Abstract
Introduction
Although the use of combustible cigarettes has decreased in many urban regions of America, the use of electronic nicotine delivery systems (ENDS) has dramatically increased. ENDS, or electronic cigarettes (e-cigarettes), differ from combustible cigarettes given that there are no restrictions on flavorant additives in e-liquids. With 95% of ENDS users vaping flavored e-liquids, it is critical to understand how flavors alter vaping-related behaviors. We have previously shown that menthol and green apple flavors enhance nicotine reward-related behavior in a mouse model and in the present study have investigated how menthol and green apple flavors alter e-Vape self-administration behavior in male mice.
Methods
Adult C57/BL6J male mice were used in vapor-inhalation self-administration assays. Mice were assigned vaping e-liquids (6 mg/mL nicotine with or without menthol or green apple flavor) to escalate on a fixed-ratio 1 (FR1) schedule in daily 3-hour sessions to examine initiation-related behaviors. Following escalation, mice were transitioned to a FR3 and progressive ratio schedules in 3-hour sessions to examine reinforcement-related behaviors.
Results
Here we observed that male mice exhibited increased rates of self-administration escalation on a FR1 schedule when assigned to flavored e-liquids. Upon transition to FR3, mice continued to exhibit enhanced levels of reinforcement with flavored e-liquids. We also observed that mice self-administer zero-nicotine green apple flavored e-liquids.
Conclusions
These data provide additional evidence that ENDS flavors enhance vaping-related initiation and reinforcement-related behavior and promote the need to continue investigating the role ENDS flavors play in vaping-related behaviors.
Implications
There has been much discussion recently regarding the impact of flavors on vaping-related behavior. Our study here shows that flavors significantly enhance the acquisition and reinforcement of vaping-related behavior. This suggests that flavors in electronic nicotine delivery systems significantly increase the risk of addiction-related behaviors among users of vaping products.
Introduction
Since the inception of electronic nicotine delivery systems (ENDS) in 2003,1 ENDS have gained popularity among life-long cigarette smokers as a “safer” alternative to smoking, as well as teens naive to smoking. ENDS use continues to rise among the adolescent population, with a 135% increase among high school students from 2017 to 2019,2 and a 114% increase among middle school students within the past year alone.2 Ongoing research has demonstrated the numerous harmful chemical constituents found in ENDS, including heavy metals, cancer-causing chemicals, nicotine, and flavoring agents.3–7 Most flavoring agents used are “generally recognized as safe” (GRAS), although this provision applies only to food or drink. GRAS-labeled flavorants have chiefly been studied in conditions subjected to first-pass elimination mechanisms. Vaping, similar to smoking, presents a distinct pharmacokinetic profile due to the rapid delivery of inhaled chemicals to the brain.8 Furthermore, Flavor and Extracts Manufacturers Association (FEMA), the organization responsible for verifying the flavoring substances safe for the Food and Drug Administration (FDA), has stated that they do not evaluate flavorants for contact through inhalation; they only evaluate for ingestion exposure.9 With over 7700 ENDS flavors to choose from and the increased use of zero-nicotine flavored e-liquids, ENDS flavors have become a growing concern.10,11
A primary reason that flavors were banned in combustible cigarettes was the understanding that flavors increase smoking initiation, especially in adolescents.12,13 We, and others, have previously shown that menthol enhances nicotine reward-related and reinforcement-related behavior14–16. Additionally, we have shown that green apple flavor enhances nicotine reward-related behavior and is rewarding by itself in male mice only.17 The mechanisms for these flavorant-induced effects on reward-related behavior is not dependent on odorant and tastant effects as these previous reports have established that flavorants in both cultured neuron16 and brain slice preparations17 trigger changes in ventral tegmental area dopamine and GABA neuron function in a manner that contributes to increases in dopamine neuron excitability. Thus, there is sufficient evidence that ENDS flavors exert an effect that goes beyond both odorant and tastant effects to alter neurons of the reward pathway directly and contribute to changes in behavior. For this reason, we examined how mice escalate to nicotine vapor inhalation with or without menthol or green apple, two very popular flavors among ENDS users. We also investigated the effect of green apple flavor alone, as our recent investigations demonstrated this flavor alters reward-related behavior and midbrain neuron function in the absence of nicotine.17 To examine the reinforcement-related behavior of various ENDS flavors, we have utilized a novel vapor-inhalation (e-Vape) self-administration paradigm to accurately and efficiently model human vaping in a noninvasive manner. Using e-Vape self-administration, we report that male mice on a fixed-ratio 1 (FR1) schedule will escalate in their self-administration behavior with menthol + nicotine, green apple + nicotine, and green apple-only e-liquids, and will maintain reinforcement-related behavior on an FR3 schedule. These ENDS flavors were verified to act in a nAChR-mediated manner, demonstrated by pretreatment injections of the nAChR antagonist, DhβE, into mice prior to self-administration assays. The finding that flavored ENDS alter vaping-related behaviors by increasing the rate of initiation through self-administration escalation and eliciting greater reinforcement-related behavior demonstrates the need to examine ENDS flavors for their role in enhancing vaping-related nicotine addiction.
