Abstract
Almost two decades ago strain and strain rate imaging were proposed as a new, potentially more sensitive modality for quantifying both regional and global myocardial function. Until now, however, strain and strain rate imaging have been slow to be incorporated into everyday clinical practice. More recently, two dimensional strain has been claimed as of greater clinical utility, given that it is angle independent, with improved feasibility and reproducibility as compared to tissue Doppler strain. Nevertheless, speckle tracking strain is reliant on 2D image quality and frame rates. Three dimensional speckle tracking could eliminate the problem of through-plane motion inherent in 2D imaging, but 3D strain is currently limited by low frame rates. Another limitation of strain imaging is that the results are dependent on the ultrasound machine on which analyses are performed, with variability in measurements between different vendors. Despite the diagnostic and prognostic advantages of 2D strain, there is a lack of specific therapeutic interventions based on strain and a paucity of long-term large-scale randomized trial evidence on cardiovascular outcomes. After overabundant literature the same definition of normal cut-off values is controversial and not univocal. Further studies are needed, involving both manufacturers and medical professionals, on the additive contribution, possibly different case by case, of interfering and artifactual factors, aside from myocardial function per se. These artifactual determinants and motion artifacts components could be dominant in individual cases and should always be taken into account in the clinical decision making process in a single case.
Keywords: ventricular function, strain and strain rate echocardiography, global and regional function, non-invasive cardiac function by ultrasound
Introduction
Almost two decades ago strain and strain rate imaging were proposed as a new, potentially more sensitive modality for quantifying both regional and global myocardial function [1]. After a decade, however, the clinical role of strain imaging in echocardiography was still considered as “emerging” [2]. In fact, despite significant promise, strain and strain rate imaging were reported as technically challenging, with a signal to noise ratio potentially affected by a wide range of factors [2]. As a result, strain and strain rate imaging have been very slow to be incorporated into everyday clinical practice [2]. Despite the reported diagnostic and prognostic advantages of strain values, there is also a lack of specific therapeutic interventions based on strain and a paucity of long-term large-scale randomized trial evidence on cardiovascular outcomes in different physiological conditions and cardiac diseases, including acute and chronic coronary artery atherosclerotic diseases [3–28]. On the other hand, incorporation of 2D Global Longitudinal Strain (GLS) in routine patient evaluation and clinical decision making is reported to be imminent [3].
Strain and strain rate imaging
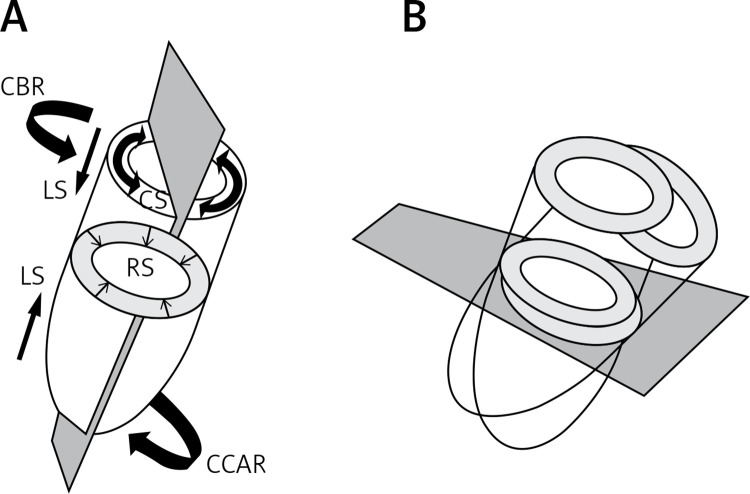
The left ventricle works by contraction and relaxation of muscular fibers organized in layers [3]. Left ventricular subendocardial and subepicardial fibers are arranged longitudinally, forming a spiral around the ventricle. Subepicardial fibers are oriented clockwise, subendocardial counterclockwise, while mid myocardial fibers are oriented in a circumferential manner [3]. The contraction and relaxation of these various groups of fibers results in a complex “deformation” of the LV myocardium in systole and diastole. Echocardiographic strain imaging can quantify this “deformation” by assessing regional and global myocardial function through quantification of longitudinal, radial and circumferential contraction, and basal and apical rotation, from which twisting and untwisting functions are calculated [3, 5, 9] (Figures 1 A, B).
Figure 1.
