Abstract
Phospholipase Cβ (PLCβ) enzymes are peripheral membrane proteins required for normal cardiovascular function. PLCβ hydrolyzes phosphatidylinositol 4,5-bisphosphate, producing second messengers that increase intracellular Ca2+ level and activate protein kinase C. Under basal conditions, PLCβ is autoinhibited by its C-terminal domains and by the X–Y linker, which contains a stretch of conserved acidic residues required for interfacial activation. Following stimulation of G protein-coupled receptors, the heterotrimeric G protein subunit Gαq allosterically activates PLCβ and helps orient the activated complex at the membrane for efficient lipid hydrolysis. However, the molecular basis for how the PLCβ X–Y linker, its C-terminal domains, Gαq, and the membrane coordinately regulate activity is not well understood. Using compressed lipid monolayers and atomic force microscopy, we found that a highly conserved acidic region of the X–Y linker is sufficient to regulate adsorption. Regulation of adsorption and activity by the X–Y linker also occurs independently of the C-terminal domains. We next investigated whether Gαq-dependent activation of PLCβ altered interactions with the model membrane. Gαq increased PLCβ adsorption in a manner that was independent of the PLCβ regulatory elements and targeted adsorption to specific regions of the monolayer in the absence of the C-terminal domains. Thus, the mechanism of Gαq-dependent activation likely includes a spatial component.
Phospholipase C (PLC) enzymes are peripheral membrane proteins that hydrolyze the plasma membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the second messengers inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG).1,2 IP3 binds to receptors in the endoplasmic or sarcoplasmic reticulum, releasing calcium from intracellular stores.1,2 DAG remains in the membrane and, together with increased Ca2+, activates protein kinase C (PKC). These events activate numerous downstream pathways, including those involved in cell survival and proliferation.1,3,4 PLCβ enzymes have low basal activity and are stimulated up to ~60-fold through direct interactions with the heterotrimeric G protein subunits Gαq and Gβγ, which are released upon activation of Gq- and Gi-coupled receptors, respectively.1,5 An increased level of PLCβ expression and/or activation is associated with vascular smooth muscle contraction, cardiac arrhythmias, hypertrophy, and heart failure.6-12
PLC enzymes, including PLCβ, share a highly conserved set of core domains, including an N-terminal pleckstrin homology (PH) domain, four tandem EF hand repeats, a catalytic TIM barrel domain that is split by the autoinhibitory X–Y linker, and a C2 domain (Figure 1A).1,13 The defining feature of the PLCβ subfamily is an ~400-amino acid C-terminal extension immediately following the C2 domain, which is subdivided into proximal and distal C-terminal domains (CTDs).1,13 The proximal CTD is required for activation by Gαq and autoinhibition,14,15 while the distal CTD contributes to membrane association and maximum basal and Gαq-stimulated activity.16,17
Figure 1.
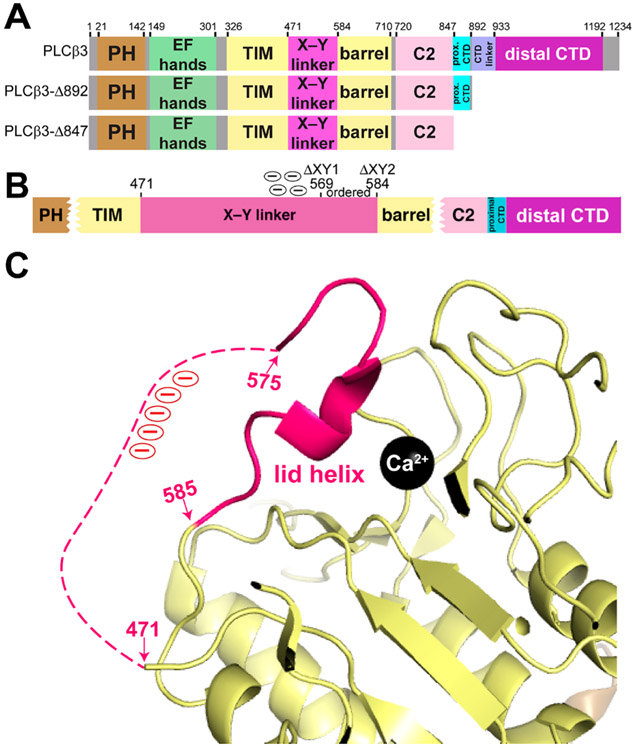
PLCβ3 domain architecture and autoinhibition by the X–Y linker. (A) Domain diagrams of the PLCβ3 variants used in this study. Numbers above the diagram correspond to amino acids at domain boundaries. The full-length enzyme consists of residues 1–1234, while the C-terminal truncation variants remove the distal CTD (PLCβ3-Δ892) or both the proximal and distal CTD (PLCβ3-Δ847). (B) Schematic of the PLCβ3 X–Y linker. Internal deletions used in this study remove the unconserved N-terminus and the acidic stretch (ΔXY1, residues 471–569) or the entire X–Y linker (ΔXY2, residues 471–584). (C) The C-terminus of the X–Y linker occludes the active site (Protein Data Bank entry 4GNK).16 Domains are colored as in panel A. The disordered region of the X–Y linker is shown as a dashed hot pink link, and the acidic region is labeled with circled minus signs (red). The catalytic Ca2+ ion is shown as a black sphere.
The basal activity of PLCβ is regulated by three known elements: the X–Y linker, the Hα2′ helix in the proximal CTD, and the distal CTD.13,14,18,19 The X–Y linker is largely unconserved in sequence and in length, with the exception of its C-terminus, which features a highly conserved 10–15-residue acidic stretch and a short α helix (Figure 1B,C).1,13,18 This helix interacts with residues adjacent to the active site and acts as a lid, preventing the substrate from binding to the active site (Figure 1B,C).1,18,20 Electrostatic repulsion between the negatively charged membrane and the acidic region of the X–Y linker ejects the lid helix and exposes the active site. Catalysis is likely further promoted by favorable electrostatic interactions between the TIM barrel domain and the membrane.21 The Hα2′ helix in the proximal CTD must also be displaced for maximum lipase activity.1,13,14,18 Under basal conditions, Hα2′ is bound to a cleft between the TIM barrel and C2 domains, and mutations that disrupt this interface increase basal activity and decrease the efficacy of Gαq-dependent activation.14,16 In addition, Hα2′ protrudes into the predicted membrane binding plane, where it sterically hinders membrane association.14,19 However, whether this helix is displaced through an interfacial mechanism has not been established. Finally, the distal CTD increases membrane association as well as basal and G protein-stimulated activity.19,22-25 Mutations of highly conserved basic clusters on the surface of the domain or deletion of the entire domain decreases basal activity, Gαq-dependent activation, and the level of membrane association.18,20 The distal CTD also interacts with the TIM barrel in solution, which may help regulate the distribution of membrane-bound and cytosolic populations of PLCβ.13 More recently, the PLCβ3 distal CTD was shown to target the enzyme to specific regions of PIP2-containing lipid monolayers, consistent with its reported PIP2 binding site.11,19
PLCβ is activated through direct binding of the heterotrimeric G protein subunits Gαq and Gβγ. Gαq allosterically activates PLCβ by binding to Hα1/Hα2 in the proximal CTD, displacing the autoinhibitory Hα2′ helix from the core.16,18 Gαq also interacts with the membrane via its palmitoylated N-terminus and with the PLCβ distal CTD, and these combined integrations are proposed to facilitate interfacial activation.16,18 Thus, maximum PIP2 hydrolysis by PLCβ requires multivalent interactions among Gαq, PLCβ, and the membrane.15 In contrast, the mechanism of Gβγ-mediated activation is largely unknown but is proposed to prolong the dwell time of the Gβγ–PLCβ complex at the membrane.5,24-33 Both Gαq-dependent activation and Gβγ-dependent activation are dependent upon the lipidation of the heterotrimeric G protein subunit.34-36 The differences in the type of lipid modification on the G protein may also contribute to regulation. Palmitoylated Gαq may help localize PLCβ to raftlike regions of the membrane, which are also enriched with PIP2. In contrast, the prenylated Gγ subunit would exclude Gβγ and its activated complexes from raftlike regions.26-28
PLCβ enzymes must interact with the membrane for efficient catalysis, and under basal conditions, membrane association is hindered by the X–Y linker and proximal CTD.37-41 While heterotrimeric G proteins stimulate activity, in part by displacing autoinhibitory elements and/or facilitating interfacial activation, this is insufficient for maximum lipase activity. Activation is also unlikely to involve recruitment to the membrane, as heterotrimeric G proteins have been reported to impact PLCβ membrane association only when the effective Kd is smaller than the membrane partition coefficient. Thus, the membrane itself contributes to PLCβ activation. In this study, we used biochemical assays and atomic force microscopy (AFM) on compressed lipid monolayers to begin characterizing how the PLCβ regulatory elements and Gαq regulate adsorption and activation. Specifically, the role of the X–Y linker and the contribution of interfacial activation to both basal and Gαq-stimulated activity were investigated using a series of PLCβ3 domain deletion variants. We found that PLCβ3 variants lacking the X–Y linker, in whole or in part, had increased levels of monolayer adsorption through a mechanism independent of the proximal and distal CTDs. The addition of activated Gαq to the model membrane system was sufficient to further increase adsorption of protein to specific regions of the monolayer, suggesting that Gαq-dependent activation of PLCβ includes a spatial component.
