining optimal ways to support people following discharge from SUD treatment with pharmacotherapies. This has been a largely neglected group of smokers, yet if given encouragement, they will take up nicotine replacement treatment. Adherence to treatment was high amongst both NVP and NRT groups, but higher for those using NVPs. There are two important implications from these results. Firstly, contrary to research that suggests people with SUD prefer not to use NRT,20 this study found that 70% of NRT users were still using combination NRT at 12-week follow-up. This result is promising, and likely related to the level of behavioral support provided to participants from the telephone Quitline. Providing choices for types of NRT seems important given the variability in acceptability levels by NRT type. Secondly, that 80% of participants in the NVP group were still using their devices suggests that NVPs may be an acceptable form of providing nicotine to smokers with SUD. The effectiveness of NRT for people with SUD has not been as positive as it has been for the general population,44 and more trials of nicotine therapies are needed.
It should be noted that in Australia, NVPs are banned from use within smoke-free health services. Consequently, participants in the current trial received an hour of training in how to use their NVP device, but this had to occur in a private room and no nicotine liquid was used during training. No NVPs were able to be used while participants were still in treatment. All research-supplied nicotine products were provided at discharge from the facility, limiting the ability to properly train and supervise NVP use. The potency of the intervention may be enhanced if people are provided with the NVPs while in treatment and are using the devices more routinely and efficiently once they are discharged. Further qualitative research exploring the views of staff and clients of SUD treatment services on NVP use while in treatment would help determine the feasibility of implementing them during treatment.
Strengths and Limitations
The QuitNic trial was designed to aid in the development of a larger powered randomized controlled trial.43 As a pilot trial, QuitNic is exploratory in nature and has a number of limitations. The biggest design challenge in the current study was attrition, with only 50% of participants retained. Given the sample was drawn from clients leaving a SUD treatment facility and returning to their community, in some cases to unstable environments, it is not surprising that it was difficult to maintain contact with many participants. Future trials should invest in strategies for minimizing attrition such as financial reimbursement, reminder systems, and collection of detailed contact information from significant others.45 This pilot study was not funded to include these retention strategies. Also, allowing commencement of nicotine product use while in treatment may aid retention. Also, self-reported withdrawal symptoms at baseline may be related to withdrawal from drugs other than nicotine. This study only collected short-term outcomes, at weeks 6 and 12 of a 12-week intervention phase, and future trials should also collect outcomes at longer term follow-up. Furthermore, future studies could adopt a less stringent definition of quit success than no more than 5 cigarettes post-discharge. Allowing some leeway and looking at eventual success over the period people continue to engage with the aids would give a better picture of their long-term potential. Reliance on self-report alone is also a study limitation. Given this pilot study has demonstrated high treatment adherence, high acceptability, positive trends in outcomes and relatively good retention, larger effectiveness, and safety trials are likely to be feasible.
In conclusion, the results have significant public health implications for providing quit support following discharge from SUD services, and further research is required to identify the most effective smoking cessation treatments for this population to achieve long-term abstinence from cigarettes.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, is available online at https://academic.oup.com/ntr.
Funding
This study was supported by a VicHealth Innovation Research Grant (2016-0096).
Declaration of Interests
None to declare.
Acknowledgments
We are grateful for the assistance and participation of staff and clients of Wellington House, Turning Point, Melbourne and the Quitline Victoria counselors.
References
- 1. Guydish J, Passalacqua E, Pagano A, et al. An international systematic review of smoking prevalence in addiction treatment. Addiction. 2016;111(2):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275(14):1097–1103. [DOI] [PubMed] [Google Scholar]
- 3. Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev Med. 1994;23(1):61–69. [DOI] [PubMed] [Google Scholar]
- 4. Randall D, Degenhardt L, Vajdic CM, et al. Increasing cancer mortality among opioid-dependent persons in Australia: A new public health challenge for a disadvantaged population. Aust N Z J Public Health. 2011;35(3):220–225. [DOI] [PubMed] [Google Scholar]
- 5. Richter KP, Arnsten JH. A rationale and model for addressing tobacco dependence in substance abuse treatment. Subst Abuse Treat Prev Policy. 2006;1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bandiera FC, Anteneh B, Le T, Delucchi K, Guydish J. Tobacco-related mortality among persons with mental health and substance abuse problems. PLoS One. 2015;10(3):e0120581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: Systematic review and meta-analysis. BMJ. 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skelton E, Tzelepis F, Shakeshaft A, et al. Smoking cessation care provision in Australian alcohol and other drug treatment services: A cross-sectional survey of staff self-reported practices. J Subst Abuse Treat. 2017;77:101–106. [DOI] [PubMed] [Google Scholar]
- 10. Knudsen HK Implementation of smoking cessation treatment in substance use disorder treatment settings: A review. Am J Drug Alcohol Abuse. 2017;43(2):215–225. [DOI] [PubMed] [Google Scholar]
