Abstract
Introduction
This study examined the association between the introduction of an e-cigarette and subsequent change in cigarette smoking among smokers who were not immediately interested in quitting.
Aims and Methods
The Moment Study was a 21-day intensive longitudinal study with an online follow-up survey at 30 days. After observing baseline cigarette smoking for 1 week, participants received 10 cigalike e-cigarettes on study days 6 and 13. Participants reported cigarettes per day, e-cigarette puffs per day, and e-cigarette satisfaction using text-message-based surveys.
Results
The sample of 96 daily smokers was majority female (53.1%), African American (67.7%), and non-Hispanic (95.8%). When e-cigarettes were provided (day 6), average cigarettes per day dropped by 1.82 cigarettes (p < .0001). The within-person e-cigarette puff effect on daily cigarette smoking was significantly negative (β = −0.023; p = .005); a participant who consumed 100 more e-cigarette puffs in a day than usual for that person was expected to smoke 2.3 fewer cigarettes that day, but this was only true for non-menthol smokers (p = .006). Smokers older than 45 and those who started smoking at a younger age rated e-cigarettes as less satisfying (ps < .05). Participants with greater than the median reported satisfaction were 6.5 times more likely to use an e-cigarette at follow-up.
Conclusions
Giving e-cigarettes to smokers who did not intend to quit reduced their cigarette smoking on days when they used e-cigarette more frequently, but this relationship did not hold for menthol smokers. Satisfaction with e-cigarette use was predictive of continued use 30 days later.
Implications
A greater amount of cigalike e-cigarette use resulted in less smoking among adult daily smokers without immediate plans to quit, but a lack of nicotine delivery and satisfaction for these devices may have limited their utility as a replacement for cigarette smoking, especially among menthol smokers. The global concept of “satisfaction” may be an important driver of e-cigarette use among adult smokers.
Introduction
In 2015, nearly 70% of U.S. cigarette smokers reported that they wanted to quit smoking completely; 55.4% made at least one quit attempt, but only 7.4% successfully quit for at least 6 months.1 Therefore, it is not surprising that the highest prevalence of past 30-day e-cigarette use is among current cigarette smokers, who most commonly report e-cigarette use to quit smoking.2 Some systematic reviews of randomized controlled trials of e-cigarettes for smoking cessation tentatively conclude that e-cigarette use promotes smoking cessation, but highlight the need for continued research and improved reporting.3–6 A recent multicenter randomized controlled trial found that e-cigarettes were more effective cessation aids than nicotine replacement therapy when both aids were accompanied by behavioral support.7 Other systematic reviews and meta-analyses of observational research for smoking reduction or cessation conclude that e-cigarettes are associated with reduced likelihood of quitting among smokers.8,9
Despite the conflicting evidence on e-cigarette’s utility as a cessation device, smokers have adopted e-cigarette use as a means to quit or reduce their harm due to inhaling tobacco smoke. Available evidence suggests that a transition from exclusive combustible tobacco smoking to exclusive e-cigarette use will reduce exposure to harmful toxicants and yield health benefits for smokers.10 In five prospective switching studies,11–15 adult smokers significantly reduced their exposure to major toxicants after the introduction of e-cigarettes. Partial switching from cigarettes to e-cigarettes reduced some, but not all, major biomarkers of harm, highlighting the importance of complete switching.13,16
An e-cigarette device’s capacity to help a person quit smoking is closely related to its ability to deliver nicotine and reduce the urge to smoke.17,18 However, craving alleviation is more complex than simply delivering nicotine. “Satisfaction” from smoking is a concept from tobacco industry research that encompasses smokers’ ratings of the subjective experience of product self-administration.19 “Satisfaction” includes craving alleviation, but also aspects of the subjective experience of product use like taste, throat hit, pull, smell, and appearance.20 For menthol smokers, taste may play an outsized role in the satisfaction they derive from smoking,21 which may translate to reduced success in smoking cessation, especially among African American smokers.22
Despite intense smoker interest in e-cigarettes and their demonstrated potential as harm minimization tools, there has not been a wholesale shift of smokers from cigarettes to e-cigarettes in the United States. Detailed, prospective information on initial e-cigarette use is critically needed to understand the effect of e-cigarette use on cigarette smoking among adult smokers. The aim of this study is to examine how the use of an entry-level e-cigarette device affected cigarette smoking among smokers who were not immediately interested in quitting, a group for whom a harm minimization approach to nicotine use might be attractive.23 We modeled changes in cigarette consumption in relation to two factors that we hypothesized would affect cigarette consumption: (1) e-cigarette puffs per day (PPD) and (2) satisfaction from the e-cigarette. We hypothesized that a greater number of e-cigarette puffs on a day would be inversely related to cigarettes smoked in that day, and that this relationship would be greater among individuals with higher than average e-cigarette satisfaction. We also hypothesized that these relationships would be different for smokers who preferred menthol as compared to non-menthol cigarettes, given the importance of the menthol sensory experience on smoking topography, satisfaction, and menthol preference highlighted in tobacco industry documents.24