Materials and Methods
Mice
All experiments were conducted in accordance with the guidelines for care and use of animals provided by the National Institutes of Health. Protocols were approved by the Institutional Animal Care and Use Committee at Marshall University. All mice used in these assays were adult (3–6 months old) male C57BL/6J mice obtained from the Jackson laboratory. Mice were housed on a standard 12/12 hour light/dark cycle and allowed food and water ad libitum. Mice were used in behavioral experiments during their light cycle between the hours of 9 am–12 pm, 12–3 pm, or 3–6 pm for morning, afternoon, and evening sessions. We detected no difference in responses during these three light-time periods. Mice were not food restricted to attain operant behavior.
Drugs
All nicotine-containing e-liquids in this work used free-base nicotine. Free-base nicotine (product number N2472-100ML, lot number 2AH0278) was obtained from Spectrum. Dihydro-β-erythroidine (DhβE) hydrobromide, a β2* nAChR antagonist (catalog number 2349), was obtained from Tocris. 30% Propylene glycol and 70% vegetable glycerin (30/70 PGVG) was obtained from La Jolla Alcohol Research, Inc. (La Jolla, CA). For these experiments, we used commercial e-liquids and e-liquids made to contain specific concentrations of nicotine and flavorants in PGVG (see Supplementary Material for full details). DhβE was dissolved in saline and injected into mice intraperitoneally, at 2 mg/kg, prior to self-administration sessions. With the exception of the nicotine dose–response, all nicotine-containing e-liquids used 6 mg/mL nicotine.
Self-administration Assays
We used a commercial vapor self-administration setup for these assays (http://www.ljari.tech, see Supplementary Material and Supplementary Figure S1). For the experiments described here, five cohorts of C57BL/6J mice were used: cohort 1 for examining commercial (store-bought) e-liquids (Figures 1 and 2A; n = 12–28); cohort 2 for examining DhβE effects on commercial e-liquids (Figure 2B and C; n = 5); cohort 3 for a nicotine dose–response using “neat” e-liquids (Figure 3A only; n = 8); cohort 4 for examining nicotine with and without flavors in “neat” e-liquids (Figures 3B and C and 4; n = 9); and cohort 5 for assessing plasma cotinine concentrations (n = 10). Due to word limit constraints, the use of these five cohorts of mice and their specific protocols is described in detail in Supplementary Material.
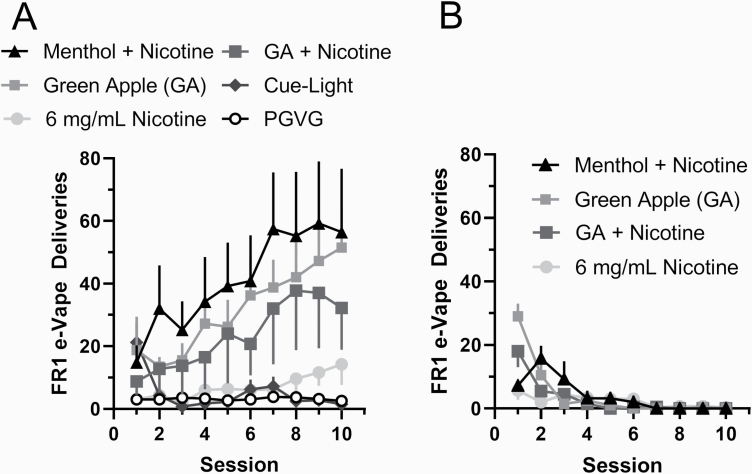
Figure 1.