MATERIALS AND METHODS
Protein Expression, Purification, and Mutagenesis.
cDNA encoding N-terminally His-tagged Homo sapiens PLCβ3 (UniProt entry Q01970 and residues 10–1234) and C-terminal truncation variants PLCβ3-Δ847 (residues 10–847) and PLCβ3-Δ892 (residues 10–892) were subcloned into pFastBac Dual (Invitrogen) and expressed and purified as previously described.19 The ΔXY1 (residues 471–569) and ΔXY2 (residues 471–584) deletions were generated using site-directed mutagenesis (Stratagene, San Diego, CA) and sequenced over the entire open reading frame.18 All PLCβ3 variants were purified as previously described.19 We attempted to express and purify the PLCβ3-Δ847 ΔXY1 variant, but despite expression trials using multiple baculoviruses and High5 and Sf9 cells,13 the protein was not expressed at a sufficiently high yield for purification.
The cDNA encoding murine Gαq (UniProt entry P21279 and residues 7–359) was subcloned into pFastbac HTA (Invitrogen), co-transfected with Ric-8A-GST42 in baculovirus-infected High5 cells, and expressed and purified as previously described, with some modifications.13,19,42 Following elution from the Ni-NTA column, the protein was concentrated to 1 mL, filtered through a 0.2 μm filter (Millipore), and applied to tandem Superdex S200 columns (GE Healthcare) pre-equilibrated with Gαq S200 activation buffer [20 mM HEPES (pH 8), 100 mM NaCl, 2 mM DTT, 5 mM MgCl2, 10 μM GDP (pH 8), 30 μM AlCl3, and 10 mM NaF].14,16,18
Gαq–PLCβ3 Variant Complex Formation.
Activated Gαq (Gαq·GDP-ALF4−) was incubated with purified PLCβ3 variants in a 1:1.5 molar ratio in buffer A [20 mM HEPES (pH 8), 200 mM NaCl, 2 mM DTT, 0.9 mM CaCl2, 5 mM MgCl2, 10 mM NaF, 30 μM AlCl3, and 50 μM GDP (pH 8)] for 30 min on ice. The reaction mixture was concentrated to 500 μL and loaded on a Superdex S200 column equilibrated with buffer A. Fractions containing the Gαq–PLCβ3 variant complexes were identified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, pooled, concentrated in an Amicon concentrator (Millipore), and flash-frozen in liquid nitrogen.16
Formation of Compressed Lipid Monolayers.
Chicken egg white phosphatidylethanolamine (PE) and porcine brain phosphatidylinositol 4,5-bisphosphate (PIP2) or porcine brain phosphatidylserine (PS) from Avanti Polar Lipids (Alabaster, AL) were mixed in a 7:3 molar ratio, aliquoted, and stored under N2 prior to their use in compressed lipid monolayers as previously described,19 with one modification to the protocol. The subphase of the monolayers contained 25 mL of 20 mM H2NaPO4 (pH 8.0), and all monolayers were compressed at a rate of 56.1 mm/min.
Atomic Force Microscopy.
All samples were imaged using a Veeco MultiMode atomic force microscope equipped with a Nanoscope V controller in tapping mode as previously described, with some modifications.19 Gwyddion scanning probe microscopy data visualization software was used to perform plane flattening, median line corrections, scar artifact removal, contrast adjustment, column statistical analysis, and grain analysis.19,43 Protein adsorption was detected qualitatively by the appearance of lighter colors, corresponding to increases in height, in the false-colored AFM micrographs. Quantitative changes in the monolayer after protein incubation were determined by measuring the maximum height as a function of position.43 All AFM data were collected as line scans along the x-axis and then combined to generate a two-dimensional micrograph, where z is the second dimension. The height profile of the monolayer was determined by the maximum height sampled in regular intervals along the x-axis. The step size of the intervals was based on the full scan size and the number of data points per line. In these experiments, all samples were imaged with a scan size of 1 μm × 1 μm and a total of 512 data points per line. The distance between each data point per line is equal to the step size of the interval. Additional quantitative information was obtained by grain analysis of each micrograph, which takes the sample background into account.43 The heights for all grains on the monolayer surface were quantified and plotted as a histogram. Adsorption of protein to the monolayer changes the grain height distribution relative to that of monolayers in the absence of protein.43 The maximum height values for each micrograph and the normal distribution of height values of each grain were plotted in GraphPad Prism version 8.0.43
Differential Scanning Fluorimetry (DSF) Assays.
Melting temperature (Tm) values for all PLCβ3 variants were determined by monitoring the binding of SYPRO Orange (Molecular Probes, Eugene, OR) to hydrophobic regions of the proteins at increasing temperatures as previously described.19 At least three experiments were carried out in triplicate using proteins from three independent purifications.
Activity Assays.
Hen egg white phosphatidylethanolamine (PE) at 300 μM and soy phosphatidylinositol (PI) at 750 μM (Avanti Polar Lipids) were resuspended in CHCl3, mixed, and dried in 312 μL aliquots in borosilicate glass tubes under a low stream of N2. Lipids were sealed and stored at −20 °C until use. Liposomes were resuspended in 312 μL of sonication buffer [50 mM HEPES (pH 7), 80 mM KCl, 2 mM EGTA, and 1 mM DTT], vortexed, and incubated at room temperature for 5 min. To prepare liposomes, the resuspended lipid mixture was sonicated for two 30 s pulses using a bath sonicator (Avanti Polar Lipids). This produces largely unilamellar liposomes 15–50 nm in diameter (Avanti Polar Lipids). For basal activity measurements, each reaction mixture contained 10 μL of PLC dilution buffer [50 mM HEPES (pH 7), 3 mM EGTA, 80 mM KCl, 3 mM DTT, and 3 mg/mL BSA], 5 μL of G protein control buffer [50 mM HEPES (pH 7), 100 mM NaCl, 5 mM MgCl, 1 mM DTT, and 3 mM EGTA], and 5 μL of a CaCl2 solution [50 mM HEPES (pH 7), 3 mM EGTA, 80 mM KCl, 1 mM DTT, and 13.6 μM CaCl2]. Control reaction mixtures contained all components except CaCl2. Concentrations of the PLCβ3 variants were chosen such that activity was in the linear range over the time course (2–10 min) of the assay. Reactions were terminated by the addition of an ice-cold quench solution [50 mM HEPES (pH 7), 80 mM KCl, 210 mM EGTA, and 1 mM DTT], and mixtures incubated on ice. The final protein concentrations were 15 ng/μL PLCβ3, 15 or 20 ng/μL PLCβ3 ΔXY1, 5 ng/μL PLCβ3 ΔXY2, 15 ng/μL PLCβ3-Δ847, 30 ng/μL PLCβ3-Δ847 ΔXY2, 30 ng/μL PLCβ3-Δ892, and 30 ng/μL PLCβ3-Δ892 ΔXY2.
Gαq-dependent increases in lipase activity were measured by adding activated Gαq–PLCβ3 variant complexes to the reaction mixtures such that activity remained in the linear range from 2 to 10 min. The final protein concentrations were 20 ng/μL Gαq–PLCβ3, Gαq–PLCβ3 ΔXY1, and Gαq–PLCβ3 ΔXY2 and 30 ng/μL Gαq–PLCβ3-Δ892, Gαq–PLCβ3-Δ892 ΔXY1, and Gαq–PLCβ3-Δ892 ΔXY2.
Inositol phosphate (IP1) production was quantified using a modified version of the CisBio IP-One Gq assay kit. Following termination, 14 μL of each reaction mixture, 3 μL of d2-labeled IP1, and 3 μL of the cryptate-labeled anti-IP1 antibody (CisBio) were added to a 384-well low-volume white microplate at room temperature (Corning, Corning, NY). Positive controls contained assay buffer, d2-labeled IP1, and cryptate-labeled anti-IP1, and negative controls contained assay buffer, lysis and detection buffer, and cryptate-labeled anti-IP1. The plate was centrifuged at 1000g for 1 min, incubated at room temperature for 1 h, and read on a Synergy4 plate reader (BioTek, Winooski, VT). The concentration of IP1 was calculated from a standard curve and normalized following the manufacturer’s protocol (CisBio).21 At least four experiments were performed in duplicate with protein from three different purifications.
RESULTS
Deletions within the X–Y Linker Perturb Stability and Activity.