A – Schematic representation of longitudinal, radial and circumferential strain. Basal and apical rotation are also depicted, from which twisting and untwisting are calculated. Echocardiographic apical and short axis imaging planes are represented in gray. B – While the imaging plane is maintained in a fixed position, the heart is moving through the plane during the cardiac cycle, due to contraction, relaxation, translational, rocking or swinging motion, or even dyssynchrony (LS – longitudinal strain, RS – radial strain, CS – circumferential strain, CBR – clockwise basal rotation, CCAR – counter clockwise apical rotation)
Strain (ε) is a dimensionless measure of tissue deformation calculated as, ε = (L – L0)/L0, where L is the final length and L0 the original length. Strain is negative with shortening and positive with lengthening. As the LV contracts, myocardial fibers shorten in the longitudinal and circumferential plane (i.e. negative strain) and thicken or lengthen in the radial direction (positive strain). Strain rate (unit s–1) is the rate at which this deformation occurs, i.e. change in velocity between two points divided by distance between the points [3]. At first Doppler derived strain values were obtained [3]. More recently two dimensional strain has been claimed as of greater clinical utility, given that it is angle independent and has improved feasibility and reproducibility compared to tissue Doppler strain [3]. Nevertheless, speckle tracking strain is reliant on 2D image quality and frame rates. Three dimensional speckle tracking could eliminate the problem of through-plane motion inherent in 2D imaging, but 3D strain is currently limited by low frame rates [3]. Results of strain imaging also depend on the ultrasound machine on which analyses are performed, with variability in measurements between different vendors [4, 6–8, 10, 17, 19, 20, 29, 30].
Why is clinical utility of strain and strain rate imaging so widely suggested, but its clinical impact in a single case is still so uncertain?
One possibility is that intrinsic technical limitations have been underestimated. A basic assumption underlying 2D speckle tracking is that in-plane displacements of tissue correspond to the displacements of local patterns in the gray scale distribution of a 2D echocardiographic clip [5]. However, it should be appreciated that this may not always be the case. For example, through-plane displacement of a tapering, helically structured or otherwise obliquely angulated form could be misinterpreted, both visually and by speckle tracking, as in-plane deformation or displacement in a 2D sequence of images [5] (Figure 1 B). This off-plane limitation issue is known to be more critical in short axis than in apical views. In fact, speckle tracking works in general better along the ultrasound beam than across beams. This is supported by the fact that in children GLS agreement between vendors was more robust (intraclass correlation coefficient = 0.88–0.9) than global circumferential strain (GCS) (ICC = 0.75–0.82), and this difference is exaggerated in specific subanalyses [6]. GLS at age < 3 years was generally associated with lower intervendor agreement (ICC = 0.62–0.92 vs. 0.9–0.95 for > 3 years old). Intervendor agreement for GCS was also lower for patients < 3 years old (ICC, 0.67–0.94 vs. 0.78–0.86 for > 3 years old), and additionally GCS agreement was lower in the HR > 100 BPM subgroup compared with patients with HR < 100 BPM (ICC, 0.54–0.91 vs. 0.77–0.9, respectively). Both GLS and GCS agreements were lower in the EF > 55% subgroup [6]. Again, GCS appears to be a less robust measure than GLS even in the intrareader and interreader comparison [6–8]. Overall, the current LV twist data obtained during a variety of physiological perturbations show marked interstudy heterogeneity, which may tempt some researchers to conclude that measuring LV twist may not be of value in the clinical setting. Indeed, the current lack of normative values for LV twist and the untwisting rate in relation to the prevailing hemodynamic load prevents these markers from being used routinely as clinical indicators [9]. Furthermore, due to the beam divergence with increasing depth in a sector image, tracking across beams works better in regions close to the transducer than in the far field of the image [5]. Tracking quality may be suboptimal if regions of the myocardium are poorly visualized, if stationary image artifacts (reverberations) compromise speckle recognition or if spatial or temporal resolution of the image acquisition is insufficient [5].
3D speckle tracking
When suspicion of artifacts due to through-plane motion arises, 3D imaging could be used, if available, to avoid potential misinterpretation. The user should however take into account the fact that 3D speckle tracking has the same intervendor variability limitations that affect 2D speckle tracking and has lower temporal and spatial resolution than 2D imaging [5, 10]. Three-dimensional STE-derived measurements of myocardial deformation are highly dependent on the choice of both imaging equipment and analysis software, and the discordance levels are beyond the intrinsic measurement variability of any of the tested combinations of hardware and software [10–12]. It has also been reported that coronary tortuosity in apparently healthy individuals could affect strain parameters, but 3D derived LV GLS values showed only 81.3% specificity and a sensitivity as low as 56.7% in the ability to detect the presence of coronary tortuosity [13]. Nevertheless, the authors conclude that their results indicate that patients with coronary tortuosity may have subclinical LV longitudinal deformation abnormalities even if they are apparently healthy [13]. An even more complex 3D strain approach has been utilized for studying left atrial strain [14] and right ventricular strain [15]. For 3D left atrial strain the numerous parameters were mainly descriptive and difficult to implement in a clinical arena due to the great heterogeneity of the results [14]. In fact, there are too many strain parameters for which we do not know the real physiopathological meaning and which need further research [14].
For 3D right ventricular strain each value should be interpreted with caution while considering the loading condition of the patients [15]. For left ventricular dyssynchrony indices in three-dimensional speckle-tracking echocardiography, sub-optimal image quality compromised the reliability of 3D-STE derived dyssynchrony indices but did not introduce systematic bias in healthy individuals [16]. Even with optimal quality images, only 3D-STE indices based on volume, circumferential strain and principal tangential strain showed acceptable test-retest reliability [16].
