Abstract
Corticotropin-releasing hormone (CRH) is a key regulator of the stress response. This peptide controls the hypothalamic-pituitary adrenal (HPA) axis as well as a variety of behavioral and autonomic stress responses via the two CRH receptors, CRH-R1 and CRH-R2. The CRH system also includes an evolutionarily conserved CRH-binding protein (CRH-BP), a secreted glycoprotein that binds CRH with subnanomolar affinity to modulate CRH receptor activity. In this review, we discuss the current literature on CRH-BP and stress across multiple species, from insects to humans. We describe the regulation of CRH-BP in response to stress, as well as genetic mouse models that have been utilized to elucidate the in vivo role(s) of CRH-BP in modulating the stress response. Finally, the role of CRH-BP in the human stress response is examined, including single nucleotide polymorphisms in the human CRHBP gene that are associated with stress-related affective disorders and addiction.
Keywords: Corticotropin-releasing hormone, Corticotropin-releasing factor, Stress, CRH receptors, Glucocorticoids, CRH-Binding Protein
Lay summary
The stress response is controlled by corticotropin-releasing hormone (CRH), acting via CRH receptors. However, the CRH system also includes a unique CRH-binding protein (CRH-BP) that binds CRH with an affinity greater than the CRH receptors. In this review, we discuss the role of this highly conserved CRH-BP in regulation of the CRH-mediated stress response from invertebrates to humans.
All living organisms experience stress, a bodily response to environmental or homeostatic demands. Stressors can take many different forms, from physiological to psychological challenges. The major sensory systems relay stressor-relevant information to the brain, which recruits both neural (autonomic nervous system (ANS)) and neuroendocrine (hypothalamic-pituitary-adrenal (or interrenal; hereafter referred to as HPA/I)) systems to respond and minimize cost to the organism and maintain homeostasis.
Physical stressors (i.e. hemorrhage, pain, predator) rapidly engage both the ANS and HPA systems. The ANS increases heart rate and blood pressure allowing for a rapid response within seconds, while the HPA axis is a bit slower, resulting in increased glucocorticoid secretion within minutes. Psychogenic stressors (i.e. social stress) require processing in the forebrain and can occur in response to or in anticipation of stressful events. The acute stress response is an adaptive mechanism to allow the organism to both respond to the environmental or physiological changes and to return to homeostasis. The duration and magnitude of the stress response differ markedly based on the stressor and the stress history, genetic background and life events of the organism. In contrast, chronic stress can be maladaptive and is linked to a variety of pathological states including depression, anxiety, cognitive impairment, and heart disease.
The neuroendocrine HPA axis mediates the endocrine component of the stress response and is controlled, in large part, by corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor (CRF). Stressors signal via a variety of direct and indirect pathways to the CRH neurons in the preoptic area (POA) in non-mammalian vertebrates or paraventricular nucleus of the hypothalamus (PVN) in mammals, signaling for release of CRH into the hypophyseal portal system. CRH travels to the anterior pituitary where it stimulates corticotropes to release ACTH into the blood. ACTH acts on the adrenal cortex to increase synthesis and release of glucocorticoids (corticosterone or cortisol) that mediate the metabolic and immune responses to stress and then help to limit the response via negative feedback. CRH is the key hypothalamic regulator in the HPA axis, but other hypothalamic hormones such as vasopressin can also play a role in ACTH release. CRH also mediates other behavioral, autonomic and immune responses to stress via its expression and signaling in many other regions of the brain.
CRH family of peptides:
CRH, a 41-amino acid peptide, was first isolated and characterized from sheep hypothalami by Vale and colleagues in 1981 (Vale, Spiess, Rivier, & Rivier, 1981). Studies in fish and frog also isolated peptides similar to CRH (urotensin I and sauvagine) (Ichikawa, McMaster, Lederis, & Kobayashi, 1982; Lederis, Letter, McMaster, Moore, & Schlesinger, 1982; Montecucchi & Henschen, 1981), but then also identified CRH orthologues (Okawara et al., 1988; Stenzel-Poore, Heldwein, Stenzel, Lee, & Vale, 1992), suggesting the presence of additional CRH-like peptides in mammals as well (Figure 1). Not surprisingly, in 1995, the first orthologue of urotensin was cloned in mammals and named urocortin (now called urocortin 1 (UCN1)) (Vaughan et al., 1995). In 2001, genomic analysis led to the identification of two other CRH-like peptides, named urocortin 2 (UCN2) and urocortin 3 (UCN3) (Hsu & Hsueh, 2001; Lewis et al., 2001; Reyes et al., 2001). Evolutionary studies suggest that the vertebrate progenitor had two “CRH-like” peptide genes, CRH/Urotensin1-like and Ucn 2/3-like, that served as the founders for the two separate vertebrate subfamilies CRH/UCN1 and UCN2/UCN3. Genome duplications of these two subfamilies eventually resulted in the 4 paralogous CRH-like peptides found in mammals (CRH, UCN1, UCN2, UCN3, see Figure 1). A CRH2 gene is present in some species, but has been lost in placental mammals and teleost fish. Invertebrates (insects) also possess CRH-like homologues known as diuretic hormones (DH). Several recent reviews have described the origins of the vertebrate CRH family of peptides and compare many of these peptides sequences across a variety of species (Cardoso, Bergqvist, Felix, & Larhammar, 2016; Endsin, Michalec, Manzon, Lovejoy, & Manzon, 2017; Lovejoy & Barsyte-Lovejoy, 2010; Lovejoy, Chang, Lovejoy, & del Castillo, 2014).
Figure 1.
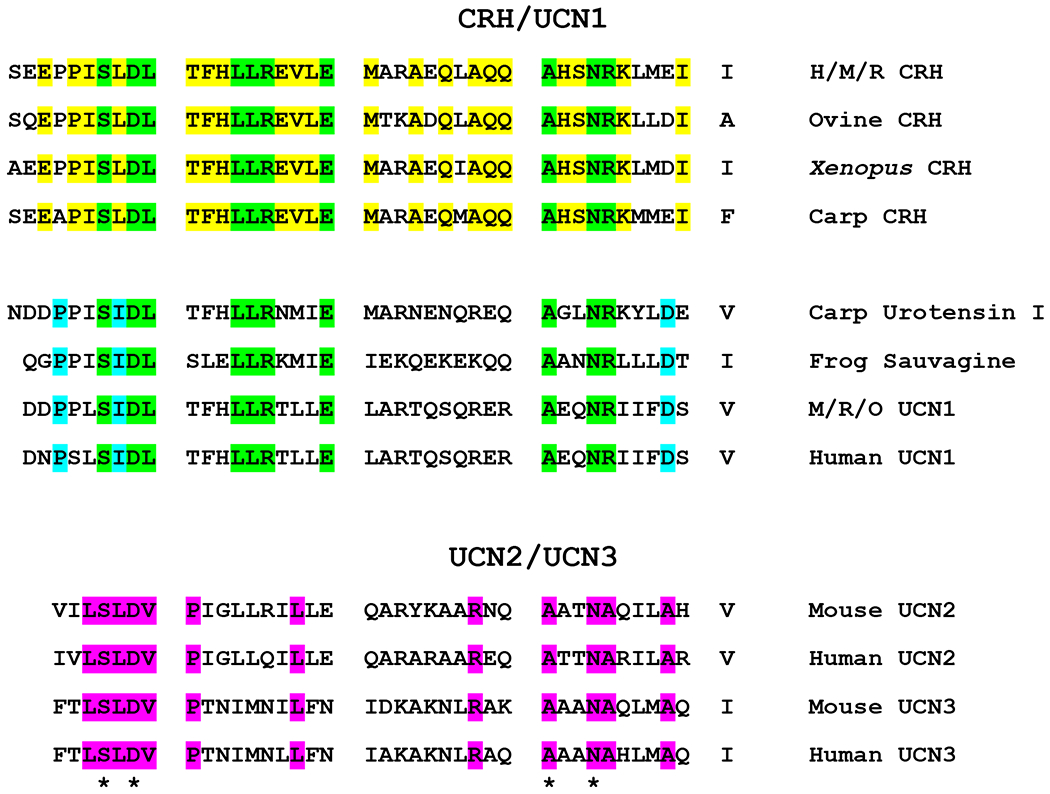
Alignment of amino acid sequences of members of the CRH/UCN1 and UCN2/UCN3 families. Conserved amino acid residues are color-coded as follows: yellow for CRH (between species); blue for UCN1/urotensin/sauvagine; green for residues common between CRH and UCN1/urotensin/sauvagine peptides; pink for UCN2/UCN3. Amino acids common between all peptides listed are marked with an asterisk (*). Abbreviations listed: M, mouse; R, rat; H, human; O, ovine. The accession numbers are as follows: Human CRH, NP_000747.1; Mouse CRH, NP_991338.1; Rat CRH, P01143; Ovine CRH, P01142; Xenopus CRH, P49188; Carp CRH, CAC84859; Carp Urotensin 1, P01146; Painted-Belly Leaf Frog Sauvagine, P01144; Mouse UCN1, P81615; Rat UCN1, P55090; Ovine UCN1, AAC27288; Human UCN1, NP_003344; Mouse UCN2, Q99ML8.2; Human UCN2, NP_149976; Mouse UCN3, Q924A4.1; Human UCN3, NP_444277.
The distribution of expression of CRH, UCN1, UCN2, and UCN3 has been widely reviewed, with expression localized not only in the central nervous system, but also in the periphery (reviewed in Fekete & Zorrilla, 2007). Within the brain, CRH is expressed in the limbic system (hippocampus, nucleus accumbens, BNST, amygdala), hypothalamus, thalamus, cortex, cerebellum and hindbrain in mammals and non-mammalian species (Owens & Nemeroff, 1991). This conserved pattern of expression suggests strong positive selection on the tissue- and cell-type specific roles of CRH in the vertebrate stress response.
CRH receptors:
The CRH system in vertebrates includes two receptors (CRH-R1 and CRH-R2). These CRH receptors were cloned and characterized from human and rodent in the mid-1990s, and are members of the class B1 subfamily (secretin-like receptor family) of G protein-coupled receptors (GPCRs) (Chang, Pearse, O’Connell, & Rosenfeld, 1993; R. Chen, Lewis, Perrin, & Vale, 1993; Kishimoto, Pearse, Lin, & Rosenfeld, 1995; Liaw et al., 1996; Lovenberg et al., 1995; M. Perrin et al., 1995; M. H. Perrin, Donaldson, Chen, Lewis, & Vale, 1993; Stenzel et al., 1995; Vita et al., 1993). The two receptors are encoded from distinct genes, but show approximately 70% identity at the amino acid level with the largest divergence in the N-terminal extracellular domain. CRH-R1 and CRH-R2 couple predominantly to Gαs, but both receptors have also been shown to activate other G proteins as well, with coupling varying somewhat by tissue or cell-type (reviewed in Dautzenberg & Hauger, 2002; Hillhouse & Grammatopoulos, 2006).
CRH-R1 and CRH-R2 show differential expression patterns in the brain and periphery (reviewed in Hillhouse & Grammatopoulos, 2006; Van Pett et al., 2000). In mammals, CRH-R1 is quite widely expressed in the brain and in anterior pituitary corticotropes and lactotropes (Mano-Otagiri, Nemoto, Yamauchi, Kakinuma, & Shibasaki, 2016; Westphal, Evans, & Seasholtz, 2009), with lower expression in a variety of peripheral tissues. Thus, CRH-R1 is the key receptor for CRH-mediated ACTH release in pituitary in response to stress. CRH-R2 has a more limited expression profile in the brain, but is more widely expressed in the periphery. Mammals have multiple splice isoforms of CRH-R2, with CRH-R2alpha and CRH-R2beta expressed in human and rodent, while CRH-R2gamma is detected only in human brain. These splice variants have different N-termini, but the same transmembrane and intracellular domains. CRH-R2alpha is predominantly localized in the brain while CRH-R2beta is more widely expressed in peripheral tissues including heart, thymus and spleen.