Interfacial activation is thought to require the acidic stretch within the X–Y linker. To determine whether this region is sufficient for autoinhibition or if the entire X–Y linker is required, we expressed and purified a series of PLCβ3 variants lacking the unconserved N-terminus and acidic stretch of the X–Y linker (residues 471–569, termed ΔXY1) or the entire X–Y linker (residues 471–584, termed ΔXY2). These deletions were introduced in the background of PLCβ3 and two previously characterized C-terminal truncations, PLCβ3-Δ892 and PLCβ3-Δ847, which lack the distal CTD and the proximal and distal CTDs, respectively (Figure 1A,B).14,18,19 DSF was used to determine whether these deletions altered the melting temperature (Tm) of the PLCβ3 variants. PLCβ3 had a Tm of 55.5 ± 0.2 °C, while the Tms of PLCβ3 ΔXY1 and PLCβ3 ΔXY2 were increased by ~3 °C (Table 1 and Figure S1). PLCβ3-Δ892 had a Tm of 59.4 ± 0.1 °C, but the Tm of PLCβ3-Δ892 ΔXY1 was decreased by ~2.5 °C and was ~5 °C lower for PLCβ3-Δ892 ΔXY2 (Table 1 and Figure S1). Finally, PLCβ3-Δ847 had the lowest thermal stability of the PLCβ3 variants, consistent with loss of the proximal CTD, with a Tm of 53.3 ± 0.1 °C. Deletion of the X–Y linker in PLCβ3-Δ847 ΔXY2 decreased the Tm by an additional ~2 °C (Table 1 and Figure S1).
Table 1.
Thermal Stabilities of PLCβ3 Variants
| PLCβ3 variant | Tm (°C)a |
|---|---|
| PLCβ3 | 55.5 ± 0.2 |
| PLCβ3 ΔXY1 | 58.7 ± 0.1 |
| PLCβ3 ΔXY2 | 58.5 ± 0.1b |
| PLCβ3-Δ847 | 53.3 ± 0.1 |
| PLCβ3-Δ847 ΔXY2 | 48.2 ± 0.2c |
| PLCβ3-Δ892 | 59.4 ± 0.1 |
| PLCβ3-Δ892 ΔXY1 | 56.8 ± 0.1d |
| PLCβ3-Δ892 ΔXY2 | 57.4 ± 0.2d |
Data represent the average of at least three experiments ± SEM.
Significant relative to PLCβ3 (*p ≤ 0.05).
Significant relative to PLCβ3-Δ847 (****p ≤ 0.0001).
Significant relative to PLCβ3-Δ892 (****p ≤ 0.0001).
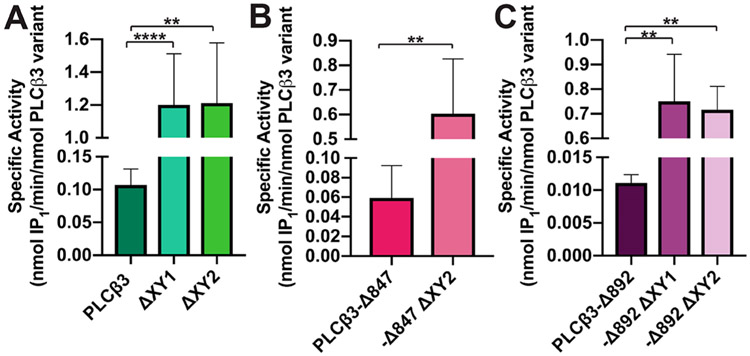
The basal activity of the PLCβ3 variants was then measured using a modified version of the CisBio IP-One assay, wherein PI was incorporated into liposomes, and the amount of inositol phosphate (IP1) produced was quantified.21,44-46 PLCβ3 had a specific activity of 0.11 ± 0.01 nmol of IP1 min−1 (nmol of PLCβ3 variant)−1 (Figure 2A and Table 2), while PLCβ3 ΔXY1 and PLCβ3 ΔXY2 had ~11-fold higher basal activity [1.20 ± 0.11 and 1.20 ± 0.16 nmol of IP1 min−1 (nmol of PLCβ3 variant)−1, respectively], consistent with deletions in the X–Y linker relieving autoinhibition (Figure 2A and Table 2). PLCβ3-Δ847 has basal activity that is ~2-fold lower than that of PLCβ3, due to loss of membrane association by the distal CTD. PLCβ3-Δ847 ΔXY2 increased activity ~10-fold over that of PLCβ3-Δ847 (Figure 2B and Table 2). Finally, PLCβ3-Δ892 had the lowest specific activity at 0.01 ± 0.001 nmol of IP1 min−1 (nmol of PLCβ3 variant)−1, due to autoinhibition by both the X–Y linker and the proximal CTD. Deletion of the acidic stretch or the entire linker in this variant increased basal activity ≳60-fold compared to that of PLCβ3-Δ892 (Figure 2C and Table 2). The modest changes in Tm, together with the measurable basal activity, demonstrate the deletions within the X–Y linker do not compromise the structure of the PLCβ3 variants. In addition, deletion of the acidic stretch in the X–Y linker increases basal activity to the same extent as deletion of the entire X–Y linker.
Figure 2.
Deletions within the X–Y linker increase basal specific activity. Deletion of the X–Y linker, in whole or in part, in the background of (A) PLCβ3 or (B) PLCβ3-Δ847 increases basal activity ~10–11-fold. PLCβ3-Δ847 has a basal activity that is lower than that of PLCβ3 due to the absence of the distal CTD. (C) PLCβ3-Δ892 has the lowest basal activity of the PLCβ3 variants tested, with deletions in the X–Y linker increasing activity ~65-fold. Data shown represent the average of at least four individual experiments in duplicate ± SEM (****p ≤ 0.0001; **p ≤ 0.01).
Table 2.
Basal Activity of PLCβ3 Variantsa
| PLCβ3 variant | basal specific activity [nmol of IP1 min−1 (nmol of PLCβ3)−1]a | x-fold increase relative to the wild-type value |
|---|---|---|
| PLCβ3 (wt) | 0.11 ± 0.01 | 1 |
| PLCβ3 ΔXY1 | 1.20 ± 0.11b | 11 |
| PLCβ3 ΔXY2 | 1.20 ± 0.16c | 11 |
| PLCβ3-Δ847 (wt) | 0.06 ± 0.01 | 1 |
| PLCβ3-Δ847 ΔXY2 | 0.60 ± 0.09d | 10 |
| PLCβ3-Δ892 (wt) | 0.01 ± 0.001 | 1 |
| PLCβ3-Δ892 ΔXY1 | 0.75 ± 0.19e | 68 |
| PLCβ3-Δ892 ΔXY2 | 0.72 ± 0.10e | 65 |
The data shown represent at least four experiments performed in duplicate ± SEM.
Significant relative to PLCβ3 (****p ≤ 0.0001).
Significant relative to PLCβ3 (**p ≤ 0.01).
Significant relative to PLCβ3-Δ847 (**p ≤ 0.01).
Significant relative to PLCβ3-Δ892 (**p ≤ 0.01).
The Acidic Stretch of the X–Y Linker Regulates Adsorption to Lipid Monolayers.
Deletions within the PLCβ3 X–Y linker increase basal activity, potentially by exposing the active site and eliminating unfavorable interactions between the acidic stretch of the X–Y linker and liposomes. To determine whether the increased activity of the ΔXY variants is due, at least in part, to increased membrane binding, the PLCβ3 variants were added to the subphases of compressed PE/PIP2 lipid monolayers and allowed to incubate, and then the monolayer containing adsorbed protein was visualized by AFM.19 The composition of the monolayer (7:3 PE:PIP2 ratio) was chosen to be similar to the liposome composition used in a well-established assay to measure in vitro PLC activity.14,47 This composition was also previously used to investigate the roles of the PLCβ3 proximal and distal CTDs in protein adsorption and activation.19 In the absence of protein, the monolayers have clustered, elevated features that likely correspond to regions enriched with PIP2. This clustering phenomenon has been previously reported in other model membrane systems that contain PIP2.19,48-50 These studies were also carried out in the absence of free Ca2+, which prevents Ca2+-dependent clustering of PIP2 in compressed lipid monolayers and decreases the activity of the PLCβ3 variants.51,52 Thus, changes in the monolayer can be more directly correlated to protein adsorption.
For these experiments, adsorption to the monolayer was detected as changes in the appearance of the monolayer surface, where taller features are shown in lighter colors in the false-colored AFM micrographs. The relative heights of these surface features were quantified by measuring the maximum height above the supporting surface as a function of position, and the number of surface features within a specific height range was quantified using grain analysis of the micrograph (Figure 3).
Figure 3.
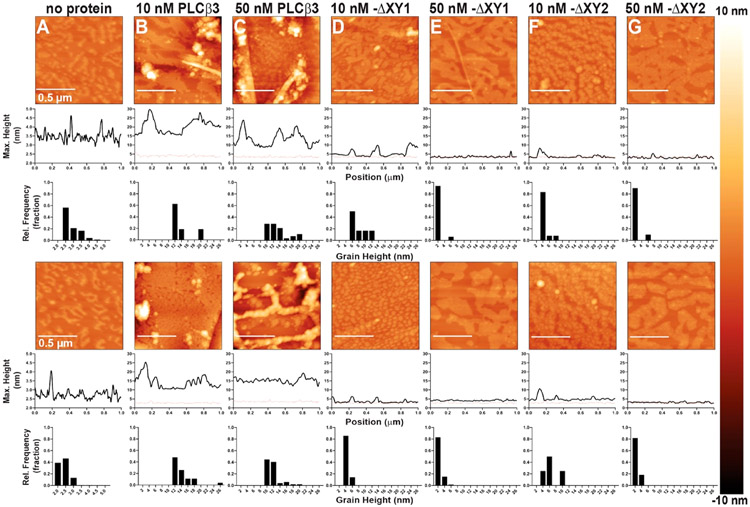
Representative AFM tapping mode images of compressed PE:PIP2 monolayers incubated with PLCβ3 DXY variants. Two representative images of each condition are shown, with sample variations most likely due to the lipid distribution in the monolayer and/or Langmuir–Schaefer transfer to the HOPG substrate. Topographical changes upon addition of protein are detected as changes in the appearance of the monolayer surface, with taller features shown in lighter colors, and increases in the height and/or size of surface features. The maximum heights above the HOPG surface as a function of position and the grain analysis for the relative height frequency of surface features are quantified below each micrograph. For comparison, a representative height profile from a compressed monolayer in the absence of protein is shown on each height profile as a dashed red line. Monolayers (A) in the absence of protein or incubated with (B) 10 nM PLCβ3, (C) 50 nM PLCβ3, (D) 10 nM PLCβ3 ΔXY1, (E) 50 nM PLCβ3 ΔXY1, (F) 10 nM PLCβ3 ΔXY2, or (G) 50 nM PLCβ3 ΔXY2.