(A) Mice naive to vapor exposure were escalated on designated e-liquids in e-Vape self-administration assays using 10 daily 3-h FR1 sessions (3-s puff with 60-s timeout) (n = 4 mice/group) with the indicated commercial e-liquids. Where not indicated, nicotine is 6 mg/mL. Data are mean (± SEM) e-Vape deliveries for each session. (B) Mean inactive nose-pokes for mice during sessions described in (A).
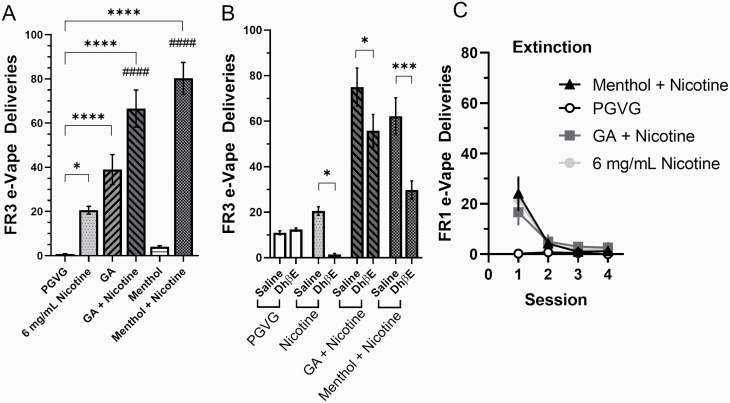
Figure 2.
(A) Mice on a FR3 schedule were assigned commercial e-liquids during a 3-h e-Vape self-administration session, n = 12 mice/group. (B) Mice were intraperitoneally injected with saline or 2 mg/kg DhβE immediately prior to FR3 self-administration sessions. Two-way ANOVA with post hoc Bonferroni; n = 5 mice. (C) All mice were assigned PGVG on a FR1 schedule to examine extinction-related behavior. Data are mean (± SEM) e-Vape deliveries for each session. Where not indicated, nicotine is 6 mg/mL. *p < .05; **p < .01; ***p < .005. ####p < .0001; vs. 6 mg/mL nicotine, one-way ANOVA with post hoc Tukey.
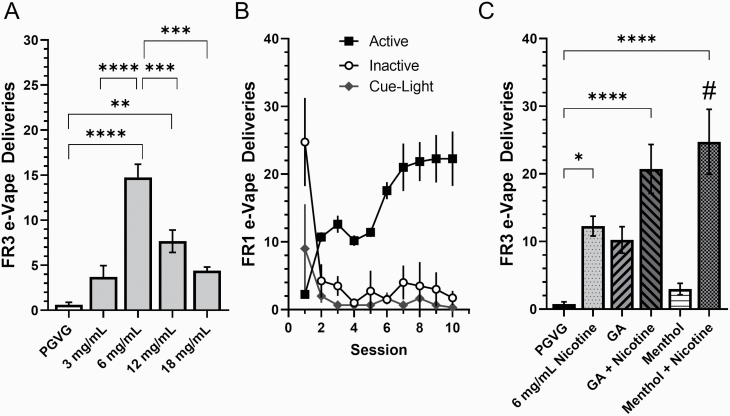
Figure 3.
(A) Dose–response of nicotine only (unflavored) at concentrations of 0 (PGVG only), 3, 6, 12, and 18 mg/mL in e-Vape self-administration 3-h FR3 sessions (n = 8 mice). (B) Adult male mice were escalated on “neat” (−)-menthol + nicotine on a FR1 schedule (10 daily 3-h sessions). Data are mean (± SEM) FR1 e-Vape deliveries per session. (C) Mice were placed on a FR3 schedule to examine earned e-Vape deliveries for “neat” nicotine with or without flavors. Where not indicated, nicotine is 6 mg/mL. (A, C) Data are mean (± SEM) FR3 e-Vape deliveries for each condition. *p < .05; **p < .01; ***p < .005; ****p < .001. #p < .05; vs. 6 mg/mL nicotine. One-way ANOVA with post hoc Tukey.