Interestingly, the CRH family of ligands has differing affinities for the two CRH receptors. CRH and UCN1 both bind with nanomolar affinity to CRH-R1 (Jahn, Tezval, van Werven, Eckart, & Spiess, 2004). UCN1 also binds with nanomolar affinity to CRH-R2, while CRH has a decreased affinity (by about 25 fold) for CRH-R2 as compared to CRH-R1 (Jahn et al., 2004). UCN2 and UCN3 bind preferentially to CRH-R2. While the affinities vary slightly, this general scheme appears to hold true across vertebrates.
This divergence of the CRH receptor into CRH-R1 and CRH-R2 appears to have occurred early in vertebrate evolution. As noted above, homologues of the vertebrate CRH peptide, such as diuretic hormone (DH), have been identified in invertebrates. DH binds to specific receptors (DH receptors) that exhibit a high degree of homology to the earliest vertebrate CRH receptor. Hence the presence of CRH/DH and CRH-R/DH-R systems over 500 million years suggests that this system arose early and has persisted in metazoan genomes due to the important physiological role of this peptide during evolution (Cardoso et al., 2016; Endsin et al., 2017; Lovejoy & Barsyte-Lovejoy, 2010; Lovejoy et al 2014).
CRH-Binding Protein (CRH-BP)
Strikingly, the CRH family also includes a unique high affinity CRH-binding protein. This protein is distinct from the receptors and has been evolutionarily conserved from the honeybee to humans (reviewed in Huising et al., 2008; Westphal & Seasholtz, 2006). Throughout evolution, genome doublings have resulted in multiple CRH ligands and receptors, but only one CRH-BP has been found in any species. This suggests that any duplications of the CRH-BP gene were lost during evolution. The high homology of the CRH-BP from honeybee to human suggests that its function(s) also has been evolutionarily conserved.
The CRH-BP was originally identified in human plasma as a molecule that interfered with the CRH radioimmunoassay (Orth & Mount, 1987; Suda et al., 1988). The CRH-BP was purified from human plasma and shown to be a 37 kDa glycoprotein (Behan, Linton, & Lowry, 1989; Orth & Mount, 1987; Suda et al., 1988). Several years later, it was cloned from human liver and rat brain (Potter et al., 1991). The CRH-BP cDNA encodes a 322 amino acid protein including a signal sequence for targeting to the ER, but no hydrophobic transmembrane sequences. Hence the CRH-BP appears to traffic through the ER/Golgi/secretory pathway to function as a secreted protein, consistent with its original isolation from human plasma. Studies in neuroendocrine cells and primary neuronal cultures suggest that it can be secreted via both constitutive and regulated secretory pathways (Behan, Maciejewski, Chalmers, & De Souza, 1995; Blanco, Zúñiga, Andrés, Alvarez, & Gysling, K., 2011; Westphal, N.J., & Seasholtz, A.F., 2005). While biochemical subcellular fractionation studies have suggested a membrane association for brain CRH-BP (Behan, et al. 1996) the potential mechanism for this association is unclear as the CRH-BP has no transmembrane domains and no obvious signal motifs for modifications that could provide plasma membrane anchorage.
The tertiary structure of the CRH-BP has not been determined, but it is highly folded with 10 cysteine residues in 5 consecutive disulfide bonds that have been conserved in all vertebrate CRH-BPs. The invertebrate honeybee CRH-BP contains the first 8 cysteines at similarly spaced intervals in the sequence, suggesting that the first 4 disulfide bonds are conserved. All vertebrate sequences also contain a highly conserved N-linked glycosylation site. Other potential sites for post-translational modifications (i.e. phosphorylation) are present, but these modifications have not been studied in vivo.
The vertebrate CRH-BP binds both CRH and UCN1 with sub-nanomolar affinity (greater than affinity of CRH receptors for CRH and UCN1), but has variable or much lower affinity for UCN2 and UCN3, respectively (Jahn et al., 2004). Various CRH peptides have been utilized to determine the binding properties of CRH to the CRH-BP, and CRH6-33 and CRH9-33 were found to bind to CRH-BP with an affinity almost equal to the endogenous CRH (Sutton et al., 1995), without significant binding to CRH receptors.
Hence, the N- and C-terminus of the CRH peptide are not required for binding to CRH-BP, but are critical for binding (C-terminus) to CRH receptors and receptor activation (N-terminus) (reviewed in (Hillhouse & Grammatopoulos, 2006). This distinct difference makes CRH6-33 or CRH9-33 a useful ligand to bind to CRH-BP and displace endogenous ligand (often called a CRH-BP competitive ligand inhibitor or CRH-BP antagonist), without significant binding of CRH6-33 to the CRH receptors. Similarly, rodent and human(h) CRH-BP bind h/r/mCRH with sub-nanomolar affinity (IC50=0.5 nM), but bind ovine (o) CRH with much lower affinity (IC50= 470 nM); in contrast, both hCRH and oCRH bind to CRH-R1 with similar high affinity (IC50= 1-1.6 nM) (Eckart et al., 2001). Consistent with these results, studies by Sutton et al. (1995) showed that amino acids 22, 23 and 25 of the CRH peptide (3 of the 7 amino acid differences between ovine and hCRH) are key for binding to the CRH-BP, while not important for binding to the CRH receptor. Huising and colleagues (2008) recently performed a comprehensive alanine scan of 76 evolutionarily conserved amino acid residues on CRH-BP, allowing them to determine that CRH and UCN1 bind with high affinity to distinct binding surfaces of CRH-BP.
The sites of CRH-BP expression are of course key to its potential roles in vivo. In humans, it is expressed in liver, pituitary, brain and placenta (reviewd in Westphal & Seasholtz, 2006). The liver and placental expression appears to be specific to humans and a subset of higher primates (Bowman et al., 2001; Potter et al., 1991), with most other mammals showing expression only in brain, pituitary, and very low levels of BP expressed in a few other peripheral tissues. In the mouse pituitary, CRH-BP is expressed in corticotropes, lactotropes, and gonadotropes in females, with expression only detected at low levels in corticotropes in male mouse pituitary. In the brain, CRH-BP is widely expressed in cerebral cortex, hippocampus, the amygdaloid complex, BNST, ventral tegmental area (VTA), olfactory bulb, and a variety of sensory relays (Potter et al., 1992). CRH and CRH-BP immunoreactivity co-localize in a number of regions, including BNST and CeA, suggesting sites for interactions (Potter et al., 1992). Similarly, CRH-BP is also expressed in a number of CRH target sites including the anterior pituitary corticotropes and lactotropes, where CRH-BP and CRH receptors are often co-localized (Potter et al., 1992; Stinnett, Westphal, & Seasholtz, 2015; Westphal, Evans, & Seasholtz, 2009).
CRH-BP expression has also been characterized in non-mammalian vertebrates and invertebrates. In Xenopus laevis tadpoles, CRH-BP is detected in brain, pituitary, liver and tail (Valverde, Seasholtz, Cortright, & Denver, 2001), while in fish, it has been detected in the POA, hypothalamus, telencephalon, pituitary, skin and gills (Alderman & Bernier, 2007, 2009; Mazon, Verburg-van Kemenade, Flik, & Huising, 2006). In honeybee, CRH-BP is expressed in the head and in the abdomen where it may regulate water balance (Huising & Flik, 2005). These other sites of expression may suggest additional/alternate roles for the CRH-BP in these organisms.
Multiple roles have been suggested for the vertebrate CRH-BP (Figure 2). Early studies indicated that approximately 40-60% of CRH in human brain is bound by CRH-BP, suggesting a role for CRH-BP in limiting the bioavailability of CRH and reducing CRH receptor activation (Behan et al., 1995; Behan et al., 1997). Similarly, a variety of experiments in anterior pituitary cells showed that CRH-BP attenuates the ACTH-releasing activity of CRH at CRH-R1 in anterior pituitary (an inhibitory role) (Cortright, Nicoletti, & Seasholtz, 1995; Potter et al., 1991; Sutton et al., 1995). CRH-BP also blocks the CRH-mediated increases in cAMP-luciferase activity in CRH-R1 expressing transfected cells (Huising et al., 2008). However, studies using the CRH6-33 ligand inhibitor in the ventral tegmental area of rats suggest that CRH-BP may be required for CRH activity at CRH-R2 (an enhancing role) in this region (Ungless et al., 2003; B. Wang et al., 2007) In yet another study, icv injection of CRH6-33 has been shown to activate fos expression not only in CRH receptor-expressing cells (via increased free CRH levels), but also in CRH-BP-expressing cells that do not express CRH receptors, suggesting CRH-BP actions that may be independent of CRH receptor (CRH-R independent role) (Chan, Vale, & Sawchenko, 2000). Finally, recent studies suggest that CRH-BP may act as an escort protein to traffic CRH-R2alpha to the cell surface (trafficking role) (Slater et al., 2016). Ultrastructural analyses in brain and pituitary also suggest that the function of CRH-BP may vary in different cellular contexts (Peto, Arias, Vale, and Sawchenko, 1999). Hence, the CRH-BP may mediate multiple roles that may be tissue-, cell-, or CRH-receptor-subtype specific. However, its role in the regulation and control of the stress response may be its most important role in the evolutionary perspective. Hence, in this review, we will focus on the regulation of CRH-BP by diverse environmental and physiological stressors in both vertebrates and invertebrates and its potential role in modulation of the vertebrate stress response, both in the HPA/I axes and other sites within the brain.
Figure 2.
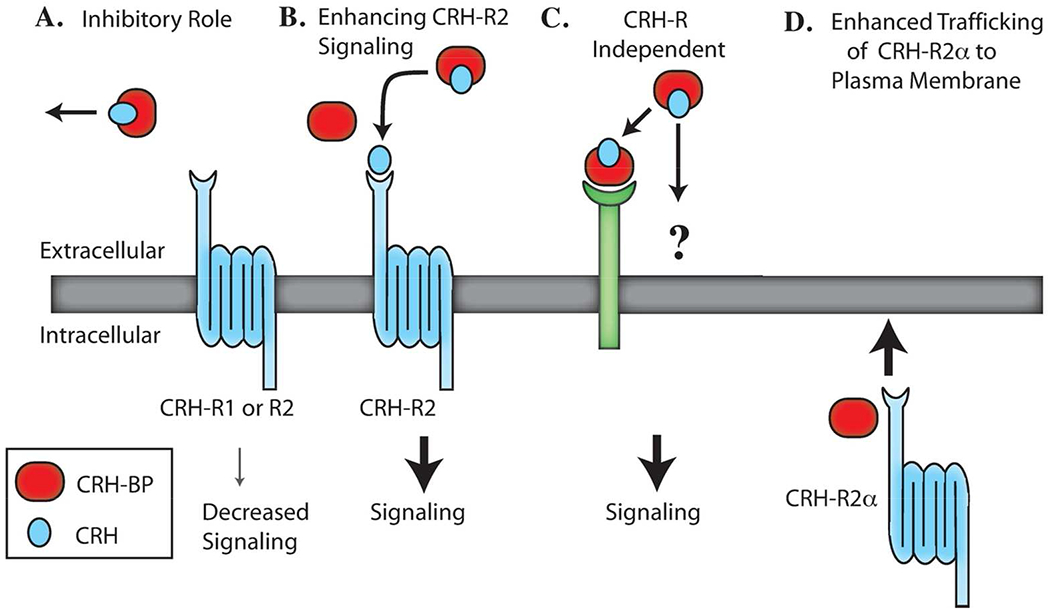
Potential roles for CRH-BP. A. CRH-BP may bind CRH and inhibit CRH receptor activation and downstream signaling. B. CRH-BP may bind CRH and enhance activation of CRH-R2 and downstream signaling. C. CRH-BP may have CRH receptor-independent signaling roles. D. CRH-BP may act as an escort protein to traffic CRH-R2± to the plasma membrane. This figure is modified from Westphal and Seasholtz, 2006.