Compressed lipid monolayers incubated with 10 nM PLCβ3 showed an increase in the maximum height of surface features, as illustrated by the lighter puncta on the surface and the increased height of surface features from ~4 nm on the monolayer alone to ~20 nm after addition of protein. Adsorption of protein is also observed in the grain height distribution, where a majority of surface features are ~10–20 nm in height (Figure 3A,B and Figure S2). Addition of 50 nM PLCβ3 to the subphase increased the number of elevated surface features in the grain analysis but did not further increase the height of the surface features compared to that of the 10 nM PLCβ3 monolayers (Figure 3A-C and Figure S2). In contrast, monolayers incubated with 10 nM PLCβ3 ΔXY1 had a more uniform appearance, with smaller surface features only ~4–6 nm in height (Figure 3B-D and Figure S2). Monolayers incubated with 50 nM PLCβ3 ΔXY1 lacked clear surface features, as supported by the uniform absence of light puncta in the micrographs, and had a further decrease in the height frequency distribution to ~2 nm, relative to that of monolayers incubated with lower concentrations of PLCβ ΔXY1 (Figure 3D,E and Figure S2). Similar trends were observed for monolayers incubated with PLCβ3 ΔXY2 (Figure 3F,G and Figure S2).
The more uniform adsorption of PLCβ3 ΔXY1 and PLCβ3 ΔXY2 to monolayers could be due to local depletion of the PIP2 clusters caused by their higher basal activity.19,39,48-50 Therefore, monolayers were incubated with 10 nM PLCβ3 ΔXY1 or 10 nM PLCβ3 ΔXY2 for shorter times (Figure 4). After 5 min, monolayers incubated with 10 nM PLCβ3 ΔXY1 had large elevated surface features ~8–20 nm in height (Figure 4A), similar in size and appearance to features observed on monolayers incubated with PLCβ3 (Figure 3B,C and Figure S2). Similar trends were observed for monolayers incubated with 10 nM PLCβ3 ΔXY2, with surface features ~12–22 nm in height (Figure 4B). At longer incubation times, the height of the surface features decreased by ~10 nm on monolayers incubated with either PLCβ3 ΔXY variant (Figure 4C), but the features can still be clearly resolved in the micrographs. After 20 min, the monolayers incubated with PLCβ3 ΔXY1 or PLCβ3 ΔXY2 have a more uniform appearance, with the majority of surface features being ~6–7 nm in height (Figure 4E,F). Given the time-dependent decrease in the height of surface features, a possible explanation for these results is that the PLCβ3 ΔXY variants deplete PIP2 clusters from the monolayer.
Figure 4.
Representative AFM tapping mode images of compressed PE:PIP2 monolayers incubated with PLCβ3 ΔXY variants. Changes in the maximum height and height frequency distributions due to protein adsorption are quantified below each micrograph. For comparison, a representative height profile from a compressed monolayer in the absence of protein is shown on each height profile as a dashed red line. Monolayers incubated with 10 nM PLCβ3 ΔXY1 (A) and 10 nM PLCβ3 ΔXY2 (B) for 5 min, 10 min (C and D, respectively), or 20 min (E and F, respectively).
The X–Y Linker Increases Adsorption in a Manner That Is Independent of the Proximal CTD.
The proximal CTD negatively regulates adsorption by sterically preventing interactions between the active site and the membrane.14,18,19 However, whether the X–Y linker and the proximal CTD coordinately regulate adsorption is unknown. To investigate the roles of these two regulatory elements, monolayers were incubated with increasing concentrations of the PLCβ3-Δ847 and PLCβ3-Δ892 ΔXY variants.
At all concentrations tested, PLCβ3-Δ847 and PLCβ3-Δ847 ΔXY2 adsorbed nonspecifically to the monolayer, consistent with the ~2–4 nm changes in height across the surface and the lack of well-defined, elevated features on the surface (Figure 5 and Figure S3). PLCβ3-Δ892 also adsorbed uniformly to the monolayer, but to a lesser extent than PLCβ3-Δ847, due to the presence of the proximal CTD (Figures 5 and 6 and Figures S3 and S4). Even at 50 nM PLCβ3-Δ892, the surface features vary in height by only ~1 nm (Figure 6B,C and Figure S4). In contrast, monolayers incubated with 10 nM PLCβ3-Δ892 ΔXY1 show increased levels of protein adsorption, with an overall lighter appearance and an increased number of features ~3–5 nm in height (Figure 6D,E and Figure S4). At 50 nM PLCβ3-Δ892 ΔXY1, the micrographs are lighter in color and nearly uniform in height compared to PLCβ3-Δ892 or lower concentrations of PLCβ3-Δ892 ΔXY1 (Figure 6B-E and Figure S4). Similar trends were observed for monolayers incubated with PLCβ3-Δ892 ΔXY2 (Figure 6F,G and Figure S4). Therefore, deletion of the acidic stretch increases the level of adsorption to the same extent as deletion of the entire X–Y linker, independent of the proximal CTD (Figures 5 and 6 and Figures S3 and S4).
Figure 5.
Representative AFM tapping mode images of PE:PIP2 monolayers incubated with PLCβ3-Δ847 or PLCβ3-Δ847 ΔXY2. Two representative images of each condition are shown, with sample variations most likely due to the lipid distribution in the monolayer and/or Langmuir–Schaefer transfer to the HOPG substrate. Topographical changes upon addition of protein are detected as changes in the appearance of the monolayer surface, where taller features are shown in lighter colors, and increases in the height and/or size of surface features. The maximum heights above the HOPG surface as a function of position and the grain analysis for the relative height frequency of surface features are quantified below each micrograph. For comparison, a representative height profile from a compressed monolayer in the absence of protein is shown on each height profile as a dashed red line. Monolayers (A) in the absence of protein or incubated with (B) 10 nM PLCβ3-Δ847, (C) 50 nM PLCβ3-Δ847, (D) 10 nM PLCβ3-Δ847 ΔXY2, or (E) 50 nM PLCβ3-Δ847 ΔXY2.
Figure 6.
Representative AFM tapping mode images of PE:PIP2 monolayers incubated with PLCβ3-Δ892 ΔXY variants. Two representative images of each condition are shown, with sample variations most likely due to the lipid distribution in the monolayer and/or Langmuir–Schaefer transfer. Topographical changes are detected as changes in the appearance of the monolayer surface, with taller features shown in lighter colors, and increases in the height and/or size of surface features. The maximum heights above the HOPG surface as a function of position and the grain analysis for the relative height frequency of surface features are quantified below each micrograph. For comparison, a representative height profile from a compressed monolayer in the absence of protein is shown as a dashed red line. Monolayers (A) in the absence of protein or incubated with (B) 10 nM PLCβ3-Δ892, (C) 50 nM PLCβ3-Δ892, (D) 10 nM PLCβ3-Δ892 ΔXY1, (E) 50 nM PLCβ3-Δ892 ΔXY1, (F) 10 nM PLCβ3-Δ892 ΔXY2, and (G) 50 nM PLCβ3-Δ892 ΔXY2.
Gαq Increases Adsorption to Compressed Lipid Monolayers.
Gαq allosterically activates PLCβ by displacing Hα2′ from the core and could facilitate interfacial activation by increasing the number of interactions with the membrane.13,14 To evaluate the contribution of interfacial activation to Gαq-dependent activation of PLCβ, stoichiometric Gαq–PLCβ3 and Gαq–PLCβ3 Δ892 complexes were isolated by size exclusion chromatography and their basal activity and adsorption to compressed lipid monolayers were evaluated.