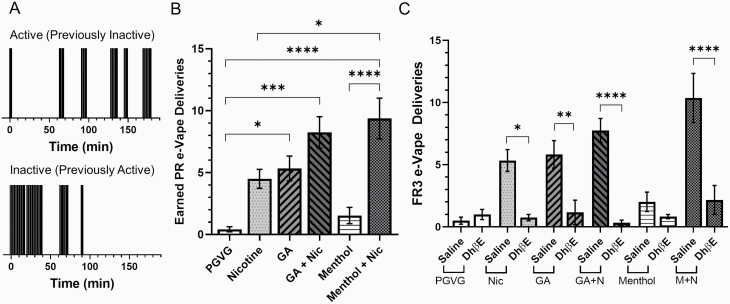
Figure 4.
(A) Raster plots that show the number of nose-pokes on the active/inactive sides for one mouse on the first day when the nose-poke assignments were reversed to examine side bias. (B) Mice were place on a PR schedule and assigned nicotine with or without flavors (n = 9). (C) Mice were pre-injected with saline or 2 mg/kg DhβE prior to a FR3 self-administration session (n = 4–6/condition). (B and C) Data are mean (± SEM) e-Vape deliveries per mouse. In all cases, nicotine is 6 mg/mL. *p < .05; **p < .01; ***p < .005; ****p < .001; one-way ANOVA with post hoc Tukey.
In general, adult (3 months old), male mice began vapor self-administration on an FR1 schedule on a Monday for 10 daily 3-hour sessions, with a weekend abstinence (Supplementary Figure S2). Mice were singly placed into air-tight operant chambers that contained two nose-pokes (one active and one inactive) (Supplementary Figure S1). Nose-pokes in the active hole resulted in a 3-second delivery of vaporized e-liquids through the vapor entrance port with a 60-second timeout. During the timeout, a yellow cue-light remained on. Inactive nose-pokes were recorded with no consequences.
Following FR1 escalation, mice were transitioned to a FR3 schedule where they were maintained on an e-liquid for four consecutive days (starting on a Monday) to reach stable responding and rebaselined to their original FR1 assigned e-liquid on day 5 (Friday). Mice were used in a within-subject, Latin square design to test multiple e-liquids. When DhβE (2 mg/kg) was used, mice were given intraperitoneal injections immediately prior to 3-hour e-Vape self-administration sessions (2 days with saline, 2 days with DhβE). For progressive ratio (PR) assays, the following equation: 2(2n/9) was used to determine the number of active nose-pokes required for e-Vape delivery. Similar to FR1/FR3, PR sessions lasted 3 hour. Once again, the methods are provided in more detail within Supplementary Material.
Statistical Analysis
All results are presented as mean ± SEM, and all statistical analyses were performed using GraphPad Prism. To examine the effect of nicotine and flavorants on escalation of e-Vape self-administration behavior, we used a one-way repeated-measures analysis of variance (ANOVA) (Figure 1, Supplementary Table S1). Nicotine dose–response data were analyzed using a one-way repeated-measures ANOVA with nicotine dose being the within-subject factor (Figure 3). FR3 e-Vape responding between e-liquids was analyzed with a one-way repeated-measures ANOVA (Figures 2 and 3) or a two-way ANOVA for comparisons among e-liquids and DhβE antagonism (Figure 2 and 4). All means comparisons included the testing of each treatment group with all others within an experiment using a post hoc Tukey or Bonferroni (selected post hoc tests are indicated in the figure legends).
Results
Effect of Flavors on Escalation and Reinforcement of Self-administration Behavior
We first investigated self-administration behavior in adult male mice (cohort 1) that were assigned commercial (store-bought) e-liquids. Mice were assigned PGVG (control), 6 mg/mL nicotine, menthol + nicotine, green apple + nicotine, menthol (zero nicotine), green apple (zero nicotine), and finally a cue-light only control (n = 4 each; Figure 1A). For ten 3-hour sessions, mice were allowed to self-administer their assigned e-liquid on an FR1 schedule (Figure 1A). Following the 10 sessions, menthol + nicotine and green apple alone produced the highest number of FR1 deliveries (41.4 and 31.7 average FR1 deliveries, respectively), followed by green apple + nicotine and nicotine alone (23.6 and 6.93 average FR1 deliveries, respectively). For both PGVG and cue-light only groups, mice gradually reduced the number of responses as the sessions continued. A one-way ANOVA and post hoc Tukey means comparison revealed that menthol + nicotine, green apple, and green apple + nicotine were significantly different from PGVG (Supplementary Table S1). We also observed that nose-pokes on the inactive side progressively decreased as FR1 sessions continued (Figure 1B). Additionally, we assigned mice to escalate on menthol alone on a FR1 schedule, but mice exhibited behavior similar to extinction (Supplementary Figure S3).