Stress and CRH-BP in Invertebrates
The identification and sequencing of the invertebrate orthologue of CRH-BP from honeybees (Huising & Flik, 2005, Liu, Yu, Meng, Guo, & Xu, 2011) and crustacean (Lee, Rhee, Raisuddin, Gi Park, & Lee, 2008) highlighted the structural conservation of this protein over several hundred million years. However, very few studies have focused on CRH-BP and stress in invertebrates (Table 1). Copepods, a crustacean, exposed to changes in salinity or elevated temperature showed increased CRH-BP mRNA. (Lee, Rhee, Raisuddin, Gi Park, & Lee, 2008). Chinese honeybees subjected to UV light, heat or cold also exhibited increased CRH-BP mRNA in the head in a time-dependent manner (Liu, Yu, Meng, Guo, & Xu, 2011). These studies demonstrate the positive regulation of invertebrate CRH-BP mRNA by stress, despite the lack of an equivalent HPA/I axis as observed in vertebrates (Barton & Iwama, 1991; Campbell, Satoh, & Degnan, 2004).
Table 1.
Stress regulation of CRH-BP in invertebrates and non-mammalian vertebrates
| Species / Model | Stressor | Region | Stress duration | Effect on BP mRNA | Reference |
|---|---|---|---|---|---|
|
INVERTEBRATES | |||||
| Honeybee | UV light | Head | 1-4 hrs | Increase | Liu et al (2011) |
| Cold | Head | 1-4 hrs | Increase | Liu et al (2011) | |
| Heat | Head | 1-4 hrs | Increase | Liu et al (2011) | |
| Copepod | Heat | Whole body | 90 min heat stress | Increase | Lee et al (2008) |
| Salinity | Whole body | 12 hr salinity | Increase | Lee et al (2008) | |
|
NON-MAMMALIAN VERTEBRATES | |||||
| Gilthead sea bream | Salinity | Brain | up to 24 hrs | Increase | Martos-Sitcha et al (2014) |
| Sea lamprey | Salinity | Brain | 30 min (for 24 hrs) | No effect | Endsin et al (2017) |
| Heat | Tail | 6 hrs | No effect | Boorse et al (2006) | |
| Sole | Air | Hypothalamus | 30 sec | No effect | Lopez-Olmeda et al (2013) |
| Carp | Hypoxia | POA | 24 hrs | Decrease | Bernier et al (2012) |
| Rainbow trout | Hypoxia | Whole larvae | 90 min or 24 hrs | Decrease in CRHBP (28 & 42 dpf) | Fuzzen et al (2011) |
| Hypoxia | Telencephalon / POA | 8 or 24 hrs | Increase in Telen/POA @ 24 hrs | Alderman et al (2008) | |
| Hypoxia | Tail | 6 hrs | Decrease | Boorse et al (2006) | |
| Carp | Infection | Gills & skin | 3 week s | Decrease | Mazon et al (2006) |
| Rainbow trout | Physical disturbance | Pituitary | Single or repeated (3x) | Increase (repeated) | Doyon et al (2005) |
| Carp | Restraint | Hypothalamus | 30 min or 24 hrs | Increase (24 hr restraint) | Huising et al (2004); Pijanowski et al (2015) |
| Restraint | Pituitary | 24 hrs | No effect | Huising et al (2004); Pijanowski et al (2015) | |
| Restraint | Gills & skin | 24 hrs | Decrease | Mazon et al (2006) | |
| Rainbow trout | Confinement or isolation | Pituitary | 4, 24 or 72 hrs | No effect | Doyon et al (2005) |
| Cichlid | Social dominance | Brain or pituitary | 4 weeks | Lower in dominant (pit), no effect in brain | Chen et al (2008) |
| Rainbow trout | Social stress | Telencephalon / POA | 5 days | No effect | Jeffrey et al (2012) |
| Social stress | Telencephalon / hypothalamus | 8 or 24 hrs | Increase in telen of subord @ 24 hr, Decrease in hypothal of domin | Alderman et al (2008) | |
| Social stress + restraint | POA | 5 days (dominance), 1 hr (restraint) | Increase (restraint) | Jeffrey et al (2014) | |
| Gilthead sea bream | Starvation | Brain | up to 3 weeks | Decrease | Martos-Sitcha et al (2014) |
| Sole | Food deprivation | Whole larvae | 3-12 days | Decrease | Wunderink et a, (2012) |
| Rainbow trout | Stocking density + decrease water | Telencephalon | 30 min (water level) | No effect | Moltesen et al (2016) |
| Sole | Stocking density + hypersalinity | Brain | 33 days (stocking density) / 1-7 days (salinity) | No effect (stocking density). Increase (high salinity, 24 hrs) | Wunderink et al (2011) |
| Salmon | UCS | POA | 23 days | No effect | Madaro et al (2015) |
| Zebrafish | UCS | Telencephalon | 21 days | No effect | Manuel et al (2014) |
Stress and CRH-BP in Non-Mammalian Vertebrates
Numerous groups have investigated the CRH stress system, including the CRH-BP, in non-mammalian vertebrate species including fish, lamprey, and frogs. In these species, the stress response is conveyed through the hypothalamic-pituitary-interrenal (HPI) axis, which is analogous to the mammalian HPA axis. Not surprisingly, a wide range of stressful stimuli can lead to elevated glucocorticoid levels in these organisms [see (Barton & Iwama, 1991) for review] including changes in aquaculture (salinity, oxygenation, temperature), physical handling, social dominance, heavy metals and pollutants. The stress-induced regulation of CRH-BP in fish, lamprey and frogs appears to be dependent on the type and duration of stress and anatomical site of expression. Table 1 highlights these changes across species, grouped by similar stressors. Exposure to air or hypoxia (altering the oxygen levels in the water) has mixed effects on CRH-BP. A 30-sec air exposure in Senegalese sole had no effect on CRH-BP mRNA (Lopez-Olmeda et al., 2013). Hypoxia for 24 hrs decreased CRH-BP in carp (Bernier, Gorissen, & Flik, 2012) as well as in whole rainbow trout larvae 28 and 42 days post fertilization (dpf; Fuzzen, Alderman, Bristow, & Bernier, 2011), whereas in juvenile rainbow trout there was an increase after 24 hrs of hypoxia (Alderman, Raine, & Bernier, 2008). The effect in larvae is of interest since the exposure and sampling occurred prior to the maturation of the stress (HPI axis) response.
Salt water fish are sensitive to changes in salinity, and while they can adapt to gradual changes in salt concentration, sudden (acute) changes are perceived as a stress (Mancera, Fernandez-Llebrez, Grondona, & Perez-Figares, 1993). CRH-BP mRNA in the whole brain of gilthead sea bream placed in either high or low salinity increased after 12 hrs and remained elevated after 24 hrs in the high salt water group (Martos-Sitcha et al., 2014). This is in agreement with the effect observed in copepods (see Invertebrates above; (Lee et al., 2008)). In contrast, altering the salinity of sea lamprey had no effect (Endsin et al., 2017).
Restraint or confinement is also a powerful stressor in fish. Restraint for 24 hrs results in an increase in CRH-BP mRNA in carp hypothalamus, while there was no effect on pituitary CRH-BP mRNA (Huising et al., 2004; Pijanowski et al., 2015). In contrast, CRH-BP mRNA in the periphery (skin and gills) was decreased by 24 hrs of restraint (Mazon et al., 2006). These prolonged restraints could also be considered a form of social stress as the fish are isolated. For rainbow trout that have been either confined or isolated, pituitary CRH-BP mRNA is not different from controls (Doyon, Trudeau, & Moon, 2005).
Social hierarchies are formed in many species and can be stressful for various ranked individuals. Short-term social stress (8-24 hrs) in rainbow trout resulted in an increase of CRH-BP mRNA in the telencephalon of subordinate fish after 24 hrs, but not 8 hrs, and a decrease in the hypothalamus of dominant fish (Alderman et al., 2008). Prolonged social stress (5 days) had no effect on CRH-BP mRNA in rainbow trout (Jeffrey, Esbaugh, Vijayan, & Gilmour, 2012; Jeffrey, Gollock, & Gilmour, 2014). In African cichlids, 4 weeks of social stress resulted in lower levels of pituitary CRH-BP mRNA of dominant cichlids (C. C. Chen & Fernald, 2008).
Fewer studies have been performed for other physiological or immunological stressors. Repeated chasing of rainbow trout to exhaustion yielded an increase in pituitary CRH-BP mRNA (Doyon et al., 2005). Food deprivation or starvation resulted in a decrease of CRH-BP mRNA in gilthead sea bream (Martos-Sitcha et al., 2014) and sole larvae (Wunderink et al., 2012). Stocking density and unpredictable chronic stress (UCS) had no effect on CRH-BP mRNA levels in hypothalamus or telencephalon (Madaro et al., 2015; Manuel et al., 2014; Moltesen et al., 2016; Wunderink et al., 2011). Immune stress by infection decreased CRH-BP mRNA in the gills of carp (Mazon et al., 2006).
In Xenopus laevis, CRH-BP is expressed not only in the brain, but also in the tadpole tail where its expression is increased during spontaneous or thyroid hormone-induced metamorphosis (Z. Wang & Brown, 1993). CRH acts as a cytoprotectant in pre-metamorphic tadpole tail, and overexpression of tail CRH-BP attenuates the CRH bioactivity, promoting the tail regression normally required for remodeling of the tail (Boorse, Kholdani, Seasholtz, & Denver, 2006). In contrast, environmental stressors including hypoxia increased CRH and decreased CRH-BP in pre-metamorphic tadpole tail explants (Boorse et al., 2006). These results suggest that the CRH peptide system and CRH-BP work in concert to maintain viability of the tail until metamorphosis, when CRH-BP is increased by thyroid hormone and remodeling is required (Boorse et al., 2006). The utilization of CRH and CRH-BP in this developmental response is independent of its role in HPA/I activity, and suggests alternative roles for the CRH/CRH-R/CRH-BP system in non-mammalian vertebrates.
The research surveyed in non-mammalian species shows that many factors can influence the expression of CRH-BP in response to stress. Anatomical site, duration, repetition and type of stressor, and the developmental stage of the organism all appear to play a role. It should also be noted that there have not been any studies to date that alter brain and/or pituitary CRH-BP levels to examine the response to stress in these organisms.
Stress and CRH-BP in rodents
Stress regulation of CRH-BP expression:
As observed in non-mammalian vertebrates, rodent CRH-BP expression is highly regulated by stress and metabolic state. Stress is a positive regulator of CRH-BP expression in rodents, with the pituitary and amygdala being key sites of regulation. Table 2 provides a summary of rodent CRH-BP regulation by stress, glucocorticoids, and CRH. In the male rat pituitary, acute (30 minute) restraint stress significantly increased CRH-BP steady-state mRNA levels, an effect that persisted for 2 hours after the onset of stress (McClennen, Cortright, & Seasholtz, 1998). These data are consistent with studies in mice demonstrating that acute restraint stress increases pituitary CRH-BP mRNA levels 3.2-fold in males and 11.8-fold in females (Stinnett et al., 2015). Interestingly, pituitary CRH-BP mRNA levels are 200-fold higher in female mice than males, suggesting that CRH-BP may play sex-specific roles in modulating responses to stress (Speert, McClennen, & Seasholtz, 2002; Stinnett et al., 2015). While CRH-BP mRNA levels return to baseline 4 hours after acute restraint stress, CRH-BP protein levels are elevated in the female pituitary 4-6 hours after stress (Stinnett et al., 2015). While it seems likely that the increased protein is the result of increased CRH-BP mRNA, the increase in CRH-BP protein levels could also reflect an increase in CRH-BP stability. Together, these data suggest that pituitary CRH-BP may play an important role in modulating CRH activity after prolonged or repeated stressors, especially in females compared to males.
Table 2.