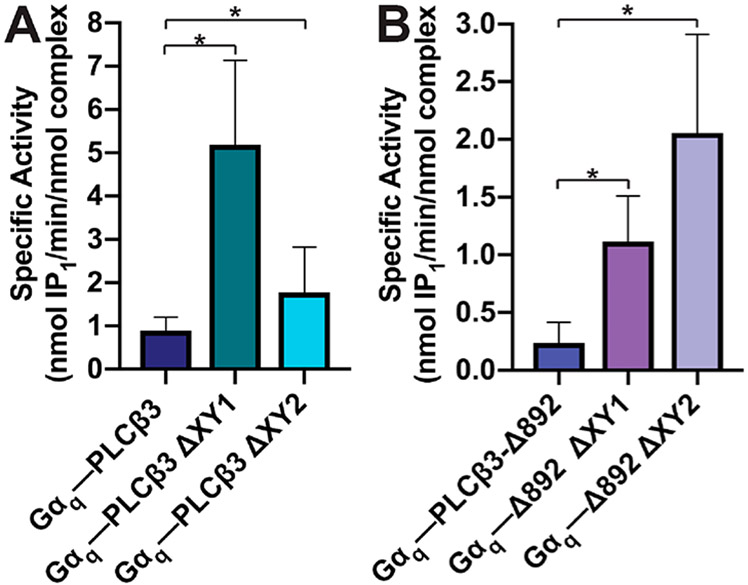
The specific activities of the Gαq–PLCβ3 variant complexes were measured using the PI liposome-based activity assay. The Gαq–PLCβ3 complex had a specific activity of 0.89 ± 0.15 nmol of IP1 min−1 (nmol of complex)−1, an ~8-fold increase over the basal activity of PLCβ3 (Figures 2A and 7A and Tables 2 and 3). Gαq–PLCβ3 ΔXY1 and Gαq–PLCβ3 ΔXY2 complexes had specific activities ~2–4-fold greater than the basal activity of the PLCβ3 ΔXY variants alone [5.2 ± 1.0 and 1.8 ± 0.40 nmol of IP1 min−1 (nmol of complex)−1, respectively] and ~2–6 fold higher than that of the wild-type Gαq–PLCβ3 complex (Figures 2A and 7A and Tables 2 and 3). The activity of the Gαq–PLCβ3-Δ892 complex was ~22-fold higher than that of PLCβ3-Δ892 [0.24 ± 0.06 nmol of IP1 min−1 (nmol of complex)−1 (Figures 2C and 7B and Tables 2 and 3)]. The activities of the Gαq–PLCβ3-Δ892 ΔXY1 and Gαq–PLCβ3-Δ892 ΔXY2 complexes [1.1 ± 0.20 nmol of IP1 min−1 (nmol of complex)−1] were ~2–3-fold higher than those of the PLCβ3-Δ892 ΔXY variants alone [2.1 ± 0.40 nmol of IP1 min−1 (nmol of complex)−1 (Figures 2C and 7B and Tables 2 and 3)] and ~5–9 fold greater than that of the Gαq–PLCβ3-Δ892 complex (Figure 7B and Table 3). As PLCβ3-Δ847 lacks the proximal CTD, it is unresponsive to Gαq-dependent activation at all concentrations and was not included in these experiments.14
Figure 7.
Deletions in the PLCβ3 X–Y linker increase the level of Gαq-dependent activation. The specific activities of Gαq–PLCβ3 variant complexes were measured using a liposome-based activity assay. (A) Gαq–PLCβ3 complexes have activities higher than those of (B) the Gαq–PLCβ3-Δ892 complexes and their ΔXY variants. This is consistent with the absence of the distal CTD in the PLCβ3-Δ892 variants. Data represent the average of at least four experiments in duplicate ± SEM (*p ≤ 0.05).
Table 3.
Gαq-Dependent Activation of PLCβ3 Variants
| variant complex |
specific activity [nmol of IP1 min−1 (nmol of complex)−1]a |
x-fold maximum activity over basald |
|---|---|---|
| Gαq–PLCβ3 | 0.89 ± 0.15 | 8 |
| Gαq–PLCβ3 ΔXY1 | 5.2 ± 1.0b | 4 |
| Gαq–PLCβ3 ΔXY2 | 1.8 ± 0.40b | 2 |
| Gαq–PLCβ3-Δ892 | 0.24 ± 0.06 | 22 |
| Gαq–PLCβ3-Δ892 ΔXY1 | 1.1 ± 0.20c | 2 |
| Gαq–PLCβ3-Δ892 ΔXY2 | 2.1 ± 0.40c | 3 |
Data represent the average of four experiments in duplicate ± SEM.
Significant relative to the Gαq–PLCβ3 complex (*p ≤ 0.05).
Significant relative to the Gαq–PLCβ3-Δ892 complex (*p ≤ 0.05).
The x-fold activation was calculated by dividing the basal activity of the Gαq–PLCβ3 ΔXY complex by the basal activity of the PLCβ3 variant (Table 2).
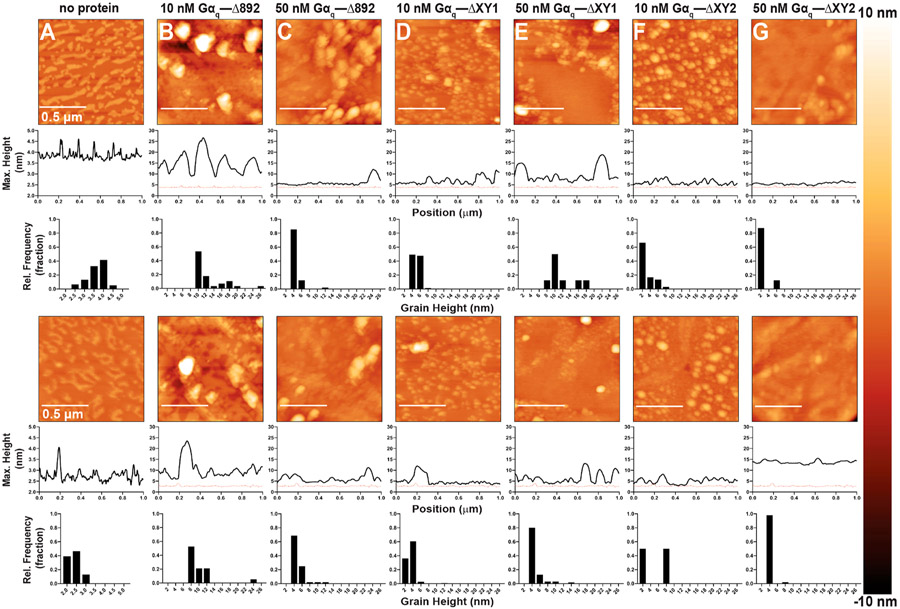
The Gαq–PLCβ3 variant complexes were then assessed for their ability to adsorb to the compressed PE/PIP2 monolayers. Addition of 10 nM Gαq–PLCβ3 complex to the subphase resulted in the formation of elevated surface features similar in appearance to those of monolayers incubated with PLCβ3 alone (Figures 3 and 8 and Figures S2 and S5). A majority of these features were ~10–16 nm in height. Increasing the concentration of the Gαq–PLCβ3 complex in the subphase also increased the height of the surface features to ~16–26 nm. Thus, inclusion of Gαq increases PLCβ3 adsorption (Figure 8B,C and Figure S5). The roles of the acidic stretch within the X–Y linker or the entire X–Y linker were then assessed in the presence of Gαq. Given that the monolayers incubated with the PLCβ3 ΔXY variants were relatively uniform in appearance, the Gαq–PLCβ3 ΔXY variant complexes may show similar trends. Alternatively, the complexes could adsorb to regions of the monolayer enriched with PIP2, as palmitoylated Gαq has been reported to preferentially localize to these regions.34-36 Monolayers incubated with 10 nM Gαq–PLCβ3 ΔXY1 had a uniform appearance, with only ~2 nm changes in height over the monolayer surface (Figure 8D and Figure S5). At higher concentrations, adsorption of the Gαq–PLCβ3 ΔXY1 complex resulted in a modest increase in the height of the small surface features to ~2–4 nm across the surface, as shown in the micrographs (Figure 8E and Figure S5). We next investigated whether deletion of the entire X–Y linker in the Gαq–PLCβ3 ΔXY2 complex altered adsorption. At low concentrations, the monolayers are largely uniform in appearance with few clustered regions ~2–4 nm in height (Figure 8F and Figure S5). Addition of 50 nM Gαq–PLCβ3 ΔXY2 to the subphase increased the number of elevated surface features with heights of ~4–6 (Figure 8F,G and Figure S5). These small changes in the monolayer surface contrast with the well-defined ~10–14 nm features observed on monolayers incubated with the wild-type Gαq–PLCβ3 complex (Figure 8 and Figure S5).
Figure 8.
Representative AFM tapping mode images of PE:PIP2 monolayers incubated with Gαq–PLCβ3 ΔXY variant complexes. Two representative images of each condition are shown, with sample variations most likely due to the lipid distribution in the monolayer and/or Langmuir–Schaefer transfer to the HOPG substrate. Topographical changes upon addition of protein are detected as changes in the appearance of the monolayer surface, where taller features are shown in lighter colors, and increases in the height and/or size of surface features. The maximum heights above the HOPG surface as a function of position and the grain analysis for the relative height frequency of surface features are quantified below each micrograph. For comparison, a representative height profile from a compressed monolayer in the absence of protein is shown on each height profile as a dashed red line. Monolayers (A) in the absence of protein or incubated with (B) 10 nM Gαq–PLCβ3, (C) 50 nM Gαq–PLCβ3, (D) 10 nM Gαq–PLCβ3 ΔXY1, (E) 50 nM Gαq–PLCβ3 ΔXY1, (F) 10 nM Gαq–PLCβ3 ΔXY2, or (G) 50 nM Gαq–PLCβ3 ΔXY2.
