Abstract
Introduction
Radiolabeled ligands for fibrillar amyloid beta (Aβ) peptides are used in positron emission tomography (PET) for dementia diagnosis. Current ligands do not discriminate parenchymal amyloid plaques from cerebral amyloid angiopathy (CAA).
Methods
We undertook neuropathological examination of 65 older people (81.6 ± 7.96 (mean ± SD) years, 27F/38M): 15 with neuropathological diagnosis of AD, 25 with neuropathological diagnosis of other neurodegenerative dementias (Lewy body dementia and Parkinson disease dementia), and 25 without significant neurodegenerative pathology.
Results
We observed CAA in non‐Alzheimer's dementia (non‐AD dementia) and control brains, of comparable extent to those with neuropathologically confirmed AD. Aβ‐positive vessel density did not differ significantly between non‐AD dementia and control groups. Across all subjects there was a highly significant correlation between vessel Aβ40 density and vessel Aβ42 density (Spearman rho = 0.855, P < .001). CAA was absent or sparse in subcortical white matter across all patient groups.
Conclusion
Our data indicate that CAA can be abundant in non‐AD brains and raise a cautionary note regarding interpretation of amyloid PET imaging.
Keywords: Alzheimer's disease, amyloid PET, cerebral amyloid angiopathy
1. INTRODUCTION
Deposits of amyloid beta (Aβ) peptides in brain tissue, described as amyloid plaques, are a cardinal feature of Alzheimer's disease (AD) neuropathology. They are frequently accompanied by deposits of Aβ in the wall of small penetrating arteries, reported as cerebral amyloid angiopathy 1 , 2 (CAA; Figure 1).
FIGURE 1.
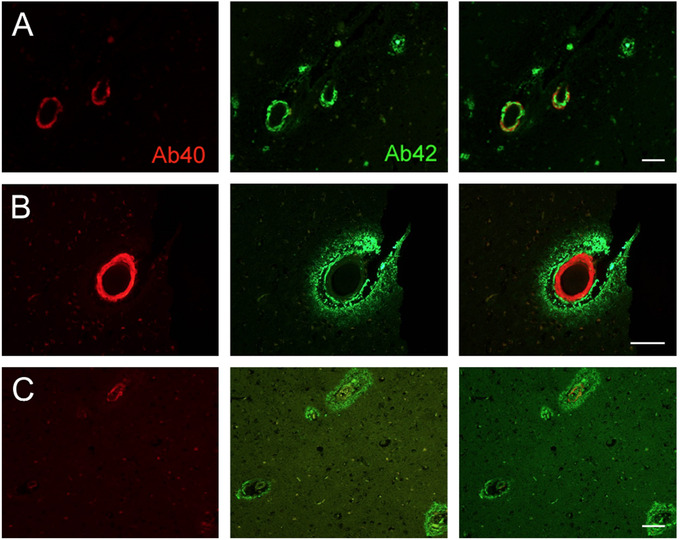
Cerebral amyloid angiopathy (CAA)–positive vessels. Examples of CAA‐positive vessels in a Control case (A; female, age 88 years), non–Alzheimer's disease (AD) neurodegenerative dementia (B; Lewy body dementia), and neuropathologically defined AD (C). Left column shows immunolabeling for amyloid beta (Aβ)40 (red), middle column for Aβ42 (green), and rightmost column merged images for both markers. Lower magnification views in A and C show examples of density of CAA‐positive vessels. Scale bar: 100 microns
Radioligands for Aβ are used in positron emission tomography (PET) to detect cerebral amyloid in living patients. 1 , 3 Currently available Aβ‐PET ligands do not discriminate amyloid plaques from CAA. In addition, it has recently been recognized that Aβ‐PET ligands bind to myelin with substantial affinity. 4 Thus Aβ‐PET has the potential to detect white matter myelin integrity. 4 In view of the diagnostic importance placed on brain Aβ‐PET, we report on the pathological distribution of CAA.
2. METHODS
We examined donated frontal cerebral cortical tissue of 65 older people, derived from the UK Brains for Dementia Research network (https://bdr.alzheimersresearchuk.org/researchers/): 15 with neuropathological diagnosis of AD (age 82.9 ± 4.76 [mean ± SD] years, 8F/7M), 25 with neuropathological diagnosis of other neurodegenerative dementias (Lewy body dementia and Parkinson disease dementia: 80.5 ± 7.05 years, 9F/16M), and 25 without significant neurodegenerative pathology (controls: 82.0 ± 10.2 years, 10F/15M). Formalin‐ fixed, paraffin‐embedded sections underwent immunofluorescent labeling with well‐ characterized antibodies 5 specific for the C‐terminal neoepitopes of Aβ40 and Aβ42. Labeled sections were examined in random order by two independent observers (JMRF, DRH) blinded to diagnoses (For further Methods, see Supplementary file and Figure S1).