Stress regulation of CRH-BP in rodents
| Regulator | Species | Region / Cell type | Stressor / Treatment | Effect on CRH-BP expression | References |
|---|---|---|---|---|---|
| Stress | Rat | Pituitary | Acute restraint stress | Increased CRH-BP mRNA | McClennen et al., 1998 |
| Mouse | Pituitary | Acute restraint stress | Increased CRH-BP mRNA and protein | Stinnett et al., 2015 | |
| Rat | Basolateral amygdala | Acute restraint stress | Increased CRH-BP mRNA | Herringa et al., 2004; Lombardo et al., 2001 | |
| Rat | Amygdala | Chronic restraint stress | Decreased CRH-BP mRNA | Pisarska et al., 2000 | |
| Rat | Amygdala | Predator stress | Increased CRH-BP mRNA | Roseboom et al., 2007 | |
| Rat | Basolateral amygdala, pituitary, and medial POA | Food deprivation (Lean and obese Zucker rats) | Increased CRH-BP mRNA in basolateral amygdala and medial POA Decreased CRH-BP mRNA in the pituitary | Timofeeva et al., 1999 | |
| Rat | Medial POA | Treadmill running | Increased CRH-BP mRNA and protein | Timofeeva et al., 2003 | |
| Rat | Amygdala and PVN | Prenatal stress | Decreased CRH-BP mRNA in PVN and amygdala | Zohar and Weinstock, 2011 | |
| Glucocorticoids | Rat | Pituitary | Adrenalectomy | Increased CRH-BP mRNA | McClennen et al., 1998 |
| Rat | Basolateral Amygdala | Corticosterone injection (sc) | No effect on CRH-BP mRNA | Herringa et al., 2006 | |
| Rat | Fetal amygdalar cells | Dexamethasone | Increased CRH-BP mRNA, protein, and promoter activity | Kasckow et al., 1999 | |
| Rat | Immortalized amygdalar cells | Dexamethasone | Increased CRH-BP mRNA and protein | Mulchahey et al., 1999 | |
| Rat | Adrenal PC12 cells | Dexamethasone | Increased CRH-BP protein | Chatzaki et al., 2002 | |
| Rat | Primary astrocytes | Dexamethasone (+TPA or forskolin) | Decreased secreted CRH-BP | Maciejewski et al., 1996 | |
| Rat | Primary astrocytes | Dexamethasone (+CRH, TPA, or forskolin) | Decreased CRH-BP mRNA and hnRNA | McClennen et al., 1999 | |
| CRH | Rat | Basolateral amygdala | CRH injection (icv) | Increased CRH-BP mRNA | Herringa et al., 2006 |
| Rat | Fetal amygdalar cells | CRH | Increased CRH-BP mRNA, protein, and promoter activity | Kasckow et al., 1999 | |
| Rat | Pituitary AtT-20 cells | CRH | Increased CRH-BP promoter activity | Cortright et al., 1997 | |
| Rat | Adrenal PC12 cells | CRH | Increased CRH-BP protein | Chatzaki et al., 2002 | |
| Rat | Primary astrocytes | CRH | Increased CRH-BP mRNA | McClennen et al., 1999 | |
Similar to pituitary studies, acute restraint stress significantly increased CRH-BP mRNA in the rat basolateral amygdala (BLA), a stress-responsive region that is a critical target site of CRH (Herringa, Nanda, Hsu, Roseboom, & Kalin, 2004; Lombardo et al., 2001). This effect is time-dependent, as enhanced CRH-BP mRNA persisted for 21 hours after the onset of stress, indicating that CRH-BP may function in the BLA to modulate future responses to stress (Herringa et al., 2004). Interestingly, repeated restraint stress had no effect on CRH-BP mRNA expression (Lombardo et al., 2001). In contrast to these data, Pisarska and colleagues (Pisarska, Mulchahey, Welge, Geracioti, & Kasckow, 2000) demonstrated that CRH-BP mRNA expression is decreased in the amygdala of aged rats after 14 days of restraint stress. These contrasting results could be due to differences in experimental parameters such as rat strain, age, and stressor duration (acute vs. chronic). In addition to restraint stress, amygdalar CRH-BP appears to be regulated by a variety of other stressors, as elevated CRH-BP mRNA levels have been observed in the amygdala after predator stress and food deprivation (Roseboom et al., 2007; Timofeeva, Deshaies, Picard, & Richard, 1999) and decreased CRH-BP mRNA expression is observed in the amygdala of prenatally stressed rats (Zohar & Weinstock, 2011).
CRH-BP regulation by CRH and glucocorticoids has also been investigated, as both of these hormones are increased in response to stress. Adrenalectomy decreased rat pituitary CRH-BP mRNA levels to about 8% of control levels, suggesting that glucocorticoids play a key role in the positive regulation of pituitary CRH-BP expression by stress (McClennen et al., 1998). The regulation of amygdalar CRH-BP expression by corticosterone was also investigated, but corticosterone administration was not found to alter CRH-BP mRNA expression in the BLA (Herringa et al., 2006). In contrast, intracerebroventricular (ICV) administration of CRH significantly increased CRH-BP mRNA expression in the BLA (Herringa et al., 2006). The regulation of CRH-BP by stress hormones was also investigated in amygdalar and pituitary cell lines in vitro. CRH-BP is positively regulated by glucocorticoids in immortalized amygdalar AR-5 cells (Mulchahey, Regmi, Sheriff, Balasubramaniam, & Kasckow, 1999) and by both glucocorticoids and CRH in rat fetal amygdalar cells (Kasckow, Regmi, Seasholtz, & Mulchahey, 1999). In pituitary AtT-20 cells, CRH positively regulated CRH-BP promoter activity (Cortright, Goosens, Lesh, & Seasholtz, 1997). Together, these data reveal that stress, likely through increased CRH and glucocorticoid release, positively regulates CRH-BP expression in the pituitary and amygdala.
Finally, in primary rat astrocyte cultures, CRH-BP is also positively regulated by CRH. However, in these cells, glucocorticoids negatively regulate CRH-, forskolin- or TPA-induced CRH-BP mRNA or protein, suggesting that glucocorticoid regulation of CRH-BP expression may be context- and cell type-dependent (Maciejewski, Crowe, De Souza, & Behan, 1996; McClennen & Seasholtz, 1999).
Together, these data indicate that CRH-BP mRNA and protein increase in the pituitary and amygdala in response to stress, particularly acute stress, likely functioning as a homeostatic mechanism to decrease activation of CRH receptors in the presence of elevated glucocorticoids and CRH.
Mouse models of altered CRH-BP expression:
The in vivo role of CRH-BP has been further investigated with mouse models of altered CRH-BP expression. These mouse models have been key to elucidating the role of CRH-BP in modulating CRH activity in the HPA axis and the central nervous system. Two mouse models of CRH-BP overexpression (Burrows et al., 1998; Lovejoy et al., 1998) and one mouse model of CRH-BP deficiency (Karolyi et al., 1999) have been generated and characterized. The first transgenic mouse model of CRH-BP overexpression (called pituitary CRH-BP overexpressors) expresses high levels of CRH-BP cDNA in gonadotropes and thyrotropes in the anterior pituitary (Burrows et al., 1998), and these mice were utilized to test the effects of elevated levels of chronically secreted pituitary CRH-BP on modulation of hypothalamic CRH activity within the HPA axis. A second transgenic mouse model of CRH-BP overexpression (called plasma CRH-BP overexpressors) expresses rat CRH-BP cDNA under the control of mouse metallothionein-I promoter, resulting in elevated CRH-BP expression in the brain, pituitary, and a variety of peripheral tissues, including the liver (Lovejoy et al., 1998). High levels of CRH-BP were also detected in the plasma of these transgenic mice, hence they were utilized to determine the effects of chronically elevated plasma CRH-BP levels on HPA axis function. Lastly, Karolyi and colleagues (Karolyi et al., 1999) generated a mouse model of CRH-BP deficiency (CRH-BP KO) by gene targeting. These mice lack CRH-BP throughout the brain and pituitary and were therefore used to test the role of this protein in modulating CRH and urocortin activity in the HPA axis and throughout the central nervous system. Interestingly, the CRH-BP KO mice display significantly increased anxiety-like behavior in the elevated plus maze compared to wildtype controls (Karolyi et al., 1999). These data are consistent with the anxiogenic effects of CRH and support the hypothesis that CRH-BP is a negative regulator of CRH activity in vivo.
To determine the effects of altered CRH-BP expression on HPA axis function, both basal and stress-induced ACTH and corticosterone levels were investigated in each mouse model described above. Surprisingly, in all three models, basal corticosterone and ACTH levels did not differ from wild-type controls (Burrows et al., 1998; Karolyi et al., 1999; Lovejoy et al., 1998). Moreover, after acute restraint stress, corticosterone and ACTH response profiles did not differ in pituitary CRH-BP overexpressing mice or CRH-BP KO mice compared to their respective controls (Burrows et al., 1998; Karolyi et al., 1999). The observation that basal and stress-induced corticosterone and ACTH levels were not significantly altered by CRH-BP deficiency or pituitary overexpression is likely explained by homeostatic changes in the CRH system that occur after chronic alterations in CRH-BP expression. In support of this, CRH and AVP mRNA levels were increased by 82% and 35%, respectively, in the PVN of unstressed pituitary CRH-BP overexpressing mice, indicating a compensatory increase in hypothalamic CRH and AVP in response to chronically elevated pituitary CRH-BP (Burrows et al., 1998).
Lipopolysaccharide (LPS), a long-lasting inflammatory stressor, was also utilized to challenge HPA function in several of the CRH-BP mouse models. In the plasma CRH-BP overexpressing mice, LPS increased plasma CRH-BP concentrations and caused a small but significant attenuation of plasma ACTH levels 3 hours post-LPS injection in transgenic males, but not females (Lovejoy et al., 1998). However, it should be noted that LPS has been shown to induce the metallothionein-I promoter and may have enhanced expression of the CRH-BP transgene and contributed to this effect (Lovejoy et al., 1998). LPS stress, which enhances HPA activity for extended periods of time, also increased pituitary CRH-BP protein levels in wild-type female mice for at least 6 hours post-administration (Stinnett et al., 2015). Interestingly, LPS stress significantly enhanced plasma corticosterone levels in female CRH-BP KO mice compared to wild-type mice at 6 hours post-injection. These data suggest that the normal increase in CRH-BP after LPS stress likely plays a role in attenuating the stress response in female mice. Hence, CRH-BP may play a particularly important role in homeostatic regulation of the HPA axis in response to long-lasting stressors.
The CRH-BP deficient mice have also been used to investigate the role of CRH-BP in stress and maternal behavior. One particular behavior that has been investigated is maternal aggression, a key component of offspring protection, which is a territorial maternal care behavior in lactating females that likely evolved to protect offspring from infanticide (Wolff, 1985; Wolff and Peterson, 1998). Maternal aggression in mice is sensitive to both stress and CRH, with both causing a reduction in maternal aggression and increased maternal neglect (Gammie, Negron, Newman, & Rhodes, 2004; Gammie & Stevenson, 2006). Mice selected for high maternal aggression display increased CRH-BP expression, suggesting that CRH-BP may play a role in offspring protection (Gammie et al., 2007). Gammie and colleagues (Gammie, Seasholtz, & Stevenson, 2008) utilized CRH-BP KO mice crossed to a mouse line bred for high maternal defense to determine the effects of CRH-BP deficiency on maternal aggression. Maternal aggression, measured by the resident intruder test, was significantly decreased in lactating CRH-BP KO mice compared to wild-type mice, consistent with previous studies showing that CRH impairs maternal aggression (Gammie et al., 2004). These data suggest that CRH-BP modulation of CRH may be necessary for offspring protection and are consistent with the observation of elevated CRH-BP in mice selected for high maternal aggression (Gammie et al., 2007).
The role of CRH-BP in maternal aggression was also investigated in rats by utilizing the CRH-BP ligand inhibitor, CRH6-33 (Klampfl et al., 2016). ICV administration of CRH6-33 increased maternal aggression, particularly threat behavior, in lactating rats. This effect was also observed after administration of CRH6-33 into the medial-posterior BNST. These data contrast with the previously described study by Gammie and colleagues (Gammie et al., 2008), which showed CRH-BP KO mice exhibit decreased maternal aggression. However, this result may be explained in part by differences in species, rodent background, and method by which CRH-BP was inhibited.