Methods
Study Overview
The Moment Study (Mixed Method E-cigarette Study) was a 21-day mixed-methods intensive longitudinal study examining how cigalike e-cigarette use affects cigarette smoking among adult smokers who did not have an immediate intention to quit smoking.25 Participants completed four in-person visits (days 0, 6, 13, and 20), followed by an online follow-up survey 30 days after the final in-person visit. The Moment Study employed multiple data streams, including random and participant-initiated ecological momentary assessments (EMAs). In the first week of the study, participants reported their cigarette smoking, mood, and craving using the EMA system. On day 6, we provided participants with two NJOY King 5-packs of cigalike e-cigarettes (3% nicotine; tobacco or menthol flavor, depending on participants’ usual flavor preference) and asked them to take at least three puffs a day to ensure that they had some exposure to the product in the first week. On day 13, we gave participants two additional NJOY King 5-packs and instructed them to use or not use the e-cigarettes as they wished. We chose this NJOY product because it was a leading cigalike brand in U.S. stores at the time of study.26 We provided cigalike e-cigarettes because they are easier to use than advanced devices and do not necessitate refilling. We assigned smokers to the same e-cigarette flavor as their usual cigarette brand because research suggested that tobacco and menthol/mint were the most popular flavors, and that new e-cigarette users were most likely to choose a flavor that was congruent with their usual brand.27–29 The Moment Study was piloted in Fall 2014 and the main study data were collected over a 26-month period between 2014 and 2016 in Washington, DC.
Recruitment and Eligibility
Participants were recruited via Craigslist, paid advertisements, flyers, and word of mouth. A research assistant called eligible individuals who completed the screener to confirm eligibility and schedule a baseline study visit. Eligible individuals had to be (1) 18 years or older, (2) reside in the Washington, DC area, (3) speak English, (4) smoking at least 8 cigarettes/day for the past 5 years, (5) record an exhaled air carbon monoxide (CO) level more than 8 parts per million at the baseline visit, and (6) interested in using an e-cigarette. Individuals could not have used an e-cigarette in the last 30 days. To reduce the complexity of the EMA reports, we excluded individuals who also used non-cigarette tobacco products. To facilitate EMA data collection, participants were required to have an iPhone or Android cell phone that allowed the installation of applications, use their cellphone daily, and have an unlimited text message plan. More than 70% of individuals who took the online screener met all of the mobile phone inclusion criteria, including nearly 50% of potential participants who were unemployed.30 Additionally, individuals could not (1) be breastfeeding or planning to become pregnant, (2) have heart disease/uncontrolled blood pressure, (3) have psychosis/suicidal thoughts, or (4) be currently enrolled in an alcohol treatment program. All criteria (except CO test) were assessed by self-report. A consort figure is presented in Supplementary Figure 1.
Measurement Instruments and Measures
Baseline and Follow-up Surveys
The baseline computer-assisted self-interview survey assessed sociodemographics, tobacco and e-cigarette use history (including the number of past 12-month quit attempts), tobacco and e-cigarette use beliefs, tobacco product and e-cigarette harm perceptions, alcohol use, and physical and mental health. The online follow-up survey assessed similar behavioral constructs as the baseline survey.
EMA Surveys
EMA surveys assessed cigarette consumption (in full cigarettes) and e-cigarette use (in estimated puffs). We collected responses via text message using participants’ personal cell phones. The EMA data collection system returned an error message to prevent skipping items or entering out-of-range values. EMA data were sent to a secure server, which a research assistant monitored and used to give feedback to participants about their EMA reporting to reduce missing data and increase data quality.
We collected two types of EMA data: participant-initiated cigarette/e-cigarette use reports and EMA system-generated random prompts. The random prompts are not included in these analyses because they assessed constructs that are outside of the scope of the current analyses, namely, mood and craving. Participant-initiated EMA texts assessed cigarette smoking (weeks 1–3) and e-cigarette puffs (weeks 2–3). We instructed participants to self-initiate a cigarette or e-cigarette use report directly after they were finished using either product (when they had “put down [the product] and did not intend to pick it up again for a while”). EMA items are presented in Supplementary Table 1.