Upon completing FR1 escalation, mice were then transitioned to a FR3 schedule and continued on their assigned e-liquid for four consecutive days to reach stable responding. Next, mice were used in a within-subject design where they were presented e-liquids (PGVG, 6 mg/mL nicotine, green apple, green apple + nicotine, menthol, or menthol + nicotine) following a Latin square design (n = 12; Figure 2A). Mice maintained their assigned e-liquid for 4 days and were rebaselined on the fifth day using their original FR1 assigned e-liquid. Using a one-way repeated-measures ANOVA, we observed a significant main effect of e-liquids on FR3 earned e-Vape deliveries (F(5, 45) = 37.7, p < .0001). Using a post hoc Tukey means comparison, we determined that 6 mg/mL nicotine, green apple, green apple + nicotine, and menthol + nicotine produced significantly more FR3 deliveries than PGVG (Figure 2A). Additionally, mice assigned green apple + nicotine or menthol + nicotine earned significantly more FR3 e-Vape deliveries compared with 6 mg/mL nicotine (Figure 2A). Similar to previous investigations,14,15 menthol alone did not result in any apparent reinforcement-related behavior as mice earned few FR3 e-Vape deliveries (Figure 2A).
We have previously shown that both menthol and green apple flavorants alter behavior through a nAChR-mediated mechanism.16,17 To confirm that these flavor-induced changes in behavior were mediated through nAChRs, we used cohort two mice (following identical preceding FR1/FR3 self-administration assays, see Supplementary Material for additional details) to determine whether the β2-containing nAChR antagonist, DhβE, reduced self-administration behavior. To do this we used a 4-day protocol where mice were divided into a between-subjects design and assigned PGVG, 6 mg/mL nicotine, green apple + nicotine, and menthol + nicotine (n = 5 each). On days 1 and 2, mice were injected with saline immediately prior to entering the self-administration chamber to establish a new baseline for FR3 responding. On days 3 and 4, mice were injected with DhβE (2 mg/kg; intraperitoneal injection) immediately prior to FR3 self-administration sessions (Figure 2B). Using a two-way ANOVA, we observed a significant effect with e-liquid assignment (interaction, F(3, 32) = 3.75, p = .021; e-liquid factor, F(3, 32) = 55.3, p < .0001; DhβE factor, F(1, 32) = 23.2, p < .0001). DhβE significantly reduced FR3 responding in mice assigned to nicotine, green apple + nicotine, and menthol + nicotine. These data suggest that nAChR-mediated mechanisms are critical to e-Vape self-administration behavior. We note that mice assigned PGVG in these assays exhibited higher FR3 e-Vape deliveries compared with those in Figure 2A. This may be attributed to the fact that these mice are different from those in Figure 2A, but their higher number of responses to PGVG may be due to an extinction-related burst as they were previously assigned to menthol + nicotine for FR1 training. Following this final series of FR3 sessions, mice were rebaselined to their assigned e-liquid during the DhβE trial to reach stable responding and then extinguished in four daily FR1 sessions with PGVG only (Figure 2C). Here we observed that mice extinguished e-Vape self-administration behavior within 2–3 days.
Nicotine Dose–Response in e-Vape Self-administration
There have been several reports that highlight the fact that ENDS e-liquids contain concentrations of nicotine and flavorants that can be vastly different from their labeled amounts.18,19 Therefore, the translational value of achieving self-administration with mice using commercial e-liquids popular in the vaping community is tremendously reduced by concerns regarding consistency of the e-liquid constituents. For this reason, we examined e-liquids made to contain specific concentrations of nicotine and flavorants (“neat” e-liquids).