Stress and CRH-BP in humans
CRH-BP expression is readily detected in brain and pituitary across vertebrate species, consistent with its role as a modulator of the stress axis. Strikingly, humans also express CRH-BP in liver and placenta (Orth & Mount, 1987; Petraglia et al., 1993; Potter et al., 1991; Trainer et al., 1998). These new sites of expression suggest other potential modulatory roles for this protein. CRH-BP produced in the liver is secreted into the plasma and is detected in both sexes, with slightly higher plasma levels in females (Trainer et al., 1998). While plasma levels of CRH are normally quite low, plasma CRH-BP readily binds this plasma CRH, preventing activation of CRH receptors and ACTH release from the pituitary (Linton et al., 1990). Interestingly, humans and some higher primates (but not rodents) also synthesize and release CRH from the placenta, especially during the second and third trimester of pregnancy; placental CRH has been hypothesized to be important in signaling the onset of parturition in humans (Campbell et al., 1987; McLean et al., 1995). Thus, plasma CRH-BP may play multiple roles in modulation of the CRH system in humans: 1) binding CRH released in response to stress or other challenges; and 2) binding CRH released by the placenta during pregnancy. In both cases, CRH-BP is thought to bind CRH (reducing free CRH levels) and attenuate CRH receptor activation in pituitary and placenta, reducing or blocking the CRH activity. However, based on these new sites of expression, the roles and regulation of human CRH-BP may be more varied than seen in rodents. Finally, while many studies in rodent address the regulation of CRH-BP mRNA levels in brain and pituitary, human plasma CRH-BP studies measure steady-state CRH-BP protein levels (released from liver) that may respond more slowly to challenges as they reflect both CRH-BP synthesis and stability.
Regulation of plasma CRH-BP:
The role of CRH-BP in the stress system of humans has not been well investigated, but glucocorticoids have been shown to regulate plasma CRH-BP levels. Forty-eight-hour oral dexamethasone treatment decreased plasma CRH-BP in healthy patients, suggesting that plasma CRH-BP is negatively regulated by glucocorticoids (Trainer et al., 1998). However, a later study by Kasckow and colleagues (2001) reported that low dose hydrocortisone administration increased plasma CRH-BP levels. This discrepancy may be due in part to different pharmacological profiles of the glucocorticoid administered or the length of administration (2 hours compared to 48 hours).
Plasma CRH-BP levels were also examined in patients with HPA axis disorders, including Cushing’s syndrome (hypercortisolism) and Addison’s disease (hypocortisolism). Plasma CRH-BP levels decreased in patients with Cushing’s syndrome and increased in patients with Addison’s compared to healthy controls, likely a result of altered CRH-BP expression in human liver (Suda et al., 1990, Ohmori et al., 1994; Trainer et al., 1998). Strikingly, CRH-BP levels returned to control levels in Addison’s patients after glucocorticoid replacement and in Cushing’s patients after surgery, consistent with negative glucocorticoid regulation of plasma CRH-BP (Suda et al., 1990, Ohmori et al., 1994; Trainer et al., 1998).
A few studies have also examined the regulation of plasma CRH-BP by CRH. Intravenous administration of hCRH decreases plasma CRH-BP levels in both healthy subjects and in patients with Cushing’s syndrome, likely due to rapid clearance of the CRH-BP:CRH complex (Trainer et al., 1998; Woods et al., 1994). As discussed above, the hCRH-BP binds hCRH with high affinity, but binds oCRH with much low affinity. This differential affinity may explain the much longer half-life of oCRH vs hCRH in human clinical studies, as CRH-BP does not bind and clear the oCRH in human plasma (Woods et al., 1994).
Plasma CRH-BP in humans may also play an important role in pregnancy. As noted above, CRH is highly expressed in placenta in the third trimester of pregnancy, and plasma CRH-BP binds the placental CRH released into the plasma, reducing its bioactivity and preventing overactivation of the pituitary and an elevated stress response (Linton et al., 1990; Saphier et al., 1992; Suda et al., 1988). Through regulatory mechanisms that are not yet identified, plasma CRH-BP levels drop in the last 3-4 weeks of pregnancy, resulting in a significant increase in “free” (not bound by CRH-BP) CRH levels. This increase in free CRH is thought to help trigger the onset of labor and may also be involved in the increased glucocorticoids that are key to organ maturation (especially lungs) before birth.
Thus, while it is clear that glucocorticoids regulate plasma CRH-BP, the effects of stress on plasma CRH-BP levels have not been well characterized in humans. One study found that CRH-BP plasma levels are elevated in inflammatory conditions such as rheumatoid arthritis and septicemia, indicating that CRH-BP may be positively regulated by inflammatory stressors (Woods et al., 1996). Moreover, CRH-BP is significantly elevated in the amniotic fluid and umbilical cord of patients with intraamniotic infection/inflammation (Florio et al., 2008). Future studies should investigate the role of additional stressors in regulating CRH-BP levels in plasma. Similarly, studies to address changes in CRH-BP mRNA in placenta and liver would also be informative, as changes in plasma CRH-BP protein levels likely reflect changes in synthesis, release, stability and clearance of this important regulatory protein.
Role of CRH-BP in vulnerability to stress and affective disorders:
The link between stress and affective disorders, such as anxiety and depression, has been well documented in both preclinical and clinical studies (McEwen, 2008). Recent studies have identified genetic associations of single nucleotide polymorphisms (SNPs) in the human CRHBP gene with stress-related psychiatric disorders and addiction (Table 3). For example, three CRHBP SNPs (rs7728378, rs10474485, and rs6453267) were associated with suicide risk as a result of childhood trauma (Roy, Hodgkinson, Deluca, Goldman, & Enoch, 2012). Interestingly, two of these SNPS, rs7728378 and rs10474485, were associated with anxiety disorders in Plains Indians and rs7728378 was also associated with alcohol use disorders in Caucasians (Enoch et al., 2008). Furthermore, rs10474485 was associated with depressive symptoms in alcohol dependent individuals (Kertes et al., 2011) and antidepressant treatment response in depressed patients (Binder et al, 2010), indicating that CRH-BP may play a role in susceptibility to depression/anxiety and alcohol abuse, and perhaps the comorbidity between these disorders. A different CRHBP SNP, rs10055255, was associated with stress-induced craving for alcohol in non-treatment-seeking heavy drinkers (Ray, 2011). Moreover, this SNP was associated with feelings of tension and negative mood in response to a guided imagery stressor. A separate study found that the same CRHBP SNP was associated with the correlation between stress-induced negative mood and negative consequences of drinking in non-treatment-seeking heavy drinkers (Tartter and Ray, 2012). Together, these data suggest that CRH-BP may play a role in vulnerability to stress-induced craving and relapse in humans, and are consistent with studies in rodents indicating a role for CRH-BP in alcohol addiction and stress-induced reinstatement to cocaine (Albrechet-Souza et al., 2015; Haass-Koffler et al., 2016; Ketchesin, Stinnett, & Seasholtz, 2016; Wang, You, Rice, & Wise, 2007).
Table 3.
CRHBP SNPs in stress-related disorders
| CRHBP SNPs | Location | Association | Population | Reference |
|---|---|---|---|---|
| rs10055255 | Intron 6 | Depressive symptoms after citalopram | Major depressive disorder (STAR*D) | Binder et al., 2010 |
| Stress-induced alcohol craving | Heavy drinkers | Ray, 2011 | ||
| Post-ICU PTSD and depressive symptoms | ICU patients | Davydow et al., 2014 | ||
| Stress-induced negative affect and negative consequences of drinking | Heavy drinkers | Tartter and Ray, 2012 | ||
| rs10473984 | 3′-Flanking | Cortisol reactivity | Three-year-old children | Sheikh et al., 2013 |
| Depressive symptoms after citalopram treatment | Major depressive disorder (STAR*D) | Binder et al., 2010 | ||
| rs10474485 | 3′-Flanking | Depressive symptoms after citalopram treatment | Major depressive disorder (STAR*D) | Binder et al., 2010 |
| Emotional state | Irritable Bowel Syndrome patients, Japan | Sasaki et al., 2016 | ||
| Childhood trauma and suicide attempt | African Americans | Roy et al., 2012 | ||
| Anxiety disorders | Plains Indians | Enoch et al., 2008 | ||
| History of depressive symptoms | Alcohol dependence, Ireland | Kertes et al., 2011 | ||
| rs1715747* | 3′-Flanking | History of depressive symptoms | Alcohol dependence, Ireland | Kertes et al., 2011 |
| Anxiety disorders, EEG alpha power | Plains Indians | Enoch et al., 2008 | ||
| Alcohol use disorders, EEG alpha power | U.S. Caucasians | Enoch et al., 2008 | ||
| rs1875999 | 3′-UTR | Major depressive disorder | Major depressive disorder, Sweden | Claes et al., 2003 |
| Anxiety disorders, EEG alpha power | Plains Indians | Enoch et al., 2008 | ||
| Alcohol use disorders | U.S. Caucasians | Enoch et al., 2008 | ||
| Suicide attempt | Schizophrenia | De Luca et al., 2010 | ||
| Major depressive disorder | Major depressive disorder, Swedish males | Van Den Eede et al., 2007 | ||
| rs7728378 | Intron 6 | Major depressive disorder | Major depressive disorder, Swedish males | Van Den Eede et al., 2007 |
| Major depressive disorder | Major depressive disorder, Sweden | Claes et al., 2003 | ||
| Anxiety disorders, EEG alpha power | Plains Indians | Enoch et al., 2008 | ||
| Alcohol use disorders, EEG alpha power | U.S. Caucasians | Enoch et al., 2008 | ||
| Childhood trauma and suicide attempt | African Americans | Roy et al., 2012 | ||
| rs6453267 | Intron 5 | Childhood trauma and suicide attempt | African Americans | Roy et al., 2012 |
| rs1500 | 3′-Flanking | Anxiety disorders, EEG alpha power | Plains Indians | Enoch et al., 2008 |
| rs3811939 | Intron 3 | Alcohol use disorders | Schizophrenia (Gottingen Research Association for Schizophrenia sample) | Ribbe et al., 2011 |
Previously called rs7704995
Table 3 provides a summary of the CRHBP SNPs that have been linked with stress-related disorders, including their location within the CRHBP gene. It should be noted that none of these SNPs are within CRH-BP protein coding sequences. However, one of the SNPs is located in the 3’UTR and could affect RNA stability, while several other SNPs are in intronic or 3’flanking sequences that could influence transcriptional control or transcript splicing. Future studies will determine whether these CRHBP SNPs correlate with altered CRH-BP levels and whether they can be used as markers for vulnerability or resistance to stress-related affective disorders and/or addiction.
Summary:
The CRH-BP is a unique high affinity binding protein that modulates CRH-mediated activation of CRH receptors in the brain and periphery. The CRH-BP has been highly conserved across evolution, from crustaceans to humans. In invertebrates, its role is not yet well defined, but it likely binds the invertebrate DH family of CRH-like peptides that are involved in diuresis and feeding. In vertebrates, we see the development of the HPI and HPA axes and the high affinity binding of CRH-BP to the evolving CRH peptide family of ligands, especially those in the CRH-UCN1 family involved in the stress response. In both non-mammalian and mammalian vertebrates, pituitary and brain CRH-BP expression are predominantly increased by stress, often in a stressor- and time-dependent fashion. This increased CRH-BP expression, especially within the HPA axis, appears to act as a mediator of negative feedback by binding CRH and decreasing CRH-R1 activation and signaling. The temporal profile of the increase in CRH-BP does not appear to blunt the ACTH or glucocorticoid response to a brief acute stressor. However, the increased CRH-BP appears to play a larger, more significant role in negative feedback to more prolonged, or potentially repeated stressors. Future studies should conditionally and site-specifically manipulate CRH-BP expression to further elucidate its role in modulating the response to acute and chronic stress with particular attention to sex, age and stress-state of the animal.