Analyses
First, we used a linear mixed-effects model to examine the change in cigarettes per day (CPD) before and after the provision of cigalike e-cigarettes (ie, a two-part growth curve model). The response variable is Wcpd (average CPD, winsorized), and the fixed effects are Daypreecig, which represents the slope before participants received e-cigarettes; Pstecig, which represents the sudden change in CPD after the provision of e-cigarettes; and Daypstecig, which represents the slope after the provision of e-cigarettes. The fixed-effect part of the model is written as:
Four subject-level random effects—one for each intercept and slope—are also included in the model.
Next, we used a linear mixed-effects model to understand whether a person’s e-cigarette use affected his or her cigarette use on that same day and whether these relationships are different for those who found the e-cigarettes more satisfying than the average study participant, as well as for those who preferred menthol cigarettes. This linear effects model only considered observations after e-cigarettes were introduced (ie, the first week of data was omitted). The response variable is Wcpd, and the fixed effects are the within-person effect of using e-cigarettes (=ecigppd – subject-specific mean ecigppd), the between-person effect of using e-cigarettes (=subject-specific mean ecigppd – sample mean ecigppd), the within-person effect of satisfaction (avg_ecig_satisfy – subject-specific mean avg_ecig_satisfy), the between-person effect of satisfaction (subject-specific mean avg_ecig_satisfy – sample mean avg_ecig_satisfy), the day of the study, age, education level (educ), sex, and menthol preference (menthol). We included an interaction term between the e-cigarette and satisfaction within-person variables and also between menthol preference and the within-person e-cigarette variable. A random intercept for each subject is also included in the model. The fixed-effect part of the model is written as:
Finally, we used a logistic regression model to investigate whether a participant’s average daily satisfaction with the e-cigarette during the 14 days of intensive observation predicted continued use at the 30-day follow-up. The response variable is past30dayecig dichotomized (0 vs. >0, dichotomized in this manner because there were many zeros and other values spread between 1 and 30), and the important predictor variable is average daily satisfaction (dichotomized at the median = 5.59). The other predictor variables in the logistic regression model include age, education level, sex, menthol preference, average cigarette usage per day during the study (Wcpd), and average e-cigarette PPD during the study (ecigppd) (daily average totals are combined into a single value, which is the average of the averages).
We evaluated the effect of including the number of quit attempts in the past 12 months (both as a binary and continuous measure) in the second and third models, but did not include it in the final model as its inclusion did not affect our conclusions. Analyses were conducted in Stata/SE 15.1. and R version 3.3.2.
Results
The final sample of 96 participants was majority female (53.1%), African American (67.7%), and non-Hispanic (95.8%). Nearly 40% were 45–64 years old, and the majority (68.8%) had completed at least some college. Most participants were menthol smokers (75%), and nearly 60% had a Fagerstrom score of 6–7 (high nicotine dependence). Half of the participants had their first cigarette between the ages of 14 and 18 (Table 1). Participants reported 9534 cigarette and 2926 e-cigarette use instances during 21 days of observation.
Table 1.
Baseline Sociodemographic and Tobacco Use Characteristics of Study Participants Overall and by Mean E-cigarette Satisfaction (N = 96)
| Overall | Mean e-cigarette satisfactiona | |||
|---|---|---|---|---|
| N | % | Mean | p b | |
| Sex | ||||
| Female | 51 | 53.1 | 6.60 | .86 |
| Male | 45 | 46.9 | 6.62 | |
| Race | ||||
| African American/black | 65 | 67.7 | 6.64 | .47 |
| Non-African American/black | 31 | 32.3 | 6.53 | |
| Hispanic ethnicity | ||||
| No | 92 | 95.8 | 6.63 | .24 |
| Yes | 4 | 4.2 | 6.07 | |
| Age group | ||||
| 18–29 | 26 | 27.1 | 6.72 | .03 |
| 30–44 | 32 | 33.3 | 6.82 | |
| 45–64 | 38 | 39.6 | 6.39 | |
| Educational attainment | ||||
| High school graduate or lower | 30 | 31.2 | 6.54 | .53 |
| Some college or higher | 66 | 68.8 | 6.64 | |
| Menthol preference | ||||
| No | 24 | 25.0 | 6.46 | .21 |
| Yes | 72 | 75.0 | 6.65 | |
| Fagerström Test for Cigarette Dependence score | ||||
| <5 | 16 | 16.7 | 6.28 | .94 |
| 6–7 | 57 | 59.4 | 6.77 | |
| 8–10 | 23 | 24.0 | 6.42 | |
| Age of first cigarette | ||||
| <14 | 31 | 32.3 | 6.27 | <.001 |
| 14–18 | 48 | 50.0 | 6.74 | |
| 19–24 | 12 | 12.5 | 6.26 | |
| 24–55 | 5 | 5.2 | 7.66 | |
| Enjoy smoking | ||||
| <5 | 26 | 27.1 | 6.51 | .49 |
| 6–7 | 24 | 25.0 | 6.99 | |
| 8–10 | 46 | 47.9 | 6.45 | |
| Confidence to quit smoking | ||||
| ≤4 | 25 | 26 | 6.31 | <.001 |
| 5–7 | 42 | 43.8 | 6.51 | |
| 8–10 | 29 | 30.2 | 7.05 | |
| Average cigarettes per day | ||||
| ≤5 | 28 | 29.2 | 6.68 | .10 |
| 6–9 | 37 | 38.5 | 6.51 | |
| 10+ | 31 | 32.3 | 6.33 |
aMean e-cigarette satisfaction assessed during the two weeks of observation using participant-initiated reports.