First, we decided to examine the dose–response of nicotine only (zero flavorants) in mice using 0 (PGVG), 3, 6, 12, and 18 mg/mL nicotine (n = 8; Figure 3A). Cohort 3 mice (see Supplementary Figure S2) were allowed to escalate e-Vape self-administration behavior using 6 mg/mL nicotine during 10 daily 3-hour FR1 sessions identical to those used for mice assigned commercial e-liquids described above (cohort 1). Following 10 days of FR1 escalation, mice were transitioned to an FR3 schedule and maintained on 6 mg/mL nicotine for 4 days to reach stable responding. Following this, mice were used in a within-subject design and presented the different nicotine concentrations using a Latin square design (Figure 3A). A one-way repeated-measures ANOVA, with nicotine dose set as within-subject factor, resulted in a main effect on e-Vape responding (F(4, 36) = 22.3, p < .0001). Using a post hoc Tukey means comparison, we compared all five doses and observed that only 6 and 12 mg/mL nicotine were significantly different from PGVG (p < .0001 and p = .0025, respectively). Six milligrams per milliliter nicotine resulted in the highest number of FR3 e-Vape deliveries and was also significantly different from 3, 12, and 18 mg/mL nicotine (p < .0001, .0007, <.0001, respectively; Figure 3A). Six milligrams per milliliter nicotine is also preferred by human ENDS users who are new to nicotine use.18
Using “Neat” E-liquids, Mice Exhibit Vapor Self-administration Trends Similar to Prior Reports
Based on prior analytical chemistry reports on ENDS e-liquid compositions, menthol and green apple flavors are present at concentrations of 15 mg/mL.18 Furthermore, green apple e-liquids were in many cases constituted of hexyl acetate, ethyl acetate, and methylbutyl acetate.18,19 Accordingly, we made menthol e-liquids using 15 mg/mL (−)-menthol and green apple e-liquids using 15 mg/mL green apple (hexyl acetate, ethyl acetate, and methylbutyl acetate at a 2.5:1:1 ratio) in PGVG. In e-liquids containing nicotine, we used 6 mg/mL based on the peak response we noted (Figure 3A). Similar to assays described with commercial e-liquids, mice escalated on a FR1 schedule for ten 3-hour e-Vape self-administration sessions using a protocol identical to that used for commercial e-liquids (n = 9; Figure 3B). Next, mice were transitioned to a FR3 schedule for 4 days on their assigned e-liquid to reach stable responding. Following this, mice were used in a within-subject design to examine PGVG, 6 mg/mL nicotine, green apple, green apple + nicotine, menthol, and menthol + nicotine. Mice were assigned e-liquids using a Latin square design, maintained their e-liquid for 4 days, and were rebaselined using menthol + nicotine on the fifth day. The final 2 days of FR3 responding on each e-liquid were averaged to calculate the number of FR3 e-Vape deliveries (Figure 3C). Using the “neat” e-liquids, we observed a trend in FR3 responding that was similar to the commercial e-liquids. Using a one-way repeated-measures ANOVA, we noted a significant effect of e-liquid treatment (F(5, 42) = 12.7, p < .0001). Using post hoc Tukey means comparison, we noted a significant difference between mice assigned 6 mg/mL nicotine, green apple + nicotine, and menthol + nicotine when compared with PGVG (p = .033, <.0001, and <.0001, respectively, Figure 3C). Additionally, we noted a significant difference between menthol + nicotine versus nicotine alone (p = .017). Although green apple + nicotine produced a 68.8% increase in FR3 responding versus nicotine (mean FR3 e-Vape deliveries of 20.7 and 12.3, respectively), these two conditions were not statistically different from each other (p = .213). In order to determine whether our e-Vape methods produce relevant plasma cotinine concentrations in mice, we utilized a plasma cotinine ELISA assay and a yoked paradigm (see Supplementary Results).
Following FR3 responding, mice were then moved to a PR schedule. Here, required nose-pokes were determined by the equation, PR = 2(2n/9), where “n” is the number of earned e-Vape deliveries within the session. PR responses were maintained for 2 days on a mouse’s prior assigned active nose-poke. On the next 2 days, the active and inactive nose-pokes were switched to examine the presence of a side bias. Upon switching the active nose-poke assignment, mice gradually switched responding from the inactive (previously active) to the active (previously inactive) nose-poke during their 3-hour PR session (Figure 4A, Supplementary Figure S4). This supports the fact that the mice have learned the self-administration behavior and do not have a side bias toward operant behavior. When all 4 days of PR were averaged, we noted a trend that was similar to FR3 e-Vape deliveries (Figure 4B), and using a one-way repeated-measures ANOVA, we noted a significant main effect (F(5, 40) = 10.9, p < .0001). Similar to FR3 sessions, we noted a significant difference between green apple, green apple + nicotine, and menthol + nicotine when compared with PGVG (Figure 4B). Additionally, menthol + nicotine resulted in significantly more earned PR deliveries when compared with nicotine alone. Finally, we used DhβE to examine the role of β2* nAChRs in reinforcement-related behaviors of mice assigned to these “neat” e-liquids (Figure 4C). Using a two-way ANOVA, we detected a significant effect of e-liquid and pretreatment (interaction, F(5, 54) = 5.40, p = .0004; e-liquid factor, F(5, 54) = 7.50, p < .0001; DhβE factor, F(1, 54) = 52.3, p < .0001). With nicotine, green apple, green apple + nicotine, and menthol + nicotine, we detected a significant difference between saline and DhβE pretreated mice (Figure 4C). We detected no difference with mice assigned to PGVG or menthol alone.