Even with 2D-STE, assessment of dyssynchrony parameters was vendor specific and not applicable outside the context of the implemented platform [17]. We also have to remember that unless properly addressed, the intervendor discordance combined with the relatively low reproducibility and uncertain clinical meaning and utility in a particular case may hinder future dissemination of 3D STE [10]. However, it has been suggested that this problem could be solved in the future by developing standardized methodology via collaborative intervendor efforts that would eventually result in clinically useful, vendor-independent measurements [10].
Intervendor variability
The local frame-by-frame tracking is based on the search of maximum likelihood between two local speckle patterns in two consecutive frames. All kinds of ultrasound noise reduce the tracking quality. Good image quality enhances the clarity of speckle patterns and improves accuracy and robustness of their detection. It is therefore important to note that the acquisition of standardized image planes in optimized quality is essential for reducing inter- and intraobserver variability of tracking data [4, 5]. The most critical limitation in the tracking techniques is the temporal stability of tracking patterns [4, 5]. The ultrasound speckle patterns are generated by the interference of the ultrasound waves reflected from tissue structures. Speckle patterns are not stable temporally, not only due to through-plane motion, but also to physiological changes of living tissue structures and changes of interrogation angles between moving tissue and the ultrasonic beam [4, 5] (Figure 1 B). The accumulation of small random errors in the detection of speckle patterns along the tracking process can then lead to inaccurate tracking results [4, 5]. The general impression is that limitations have been declared, but no extensive research on the factors which can influence and affect the calculation of strain parameters has been performed. Intervendor variability is significant and difficult to be accepted by a clinician who has to deal with the clinical decision making in a single patient. From a physiological point of view it is also not so clear how we can explain such different results obtained by different machines which are in any case measuring the same parameters in the same patients. Obviously different software packages are proprietary for different vendors and not accessible to check how they work. It is thus not so clear which assumptions are at the base of software calculations for each vendor.
Standardization of technology has been extensively searched [4, 5, 18–20], but only GLS has found wider clinical acceptance. In contrast to GLS, RV or LV segmental or regional longitudinal strain measurements, or circumferential or radial strain, have a higher variability on top of the known intervendor bias [21–25]. Not only do the average strain values differ among vendors, but also the measurement variability is different [23, 26]. This leads to dramatic differences in the lower limits of normality for different companies [27]. Normal values for GLS depend on the definition of the measurement position in the myocardium, the vendor, and the version of the analysis software, resulting in considerable heterogeneity in the published literature [27]. Lang et al. reached a consensus, in the writing committee of the American Society of Echocardiography and the European Association of Cardiovascular Imaging, that differences among vendors and software packages are still too large to recommend universal normal values and lower limits of normal [27]. This could also be partly explained by regional nonuniformity of even the normal adult left ventricle [28]. Another difference among vendors is how their software is layer specific (i.e. subendocardium, mid wall and subepicardium) and how this can affect the behavior of speckle tracking, also taking into account that physiological interpretation of different layer specific results are not always so consistent and clinically meaningful [6, 29–31]. Aside from all this technology advancement it is interesting to find that an M-mode index based on mitral annular plane systolic excursion (MAPSE) is a reproducible and reliable measurement which can be used as a potential index in place of GLS at least in the critically ill population [32] and in aortic stenosis [33].
The definition of normal values
Another difficulty may derive from the definition of normal values and how this can interfere with the ability to detect subtle left ventricular systolic dysfunction. Normal GLS for most echocardiographic systems is reported between –18% and –25% in healthy individuals, variation which in part may be explained by inter-software and intervendor variability [34]. Technically, good recordings can be achieved along any axis, but interpretation of radial and circumferential strains are complicated by substantial transmural non-uniformity in the normal left ventricle [34]. Different cut-off GLS values to separate abnormality are suggested in different diseases and different reported series [34]. In a meta-analysis of LV strain the narrowest confidence intervals were for GLS and global circumferential strain, but individual studies have shown a broad range of strain in apparently normal subjects [35]. Variations between different normal ranges seem to be associated with differences in systolic blood pressure, emphasizing that this should also be considered in the interpretation of strain [35].
For cardiotoxicity during chemotherapy a relative decrease in GLS > 15% compared with baseline is reported to be likely of clinical significance [34, 36]. However, although strain imaging may detect sub-clinical myocardial changes in this setting, the value of these changes in predicting clinical outcome is still unknown [34]. A combination of strain imaging with ultrasensitive troponin has been proposed [34]. It is also recommended to measure GLS in addition to LVEF, which will be helpful in cases when LVEF is in the lower normal range and it is difficult to conclude about systolic function [34]. In such cases, the finding of subnormal strain should result in closer monitoring of cardiac function. Not all sub-clinical reduction in LV function may progress to significant dysfunction or heart failure, and there is need for studies which can help to define criteria for clinically relevant changes in strain [34]. Furthermore, it has also been stated that there is no evidence that changes in strain should prompt changes in oncology treatment at this time [36]. Studies are often retrospective and increasing cumulative anthracycline dose during cancer treatment correlates with subclinical cardiac dysfunction in childhood cancer survivors, but with wide dispersion of individual data [37]. Most recently EF alone has been used in a contemporary paper on cardiotoxicity [38].