Gαq Increases Specific Adsorption to the Monolayer in the Absence of the Distal CTD.
We next assessed whether Gαq increased and/or altered the distribution of PLCβ3-Δ892 and its ΔXY variants at the monolayer. As PLCβ3-Δ892 lacks the distal CTD, the addition of Gαq could increase the level of adsorption, resulting in taller and/or more extended features compared to monolayers incubated with PLCβ3-Δ892. Gαq could also promote adsorption to PIP2-enriched regions of the monolayer via its palmitoylated N-terminus. Addition of 10 nM Gαq–PLCβ3-Δ892 to the subphase resulted in the formation of large, elevated surface features ~10–14 nm in height, similar in appearance to monolayers incubated with the Gαq–PLCβ3 complex (Figures 8B,C and 9B and Figures S5 and S6). At higher concentrations of the Gαq–PLCβ3-Δ892 complex, the monolayers retained large, elevated clusters, but the height of these features decreased to ~8–12 nm (Figure 9C and Figure S6). This contrasts with the monolayers incubated with PLCβ3-Δ892 alone, which have a uniform appearance (Figure 6B,C), suggesting that Gαq may contribute to the spatial distribution of the protein complex at the monolayer.
Figure 9.
Representative AFM tapping mode images of PE:PIP2 monolayers incubated with Gαq–PLCβ3 Δ892 ΔXY variant complexes. Two representative images of each condition are shown, with sample variations most likely due to the lipid distribution in the monolayer and/or LangmuirαSchaefer transfer to the HOPG substrate. Topographical changes upon addition of protein are detected as changes in the appearance of the monolayer surface, where taller features are shown in lighter colors, and increases in the height and/or size of surface features. The maximum heights above the HOPG surface as a function of position and the grain analysis for the relative height frequency of surface features are quantified below each micrograph. For comparison, a representative height profile from a compressed monolayer in the absence of protein is shown on each height profile as a dashed red line. Monolayers (A) in the absence of protein or incubated with (B) 10 nM Gαq–PLCβ3 Δ892, (C) 50 nM Gαq–PLCβ3 Δ892, (D) 10 nM Gαq–PLCβ3 Δ892 ΔXY1, (E) 50 nM Gαq–PLCβ3 Δ892 ΔXY1, (F) 10 nM Gαq–PLCβ3 Δ892 ΔXY2, or (G) 50 nM Gαq–PLCβ3 Δ892 ΔXY2.
The ability of Gαq to alter the adsorption and/or distribution of PLCβ3-Δ892 ΔXY variants to the monolayer was then assessed. Addition of 10 nM Gαq–PLCβ3-Δ892 ΔXY1 also resulted in the formation of small, clustered features ~4–6 nm in height (Figure 9D and Figure S6). Higher concentrations of the Gαq–PLCβ3-Δ892 ΔXY1 complex further increased the level of adsorption and the height of the features to ~4–10 nm (Figure 9E and Figure S6). These features are taller than those observed in monolayers incubated with the Gαq–PLCβ3-Δ892 complex, again demonstrating that adsorption increases when the acidic stretch of the X–Y linker is removed (Figure 9A-E and Figure S6). Addition of 10 nM Gαq–PLCβ3-Δ892 ΔXY2 to the monolayer increased the number of clustered regions ~2–8 nm in height (Figure 9F and Figure S6). However, at higher concentrations of the Gαq–PLCβ3-Δ892 ΔXY2 complex, the monolayers lacked well-defined surface features and had a more uniform appearance (Figure 9E and Figure S6). This could be due to depletion of PIP2 from the monolayer, given the significantly higher basal activity of the Gαq–PLCβ3-Δ892 ΔXY2 complex (Figure 7 and Table 3).
DISCUSSION
PLCβ enzymes are maintained in a catalytically quiescent state by their X–Y linker and CTDs. The X–Y linker regulates activity through an interfacial mechanism that requires a highly conserved stretch of acidic residues. The Hα2′ helix in the proximal CTD also inhibits interactions between the active site and the membrane. Finally, the distal CTD can interact with the rest of the PLCβ core, partitioning the enzyme between the membrane and cytosolic populations. However, whether the acidic stretch within the X–Y linker is responsible for the autoinhibitory function of this element and whether or how the X–Y linker and CTDs coordinately regulate membrane association and activity remain unclear.
To establish whether the acidic stretch of the linker is necessary and sufficient for adsorption in a manner that is independent of the CTDs, a series of PLCβ3 variants lacking the disordered N-terminus and acidic stretch (ΔXY1 variants) or the entire linker (ΔXY2 variants) in the background of PLCβ3 variants lacking the distal and/or proximal CTDs (Figure 1) were assessed for stability and activity. While deletions within the X–Y linker caused modest changes in Tm values, deletion of the acidic stretch was sufficient to increase basal activity to the same extent as deletion of the entire linker in all PLCβ3 backgrounds (Figure 1 and Tables 1 and 2). The increased activity could be due to an increased number of lipid interactions, which can then be detected as changes in the topographical features of compressed PE/PIP2 monolayers using AFM. PLCβ3 adsorbed to specific regions of the monolayer, forming large, clearly defined elevated features on the surface (Figure 3B,C and Figure S2). These features likely correspond to the protein adsorbing specifically to regions of the monolayer enriched with PIP2. To determine whether these elevated clusters are dependent upon the presence of PIP2, we repeated the experiment using compressed lipid monolayers containing 70% PE and 30% phosphatidylserine (PS), in place of PIP2. Even upon addition of 50 nM PLCβ3, the PE/PS monolayers had a uniform appearance, further supporting specific adsorption of PLCβ3 to PIP2-containing monolayers (Figure S7). Deletion of the acidic stretch in the X–Y linker or the entire X–Y linker in PLCβ3 resulted in monolayers more uniform in height and lacking elevated clusters, consistent with nonspecific adsorption and/or depletion of PIP2 clusters (Figure 2 and Table 2).19 To distinguish between these possibilities, the PLCβ3 ΔXY variants were incubated for shorter times in the monolayer subphases (Figure 4). At shorter time points, elevated clustered features were clearly visible, suggesting that the flatter monolayers observed at long incubation times are likely due to PIP2 depletion (Figure 4).
PLCβ3-Δ847, which lacks the proximal and distal CTDs, and PLCβ3-Δ892, which lacks only the distal CTD, adsorbed uniformly to the monolayer (Figures 5 and 6 and Figures S3, S4, and S7). Deletion of the X–Y linker in these variants, in whole or in part, increased the level of adsorption but did not alter the spatial distribution (Figures 5 and 6 and Figures S3 and S4). In all cases, deletion of the acidic stretch increases the level of monolayer adsorption to an extent similar to that seen upon deletion of the entire X–Y linker, demonstrating that the acidic stretch is responsible for autoinhibition by the X–Y linker (Figures 3, 5, and 6 and Figures S2-S4).
We next investigated whether the heterotrimeric G protein subunit Gαq altered adsorption and/or spatial localization to the model membrane. The inclusion of Gαq increases adsorption of both PLCβ3 and PLCβ3-Δ892 to the monolayer, as compared to the PLCβ3 variants alone (Figures 3, 6, 8, and 9 and Figures S2 and S4-S6). While previous studies have found that Gαq does not increase the affinity of PLCβ for the membrane, Gαq may stabilize PLCβ at the monolayer, increasing the protein.22,25-27 Our results suggest that Gαq also promotes adsorption of PLCβ3 to specific regions of the monolayer. We previously showed that the PLCβ3 distal CTD was required for specific adsorption to regions likely enriched in PIP2, while PLCβ3 variants lacking the distal CTD adsorbed nonspecifically.19 Monolayers incubated with the Gαq–PLCβ3-Δ892 complex had clearly defined, elevated features on the surface (Figure 9B,C and Figure S6), similar to those observed on PLCβ3 and Gαq–PLCβ3 monolayers (Figures 3 and 8 and Figures S2 and S5). Thus, Gαq appears to rescue specific adsorption in the absence of the distal CTD.
As Gαq increases specific adsorption of PLCβ3, an additional component in its activation of PLCβ may include targeting of the complex to regions enriched with PIP2 (Figure 10). Recent studies have found that PIP2 is highly concentrated at the rim of caveolae, and these regions also have increased levels of Gαq-dependent PLCβ activation.53,54 In the cell, this localization could in turn regulate other signaling proteins and processes dependent upon PIP2, including ion channel and transporter activity, cell motility, and vesicular trafficking.55-62 PLCβ is also regulated by the Gβγ heterodimer, but the prenylation of the Gγ subunit would result in exclusion from PIP2-enriched regions,22,26-28 which may impact the amplitude or duration of PLCβ-dependent PIP2 hydrolysis. Future studies exploring the spatial component of PLCβ activity, alone and following GPCR stimulation, will be essential for understanding the role of the membrane in these dynamic processes.
Figure 10.