Discussion
The goal of this study was to establish our vapor self-administration assays and examine the effects of ENDS flavors on vaping-related behaviors in a mouse model. With the growing popularity of flavored ENDS and the plethora of flavor options,10,20 it is important to establish a model relevant to human vaping that can then be used in conjunction with high-resolution neurobiology and neurophysiological assays. Given our previous studies16,17 and the current popularity among ENDS users, we chose to begin our studies with menthol and green apple flavor, with and without nicotine. Prior research regarding flavors in rodent self-administration behavioral assays reported enhanced nicotine reward- and reinforcement-related behavior; however, this data were compiled using intravenous self-administration14,15 or conditioned place preference.16,17 E-Vape self-administration provides a unique methodology to study vaping-related behaviors in mice, without surgery and with the potential of using identical vaping products used by humans. Although the novelty and translational value of using commercial e-liquids is an option, the unavoidable problem with this is the discrepancies between labeled and actual concentrations of nicotine and flavorants. Thus, we made “neat” e-liquids to contain specific concentrations of nicotine and flavorants to ensure consistency. These e-liquids and our operant exposure paradigm produced cotinine levels that are consistent with previously reported studies using vapor inhalation.21–23
In this study, we note that flavored ENDS (menthol + nicotine, green apple + nicotine, and green apple alone) enhance the rate of acquisition of self-administration behavior through a FR1 schedule compared with nicotine alone or PGVG (Figure 1). In humans, we may consider a finding like this to convey flavors may enhance the initiation of ENDS use by masking the harshness of nicotine and providing a more palatable taste.24,25 However, mice experience taste and odor in a distinctly different manner than humans. Thus, it would be more accurate to rely on our previous publications that used administration routes that bypassed odorant and tastant responses (intraperitoneal injections) to document flavorant-induced changes in ventral tegmental area dopamine and GABA neuron firing.16,17 Thus, this enhancement in escalation, FR3 responding, and PR responding may be indicative of enhancements in the neurons that mediate the nicotine reward pathway. We also note that our results with menthol, do mirror the results obtained using intravenous self-administration paradigms that examined menthol + nicotine.14,15 We also note that our dose–response with vaporized nicotine was observed to exhibit an inverted-U dose response curve that is similar to previous nicotine intravenous self-administration paradigms.26,27 In using DhβE, we determined that the self-administration behavior we observed is mediated through nAChR activation. We note the differences between the use of commercial e-liquids (flavored e-liquids were not blocked 100%) and “neat” e-liquids (responding was eliminated largely). This may be due to the fact that responding with the commercial e-liquids was approximately twofold higher than the “neat” e-liquids. This is likely due to varying concentrations of nicotine (despite labeling) or unlabeled constituents. Several analytical reports have noted that menthol and green apple e-liquids may contain additional flavorants of the vanilla flavor category,19 and this may provide additional enhancements in reinforcement-related behavior.
In summary, we have used a novel contingent vaping-related model to reproduce trends involving flavor-induced enhancement of nicotine-related behaviors.14–17 Furthermore, this study provides further evidence that flavors can enhance nicotine reinforcement-related behaviors through a nAChR mechanism. We acknowledge this study is composed of only adult male mice and it is necessary to examine these effects in both sexes and also at an adolescent time point. Since prenatal and early exposure to nicotine triggers unique changes in neurobiology,28–30 it will be important to understand if a vaping-related model causes similar changes in early exposure models. Furthermore, in this study we examine only two flavor categories. Given the large number of flavors available, it is critical to understand which flavors alter nicotine-related behavior and to also follow-up these behavioral studies by investigating changes in neurobiology and neurophysiology.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
This work was supported by National Institute on Drug Abuse (NIDA) (DA040047 to B.J.H.). Research reported in this publication was supported by NIDA and FDA Center for Tobacco Products (CTP) (DA046335 to B.J.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug administration. This work was also supported by the PhRMA Foundation (Predoctoral fellowship in Pharmacology/Toxicology to S.Y.C).