It is thus very difficult to understand what it means to search for subtle changes in left ventricular function and early dysfunction when the normality range is reported between –18% and –25% in healthy individuals [34]. The normative values of LV GLS vary according to gender, age, and system used to acquire and analyze the data. As such, current recommendations do not provide universal normal values and lower limits of normal but, as guidance, the expected value of LV GLS in a healthy individual is around –20% [3, 27, 39, 40]. Therefore, any value of LV GLS less negative than –20% could be considered pathological [39]. Women show slightly more negative (better) LV GLS compared with men, and it has been shown that LV GLS decreases (becomes less negative) with age [3, 39, 40]. In such a scale range of normality between –18% and –25% [34] subtle changes can only be considered at most as a flag to induce a more strict follow-up. It is in fact really very difficult to define the physiological meaning of a single absolute GLS value in this range. Furthermore, any subtle change of GLS value between successive tests is also very difficult to interpret in a single case and cannot be directly and clinically trusted if not accompanied by a concordant change of a different parameter of known physiological behavior and meaning, for example EF. For EF the range of scale is wider, up to 65%, with the possibility to evaluate a more gradual decrease and the ability to identify even the mid-range of EF [41]. From the clinical point of view minor changes in EF can then be managed more easily. The amplitude of scale of GLS and EF differs by a factor of around 2.8. In fact, any 1% change for a GLS value is a 5% relative change if we take a –20% GLS value for normality, while a 1% change for EF corresponds to a 1.8% relative change if we take a 55% EF cut-off value for normality. On the other hand, when changes of GLS are larger between successive examinations the interpretation is uncertain and it is very difficult to proceed to an operative clinical decision making process if not supported by concordant different physiopathological changes, due to the variety of possible factors interfering with GLS values. At the end it is not so clear what LV strain parameters can add in clinical practice, and to EF, when a decision has to be made in a single patient, due also to the great heterogeneity of the reported results and the wide overlap of standard deviation of GLS values when comparing different populations or subgroups [39, 42–49]. The management of valve disease relies to a large extent on the assessment of cardiac function. LVEF is considered to be an essential measurement, even though it is both preload- and afterload-dependent [50, 51]. The same accounts, however, for strain, as myocardial deformation depends on contractile properties of the myocardial fibers (“contractility”), but also on their loading conditions (pre- and after-load), chamber geometry, dyssynchrony, and segment interactions [50, 51]. Therefore, abnormally low strain values are not necessarily a sign of myocardial dysfunction, while normal values do not automatically exclude diseases [50, 51].
Why is the literature so overabundant in suggesting positive results, but no in depth research has been dedicated to limiting or conditioning factors?
In the last two decades many papers have been published suggesting the potential positive role of strain parameters, but conclusive contributions to their actual clinical role in the decision making process in a single patient are still lacking. Potential limitations are always reported and declared, but without adequate research on limiting or conditioning factors. Some intellectual conflict of interest and bias against negative result papers can play a role in this issue, since after so many “positive” reports it is progressively more difficult to define and accept limitations, which can potentially fundamentally modify our general perception and clinical acceptance of strain parameters.
Sengelov et al. [52] recently reported that GLS is an independent predictor of all-cause mortality in heart failure with reduced ejection fraction (HFrEF) patients, especially in male patients without atrial fibrillation. They also concluded that GLS was a superior prognosticator compared with all other echocardiographic parameters. When we look at the results of the study, mean GLS% in the group of 888 alive patients was –9.9 ±3.2% vs. –8.1 ±3.0 in the group of 177 dead patients at the median follow-up of 40 months [53]. In multivariable analysis the hazard ratio was 1.15 (1.04–1.27) with a p-value of 0.008 [52]. With such a wide overlap of GLS values between the two groups and a 15% mean prognostic superiority (range from 4% to 27%) at the population level it is very difficult to understand what the utility of a single GLS value could be when you have to made a clinical decision for a single patient at a single time window. In the Editorial Comment to this paper Lumens et al. [53] suggest that the preload dependence of systolic fiber strain may be the physiological mechanism behind the observation that the relationship between GLS and mortality is less strong in patients with atrial fibrillation compared with patients without this arrhythmia. The Editorial Comment concludes, however, that GLS has the potential to become an important diagnostic metric of global LV systolic function [53]. Great heterogeneity of the results and a wide overlap of strain values when comparing different populations or subgroups have also been shown in a variety of clinical situations [39, 42–49] and in systemic hypertension [14, 54–57].