- 11. Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014;311(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skelton E, Tzelepis F, Shakeshaft A, et al. Addressing tobacco in Australian alcohol and other drug treatment settings: A cross-sectional survey of staff attitudes and perceived barriers. Subst Abuse Treat Prev Policy. 2017;12(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brose LS, Simonavicius E, McNeill A. Maintaining abstinence from smoking after a period of enforced abstinence - systematic review, meta-analysis and analysis of behaviour change techniques with a focus on mental health. Psychol Med. 2018;48(4):669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clarke JG, Stein LA, Martin RA, et al. Forced smoking abstinence: Not enough for smoking cessation. JAMA Intern Med. 2013;173(9):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prochaska JJ, Fletcher L, Hall SE, Hall SM. Return to smoking following a smoke-free psychiatric hospitalization. Am J Addict. 2006;15(1):15–22. [DOI] [PubMed] [Google Scholar]
- 16. Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. [DOI] [PubMed] [Google Scholar]
- 17. Rüther T, Ruderer A, Wirth C, et al. Smoking cessation program for inpatients with substance use disorder: A quasi-randomized controlled trial of feasibility and efficacy. Eur Addict Res. 2016;22(5):268–276. [DOI] [PubMed] [Google Scholar]
- 18. Rohsenow DJ, Martin RA, Tidey JW, Colby SM, Monti PM. Treating smokers in substance treatment with contingent vouchers, nicotine replacement and brief advice adapted for sobriety settings. J Subst Abuse Treat. 2017;72:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rohsenow DJ, Tidey JW, Kahler CW, Martin RA, Colby SM, Sirota AD. Intolerance for withdrawal discomfort and motivation predict voucher-based smoking treatment outcomes for smokers with substance use disorders. Addict Behav. 2015;43:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson AJ, Bonevski B, Dunlop A, et al. ‘The lesser of two evils’: A qualitative study of staff and client experiences and beliefs about addressing tobacco in addiction treatment settings. Drug Alcohol Rev. 2016;35(1):92–101. [DOI] [PubMed] [Google Scholar]
- 21. Gartner C, Hall W. A licence to vape: Is it time to trial of a nicotine licensing scheme to allow Australian adults controlled access to electronic cigarettes devices and refill solutions containing nicotine? Int J Drug Policy. 2015;26(6):548–553. [DOI] [PubMed] [Google Scholar]
- 22. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: An internet survey. Drug Alcohol Rev. 2013;32(2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Academies of Sciences E, Medicine, Health, et al. Public Health Consequences of E-Cigarettes. In: Eaton DL, Kwan LY, Stratton K, eds. Washington (DC): National Academies Press (US). Copyright 2018 by the National Academy of Sciences; All rights reserved.; 2018. [PubMed] [Google Scholar]
- 24. Etter JF Gateway effects and electronic cigarettes. Addiction. 2018;113(10):1776–1783. [DOI] [PubMed] [Google Scholar]
- 25. Glasser A, Abudayyeh H, Cantrell J, Niaura R. Patterns of E-Cigarette use among youth and young adults: Review of the impact of E-Cigarettes on cigarette smoking. Nicotine Tob Res. 2019;21(10):1320–1330. [DOI] [PubMed] [Google Scholar]
- 26. Levy DT, Warner KE, Cummings KM, et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: A reality check. Tob Control. 2019;28(6):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centres for Disease Control and Prevention. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Published 2020. Accessed March 2020.
- 28. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: A randomised controlled trial. Lancet. 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 29. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of E-Cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–637. [DOI] [PubMed] [Google Scholar]
- 30. Walker N, Parag V, Verbiest M, Laking G, Laugesen M, Bullen C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: A pragmatic, randomised trial. Lancet Respir Med. 2020;8(1):54–64. [DOI] [PubMed] [Google Scholar]
- 31. Felicione NJ, Enlow P, Elswick D, Long D, Sullivan CR, Blank MD. A pilot investigation of the effect of electronic cigarettes on smoking behavior among opioid-dependent smokers. Addict Behav. 2019;91:45–50. [DOI] [PubMed] [Google Scholar]
- 32. Stein MD, Caviness C, Grimone K, Audet D, Anderson BJ, Bailey GL. An open trial of electronic cigarettes for smoking cessation among methadone-maintained smokers. Nicotine Tob Res. 2016;18(5):1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guillaumier A, Manning V, Wynne O, et al. Electronic nicotine devices to aid smoking cessation by alcohol- and drug-dependent clients: Protocol for a pilot randomised controlled trial. Trials. 2018;19(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 35. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction. 2005;100(3):299–303. [DOI] [PubMed] [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 37. Taggar JS, Lewis S, Docherty G, Bauld L, McEwen A, Coleman T. Do cravings predict smoking cessation in smokers calling a national quit line: Secondary analyses from a randomised trial for the utility of ‘urges to smoke’ measures. Subst Abuse Treat Prev Policy. 2015;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav. 2007;21(2):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184–189. [DOI] [PubMed] [Google Scholar]
- 40. Fidler JA, Shahab L, West O, et al. ‘The smoking toolkit study’: A national study of smoking and smoking cessation in England. BMC Public Health. 2011;11:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34(4):365–373. [DOI] [PubMed] [Google Scholar]
- 42. SAS Software [computer program]. Version Version 9.4. Cary, NC. USA Copyright. 2020. [Google Scholar]
- 43. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: Recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. [DOI] [PubMed] [Google Scholar]
- 44. Miller ME, Sigmon SC. Are Pharmacotherapies ineffective in opioid-dependent smokers? Reflections on the scientific literature and future directions. Nicotine Tob Res. 2015;17(8):955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: A systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.