At other sites in the mammalian brain, the CRH-BP may be playing additional roles via modulation of CRH-R1 and CRH-R2 activity in other stress-related processes, such as feeding, reproduction, and reward. A number of recent studies suggest alternative roles for the CRH-BP in CRH-R2 signaling and CRH-R2alpha trafficking; its role and regulation in these processes are active areas of investigation. Finally, in humans, CRH-BP is expressed not only in pituitary and brain where its role in HPA and other functions likely continues, but also in liver and placenta, resulting in significant plasma CRH-BP expression. This plasma CRH-BP prevents pituitary and peripheral CRH-R1 activation when endogenous plasma CRH is increased due to stress, disease, or pregnancy. Additionally, a number of CRHBP SNPs have been associated with stress and a variety of stress-related disorders, suggesting that CRH-BP could be a potential biomarker for disorders such as depression and anxiety. Thus, while the CRH-BP’s role in stress regulation may persist from non-mammalian vertebrates to man, other roles may have been added throughout evolution. These roles may be ligand-, receptor-, and/or region-specific and additional genetic and viral CRH-BP knockdown and overexpression studies will be required to further elucidate its role in physiology and disease states.
Acknowledgments
Research was supported by NIH U01 AA013641(AFS), NSF Graduate Research Fellowship F031543 (KDK), Rackham Predoctoral Fellowship (KDK), University of Michigan Office of Research (AFS) and the Molecular and Behavioral Neuroscience Institute.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, & Miczek KA (2015). Corticotropin Releasing Factor Binding Protein and CRF2 Receptors in the Ventral Tegmental Area: Modulation of Ethanol Binge Drinking in C57BL/6J Mice. Alcohol Clin Exp Res, 39(9), 1609–18. doi: 10.1111/acer.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman SL, & Bernier NJ (2007). Localization of corticotropin-releasing factor, urotensin I, and CRH-binding protein gene expression in the brain of the zebrafish, Danio rerio. J Comp Neurol, 502(5), 783–793. doi: 10.1002/cne.21332 [DOI] [PubMed] [Google Scholar]
- Alderman SL, & Bernier NJ (2009). Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen Comp Endocrinol, 164(1), 61–69. doi: 10.1016/j.ygcen.2009.04.007 [DOI] [PubMed] [Google Scholar]
- Alderman SL, Raine JC, & Bernier NJ (2008). Distribution and regional stressor-induced regulation of corticotrophin-releasing factor binding protein in rainbow trout (Oncorhynchus mykiss). J Neuroendocrinol, 20(3), 347–358. doi: 10.1111/j.1365-2826.2008.01655.x [DOI] [PubMed] [Google Scholar]
- Barton BA, & Iwama GK (1991). Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annual Review of Fish Diseases, 1, 3–26. doi: 10.1016/0959-8030(91)90019-G [DOI] [Google Scholar]
- Behan DP, Cepoi D, Fischer WH, Park M, Sutton S, Lowry PJ, & Vale WW (1996). Characterization of a sheep brain corticotropin releasing factor binding protein. Brain Res, 709(2), 265–74. Doi: 10.1016/0006-8993(95)01317-2 [DOI] [PubMed] [Google Scholar]
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, & De Souza EB (1995). Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature, 378(6554), 284–287. doi: 10.1038/378284a0 [DOI] [PubMed] [Google Scholar]
- Behan DP, Khongsaly O, Owens MJ, Chung HD, Nemeroff CB, & De Souza EB (1997) Corticotropin-Releasing Factor (CRF), CRF-Binding Protein (CRF-BP) and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brain. J Neurochem, 68(5):2053–2060. doi: 10.1046/j.1471-4159.1997.68052053.x [DOI] [PubMed] [Google Scholar]
- Behan DP, Linton EA, & Lowry PJ (1989). Isolation of the human plasma corticotrophin-releasing factor-binding protein. J Endocrinol, 122(1), 23–31. doi: 10.1677/joe.0.1220023 [DOI] [PubMed] [Google Scholar]
- Behan DP, Maciejewski D, Chalmers D, & De Souza EB (1995). Corticotropin releasing factor binding protein (CRF-BP) is expressed in neuronal and astrocytic cells. Brain Res, 698(1-2), 259–64. doi: 10.1016/0006-8993(95)01014-M [DOI] [PubMed] [Google Scholar]
- Bernier NJ, Gorissen M, & Flik G (2012). Differential effects of chronic hypoxia and feed restriction on the expression of leptin and its receptor, food intake regulation and the endocrine stress response in common carp. J Exp Biol, 215(Pt 13), 2273–2282. doi: 10.1242/jeb.066183 [DOI] [PubMed] [Google Scholar]
- Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH … & Nemeroff CB (2010). Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry, 67(4), 369–79. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- Blanco EH, Zúñiga JP, Andrés ME, Alvarez AR, & Gysling K (2011). Corticotropin- releasing factor binding protein enters the regulated secretory pathway in neuroendocrine cells and cortical neurons. Neuropeptides, 45(4):273–9. doi: 10.1016/j.npep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Kholdani CA, Seasholtz AF, & Denver RJ (2006). Corticotropin-releasing factor is cytoprotective in Xenopus tadpole tail: coordination of ligand, receptor, and binding protein in tail muscle cell survival. Endocrinology, 147(3), 1498–1507. doi: 10.1210/en.2005-1273 [DOI] [PubMed] [Google Scholar]
- Bowman ME, Lopata A, Jaffe RB, Golos TG, Wickings J, & Smith R (2001). Corticotropin-releasing hormone-binding protein in primates. Am J Primatol, 53(3), 123–130. doi: [DOI] [PubMed] [Google Scholar]
- Burrows HL, Nakajima M, Lesh JS, Goosens KA, Samuelson LC, Inui A, … Seasholtz AF (1998). Excess corticotropin releasing hormone-binding protein in the hypothalamic-pituitary-adrenal axis in transgenic mice. J Clin Invest, 101(7), 1439–1447. doi: 10.1172/JCI1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RK, Satoh N, & Degnan BM (2004). Piecing together evolution of the vertebrate endocrine system. Trends Genet, 20(8), 359–366. doi: 10.1016/j.tig.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Cardoso JC, Bergqvist CA, Felix RC, & Larhammar D (2016). Corticotropin-releasing hormone family evolution: five ancestral genes remain in some lineages. J Mol Endocrinol, 57(1), 73–86. doi: 10.1530/JME-16-0051 [DOI] [PubMed] [Google Scholar]
- Chan RK, Vale WW, & Sawchenko PE (2000). Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience, 101(1), 115–129. doi: 10.1016/S0896-6273(03)00461-6 [DOI] [PubMed] [Google Scholar]
- Chang CP, Pearse RV 2nd, O’Connell S, & Rosenfeld MG (1993). Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron, 11(6), 1187–1195. DOI: 10.1016/0896-6273(93)90230-O [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Margioris AN, & Gravanis A (2002). Expression and regulation of corticotropin-releasing hormone binding protein (CRH-BP) in rat adrenals. J Neurochem, 80(1), 81–90. DOI: 10.1046/j.0022-3042.2001.00667.x [DOI] [PubMed] [Google Scholar]
- Chen CC, & Fernald RD (2008). Sequences, expression patterns and regulation of the corticotropin-releasing factor system in a teleost. Gen Comp Endocrinol, 157(2), 148–155. doi: 10.1016/j.ygcen.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, & Vale WW (1993). Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A, 90(19), 8967–8971. doi: 10.1073/pnas.90.19.8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes S, Villafuerte S, Forsgren T, Sluijs S, Del-Favero J, Adolfsson R, & Van Broeckhoven C (2003). The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol Psychiatry, 54(9), 867–72. doi: 10.1016/S0006-3223(03)00425-6 [DOI] [PubMed] [Google Scholar]
- Cortright DN, Goosens KA, Lesh JS, & Seasholtz AF (1997). Isolation and characterization of the rat corticotropin-releasing hormone (CRH)-binding protein gene: transcriptional regulation by cyclic adenosine monophosphate and CRH. Endocrinology, 138(5), 2098–2108. doi: 10.1210/endo.138.5.5128 [DOI] [PubMed] [Google Scholar]
- Cortright DN, Nicoletti A, & Seasholtz AF (1995). Molecular and biochemical characterization of the mouse brain corticotropin-releasing hormone-binding protein. Mol Cell Endocrinol, 111(2), 147–157. doi: 10.1016/0303-7207(95)03558-O [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, & Hauger RL (2002). The CRH peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci, 23(2), 71–77. 10.1016/S0165-6147(02)01946-6 [DOI] [PubMed] [Google Scholar]
- Davydow DS, Kohen R, Hough CL, Tracy JH, Zatzick D, & Katon WJ (2014). A pilot investigation of the association of genetic polymorphisms regulating corticotrophin-releasing hormone with posttraumatic stress and depressive symptoms in medical-surgical intensive care unit survivors. J Crit Care, 29(1), 101–6. doi: 10.1016/j.jcrc.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Tharmalingam S, Zai C, Potapova N, Strauss J, Vincent J, & Kennedy JL (2010). Association of HPA axis genes with suicidal behaviour in schizophrenia. J Psychopharmacol, 24(5), 677–82. doi: 10.1177/0269881108097817. [DOI] [PubMed] [Google Scholar]
- Doyon C, Trudeau VL, & Moon TW (2005). Stress elevates corticotropin-releasing factor (CRH) and CRH-binding protein mRNA levels in rainbow trout (Oncorhynchus mykiss). J Endocrinol, 186(1), 123–130. doi: 10.1677/joe.1.06142 [DOI] [PubMed] [Google Scholar]
- Eckart K, Jahn O, Radulovic J, Tezval H, van Werven L, & Spiess J (2001). A single amino acid serves as an affinity switch between the receptor and the binding protein of corticotropin-releasing factor: implications for the design of agonists and antagonists. Proc Natl Acad Sci U S A, 98(20), 11142–11147. doi: 10.1073/pnas.211424998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endsin MJ, Michalec O, Manzon LA, Lovejoy DA, & Manzon RG (2017). CRH peptide evolution occurred in three phases: Evidence from characterizing sea lamprey CRH system members. Gen Comp Endocrinol, 240, 162–173. doi: 10.1016/j.ygcen.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, … & Goldman D (2008). Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One, 3(10):e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, & Zorrilla EP (2007). Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRH paralogs. Front Neuroendocrinol, 28(1), 1–27. doi: 10.1016/j.yfrne.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzzen ML, Alderman SL, Bristow EN, & Bernier NJ (2011). Ontogeny of the corticotropin-releasing factor system in rainbow trout and differential effects of hypoxia on the endocrine and cellular stress responses during development. Gen Comp Endocrinol, 170(3), 604–612. doi: 10.1016/j.ygcen.2010.11.022 [DOI] [PubMed] [Google Scholar]
- Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, & Stevenson SA (2007). Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav, 6(5), 432–443. doi: 10.1111/j.1601-183X.2006.00271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, & Rhodes JS (2004). Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci, 118(4), 805–814. doi: 10.1037/0735-7044.118.4.805 [DOI] [PubMed] [Google Scholar]