In the published literature minor and subtle changes of strain parameters have always been immediately and directly interpreted as subtle changes in left ventricular function and early dysfunction, without taking into account the possibility of interfering concurrent factors which could become more or less relevant in determining and explaining the results in specific conditions and in single cases. This could be the case for example for athletes, where a great heterogeneity of results is reported, difficult to interpret from the physiological point of view [58–60]. We do not know in fact, in this setting, how much the remodeling and dilatation of heart chambers, due to training and type of sport activity, could modify in a single case the through-plane displacement motion of the myocardial walls due to the changing position characteristics of the heart inside the chest during the cardiac cycle and respiration, independently from major changes in cardiac mechanics (Figure 1 B). Other controversial situations are represented by the different modifications of chest shape due to scoliosis, pectus excavatum, pectus carinatum, flat chest with straight back, flat or doming shape of the diaphragm, and so on. All these situations can modify the position and the complexity of motion of the heart inside the chest (and then also the orientation of the insonication beam) in a single case. Even the dimension and shape of cardiac chambers can be altered by compression of the chest wall, modifying motion and displacement of the heart, leading to dyssynchrony [61–63]. How much the strain results in these settings could be related then to motion artifacts and through-plane displacement motion of myocardial walls, and not necessarily to changes in cardiac mechanics, is open to debate. This is also supported by the minimal difference in absolute terms as compared to controls with a wide overlap of standard deviations. According to this hypothesis, Sonaglioni et al. have recently shown the existence of a strong linear correlation between strain alterations and progressively worse chest deformity in normal subjects with pectus excavatum; such correlation was absent in controls with normal chest shape [64]. This could suggest intraventricular dyssynchrony, rather than intrinsic myocardial dysfunction [64].
How could we make a plan trying to solve the clinical paradox of strain parameters?
It is time for a wide and open minded general discussion between manufacturers and medical professionals trying to first clarify why we are at this point with strain parameters. This could be achieved through organized institutional standardization cardiovascular imaging task forces which could and should plan and implement new unbiased research, where also industry has to contribute. This could make it possible to compare different speckle tracking software, both for 2D and 3D, and validate them by phantom and appropriately designed experimental models and situations, able to differentiate how much of each tracking system is really measuring cardiac mechanics or instead, totally or partially, could be in relation with the complexity of total heart motion and through-plane displacement of the myocardial wall in different disease specific situations and within different chest conformations. The intervendor stability of tracking software of the region of Interest for each identified myocardial segment under study should also be explored. Thickness of myocardial walls and shape and remodeling of cardiac chambers could also influence the speckle tracking sampling system and insonication direction independently of cardiac mechanics. This should also be searched, trying to further standardize different clinical situations and understand and separate pathophysiology and cardiac mechanics from artifactual or external changes.
Until now, research on strain parameters has been concentrated mostly on descriptive physiological interpretations of more and more post-processing parameters at the population level. There is now a strong need to demonstrate clinical utility of strain values in a single patient, both in the diagnostic and in the prognostic field. To reach this goal, prospective randomized clinical registry trials, both single center (single vendor) and multicenter (multivendor), should also be implemented using strain parameters to stratify at each Institution all-comer consecutive patients with different diseases, before applying specific therapeutic interventions. Each registry could have a different disease or different physiopathological and demographic baseline clinical characteristics as inclusion criteria. The primary outcome of each prospective registry could be the ability of strain parameters to predict pre-defined clinical events and to modify clinical management strategies. Accurate blind follow-up statistical analysis on the results could give many relevant answers to all our present questions on strain clinical predictive value in a single case. This type of research could also help to find, if possible, definite and univocal cut-off values for each specific clinical condition, to clearly differentiate normal from abnormal results. Another expected result should be a documented linear proportionality between decreasing strain values (less negative values) and the severity of decreased cardiac function. Subclinical cardiac dysfunction could then be clearly identified in a single patient when strain values are just immediately below a univocally accepted normal cut-off value (less negative than the cut-off). The prognostic implication of subtle individual changes below normal and/or possible recovery of normality could also be determined and assessed in the daily practice of the clinical decision making process in a single patient.