b p-values from Pearson’s chi-squared tests.
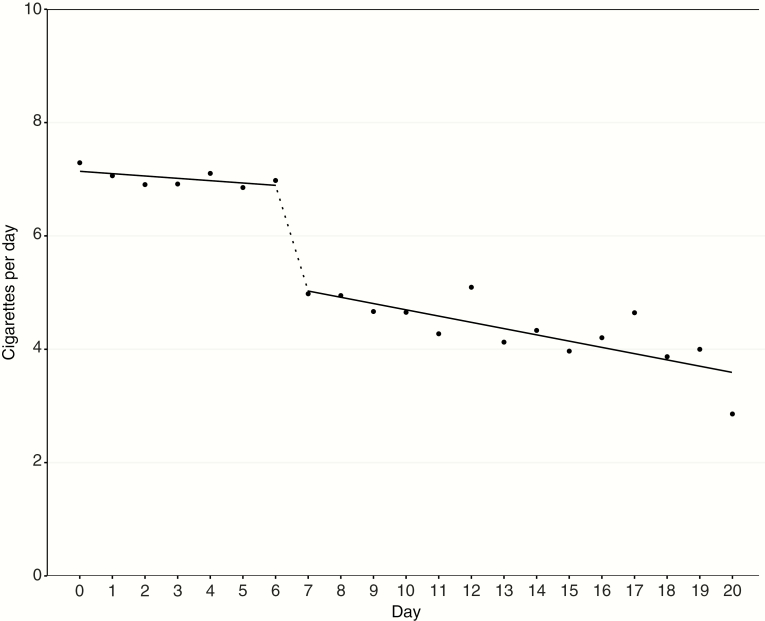
Table 2 and Figure 1 present the results of the two-part growth curve model for the relationship between CPD and study day, before and after the provision of cigalike e-cigarettes. Participants reported an average of 6.85 CPD on the first day of the study; average reported CPD did not change in week 1 of the study, before the provision of cigalike e-cigarettes (β = −0.04, p = .555). On the day that e-cigarettes were provided, the average reported CPD dropped by 1.82 cigarettes (p < .0001). After this immediate change on day 7, CPD continued to decrease by a tenth of a cigarette per day through the end of daily observation at study day 20 (β = −0.11, p < .0001).
Table 2.
Two-Part Linear Mixed Effects Model of the Relationship Between Cigarettes Per Day and Time, Before and After Provision of Cigalike E-cigarettes
| Estimate | 95% CI | p | |
|---|---|---|---|
| Intercept | 6.85 | 6.15 to 7.55 | <.0001 |
| Post-e-cigarette immediate change | −1.82 | −2.32 to −1.32 | <.0001 |
| Pre-e-cigarette change over time | −0.04 | −0.18 to 0.10 | .555 |
| Post-e-cigarette change over time | −0.11 | −0.17 to −0.05 | <.0001 |
| Intercept variance | 7.51 | 5.40 to 10.44 | — |
CI = confidence interval.
Figure 1.
Two-part linear mixed-effects model of the relationship between cigarettes per day and study day, before and after the provision of cigalike e-cigarettes.