Regulation of PLCβ adsorption by the X–Y linker and Gαq. Under basal conditions, PLCβ3 can adsorb to regions of the monolayer enriched with PIP2 via interactions between the distal CTD and the negatively charged PIP2. Unfavorable electrostatic interactions between the acidic stretch of the X–Y linker and the membrane expose the active site and facilitate substrate binding. The Hα2′ helix remains bound to the core and regulates activity by limiting adsorption to the membrane. Upon activation of Gq-coupled receptors, Gαq binds to the proximal CTD, displacing the Hα2′ helix and allosterically activating PLCβ3. Interactions among Gαq, the core, and the distal CTD promote interfacial activation to expose the active site and regulate the spatial distribution of the activation complex.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank S. Claridge (Department of Chemistry, Purdue University), J. Tesmer (Department of Biological Sciences, Purdue University), D. Thompson (Department of Chemistry, Purdue University), and V. Watts (Department of Pharmacy, Purdue University) for helpful discussions. The authors thank P. Bishop (Amy Facility, Purdue University) for training and technical assistance for the AFM studies.
Funding
This work is supported by a Showalter Foundation Award, American Heart Association Scientist Development Grant 16SDG29930017, and National Institutes of Health Grant 1R01HL141076-01 to A.M.L.
ABBREVIATIONS
- CTD
C-terminal domain
- PLC
phospholipase C
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PE
phosphatidylethanolamine
- PH
pleckstrin homology
- TIM
triosephosphate isomerase
- IP1
inositol phosphate
- IP3
inositol 1,4,5-triphosphate
- HOPG
highly ordered pyrolytic graphite
- AFM
atomic force microscopy
- DSF
differential scanning fluorimetry
- SEM
standard error of the mean.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/acs.biochem.9b00441.
Seven supplemental figures (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Kadamur G, and Ross EM (2013) Mammalian Phospholipase C. Annu. Rev. Physiol 75 (1), 127–154. [DOI] [PubMed] [Google Scholar]
- (2).Gresset A, Sondek J, and Harden TK (2012) The Phospholipase C Isozymes and Their Regulation. Subcell. Biochem 58, 61–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Shortridge RD, and McKay RR (1995) Invertebrate Phosphatidylinositol-Specific Phospholipases C and Their Role in Cell Signaling. Invertebr. Neurosci 1 (3), 199–206. [DOI] [PubMed] [Google Scholar]
- (4).McKay RR, Chen DM, Miller K, Kim S, Stark WS, and Shortridge RD (1995) Phospholipase C Rescues Visual Defect in NorpA Mutant of Drosophila Melanogaster. J. Biol. Chem 270 (22), 13271–13276. [DOI] [PubMed] [Google Scholar]
- (5).Park D, Jhon DY, Lee CW, Lee KH, and Rhee SG (1993) Activation of Phospholipase C Isozymes by G Protein Beta Gamma Subunits. J. Biol. Chem 268 (7), 4573–4576. [PubMed] [Google Scholar]
- (6).Filtz TM, Grubb DR, McLeod-Dryden TJ, Luo J, and Woodcock EA (2009) Gq-Initiated Cardiomyocyte Hypertrophy Is Mediated by Phospholipase Cβ1b. FASEB J. 23 (10), 3564–3570. [DOI] [PubMed] [Google Scholar]
- (7).Grubb DR, Crook B, Ma Y, Luo J, Qian HW, Gao X-M, Kiriazis H, Du X-J, Gregorevic P, and Woodcock EA (2015) The Atypical ‘b’ Splice Variant of Phospholipase Cβ1 Promotes Cardiac Contractile Dysfunction. J. Mol. Cell. Cardiol 84, 95–103. [DOI] [PubMed] [Google Scholar]
- (8).Atef ME, and Anand-Srivastava MB (2016) Role of PKCδ in Enhanced Expression of Gqα/PLCβ1 Proteins and VSMC Hypertrophy in Spontaneously Hypertensive Rats. PLoS One 11 (7), e0157955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Woodcock EA, Grubb DR, Filtz TM, Marasco S, Luo J, McLeod-Dryden TJ, Kaye DM, Sadoshima J, Du X-J, Wong C, McMullen JR, and Dart AM (2009) Selective Activation of the “b” Splice Variant of Phospholipase Cβ1 in Chronically Dilated Human and Mouse Atria. J. Mol. Cell. Cardiol 47 (5), 676–683. [DOI] [PubMed] [Google Scholar]
- (10).Dent MR, Dhalla NS, and Tappia PS (2004) Phospholipase C Gene Expression, Protein Content, and Activities in Cardiac Hypertrophy and Heart Failure Due to Volume Overload. Am. J. Physiol 287 (2), H719–H727. [DOI] [PubMed] [Google Scholar]
- (11).Adjobo-Hermans MJW, Goedhart J, and Gadella TWJ (2008) Regulation of PLCβ1a Membrane Anchoring by Its Substrate Phosphatidylinositol (4,5)-Bisphosphate. J. Cell Sci 121 (22), 3770–3777. [DOI] [PubMed] [Google Scholar]
- (12).Mathews JL, Smrcka AV, and Bidlack JM (2008) A Novel Gβγ-Subunit Inhibitor Selectively Modulates μ-Opioid-Dependent Antinociception and Attenuates Acute Morphine-Induced Antinociceptive Tolerance and Dependence. J. Neurosci 28 (47), 12183–12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lyon AM, and Tesmer JJG (2013) Structural Insights into Phospholipase C-β Function. Mol. Pharmacol 84 (4), 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lyon AM, Tesmer VM, Dhamsania VD, Thal DM, Gutierrez J, Chowdhury S, Suddala KC, Northup JK, and Tesmer JJG (2011) An Autoinhibitory Helix in the C-Terminal Region of Phospholipase C-β Mediates Gαq Activation. Nat. Struct. Mol. Biol 18 (9), 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, and Harden TK (2010) Kinetic Scaffolding Mediated by a Phospholipase C-β and Gq Signaling Complex. Science 330 (6006), 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lyon AM, Dutta S, Boguth CA, Skiniotis G, and Tesmer JJG (2013) Full-Length Gaq-Phospholipase C-B3 Structure Reveals Interfaces of the C-Terminal Coiled-Coil Domain. Nat. Struct. Mol. Biol 20 (3), 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, and Gelb MH (2002) Interfacial Kinetic and Binding Properties of the Complete Set of Human and Mouse Groups I, II, V, X, and XII Secreted Phospholipases A2. J. Biol. Chem 277 (50), 48535–48549. [DOI] [PubMed] [Google Scholar]
- (18).Lyon AM, Begley JA, Manett TD, and Tesmer JJG (2014) Molecular Mechanisms of PLCβ3 Autoinhibition. Structure 22 (12), 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hudson BN, Hyun S-H, Thompson DH, and Lyon AM (2017) Phospholipase Cβ3 Membrane Adsorption and Activation Are Regulated by Its C-Terminal Domains and Phosphatidylinositol 4,5-Bisphosphate. Biochemistry 56 (41), 5604–5614. [DOI] [PubMed] [Google Scholar]
- (20).Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, and Sondek J (2008) General and Versatile Autoinhibition of PLC Isozymes. Mol. Cell 31 (3), 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Esquina CM, Garland-Kuntz EE, Goldfarb D, McDonald EK, Hudson BN, and Lyon AM (2019) Intramolecular Electrostatic Interactions Contribute to Phospholipase Cβ3 Autoinhibition. Cell. Signalling 62, 109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jenco JM, Becker KP, and Morris AJ (1997) Membrane-Binding Properties of Phospholipase C-Beta1 and PhospholipaseC-Beta2: Role of the C-Terminus and Effects of Polyphosphoinositides, G-Proteins and Ca2+. Biochem. J 327 (Part2), 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, and Gierschik P (1998) Stimulation of Phospholipase C-Beta2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 17 (21), 6241–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Illenberger D, Walliser C, Nurnberg B, Diaz Lorente M, and Gierschik P (2003) Specificity and Structural Requirements of Phospholipase C-Beta Stimulation by Rho GTPases versus G Protein Beta Gamma Dimers. J. Biol. Chem 278 (5), 3006–3014. [DOI] [PubMed] [Google Scholar]
- (25).Romoser V, Ball R, and Smrcka AV (1996) Phospholipase C B2 Association with Phospholipid Interfaces Assessed by Fluorescence Resonance Energy Transfer G Protein Beta Gamma Subunit-Mediated Translocation Is Not Required for Enzyme Activation. J. Biol. Chem 271 (41), 25071–25078. [DOI] [PubMed] [Google Scholar]
- (26).Runnels LW, Jenco J, Morris A, and Scarlata S (1996) Membrane Binding of Phospholipases C-Beta 1 and C-Beta 2 Is Independent of Phosphatidylinositol 4,5-Bisphosphate and the Alpha and Beta Gamma Subunits of G Proteins. Biochemistry 35 (51), 16824–16832. [DOI] [PubMed] [Google Scholar]
- (27).Scarlata S (2002) Regulation of the Lateral Association of Phospholipase Cbeta2 and G Protein Subunits by Lipid Rafts. Biochemistry 41 (22), 7092–7099. [DOI] [PubMed] [Google Scholar]