Conclusions
In a survey of the European Association of Cardiovascular Imaging (EACVI) on 96 European Echocardiography Laboratories from 22 different countries, 96% of centers used LV global longitudinal strain (GLS) derived from STE to measure LV function, but this was reserved for selected cases including patients on chemotherapy or those with cardiomyopathy, valvular heart disease, or heart failure with preserved EF [65]. Of interest, when GLS was measured, 65% of the centers used the reference value of –18%, whilst the remainder used –20%. Several centers highlighted the limitation of variation in GLS measurements between different vendors [65]. After two decades of wide clinical application we could then accept that strain technology is measuring in fact cardiac function, but final absolute values of strain in a single case can be determined by the additive contribution, possibly different case by case, of interfering and artifactual factors, only one of which can be attributed to vendor characteristics of each machine. This can clarify the still uncertain clinical utility in a single case and support the urgent need of further research, involving both manufacturers and medical professionals, on the limiting, interfering and conditioning factors of strain parameters in different specific clinical situations and conditions. It is also important to open the mind to the possibility that strain results in a single case could derive not necessarily and uniquely from changes in cardiac mechanics, but possibly, at least in part, from artifactual and/or external chest shape determinants and total heart motion components, which could be dominant in individual cases and should always be taken into account.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Sutherland GR, Di Salvo G, Claus P, D’hooge J, Bijnens B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004;17:788–802. doi: 10.1016/j.echo.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Ng ACT. Emerging clinical role of strain imaging in echocardiography. Heart Lung Circulation. 2010;19:161–74. doi: 10.1016/j.hlc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi SJ, Altman M, Stanton T, Thomas L. Echocardiographic strain in clinical practice. Heart Lung Circulation. 2019;28:1320–30. doi: 10.1016/j.hlc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Farsalinos KE, Daraban AM, Unlu S, et al. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr. 2015;28:1171–81. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J Am Soc Echocardiogr. 2015;28:183–93. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Ramlogan S, Aly D, France R, et al. Reproducibility and intervendor agreement of left ventricular global systolic strain in children using a layer-specific analysis. J Am Soc Echocardiogr. 2020;33:110–9. doi: 10.1016/j.echo.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Risum N, Ali S, Olsen NT, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Manovel A, Dawson D, Smith B, Nihoyannopoulos P. Assessment of left ventricular function by different speckle-tracking software. Eur J Echocardiogr. 2010;11:417–21. doi: 10.1093/ejechocard/jep226. [DOI] [PubMed] [Google Scholar]
- 9.Stöhr EJ, Shave RE, Baggish AL, Weiner RB. Left ventricular twist mechanics in the context of normal physiology and cardiovascular disease: a review of studies using speckle tracking echocardiography. Am J Physiol Heart Circ Physiol. 2016;311:H633–44. doi: 10.1152/ajpheart.00104.2016. [DOI] [PubMed] [Google Scholar]
- 10.Gayat E, Ahmad H, Weinert L, Lang RM, Mor-Avi V. Reproducibility and inter-vendor variability of left ventricular deformation measurements by three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2011;24:878–85. doi: 10.1016/j.echo.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Truong VT, Phan HT, Pham KNP, et al. Normal ranges of left ventricular strain by three-dimensional speckle-tracking echocardiography in adults: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2019;32:1586–97. doi: 10.1016/j.echo.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Hioki A, Asanuma T, Masuda K, Sakurai D, Nakatani S. Detection of abnormal myocardial deformation during acute myocardial ischemia using three-dimensional speckle tracking echocardiography. J Echocardiogr. 2020;18:57–66. doi: 10.1007/s12574-019-00449-6. [DOI] [PubMed] [Google Scholar]
- 13.Dogdus M, Demir E, Cinar CS, Gurgun C. Coronary tortuosity affects left ventricular myocardial functions: a 3D-speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2020;36:627–32. doi: 10.1007/s10554-019-01760-0. [DOI] [PubMed] [Google Scholar]
- 14.Esposito G, Piras P, Evangelista A, et al. Improving performance of 3D speckle tracking in arterial hypertension and paroxysmal atrial fibrillation by using novel strain parameters. Sci Rep. 2019;9:7382. doi: 10.1038/s41598-019-43855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon A, Ahn HS, Kim GH, Cho JS, Park CS, Youn HJ. Right ventricular analysis using real-time three-dimensional echocardiography for preload dependency. J Cardiovasc Imaging. 2020;28:36–47. doi: 10.4250/jcvi.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saikhan LA, Park C, Hughes AD. Reproducibility of left ventricular dyssynchrony indices by three-dimensional speckle-tracking echocardiography: the impact of sub-optimal image quality. Front Cardiovasc Med. 6:149. doi: 10.3389/fcvm.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Everdingen WM, Maass AH, Vernooy K, et al. Comparison of strain parameters in dyssynchronous heart failure between speckle tracking echocardiography vendor systems. Cardiovasc Ultrasound. 