Table 3 presents the results of the linear mixed-effects model that predicts the number of cigarettes smoked per day. Three participants were excluded from these analyses due to missing satisfaction data. The between-person e-cigarette puff effect on daily cigarette consumption was significantly positive (β = 0.03; p = .034). In other words, a participant who consumed 100 more daily e-cigarette puffs than another participant was expected to smoke 2.8 more daily cigarettes. However, the within-person e-cigarette puff effect on daily cigarette smoking was significantly negative (β = −0.02; p = .005); a participant who consumed 100 or more e-cigarette puffs in a day than usual was expected to smoke 2.3 fewer cigarettes that day than usual. The within-person e-cigarette puff effect on daily cigarette smoking was stronger for those who preferred non-menthol cigarettes than for those who preferred menthol cigarettes (p = .006). Supplementary Figure 3 shows a very small effect (ie, a flat slope) for those who preferred menthol cigarettes, but a very large effect (ie, a steep, negative slope) for those who preferred non-menthol cigarettes. The within-person e-cigarette puff effect on daily cigarette smoking was also stronger for those who displayed a greater than usual daily e-cigarette satisfaction rating (p = .033; Supplementary Figure 2). Supplementary Figure 3 presents the region of significance for the slope of the e-cigarette puff per day within-person effect at different values of the satisfaction within-person effect. The blue dotted line is at −0.88, meaning that if the within-person satisfaction value is larger than −0.88 (ie, if there is greater than slightly below the typical satisfaction level for a particular participant, the “typical” satisfaction level is =0), then the slope of the e-cigarette puff per day within-person effect is significantly less than zero.
Table 3.
Estimated Coefficients, Standard Errors, and p-Values From the Linear Mixed Effects Model Predicting Number of Cigarettes Consumed Per Day
| Variable | Coefficient | Standard error | p |
|---|---|---|---|
| Intercept | 3.33 | 1.17 | .005 |
| Ecig WP effect | −0.02 | 0.01 | .005 |
| Ecig BP effect | 0.03 | 0.01 | .034 |
| Satisfaction WP effect | −0.07 | 0.07 | .330 |
| Satisfaction BP effect | 0.02 | 0.14 | .886 |
| Day | −0.04 | 0.02 | .069 |
| Sex (female) | −0.33 | 0.55 | .544 |
| Age | 0.01 | 0.02 | .578 |
| Education | 0.66 | 0.38 | .087 |
| Menthol preference | −0.53 | 0.68 | .434 |
| Ecig WP x satisfaction WP | −0.01 | 0.00 | .033 |
| Ecig WP x menthol preference | 0.03 | 0.01 | .006 |
WP = within-person; BP = between-person.
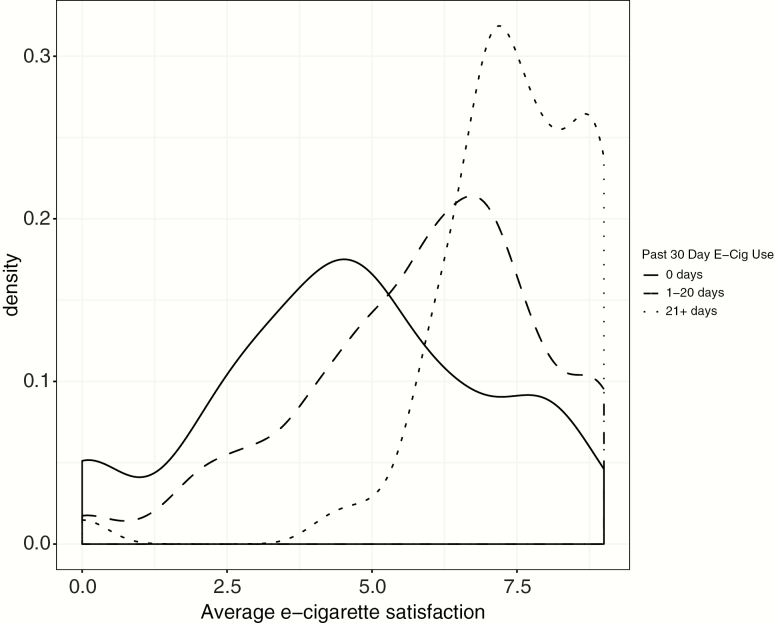
There were several sociodemographic and tobacco use history characteristics that were associated with average daily e-cigarette satisfaction during participants’ 2 weeks of cigalike e-cigarette use. Smokers older than 45 generally rated e-cigarettes as less satisfying, as did those who initiated smoking at a younger age (ps < .05). At follow-up, those who used e-cigarettes more frequently than cigarettes, those who considered themselves a “vaper,” those who used e-cigarettes at least 21 days in the past 30 days, and those who reported more general satisfaction from e-cigarettes had higher average day-level satisfaction with the cigalike e-cigarettes during the 2-week observation period (ps < .05; Supplementary Table 2). Figure 2 presents the distribution of average daily e-cigarette satisfaction ratings by frequency of past 30-day e-cigarette use at follow-up.