- Gammie SC, Seasholtz AF, & Stevenson SA (2008). Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience, 157(3), 502–512. doi: 10.1016/j.neuroscience.2008.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, & Stevenson SA (2006). Intermale aggression in corticotropin-releasing factor receptor 1 deficient mice. Behav Brain Res, 171(1), 63–69. doi: 10.1016/j.bbr.2006.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, … & Leggio L (2016). Defining the role of corticotropin releasing factor binding protein in alcohol consumption. Transl Psychiatry, 6(11), e953. doi: 10.1038/tp.2016.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Mackenrodt DB, Barlow JD, Roseboom PH, Nanda SA, & Kalin NH (2006). Corticotropin-releasing factor (CRH), but not corticosterone, increases basolateral amygdala CRH-binding protein. Brain Res, 1083(1), 21–28. doi: 10.1016/j.brainres.2006.01.122 [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, & Kalin NH (2004). The effects of acute stress on the regulation of central and basolateral amygdala CRH-binding protein gene expression. Brain Res Mol Brain Res, 131(1-2), 17–25. doi: 10.1016/j.molbrainres.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, & Grammatopoulos DK (2006). The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev, 27(3), 260–286. doi: 10.1210/er.2005-0034 [DOI] [PubMed] [Google Scholar]
- Hsu SY, & Hsueh AJ (2001). Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med, 7(5), 605–611. doi: 10.1038/87936 [DOI] [PubMed] [Google Scholar]
- Huising MO, & Flik G (2005). The remarkable conservation of corticotropin-releasing hormone (CRH)-binding protein in the honeybee (Apis mellifera) dates the CRH system to a common ancestor of insects and vertebrates. Endocrinology, 146(5), 2165–2170. doi: 10.1210/en.2004-1514 [DOI] [PubMed] [Google Scholar]
- Huising MO, Metz JR, van Schooten C, Taverne-Thiele AJ, Hermsen T, Verburg-van Kemenade BM, & Flik G (2004). Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J Mol Endocrinol, 32(3), 627–648. doi: 10.1677/jme.0.0320627 [DOI] [PubMed] [Google Scholar]
- Huising MO, Vaughan JM, Shah SH, Grillot KL, Donaldson CJ, Rivier J, … Vale WW (2008). Residues of corticotropin releasing factor-binding protein (CRH-BP) that selectively abrogate binding to CRH but not to urocortin 1. J Biol Chem, 283(14), 8902–8912. doi: 10.1074/jbc.M709904200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, McMaster D, Lederis K, & Kobayashi H (1982). Isolation and amino acid sequence of urotensin I, a vasoactive and ACTH-releasing neuropeptide, from the carp (Cyprinus carpio) urophysis. Peptides, 3(5), 859–867. doi: 10.1016/0196-9781(82)90028-6 [DOI] [PubMed] [Google Scholar]
- Jahn O, Tezval H, van Werven L, Eckart K, & Spiess J (2004). Three-amino acid motifs of urocortin II and III determine their CRH receptor subtype selectivity. Neuropharmacology, 47(2), 233–242. doi: 10.1016/j.neuropharm.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Jeffrey JD, Esbaugh AJ, Vijayan MM, & Gilmour KM (2012). Modulation of hypothalamic-pituitary-interrenal axis function by social status in rainbow trout. Gen Comp Endocrinol, 176(2), 201–210. doi: 10.1016/j.ygcen.2012.01.016 [DOI] [PubMed] [Google Scholar]
- Jeffrey JD, Gollock MJ, & Gilmour KM (2014). Social stress modulates the cortisol response to an acute stressor in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol, 196, 8–16. doi: 10.1016/j.ygcen.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Karolyi IJ, Burrows HL, Ramesh TM, Nakajima M, Lesh JS, Seong E, … Seasholtz AF (1999). Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc Natl Acad Sci U S A, 96(20), 11595–11600. doi: 10.1073/pnas.96.20.11595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasckow JW, Regmi A, Seasholtz AF, & Mulchahey JJ (1999). Regulation of corticotropin-releasing factor-binding protein expression in amygdalar neuronal cultures. J Neuroendocrinol, 11(12), 959–966. doi: 10.1046/j.1365-2826.1999.00413.x [DOI] [PubMed] [Google Scholar]
- Kertes DA, Kalsi G, Prescott CA, Kuo PH, Patterson DG, Walsh D, … Riley BP (2011). Neurotransmitter and neuromodulator genes associated with a history of depressive symptoms in individuals with alcohol dependence. Alcohol Clin Exp Res, 35(3), 496–505. doi: 10.1111/j.1530-0277.2010.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchesin KD, Stinnett GS, & Seasholtz AF (2016). Binge Drinking Decreases Corticotropin-Releasing Factor-Binding Protein Expression in the Medial Prefrontal Cortex of Mice. Alcohol Clin Exp Res, 40(8), 1641–50. doi: 10.1111/acer.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Pearse RV 2nd, Lin CR, & Rosenfeld MG (1995). A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci U S A, 92(4), 1108–1112. doi: 10.1073/pnas.92.4.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM, Schramm MM, Stinnett GS, Bayerl DS, Seasholtz AF, & Bosch OJ (2016). Brain CRH-binding protein modulates aspects of maternal behavior under stressful conditions and supports a hypo-anxious state in lactating rats. Horm Behav, 84, 136–144. doi: 10.1016/j.yhbeh.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Lederis K, Letter A, McMaster D, Moore G, & Schlesinger D (1982). Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science, 218(4568), 162–165. doi: 10.1126/science.6981844 [DOI] [PubMed] [Google Scholar]
- Lee KW, Rhee JS, Raisuddin S, Gi Park H, & Lee JS (2008). A corticotropin-releasing hormone binding protein (CRH-BP) gene from the intertidal copepod, Tigriopus japonicus. Gen Comp Endocrinol, 158(1), 54–60. doi: 10.1016/j.ygcen.2008.05.002 [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, … Vale WW (2001). Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRH) family with high affinity for the CRH2 receptor. Proc Natl Acad Sci U S A, 98(13), 7570–7575. doi: 10.1073/pnas.121165198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw CW, Lovenberg TW, Barry G, Oltersdorf T, Grigoriadis DE, & De Souza EB (1996). Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology, 137(1):72–7. doi: 10.1210/endo.137.1.8536644 [DOI] [PubMed] [Google Scholar]
- Liu L, Yu X, Meng F, Guo X, & Xu B (2011). Identification and characterization of a novel corticotropin-releasing hormone-binding protein (CRH-BP) gene from Chinese honeybee (Apis cerana cerana). Arch Insect Biochem Physiol, 78(3), 161–175. doi: 10.1002/arch.20451 [DOI] [PubMed] [Google Scholar]
- Lombardo KA, Herringa RJ, Balachandran JS, Hsu DT, Bakshi VP, Roseboom PH, & Kalin NH (2001). Effects of acute and repeated restraint stress on corticotropin-releasing hormone binding protein mRNA in rat amygdala and dorsal hippocampus. Neurosci Lett, 302(2-3), 81–84. Doi: 10.1016/S0304-3940(01)01680-9 [DOI] [PubMed] [Google Scholar]
- Lopez-Olmeda JF, Blanco-Vives B, Pujante IM, Wunderink YS, Mancera JM, & Sanchez-Vazquez FJ (2013). Daily rhythms in the hypothalamus-pituitary-interrenal axis and acute stress responses in a teleost flatfish, Solea senegalensis. Chronobiol Int, 30(4), 530–539. doi: 10.3109/07420528.2012.754448 [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Aubry JM, Turnbull A, Sutton S, Potter E, Yehling J, … Vale WW (1998). Ectopic expression of the CRH-binding protein: minor impact on HPA axis regulation but induction of sexually dimorphic weight gain. J Neuroendocrinol, 10(7), 483–491. doi: 10.1046/j.1365-2826.1998.00206.x [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, & Barsyte-Lovejoy D (2010). Characterization of a corticotropin-releasing factor (CRH)/diuretic hormone-like peptide from tunicates: insight into the origins of the vertebrate CRH family. Gen Comp Endocrinol, 165(2), 330–336. doi: 10.1016/j.ygcen.2009.07.013 [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Chang BS, Lovejoy NR, & del Castillo J (2014). Molecular evolution of GPCRs: CRH/CRH receptors. J Mol Endocrinol, 52(3), T43–60. doi: 10.1530/JME-13-0238 [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, & Oltersdorf T (1995). Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A, 92(3), 836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski D, Crowe PD, De Souza EB, & Behan DP (1996). Regulation of corticotropin-releasing factor-binding protein expression in cultured rat astrocytes. J Pharmacol Exp Ther, 278(2), 455–461. https://www.ncbi.nlm.nih.gov/pubmed/8768691 [PubMed] [Google Scholar]
- Madaro A, Olsen RE, Kristiansen TS, Ebbesson LO, Nilsen TO, Flik G, & Gorissen M (2015). Stress in Atlantic salmon: response to unpredictable chronic stress. J Exp Biol, 218(Pt 16), 2538–2550. doi: 10.1242/jeb.120535 [DOI] [PubMed] [Google Scholar]
- Mancera JM, Fernandez-Llebrez P, Grondona JM, & Perez-Figares JM (1993). Influence of environmental salinity on prolactin and corticotropic cells in the gilthead sea bream (Sparus aurata L.). Gen Comp Endocrinol, 90(2), 220–231. doi: 10.1006/gcen.1993.1077 [DOI] [PubMed] [Google Scholar]
- Mano-Otagiri A, Nemoto T, Yamauchi N, Kakinuma Y, & Shibasaki T (2016). Distribution of corticotropin-releasing factor type 1 receptor-like immunoreactivity in the rat pituitary. J Neuroendocrinol. doi: 10.1111/jne.12440 [DOI] [PubMed] [Google Scholar]
- Manuel R, Gorissen M, Zethof J, Ebbesson LO, van de Vis H, Flik G, & van den Bos R (2014). Unpredictable chronic stress decreases inhibitory avoidance learning in Tuebingen long-fin zebrafish: stronger effects in the resting phase than in the active phase. J Exp Biol, 217(Pt 21), 3919–3928. doi: 10.1242/jeb.109736 [DOI] [PubMed] [Google Scholar]
- Martos-Sitcha JA, Wunderink YS, Straatjes J, Skrzynska AK, Mancera JM, & Martinez-Rodriguez G (2014). Different stressors induce differential responses of the CRH-stress system in the gilthead sea bream (Sparus aurata). Comp Biochem Physiol A Mol Integr Physiol, 177, 49–61. doi: 10.1016/j.cbpa.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Mazon AF, Verburg-van Kemenade BM, Flik G, & Huising MO (2006). Corticotropin-releasing hormone-receptor 1 (CRH-R1) and CRH-binding protein (CRH-BP) are expressed in the gills and skin of common carp Cyprinus carpio L. and respond to acute stress and infection. J Exp Biol, 209(Pt 3), 510–517. doi: 10.1242/jeb.01973 [DOI] [PubMed] [Google Scholar]
- McClennen SJ, Cortright DN, & Seasholtz AF (1998). Regulation of pituitary corticotropin-releasing hormone-binding protein messenger ribonucleic acid levels by restraint stress and adrenalectomy. Endocrinology, 139(11), 4435–4441. doi: 10.1210/endo.139.11.6311 [DOI] [PubMed] [Google Scholar]
- McClennen SJ, & Seasholtz AF (1999). Transcriptional regulation of corticotropin-releasing hormone-binding protein gene expression in astrocyte cultures. Endocrinology, 140(9), 4095–4103. doi: 10.1210/endo.140.9.6978 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol, 583(2-3), 174–185. doi: 10.1016/j.ejphar.2007.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltesen M, Laursen DC, Thornqvist PO, Andersson MA, Winberg S, & Hoglund E (2016). Effects of acute and chronic stress on telencephalic neurochemistry and gene expression in rainbow trout (Oncorhynchus mykiss). J Exp Biol, 219(Pt 24), 3907–3914. doi: 10.1242/jeb.139857 [DOI] [PubMed] [Google Scholar]
- Montecucchi PC, & Henschen A (1981). Amino acid composition and sequence analysis of sauvagine, a new active peptide from the skin of Phyllomedusa sauvagei. Int J Pept Protein Res, 18(2), 113–120. DOI: 10.1111/j.1399-3011.1981.tb02047.x [DOI] [PubMed] [Google Scholar]
- Mulchahey JJ, Regmi A, Sheriff S, Balasubramaniam A, & Kasckow JW (1999). Coordinate and divergent regulation of corticotropin-releasing factor (CRH) and CRH-binding protein expression in an immortalized amygdalar neuronal cell line. Endocrinology, 140(1), 251–259. doi: 10.1210/endo.140.1.6406 [DOI] [PubMed] [Google Scholar]
- Okawara Y, Morley SD, Burzio LO, Zwiers H, Lederis K, & Richter D (1988). Cloning and sequence analysis of cDNA for corticotropin-releasing factor precursor from the teleost fish Catostomus commersoni. Proc Natl Acad Sci U S A, 85(22), 8439–8443. Doi: 10.1073/pnas.85.22.8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth DN, & Mount CD (1987). Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma. Biochem Biophys Res Commun, 143(2), 411–417. doi: 10.1016/0006-291X(87)91369-6 [DOI] [PubMed] [Google Scholar]