2017;15:25. doi: 10.1186/s12947-017-0116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 19.Nagata Y, Takeuchi M, Mizukoshi K, et al. Intervendor variability of two-dimensional strain using vendor-specifific and vendor-independent software. J Am Soc Echocardiogr. 2015;28:630–41. doi: 10.1016/j.echo.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Marwick TH, Fukuda N, et al. Improvement in strain concordance between two major vendors after the strain standardization initiative. J Am Soc Echocardiogr. 2015;28:642–8. doi: 10.1016/j.echo.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Il’Giovine ZJ, Mulder H, Chiswell K, et al. Right ventricular longitudinal strain reproducibility using vendor-dependent and vendor-independent software. J Am Soc Echocardiogr. 2018;31:721–32. doi: 10.1016/j.echo.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Khuffash A, Schubert U, Levy PT, Nestaas E, de Boode WP. Deformation imaging and rotational mechanics in neonates: a guide to image acquisition, measurement, interpretation, and reference values. Pediatric Res. 2018;84:S30–45. doi: 10.1038/s41390-018-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirea O, Pagourelias ED, Duchenne J, et al. Variability and reproducibility of segmental longitudinal strain measurement. A Report From the EACVI-ASE Strain Standardization Task Force. J Am Coll Cardiol Img. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Zito C, Longobardo L, Citro R, et al. Ten years of 2D longitudinal strain for early myocardial dysfunction detection: a clinical overview. BioMed Res Int. 2018;2018:8979407. doi: 10.1155/2018/8979407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Saikhan L, Park C, Hardy R, Hughes A. Prognostic implications of left ventricular strain by speckle-tracking echocardiography in the general population: a meta-analysis. Vasc Health Risk Manag. 2019;15:229–51. doi: 10.2147/VHRM.S206747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amzulescu MS, De Craene M, Langet H, et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20:605–19. doi: 10.1093/ehjci/jez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Bogaert J, Rademakers FE. Regional nonuniformity of normal adult human left ventricle. Am J Physiol Heart Circ Physiol. 2001;280:H610–20. doi: 10.1152/ajpheart.2001.280.2.H610. [DOI] [PubMed] [Google Scholar]
- 29.Ancedy Y, Ederhy S, Lang S, et al. Multilayer global longitudinal strain in patients with cancer: a comparison of two vendors. Arch Cardiovasc Dis. 2018;111:285–96. doi: 10.1016/j.acvd.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Unlu S, Mirea O, Duchenne J, et al. Comparison of feasibility, accuracy, and reproducibility of layer-specific global longitudinal strain measurements among five different vendors: a report from the EACVI-ASE strain standardization task force. J Am Soc Echocardiogr. 2018;31:374–80. doi: 10.1016/j.echo.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Rosner A, How OJ, Aarsæther E, et al. High resolution speckle tracking dobutamine stress echocardiography reveals heterogeneous responses in different myocardial layers: implication for viability assessments. J Am Soc Echocardiogr. 2010;23:439–47. doi: 10.1016/j.echo.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Huang SJ, Ting I, Huang AM, Slama M, McLean AS. Longitudinal wall fractional shortening: an M-mode index based on mitral annular plane systolic excursion (MAPSE) that correlates and predicts left ventricular longitudinal strain (LVLS) in intensive care patients. Critical Care. 2017;21:292. doi: 10.1186/s13054-017-1876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luszczak J, Olszowska M, Drapisz S, et al. Assessment of left ventricle function in aortic stenosis: mitral annular plane systolic excursion is not inferior to speckle tracking echocardiography derived global longitudinal peak strain. Cardiovasc Ultrasound. 2013;11:45. doi: 10.1186/1476-7120-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular straIn: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Banchs J, Mousavi N, et al. Contemporary role of echocardiography for clinical decision making in patients during and after cancer therapy. J Am Coll Cardiol Img. 2018;11:1122–31. doi: 10.1016/j.jcmg.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Wolf CM, Reiner B, Kühn A, et al. Subclinical cardiac dysfunction in childhood cancer survivors on 10-years follow-up correlates with cumulative anthracycline dose and is best detected by cardiopulmonary exercise testing, circulating serum biomarker, speckle tracking echocardiography, and tissue doppler imaging. Front Pediatr. 2020;8:123. doi: 10.3389/fped.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41:1720–9. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 39.Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail. 2017;19:307–13. doi: 10.1002/ejhf.694. [DOI] [PubMed] [Google Scholar]
- 40.Petitto M, Esposito R, Sorrentino R, et al. Sex-specific echocardiographic reference values: the women’s point of view. J Cardiovasc Med. 2018;19:527–35. doi: 10.2459/JCM.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 41.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 42.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–74. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Zuo H, Zhang Y, Ma F, et al. Myocardial deformation pattern differs between ischemic and non-ischemic dilated cardiomyopathy: the diagnostic value of longitudinal strains. Ultrasound Med Biol. 2020;46:233–43. doi: 10.1016/j.ultrasmedbio.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, Zhang B, Zhang Y, Dong Y, Zhang R. Ultrasonic image analysis of longitudinal strain in uremic patients with preserved left ventricular ejection fraction. BioMed Eng OnLine. 2018;17:112. doi: 10.1186/s12938-018-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaeboe LG, Edvardsen T. Echocardiographic assessment of left ventricular systolic function. J Echocardiogr. 2019;17:10–6. doi: 10.1007/s12574-018-0405-5. [DOI] [PubMed] [Google Scholar]