Figure 2.
Distribution of daily average e-cigarette satisfaction ratings by frequency of e-cigarette use in the past 30 days at follow-up.
Seventy-eight participants who completed the EMA portion of the study completed the 30-day follow-up survey. At follow-up, 60.3% of participants reported that they smoked fewer cigarettes than at the beginning of the study, but few said they used e-cigarettes with the same (10.3%) or greater (14.1%) frequency than during the study. Most identified as a smoker (59.0%), though 6 identified as a vaper and 18 identified as a smoker and vaper. The majority (56.4%) reported smoking all 30 days in the past month at follow-up and reported using e-cigarettes on 1–20 of the last 30 days (57.7%). Additionally, most reported getting less satisfaction from the e-cigarette as compared to the cigarette (42.3%) at follow-up (Supplementary Table 2).
At follow-up, participants’ average daily e-cigarette satisfaction during the 2 weeks of observation was the only significant predictor (p = .003) of continued e-cigarette use at 30 days. Participants whose average daily e-cigarette satisfaction (average of daily averages) was greater than 5.59 (the median average daily satisfaction level during the 2 weeks of intensive observation) were more than 6.5 times more likely to use an e-cigarette at 30-day follow-up compared to participants whose average daily e-cigarette satisfaction was less than 5.59.
Discussion
In this diverse sample of adult cigarette smokers without immediate plans to quit smoking, greater use of cigalike e-cigarettes resulted in a reduction in cigarette smoking, but only for non-menthol smokers. Examining longitudinal survey data, Buu et al.31 also found that a higher frequency of e-cigarette use was associated with more smoking reduction 1 year later; however, this analysis was not able to investigate how menthol smokers responded to menthol-flavored e-cigarettes. In prior analyses of interview data from a subsample of African American menthol smokers in this study, Smiley et al.20 found that the menthol NJOY cigalike e-cigarettes produced an overwhelming and unpleasant menthol taste. These menthol smokers also described the menthol NJOY cigalike e-cigarettes’ throat hit as too harsh compared to their regular brand of cigarettes and reported that using the device caused them to cough. Triangulating conclusions from the qualitative and quantitative Moment Study data streams, it appears that the sensory experience of using the menthol NJOY cigalike e-cigarettes was not reinforcing for menthol smoking participants and discouraged continued use. Our findings emphasize the importance of flavor and subjective experience in facilitating smoking reduction or switching among menthol smokers.
It is not surprising that participants were unable to completely switch to the NJOY cigalike e-cigarette during this study. Evaluations of similar cigalike e-cigarettes indicate that these devices do not alleviate the urge to smoke to the same degree as more sophisticated devices.32,33 In a qualitative investigation of smokers using e-cigarettes to quit smoking, Notley et al.34 identified a dual-use period of “sliding” toward smoking cessation that was particularly salient for participants who did not actively want to quit smoking; perhaps the observed gradual decrease in cigarette consumption after the provision of e-cigarettes has captured this process in our sample of smokers without immediate plans to quit. Participants may have decreased their cigarette smoking to a greater degree if they had been provided a device with greater capacity to reduce smoking urges. Again, it is also important to remember that smokers in this study were not making a deliberate choice to switch to e-cigarettes. Though several participants indicated that they were considering quitting at some point in the future, we might have observed different results if participants were more motivated to switch or had received instruction on how to use an e-cigarette to quit.
We found that higher ratings of e-cigarette satisfaction predicted more frequent use 30 days later. Findings from the qualitative interviews indicated that smokers considered more than craving alleviation when they rated satisfaction with the e-cigarette; they also considered taste, pull, throat hit, and smell as part of “satisfaction.” In a separate analysis of interview data from the parent study, study participants indicated that e-cigarettes offered a relative advantage over cigarettes in that e-cigarette use was allowed in spaces where smoking was prohibited and was more socially accepted, especially as a signal of a smoking cessation attempt.35 While craving alleviation is a central aspect of an e-cigarette’s capacity as a smoking cessation aid, these other aspects of satisfaction should also be considered when researching switching from cigarette smoking to e-cigarette use.