3. RESULTS
Among cases with neuropathologically confirmed AD, a significant density of CAA was present in most cases (87% with Aβ42 positive CAA, 93% with Aβ40 positive CAA) (Figure 2). Significant density of CAA was also seen in a substantial fraction of non‐AD dementia cases (34% with Aβ42 positive CAA, 51% Aβ40 positive CAA) and in control subjects (20% Aβ42, 40% Aβ40). In each group, some individuals had undetectable or very little CAA (Figure 2). Vessel Aβ40 was observed with higher density (positive vessels/cm2) in AD subjects compared to non‐AD dementia subjects or controls (P = .006, P = .001, respectively; independent‐sample Kruskal‐Wallis test; Figure 2). Aβ42 vessel density was also higher in AD relative to non‐AD dementia or controls (P = .011, P = .002). Aβ‐positive vessel density did not differ significantly between non‐AD and control groups (Figure 2). Across all subjects there was a highly significant correlation between vessel Aβ40 and vessel Aβ42 density (Spearman rho = 0.855, P < .001). Across all patient groups, CAA was undetectable or very sparse in subcortical white matter.
FIGURE 2.
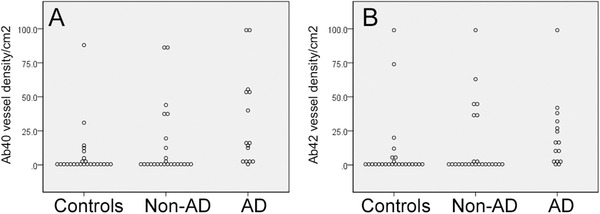
Cerebral amyloid angiopathy (CAA) is not specific to Alzheimer's disease (AD). Density of small arterial vessels positive for amyloid beta (Aβ)40 (A) or Aβ42 (B) in older people without neurodegenerative pathology (Controls), with neurodegenerative dementia other than AD (Non‐AD), or with neuropathologically confirmed AD
RESEARCH IN CONTEXT
Systematic review: Currently available amyloid beta (Aβ) positron emission tomography (PET) ligands do not discriminate cerebral amyloid plaques from amyloid angiopathy. It has recently been recognized that these ligands bind to myelin with substantial affinity.
Interpretation: We examined donated frontal cerebral cortical tissue of 65 older people: 15 with neuropathological diagnosis of Alzheimer's disease (AD), 25 with neuropathological diagnosis of other neurodegenerative dementias (Lewy body dementia and Parkinson disease dementia), and 25 without significant neurodegenerative pathology. We observed cerebral amyloid angiopathy (CAA) in non‐AD and control brains, of comparable extent to those with neuropathologically confirmed AD. CAA was absent or sparse in subcortical white matter across all patient groups. These observations support the utilization of amyloid PET ligands as reporters on white matter integrity.
Future directions: Better identification of neuropathological targets for PET ligands.
4. DISCUSSION
These data emphasize that CAA can be abundant in non‐AD and control brains. Although Aβ‐positive vessel density was higher in AD patients compared to non‐AD or controls, CAA was by no means restricted to individuals with AD pathology. This replicates existing evidence that CAA is frequently found at autopsy in elderly individuals with or without dementia. 6 , 7 , 8 , 9 Conversely, we found CAA‐negative individuals in each patient group including AD. This suggests that the pathological processes leading to CAA are non‐identical to those causing parenchymal Aβ deposition, likely including vascular clearance pathways. 10 Our data reinforce the concept that Aβ‐PET positivity may not be specific to AD. Novel PET ligands, with pharmacological or pharmacokinetic selectivity for parenchymal versus vascular Aβ deposits, 1 , 3 may permit more‐refined patient stratification.
Our data confirm the sparseness of CAA in subcortical white matter. 2 We concur with an earlier neuropathological report that “vessels in subcortical and deep white matter were invariably spared” by CAA. 2 Aβ‐PET ligands have been used by several groups as non‐specific ligands for myelin and are hence biomarkers for white matter myelin density. 4 , 11 , 12 , 13 , 14 , 15 , 16 We suggest that CAA is unlikely to confound this approach because CAA is essentially absent from subcortical white matter. On the other hand, Aβ‐positive CAA may be an initial or secondary histopathological pattern of AD as a result of impaired perivascular clearance (clearance pathways overloaded with amyloid, for example, by mutations leading to amyloid overproduction), or inefficient amyloid transport across the blood‐brain barrier (eg, by insufficient LRP1 transporter). 17 , 18 , 19 Therefore, a positive amyloid PET in a patient with Aβ+ CAA may still indicate at least a risk for AD. 20
This study has several limitations. Notably, we do not have in‐life Aβ‐PET data for these cases, or details of apolipoprotein E (APOE) genotype. However, our data emphasize that caution is necessary in interpreting PET findings. CAA positivity is not specific to AD but the degree of brain Aβ‐PET positivity (eg, CAA positive density) may be a distinguishing factor between neurodegenerative diseases. Further studies are required to clarify Aβ‐PET usefulness in clinical settings.
CONSENT
Human Tissue Ethical Approval. Brains for Dementia Research was established as a Research Tissue Bank following approval by the UK National Research Ethics Service. All brain samples were stored in established brain banks under license from the UK Human Tissue Authority.