- Owens MJ, & Nemeroff CB (1991). Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev, 43(4), 425–473. https://www.ncbi.nlm.nih.gov/pubmed/1775506 [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, … Vale W (1995). Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A, 92(7), 2969–2973. doi: 10.1073/pnas.92.7.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, & Vale WW (1993). Cloning and functional expression of a rat brain corticotropin releasing factor (CRH) receptor. Endocrinology, 133(6), 3058–3061. doi: 10.1210/endo.133.6.8243338 [DOI] [PubMed] [Google Scholar]
- Peto CA, Arias C, Vale WW, and Sawchenko PR (1999) Ultrastructural localization of the corticotropin-releasing factor-binding protein in rat brain and pituitary. J. Comp. Neurol 413:241–254. doi: [DOI] [PubMed] [Google Scholar]
- Petraglia F, Potter E, Cameron VA, Sutton S, Behan DP, Woods RJ, … Vale W (1993). Corticotropin-releasing factor-binding protein is produced by human placenta and intrauterine tissues. J Clin Endocrinol Metab, 77(4), 919–924. doi: 10.1210/jcem.77.4.8408466 [DOI] [PubMed] [Google Scholar]
- Pijanowski L, Jurecka P, Irnazarow I, Kepka M, Szwejser E, Verburg-van Kemenade BM, & Chadzinska M (2015). Activity of the hypothalamus-pituitary-interrenal axis (HPI axis) and immune response in carp lines with different susceptibility to disease. Fish Physiol Biochem, 41(5), 1261–1278. doi: 10.1007/s10695-015-0084-3 [DOI] [PubMed] [Google Scholar]
- Pisarska M, Mulchahey JJ, Welge JA, Geracioti TD Jr., & Kasckow JW (2000). Age-related alterations in emotional behaviors and amygdalar corticotropin-releasing factor (CRH) and CRH-binding protein expression in aged Fischer 344 rats. Brain Res, 877(2), 184–190. doi: 10.1016/S0006-8993(00)02606-8 [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, & Vale WW (1991). Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature, 349(6308), 423–426. doi: 10.1038/349423a0 [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, & Vale WW (1992). The central distribution of a corticotropin-releasing factor (CRH)-binding protein predicts multiple sites and modes of interaction with CRH. Proc Natl Acad Sci U S A, 89(9), 4192–4196. doi: 10.1073/pnas.89.9.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, … Sawchenko PE (2001). Urocortin II: a member of the corticotropin-releasing factor (CRH) neuropeptide family that is selectively bound by type 2 CRH receptors. Proc Natl Acad Sci U S A, 98(5), 2843–2848. doi: 10.1073/pnas.051626398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Bakshi VP, Trentani A, Newman SM, & Kalin NH (2007). Predator threat induces behavioral inhibition, pituitary-adrenal activation and changes in amygdala CRH-binding protein gene expression. Psychoneuroendocrinology, 32(1), 44–55. doi: 10.1016/j.psyneuen.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA (2011). Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res, 35(1), 166–74. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Ribbe K, Ackermann V, Schwitulla J, Begemann M, Papiol S, Grube S, … & Ehrenreich H (2011). Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Arch Gen Psychiatry, 68(12), 1247–56. Doi: 10.1001/archgenpsychiatry.2011.100. [DOI] [PubMed] [Google Scholar]
- Roy A, Hodgkinson CA, Deluca V, Goldman D, & Enoch MA (2012). Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res, 46(1), 72–79. doi : 10.1016/j.jpsychires.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Sato N, Suzuki N, Kano M, Tanaka Y, Kanazawa M, …, & Fukudo S (2016). Associations between Single-Nucleotide Polymorphisms in Corticotropin-Releasing Hormone-Related Genes and Irritable Bowel Syndrome. PLoS One, 11(2):e0149322. doi: 10.1371/journal.pone.0149322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh HI, Kryski KR, Smith HJ, Hayden EP, & Singh SM (2013). Corticotropin-releasing hormone system polymorphisms are associated with children’s cortisol reactivity. Neuroscience, 229, 1–11. doi: 10.1016/j.neuroscience.2012.10.056 [DOI] [PubMed] [Google Scholar]
- Slater PG, Cerda CA, Pereira LA, Andres ME, & Gysling K (2016). CRF binding protein facilitates the presence of CRF type 2alpha receptor on the cell surface. Proc Natl Acad Sci U S A, 113(15), 4075–5080. doi: 10.1073/pnas.1523745113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert DB, McClennen SJ, & Seasholtz AF (2002). Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology, 143(12), 4730–4741. doi: 10.1210/en.2002-220556 [DOI] [PubMed] [Google Scholar]
- Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, & Stenzel-Poore MP (1995). Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol Endocrinol, 9(5), 637–645. doi: 10.1210/mend.9.5.7565810 [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heldwein KA, Stenzel P, Lee S, & Vale WW (1992). Characterization of the genomic corticotropin-releasing factor (CRH) gene from Xenopus laevis: two members of the CRH family exist in amphibians. Mol Endocrinol, 6(10), 1716–1724. doi: 10.1210/mend.6.10.1448118 [DOI] [PubMed] [Google Scholar]
- Stinnett GS, Westphal NJ, & Seasholtz AF (2015). Pituitary CRH-binding protein and stress in female mice. Physiol Behav, 150, 16–23. doi: 10.1016/j.physbeh.2015.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Iwashita M, Tozawa F, Ushiyama T, Tomori N, Sumitomo T, … Shizume K (1988). Characterization of corticotropin-releasing hormone binding protein in human plasma by chemical cross-linking and its binding during pregnancy. J Clin Endocrinol Metab, 67(6), 1278–1283. doi: 10.1210/jcem-67-6-1278 [DOI] [PubMed] [Google Scholar]
- Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, … Vale WW (1995). Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology, 136(3), 1097–1102. doi: 10.1210/endo.136.3.7867564 [DOI] [PubMed] [Google Scholar]
- Tartter MA, & Ray LA. (2012). A prospective study of stress and alcohol craving in heavy drinkers. Pharmacol Biochem Behav, 101(4), 625–31. doi: 10.1016/j.pbb.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Deshaies Y, Picard F, & Richard D (1999). Corticotropin-releasing hormone-binding protein in brain and pituitary of food-deprived obese (fa/fa) Zucker rats. Am J Physiol, 277(6 Pt 2), R1749–1759. https://www.ncbi.nlm.nih.gov/pubmed/10600923 [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Huang Q, & Richard D (2003). Effects of treadmill running on brain activation and the corticotropin-releasing hormone system. Neuroendocrinology, 77(6), 388–405. doi: 10.1159/000071311 [DOI] [PubMed] [Google Scholar]
- Trainer PJ, Woods RJ, Korbonits M, Popovic V, Stewart PM, Lowry PJ, & Grossman AB (1998). The pathophysiology of circulating corticotropin-releasing hormone-binding protein levels in the human. J Clin Endocrinol Metab, 83(5), 1611–1614. doi: 10.1210/jcem.83.5.4751 [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, & Bonci A (2003).Corticotropin-releasing factor requires CRH binding protein to potentiate NMDA receptors via CRH receptor 2 in dopamine neurons. Neuron, 39(3), 401–407. doi: 10.1016/S0896-6273(03)00461-6 [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, & Rivier J (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213(4514), 1394–1397.doi: 10.1126/science.6267699 [DOI] [PubMed] [Google Scholar]
- Valverde RA, Seasholtz AF, Cortright DN, & Denver RJ (2001). Biochemical characterization and expression analysis of the Xenopus laevis corticotropin-releasing hormone binding protein. Mol Cell Endocrinol, 173(1-2), 29–40. doi: 10.1016/S0303-7207(00)00437-8 [DOI] [PubMed] [Google Scholar]
- Van Den Eede F, Venken T, Del-Favero J, Norrback KF, Souery D, Nilsson LG, … , & Claes SJ (2007). Single nucleotide polymorphism analysis of corticotropin-releasing factor-binding protein gene in recurrent major depressive disorder. Psychiatry Res, 753(1), 17–25. doi: 10.1016/j.psychres.2006.12.018 [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, … Sawchenko PE (2000). Distribution of mRNAs encoding CRH receptors in brain and pituitary of rat and mouse. J Comp Neurol, 428(2), 191–212. DOI: [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, … Vale W (1995). Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature, 378(6554), 287–292. doi: 10.1038/378287a0 [DOI] [PubMed] [Google Scholar]
- Vera LM, Montoya A, Pujante IM, Perez-Sanchez J, Calduch-Giner JA, Mancera JM, … Sanchez-Vazquez FJ (2014). Acute stress response in gilthead sea bream (Sparus aurata L.) is time-of-day dependent: Physiological and oxidative stress indicators. Chronobiol Int, 31(9), 1051–1061. doi: 10.3109/07420528.2014.945646 [DOI] [PubMed] [Google Scholar]
- Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, … Ferrara P (1993). Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett, 335(1), 1–5. doi: 10.1016/0014-5793(93)80427-V [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, & Wise RA (2007). Stress-induced relapse to cocaine seeking: roles for the CRH(2) receptor and CRH-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl), 193(2), 283–294. doi: 10.1007/s00213-007-0782-3 [DOI] [PubMed] [Google Scholar]
- Wang Z, & Brown DD (1993). Thyroid hormone-induced gene expression program for amphibian tail resorption. J Biol Chem, 268(22), 16270–16278. http://www.jbc.org/content/268/22/16270.long [PubMed] [Google Scholar]
- Westphal NJ, Evans RT, & Seasholtz AF (2009). Novel expression of type 1 corticotropin-releasing hormone receptor in multiple endocrine cell types in the murine anterior pituitary. Endocrinology, 150(1), 260–267. doi: 10.1210/en.2008-0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal NJ, & Seasholtz AF (2005). Gonadotropin-releasing hormone (GnRH) positively regulates corticotropin-releasing hormone-binding protein expression via multiple intracellular signaling pathways and a multipartite GnRH response element in alphaT3-1 cells. Mol Endocrinol, 19(11), 2780–97.doi: 10.1210/me.2004-0519 [DOI] [PubMed] [Google Scholar]
- Westphal NJ, & Seasholtz AF (2006). CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci, 11, 1878–1891 [DOI] [PubMed] [Google Scholar]
- Wolff JO (1985). Maternal aggression as a deterrent to infanticide in Peromyscus leucopis and P. maniculatus. Animal Behaviour 33, L117–123. doi: 10.1016/S0003-3472(85)80125-1 [DOI] [Google Scholar]
- Wolff JO, & Peterson JA (1998). An offspring-defense hypothesis for territoriality in female mammals. Ethol Ecol Evol 10, 227–239. doi: 10.1080/08927014.1998.9522854 [DOI] [Google Scholar]
- Wunderink YS, Engels S, Halm S, Yufera M, Martinez-Rodriguez G, Flik G, … Mancera JM (2011). Chronic and acute stress responses in Senegalese sole (Solea senegalensis): the involvement of cortisol, CRH and CRH-BP. Gen Comp Endocrinol, 171(2), 203–210. doi: 10.1016/j.ygcen.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Wunderink YS, Martinez-Rodriguez G, Yufera M, Montero IM, Flik G, Mancera JM, & Klaren PH (2012). Food deprivation induces chronic stress and affects thyroid hormone metabolism in Senegalese sole (Solea senegalensis) post-larvae. Comp Biochem Physiol A Mol Integr Physiol, 162(4), 317–322. doi: 10.1016/j.cbpa.2012.03.023 [DOI] [PubMed] [Google Scholar]
- Zohar I, & Weinstock M (2011). Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol, 23(4), 320–328. doi: 10.1111/j.1365-2826.2011.02117.x [DOI] [PubMed] [Google Scholar]