Study Strengths and Limitations
This study has several important limitations and associated strengths. First, it is likely that the design of the participant-initiated cigarette and e-cigarette use reports underestimated product use. Prior research has shown that event-based reports are a less reliable method to capture use than daily diaries and scheduled prompts, often underestimating product use.36,37 Given the participant-initiated nature of the product use reports, we cannot know if a lack of reporting is due to an actual lack of use or other characteristics such as fatigue. We investigated the potential effect of fatigue on reporting by examining the distribution of missing random prompt data, assuming that if participants were becoming fatigued, they would reduce self-initiating product use reports and responding to random prompts. Random prompt completion rates were significantly higher in week 1 compared to weeks 2 and 3 (week 1 vs. week 2: t-value = 4.69, p < .0001; week 1 vs. week 3: t-value = 6.00, p < .0001); however, there were no significant differences in completion between weeks 2 and 3. Future research in this area should pursue an objective measurement of e-cigarette use behavior using smart e-cigarette or wearable devices.37,38 Second, many studies of smoking reduction/cessation follow-up participants for longer than our 30-day follow-up. As changes in CPD may take longer than 30 days to manifest, our follow-up period may not have been long enough to observe a significant change.39 Fourth, as we highlight in this research, “satisfaction” is a multifaceted construct. Given the data collection mode, we were not able to assess all potential dimensions of this complex construct. Fifth, nearly 25% of the sample was lost to follow-up at 30 days after their last study visit. Sociodemographic variables did not significantly affect the likelihood of 30-day follow-up completion. While average daily satisfaction was not related to loss to follow-up at the 30-day follow-up survey (p = .636), our conclusions concerning satisfaction and continued use of e-cigarettes could have been affected by dropout. Finally, our conclusions are limited to the use of cigalike products among smokers with similar demographic characteristics (eg, majority African American and female) to our convenience sample. Repetition of this study in a more representative sample of smokers without plans to quit using a device like JUUL might yield different results.
Conclusions
A greater amount of e-cigarette use resulted in less smoking among adult daily smokers without immediate plans to quit, but a lack of nicotine delivery and satisfaction for these devices may have limited their utility as a replacement for cigarette smoking, especially among menthol smokers. Complete switching to e-cigarettes among adult smokers who are unwilling or unable to quit smoking in other ways yields health benefits to the individual and improves public health.10 The global concept of “satisfaction” may be an important driver of e-cigarette use among adult smokers. Provision of e-cigarettes that can better address “satisfaction” through nicotine delivery and other subjective effects could prove better at smoking reduction and cessation. Future research should explore which individual differences predict satisfaction with harm minimizing products and successful switching to match products to different groups. Additional research manipulating aspects of e-cigarette design will help the field understand e-cigarette satisfaction, e-cigarette use behavior, smoking cessation, and ultimately the public health impact of these devices.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, is available online at https://academic.oup.com/ntr.
Funding
The research reported in this publication was supported by the National Institute on Drug Abuse (grant number 1R21DA036472) of the National Institutes of Health under grant number 5R21DA036472. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethics approval: The study was approved by Chesapeake IRB Pro00008526.
Declaration of Interests
JLP receives grant funding from National Institute on Drug Abuse/FDA, is also a consultant to Westat on the PATH study, and is an expert witness for the Plaintiffs in a Multi District Litigation invoking Natural American Spirit cigarettes. RN receives funding from the FDA Center for Tobacco Products via contractual mechanisms with Westat and the National Institutes of Health (NIH). Within the past 3 years, he has served as a paid consultant to the Government of Canada and has received an honorarium for a virtual meeting from Pfizer. SLS receives grant funding from the National Cancer Institute/FDA. DBA receives funding from the FDA Center for Tobacco Products via contractual mechanisms with Westat and the NIH.