- 46.Kusunose K, Fujiwara M, Yamada H, et al. Deterioration of biventricular strain is an early marker of cardiac involvement in confirmed sarcoidosis. Eur Heart J Cardiovasc Imaging. 2020;21:796–804. doi: 10.1093/ehjci/jez235. [DOI] [PubMed] [Google Scholar]
- 47.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–57. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 48.Sugahara M, Kagiyama N, Hasselberg NE, et al. the IPAC Investigators Global left ventricular strain at presentation is associated with subsequent recovery in patients with peripartum cardiomyopathy. J Am Soc Echocardiogr. 2019;32:1565–73. doi: 10.1016/j.echo.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavazzoni M, Badano LP, Vizzardi E, et al. Prognostic value of right ventricular free wall longitudinal strain in a large cohort of outpatients with left-side heart disease. Eur Heart J Cardiovasc Imaging. 2020;21:1013–21. doi: 10.1093/ehjci/jez246. [DOI] [PubMed] [Google Scholar]
- 50.Cvijic M, Voigt JU. Application of strain echocardiography in valvular heart diseases. Anatol J Cardiol. 2020;23:244–53. doi: 10.14744/AnatolJCardiol.2020.09694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voigt JU, Cvijic M. 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12:1849–63. doi: 10.1016/j.jcmg.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 52.Sengeløv M, Jørgensen PG, Jensen JS, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8:1351–9. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 53.Lumens J, Prinzen FW, Delhaas T. Longitudinal strain. “Think globally, track locally”. JACC Cardiovasc Imaging. 2015;8:1360–3. doi: 10.1016/j.jcmg.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Petre I, Onciul S, Iancovici S, et al. Left atrial strain for predicting atrial fibrillation onset in hypertensive patients. High Blood Press Cardiovasc Prev. 2019;26:331–7. doi: 10.1007/s40292-019-00326-4. [DOI] [PubMed] [Google Scholar]
- 55.Muiesan ML, Paini A, Aggiusti C, Bertacchini F, Rosei CA, Salvetti M. Hypertension and organ damage in women. High Blood Press Cardiovasc Prev. 2018;25:245–52. doi: 10.1007/s40292-018-0265-0. [DOI] [PubMed] [Google Scholar]
- 56.Xu L, Wang N, Chen X, Liang Y, Zhou H, Yan J. Quantitative evaluation of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography among young adults with essential hypertension in China. Medicine. 2018;97:e12448. doi: 10.1097/MD.0000000000012448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gil TCP, Castier MB, Gondar AFP, et al. Strain analysis of left ventricular function in the association of hypertrophic cardiomyopathy and systemic arterial hypertension. Arq Bras Cardiol. 2019;113:677–84. doi: 10.5935/abc.20190176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lord RN, Utomi V, Oxborough DL, Curry BA, Brown M, George KP. Left ventricular function and mechanics following prolonged endurance exercise: an update and meta-analysis with insights from novel techniques. Eur J Appl Physiol. 2018;118:1291–9. doi: 10.1007/s00421-018-3906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forsythe L, George K, Oxborough D. Speckle Tracking echocardiography for the assessment of the athlete’s heart: is it ready for daily practice? Curr Treat Options Cardiovasc Med. 2018;20:83. doi: 10.1007/s11936-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beaumont A, Grace F, Richards J, Hough J, Oxborough D, Sculthorpe N. Left ventricular speckle tracking-derived cardiac strain and cardiac twist mechanics in athletes: a systematic review and meta-analysis of controlled studies. Sports Med. 2017;47:1145–70. doi: 10.1007/s40279-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S, Yang J, Zhu L, et al. Left ventricular mechanics assessed by 2-dimensional speckle tracking echocardiography in children and adolescents with idiopathic scoliosis. Clin Spine Surg. 2017;30:E381–9. doi: 10.1097/BSD.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 62.Chao CJ, Jaroszewski D, Gotway M, et al. Effects of pectus excavatum repair on right and left ventricular strain. Ann Thorac Surg. 2018;105:294–301. doi: 10.1016/j.athoracsur.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Jaroszewski DE, Velazco CS, Pulivarthi VSKK, Arsanjani R, Obermeyer RJ. Cardiopulmonary function in thoracic wall deformities: what do we really know? Eur J Pediatr Surg. 2018;28:327–46. doi: 10.1055/s-0038-1668130. [DOI] [PubMed] [Google Scholar]
- 64.Sonaglioni A, Nicolosi GL, Granato A, Lombardo M, Anzà C, Ambrosio G. Reduced myocardial strain parameters in subjects with pectus excavatum: impaired myocardial function or methodological limitations due to chest deformity? Semin Thoracic Surg. 2020 doi: 10.1053/j.semtcvs.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Marsan NA, Michalski B, Cameli M, et al. EACVI survey on standardization of cardiac chambers quantification by transthoracic echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:119–23. doi: 10.1093/ehjci/jez297. [DOI] [PubMed] [Google Scholar]