Acknowledgments
We thank the users of the Vapor Research User Forum (https://vaperesearch.forumotion.com) for discussion that contributed to this work.
Declaration of Interests
None declared.
References
- 1. Bhatnagar A, Payne TJ, Robertson RM. Is there a role for electronic cigarettes in tobacco cessation? J Am Heart Assoc. 2019;8(12):e012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: Use of electronic cigarettes and any tobacco product among middle and high school students – United States, 2011–2018. MMWR Morb Mortal Wkly Rep. 2018;67(45):1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walley SC, Jenssen BP; Section on Tobacco Control Electronic nicotine delivery systems. Pediatrics. 2015;136(5):1018–1026. [DOI] [PubMed] [Google Scholar]
- 4. Murthy VH Facing Addiction in America: The Surgeojn General’s Report on Alcohol, Drugs, and Health USDHHS; 2016. https://addiction.surgeongeneral.gov/sites/default/files/surgeon-generals-report.pdf. [Google Scholar]
- 5. Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16(4):438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. St Helen G, Liakoni E, Nardone N, Addo N, Jacob P 3rd, Benowitz NL. Comparison of systemic exposure to toxic and/or carcinogenic volatile organic compounds (VOC) during vaping, smoking, and abstention. Cancer Prev Res (Phila). 2020;13(2):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benowitz NL Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hallagan J Safety Assessment and Regulatory Authority to Use Flavors – Focus on Electronic Nicotine Delivery Systems and Flavored Tobacco Products FEMA; 2018. https://www.femaflavor.org/sites/default/files/2018-05/FEMAGRAS%20Ecig%2004302018.pdf. [Google Scholar]
- 10. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: How the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28(2):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine Tob Res. 2010;12(suppl 2):S110–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahijevych K, Ford J. The relationships between menthol cigarette preference and state tobacco control policies on smoking behaviors of young adult smokers in the 2006–07 Tobacco Use Supplements to the Current Population Surveys (TUS CPS). Addiction. 2010;105(suppl 1):46–54. [DOI] [PubMed] [Google Scholar]
- 14. Biswas L, Harrison E, Gong Y, et al. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl). 2016;233(18):3417–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA. Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology. 2017;42(12):2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avelar AJ, Akers AT, Baumgard ZJ, Cooper SY, Casinelli GP, Henderson BJ. Why flavored vape products may be attractive: green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function. Neuropharmacology. 2019;158:107729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, Talbot P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep. 2019;9(1):2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(e1):e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krüsemann EJZ, Boesveldt S, de Graaf K, Talhout R. An E-Liquid flavor wheel: a shared vocabulary based on systematically reviewing e-liquid flavor classifications in literature. Nicotine Tob Res. 2019;21(10):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, Taffe MA. Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug Alcohol Depend. 2019;198:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montanari C, Kelley LK, Kerr TM, Cole M, Gilpin NW. Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats. Psychopharmacology (Berl). 2020;237(3):613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapor inhalation escalates nicotine self-administration. Addict Biol. 2014;19(4):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mead EL, Duffy V, Oncken C, Litt MD. E-cigarette palatability in smokers as a function of flavorings, nicotine content and propylthiouracil (PROP) taster phenotype. Addict Behav. 2019;91:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wickham RJ, Nunes EJ, Hughley S, et al. Evaluating oral flavorant effects on nicotine self-administration behavior and phasic dopamine signaling. Neuropharmacology. 2018;128:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pons S, Fattore L, Cossu G, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28(47):12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994. [DOI] [PubMed] [Google Scholar]
- 28. Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O’Neill HC. Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioral, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice. Neuropharmacology. 2019;149:66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polli FS, Kohlmeier FS. Prenatal nicotine exposure alters postsynaptic AMPA receptors and glutamate neurotransmission within the laterdorsal tegmentum (LDT) of juvenile mice. Neuropharm. 2018;137:71–85. [DOI] [PubMed] [Google Scholar]
- 30. Omelchenko N, Roy P, Balcita-Pedicino JJ, Poloyac S, Sesack SR. Impact of prenatal nicotine on the structure of midbrain dopamine regions in the rat. Brain Struct Funct. 2016;221(4):1939–1953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.