- (28).Gutman O, Walliser C, Piechulek T, Gierschik P, and Henis YI (2010) Differential Regulation of Phospholipase C-Beta2 Activity and Membrane Interaction by Galphaq, Gbeta1gamma2, and Rac2. J. Biol. Chem 285 (6), 3905–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Schnabel P, Camps M, Carozzi A, Parker PJ, and Gierschik P (1993) Mutational Analysis of Phospholipase C-B2. Eur. J. Biochem 217 (3), 1109–1115. [DOI] [PubMed] [Google Scholar]
- (30).Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, and Gierschik P (1992) Isozyme-Selective Stimulation of Phospholipase C-B2 by G Protein)γ-Subunits. Nature 360 (6405), 684–686. [DOI] [PubMed] [Google Scholar]
- (31).Katz A, Wu D, and Simon MI (1992) Subunits Beta Gamma of Heterotrimeric G Protein Activate Beta 2 Isoform of Phospholipase C. Nature 360 (6405), 686–689. [DOI] [PubMed] [Google Scholar]
- (32).Smrcka AV, and Sternweis PC (1993) Regulation of Purified Subtypes of Phosphatidylinositol-Specific Phospholipase C Beta by G Protein Alpha and Beta Gamma Subunits. J. Biol. Chem 268 (13), 9667–9674. [PubMed] [Google Scholar]
- (33).Lee SB, Shin SH, Hepler JR, Gilman AG, and Rhee SG (1993) Activation of Phospholipase C-Beta 2 Mutants by G Protein Alpha q and Beta Gamma Subunits. J. Biol. Chem 268 (34), 25952–25957. [PubMed] [Google Scholar]
- (34).Hepler JR, Biddlecome GH, Kleuss C, Camp LA, Hofmann SL, Ross EM, and Gilman AG (1996) Functional Importance of the Amino Terminus of G. J. Biol. Chem 271 (1), 496–504. [DOI] [PubMed] [Google Scholar]
- (35).Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, and Bourne HR (1993) Palmitoylation Is Required for Signaling Functions and Membrane Attachment of Gq Alpha and Gs Alpha. J. Biol. Chem 268 (33), 25001–25008. [PubMed] [Google Scholar]
- (36).Dietrich A, Brazil D, Jensen ON, Meister M, Schrader M, Moomaw JF, Mann M, Illenberger D, and Gierschik P (1996) Isoprenylation of the G Protein γ Subunit Is Both Necessary and Sufficient for Bγ Dimer-Mediated Stimulation of Phospholipase C. Biochemistry 35 (48), 15174–15182. [DOI] [PubMed] [Google Scholar]
- (37).Demel RA, Geurts van Kessel WSM, Zwaal RFA, Roelofsen B, and van Deenen LLM (1975) Relation between Various Phospholipase Actions on Human Red Cell Membranes and the Interfacial Phospholipid Pressure in Monolayers. Biochim. Biophys. Acta, Biomembr 406 (1), 97–107. [DOI] [PubMed] [Google Scholar]
- (38).James SR, Demel RA, and Downes CP (1994) Interfacial Hydrolysis of Phosphatidylinositol 4-Phosphate and Phosphatidylinositol 4,5-Bisphosphate by Turkey Erythrocyte Phospholipase C. Biochem. J 298 (2), 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).James SR, Paterson A, Harden TK, Demel RA, and Downes CP (1997) Dependence of the Activity of Phospholipase Cβ on Surface Pressure and Surface Composition in Phospholipid Monolayers and Its Implications for Their Regulation. Biochemistry 36 (4), 848–855. [DOI] [PubMed] [Google Scholar]
- (40).Boguslavsky V, Rebecchi M, Morris AJ, Jhon DY, Rhee SG, and McLaughlin S (1994) Effect of Monolayer Surface Pressure on the Activities of Phosphoinositide-Specific Phospholipase C-Beta.1, -Gamma.1, and -Delta.1. Biochemistry 33 (10), 3032–3037. [DOI] [PubMed] [Google Scholar]
- (41).Arduin A, Gaffney PRJ, and Ces O (2015) Regulation of PLCβ2 by the electrostatic and mechanical properties of lipid bilayers. Sci. Rep 5, 12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Chan P, Gabay M, Wright FA, Kan W, Oner SS, Lanier SM, Smrcka AV, Blumer JB, and Tall GG (2011) Purification of Heterotrimeric G Protein α Subunits by GST-Ric-8 Association Primary Characterization of Purified Gαolf. J. Biol. Chem 286 (4), 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Nečas D, and Klapetek P (2012) Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys 10 (1), 181–188. [Google Scholar]
- (44).Zhang JY, Kowal DM, Nawoschik SP, Dunlop J, Pausch MH, and Peri R (2010) Development of an Improved IP 1 Assay for the Characterization of 5-HT 2C Receptor Ligands. Assay Drug Dev. Technol 8 (1), 106–113. [DOI] [PubMed] [Google Scholar]
- (45).Bergsdorf C, Kropp-Goerkis C, Kaehler I, Ketscher L, Boemer U, Parczyk K, and Bader B (2008) A One-Day, Dispense-Only IP-One HTRF Assay for High-Throughput Screening of Galphaq Protein-Coupled Receptors: Towards Cells as Reagents. Assay Drug Dev. Technol 6 (1), 39–53. [DOI] [PubMed] [Google Scholar]
- (46).Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, Bourrier E, Ansanay H, Leroy C, Michaud A, Durroux T, Maurel D, Malhaire F, Goudet C, Pin J, Naval M, Hernout O, Chrétien F, Chapleur Y, and Mathis G (2006) D-Myo-Inositol 1-Phosphate as a Surrogate of D-Myo-Inositol 1,4,5-Tris Phosphate to Monitor G Protein-Coupled Receptor Activation. Anal. Biochem 358 (1), 126–135. [DOI] [PubMed] [Google Scholar]
- (47).Ghosh M, and Smrcka AV (2003) Assay for G Protein-Dependent Activation of Phospholipase C Beta Using Purified Protein Components. Methods Mol. Biol. Clifton NJ 237, 67–75. [DOI] [PubMed] [Google Scholar]
- (48).Levental I, Cēbers A, and Janmey PA (2008) Combined Electrostatics and Hydrogen Bonding Determine Intermolecular Interactions Between Polyphosphoinositides. J. Am. Chem. Soc 130 (28), 9025–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Redfern DA, and Gericke A (2005) PH-Dependent Domain Formation in Phosphatidylinositol Polyphosphate/Phosphatidylcholine Mixed Vesicles. J. Lipid Res 46 (3), 504–515. [DOI] [PubMed] [Google Scholar]
- (50).Redfern DA, and Gericke A (2004) Domain Formation in Phosphatidylinositol Monophosphate/Phosphatidylcholine Mixed Vesicles. Biophys. J 86 (5), 2980–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Ellenbroek WG, Wang Y-H, Christian DA, Discher DE, Janmey PA, and Liu AJ (2011) Divalent Cation-Dependent Formation of Electrostatic PIP2 Clusters in Lipid Monolayers. Biophys. J 101 (9), 2178–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wang Y-H, Collins A, Guo L, Smith-Dupont KB, Gai F, Svitkina T, and Janmey PA (2012) Divalent Cation-Induced Cluster Formation by Polyphosphoinositides in Model Membranes. J. Am. Chem. Soc 134 (7), 3387–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, and Fujimoto T (2009) A Distinct Pool of Phosphatidylinositol 4,5-Bisphosphate in Caveolae Revealed by a Nanoscale Labeling Technique. Proc. Natl. Acad. Sci U. S. A 106 (23), 9256–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Qifti A, Garwain O, and Scarlata S (2019) Mechanical Stretch Redefines Membrane Gαq-Calcium Signaling Complexes. J. Membr. Biol DOI: 10.1007/s00232-019-00063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Zhang H, Craciun LC, Mirshahi T, Rohács T, Lopes CMB, Jin T, and Logothetis DE (2003) PIP(2) Activates KCNQ Channels, and Its Hydrolysis Underlies Receptor-Mediated Inhibition of M Currents. Neuron 37 (6), 963–975. [DOI] [PubMed] [Google Scholar]
- (56).Whorton MR, and MacKinnon R (2013) X-Ray Structure of the Mammalian GIRK2-Bγ G-Protein Complex. Nature 498 (7453), 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Suh B-C, and Hille B (2005) Regulation of Ion Channels by Phosphatidylinositol 4,5-Bisphosphate. Curr. Opin. Neurobiol 15 (3), 370–378. [DOI] [PubMed] [Google Scholar]
- (58).Golebiewska U, and Scarlata S (2010) The Effect of Membrane Domains on the G Protein - Phospholipase Cβ Signaling Pathway. Crit. Rev. Biochem. Mol. Biol 45 (2), 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Gamper N, and Shapiro MS (2007) Target-Specific PIP2 Signalling: How Might It Work? J. Physiol 582 (Part 3), 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, Levine T, Kapus A, and Grinstein S (2006) Receptor Activation Alters Inner Surface Potential during Phagocytosis. Science 313 (5785), 347–351. [DOI] [PubMed] [Google Scholar]
- (61).Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, and Meyer T (2006) PI(3,4,5)P3 and PI(4,5)P2 Lipids Target Proteins with Polybasic Clusters to the Plasma Membrane. Science 314 (5804), 1458–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Hammond GRV, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, and Irvine RF (2012) PI4P and PI(4,5)P2 Are Essential but Independent Lipid Determinants of Membrane Identity. Science 337 (6095), 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.