FINANCIAL DISCLOSURES
None.
Supporting information
Supporting Information
ACKNOWLEDGMENT
We thank the tissue donors and their families. We are grateful to Professor Margaret Esiri for helpful discussions.
Alakbarzade V, French JM, Howlett DR, et al. Cerebral amyloid angiopathy distribution in older people: A cautionary note. Alzheimer's Dement. 2021;7:e12145 10.1002/trc2.12145
REFERENCES
- 1. Abrahamson EE, Stehouwer JS, Vazquez AL, et al. Development of a PET radioligand selective for cerebral amyloid angiopathy. Nucl Med Biol. 2020;S0969‐8051(20):30030‐30035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esiri MM, Wilcock GK. Cerebral amyloid angiopathy in dementia and old age. J Neurol Neurosurg Psychiatry. 1986;49:1221‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beach TG, Maarouf CL, Intorcia A, et al. Antemortem‐postmortem correlation of Florbetapir (18F) PET amyloid imaging with quantitative biochemical measures of Abeta42 but not Abeta40. J Alzheimers Dis. 2018;61:1509‐1516. [DOI] [PubMed] [Google Scholar]
- 4. Auvity S, Tonietto M, Caille F, et al. Repurposing radiotracers for myelin imaging: a study comparing 18F‐florbetaben, 18F‐florbetapir, 18F‐flutemetamol,11C‐MeDAS, and 11C‐PiB. Eur J Nucl Med Mol Imaging. 2020;47:490‐501. [DOI] [PubMed] [Google Scholar]
- 5. Howlett DR, Hortobagyi T, Francis PT. Clusterin associates specifically with Abeta40 in Alzheimer's disease brain tissue. Brain Pathol. 2013;23:623‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu D, Yang C, Wang L. Cerebral amyloid angiopathy in aged Chinese: a clinico‐neuropathological study. Acta Neuropathol. 2003;106:89‐91. [DOI] [PubMed] [Google Scholar]
- 7. Bertrand E, Lewandowska E, Stepien T, Szpak GM, Pasennik E, Modzelewska J. Amyloid angiopathy in idiopathic Parkinson's disease. Immunohistochemical and ultrastructural study. Folia Neuropathol. 2008;46:255‐270. [PubMed] [Google Scholar]
- 8. Jellinger KA, Attems J. Cerebral amyloid angiopathy in lewy body disease. J Neural Transm (Vienna). 2008;115:473‐482. [DOI] [PubMed] [Google Scholar]
- 9. Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carare RO, Aldea R, Agarwal N, et al. Clearance of Interstitial Fluid (ISF) and CSF (CLIC) group‐part of vascular Professional Interest Area (PIA): cerebrovascular disease and the failure of elimination of Amyloid‐beta from the brain and retina with age and Alzheimer's disease‐opportunities for therapy. Alzheimers Dement (Amst). 2020;12:e12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glenner GG, Eanes ED, Page DL. The relation of the properties of congo red‐stained amyloid fibrils to the ‐conformation. J Histochem Cytochem. 1972;20:821‐826. [DOI] [PubMed] [Google Scholar]
- 12. Klunk WE, Pettegrew JW, Abraham DJ. Quantitative evaluation of congo red binding to amyloid‐like proteins with a beta‐pleated sheet conformation. J Histochem Cytochem. 1989;37:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 13. Ridsdale RA, Beniac DR, Tompkins TA, Moscarello MA, Harauz G. Three‐dimensional structure of myelin basic protein. II. Molecular modeling and considerations of predicted structures in multiple sclerosis. J Biol Chem. 1997;272:4269‐4275. [DOI] [PubMed] [Google Scholar]
- 14. Stankoff B, Wang Y, Bottlaender M, et al. Imaging of CNS myelin by positron‐emission tomography. Proc Natl Acad Sci U S A. 2006;103:9304‐9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bajaj A, LaPlante NE, Cotero VE, et al. Identification of the protein target of myelin‐binding ligands by immunohistochemistry and biochemical analyses. J Histochem Cytochem. 2013;61:19‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lockhart A, Lamb JR, Osredkar T, et al. PIB is a non‐specific imaging marker of amyloid‐beta (Abeta) peptide‐related cerebral amyloidosis. Brain. 2007;130:2607‐2615. [DOI] [PubMed] [Google Scholar]
- 17. Deane R, Sagare A, Hamm K, et al. ApoE isoform‐specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002‐4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer's amyloid‐ss(1‐40) peptide from brain by LDL receptor‐related protein‐1 at the blood‐brain barrier. J Clin Invest. 2000;106:1489‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular‐mediated clearance of brain amyloid beta in Alzheimer's disease: the role, regulation and restoration of LRP1. Front Aging Neurosci. 2015;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Assema DM, Lubberink M, Bauer M, et al. Blood‐brain barrier P‐glycoprotein function in Alzheimer's disease. Brain. 2012;135:181‐189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