References
- 1. Babb S, Malarcher AM, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;56(52). [DOI] [PubMed] [Google Scholar]
- 2. Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall-Carter L. E-cigarettes and smoking cessation: evidence from a systematic review and meta-analysis. PLoS One. 2015;10(3):e0122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016;18(10):1926–1936. [DOI] [PubMed] [Google Scholar]
- 6. Gentry S, Forouhi NG, Notley C. Are electronic cigarettes an effective aid to smoking cessation or reduction among vulnerable groups? A systematic review of quantitative and qualitative evidence. Nicotine Tob Res. 2019;21(5):602–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–637. [DOI] [PubMed] [Google Scholar]
- 8. El Dib R, Suzumura EA, Akl EA, et al. Electronic nicotine delivery systems and/or electronic non-nicotine delivery systems for tobacco smoking cessation or reduction: a systematic review and meta-analysis. BMJ Open. 2017;7(2):e012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Stratton K, Kwan LY, Eaton DL, eds. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. [PubMed] [Google Scholar]
- 11. McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila). 2015;8(9):873–878. [DOI] [PubMed] [Google Scholar]
- 12. Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res. 2017;19(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pulvers K, Emami AS, Nollen NL, et al. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine Tob Res. 2018;20(2):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Czoli CD, Fong GT, Goniewicz ML, Hammond D. Biomarkers of exposure among “Dual Users” of tobacco cigarettes and electronic cigarettes in Canada. Nicotine Tob Res. 2019;21(9):1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Round EK, Chen P, Taylor AK, Schmidt E. Biomarkers of tobacco exposure decrease after smokers switch to an e-cigarette or nicotine gum. Nicotine Tob Res. 2019;21(9):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1(8):e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hajek P, Przulj D, Phillips-Waller A, Anderson R, McRobbie H. Initial ratings of different types of e-cigarettes and relationships between product appeal and nicotine delivery. Psychopharmacology (Berl). 2018;235(4):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Etter JF Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015;148:102–108. [DOI] [PubMed] [Google Scholar]
- 19. Brown & Williamson. The role of smoker-product interaction in subjective assessment. Bates #689105564. https://legacy.library.ucsf.edu/tid/amo90f00/pdf?search=%22subjective%20sensory%20experience%20satisfaction%22. Published 1981. Accessed March 23, 2020.
- 20. Smiley SL, DeAtley T, Rubin LF, et al. Early subjective sensory experiences with “Cigalike” e-cigarettes among African American menthol smokers: a qualitative study. Nicotine Tob Res. 2018;20(9):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kreslake JM, Wayne GF, Connolly GN. The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res. 2008;10(4):705–715. [DOI] [PubMed] [Google Scholar]
- 22. Food and Drug Administration. Preliminary scientific evaluation of the possible public health effects of menthol versus nonmentol cigarettes. n.d. https://www.fda.gov/media/86497/download.
- 23. Abrams DB, Glasser AM, Villanti AC, Pearson JL, Rose S, Niaura RS. Managing nicotine without smoke to save lives now: evidence for harm minimization. Prev Med. 2018;117:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yerger VB, McCandless PM. Menthol sensory qualities and smoking topography: a review of tobacco industry documents. Tob Control. 2011;20(suppl 2):ii37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pearson JL, Smiley SL, Rubin LF, et al. The Moment Study: protocol for a mixed method observational cohort study of the Alternative Nicotine Delivery Systems (ANDS) initiation process among adult cigarette smokers. BMJ Open. 2016;6(4):e011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. E-Cigarette market trends in traditional U.S. retail channels, 2012–2013. Nicotine Tob Res. 2015;17(10):1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Etter JF Characteristics of users and usage of different types of electronic cigarettes: findings from an online survey. Addiction. 2016;111(4):724–733. [DOI] [PubMed] [Google Scholar]
- 28. Etter JF Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. [DOI] [PubMed] [Google Scholar]
- 30. Harvey EJ, Rubin LF, Smiley SL, Zhou Y, Elmasry H, Pearson JL. Mobile phone ownership is not a serious barrier to participation in studies: descriptive study. JMIR Mhealth Uhealth. 2018;6(2):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buu A, Hu YH, Piper ME, Lin HC. The association between e-cigarette use characteristics and combustible cigarette consumption and dependence symptoms: results from a national longitudinal study. Addict Behav. 2018;84:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romijnders K, van Osch L, de Vries H, Talhout R. A deliberate choice? Exploring the decision to switch from cigarettes to e-cigarettes. Int J Environ Res Public Health. 2019;16(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Voos N, Kaiser L, Mahoney MC, et al. Randomized within-subject trial to evaluate smokers’ initial perceptions, subjective effects and nicotine delivery across six vaporized nicotine products. Addiction. 2019;114(7):1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Notley C, Ward E, Dawkins L, Holland R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct J. 2018;15(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smiley SL, Kierstead EC, Harvey E, Abudayyeh H, Pearson JL. An exploratory analysis of adult daily smokers’ experiences using e-cigarettes in smoke-free places. Tob Induc Dis. 2018;16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cooper MR, Case KR, Hébert ET, et al. Characterizing ENDS use in young adults with ecological momentary assessment: results from a pilot study. Addict Behav. 2019;91:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pearson JL, Elmasry H, Das B, et al. Comparison of ecological momentary assessment versus direct measurement of e-cigarette use with a bluetooth-enabled e-cigarette: a pilot study. JMIR Res Protoc. 2017;6(5):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bertz JW, Epstein DH, Preston KL. Combining ecological momentary assessment with objective, ambulatory measures of behavior and physiology in substance-use research. Addict Behav. 2018;83:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.