Abstract
Introduction
Metabolic syndrome has been recognized as a predictor of cardiovascular diseases. Epicardial fat tissue (EFT) thickness has recently been shown to be a predictor of cardiovascular diseases in metabolic syndrome patients. Endocan is a novel molecule which is considered to be an early marker of endothelial dysfunction. Our aim was to evaluate endocan serum levels for the first time in metabolic syndrome patients, in relation to EFT thickness.
Material and methods
The study included 44 patients with metabolic syndrome who had neither chronic kidney disease nor chronic inflammation and 26 healthy controls. Fasting blood samples were obtained from the groups. The serum levels of endocan were measured with a Sunred ELISA kit. EFT thickness of patients was measured by echocardiography.
Results
The serum endocan levels were significantly lower in the metabolic syndrome patients compared to the healthy controls (120.71 ±90.17 pg/ml vs. 414.59 ±277.57, p < 0.001). Metabolic syndrome patients demonstrated significantly higher EFT (p = 0.042). EFT thickness had a positive correlation with age (r = 0.397, p = 0.008) and weight (r = 0.010). However, there was no correlation with serum endocan (r = –0.021, p = 0.893) or other parameters. Regression analysis revealed that waist circumference is the parameter among metabolic syndrome criteria having the strongest relationship with serum endocan levels (β = –0.499, p = 0.21).
Conclusions
EFT thickness was high in metabolic syndrome patients and can be a useful marker for cardiovascular risk assessment. However, serum endocan levels were found to be low and there was no correlation with EFT thickness. Large sample sized prospective studies are needed to clarify the relation of endocan levels with the other clinical indicators of cardiovascular risk in metabolic syndrome.
Keywords: metabolic syndrome, adipose tissue, endothelium, proteoglycans, epicardial fat tissue thickness
Introduction
Metabolic syndrome is a common disease characterized by central obesity, insulin resistance, dyslipidemia (elevated triglycerides and low-density lipoprotein (LDL) and decreased high-density lipoprotein (HDL)), and increased blood pressure [1]. The incidence of metabolic syndrome is insidiously increasing throughout the world, which is alarming due to its cardiovascular complications [2]. For this reason many studies and meta-analyses focus on metabolic syndrome and its components.
Increased visceral adipose tissue increases the risk for endothelial dysfunction, which manifests as high blood pressure, insulin resistance and diabetes and long-term cardiovascular risk [3]. Endothelial dysfunction affects vascular homeostasis through vessel wall vasoconstriction, leukocyte adhesion, platelet activation, oxidative stress, thrombosis, coagulation and inflammation [4]. It is suggested that in the pathogenesis of atherosclerosis, endothelial dysfunction has a key role from the early lesions to thrombotic complications [5].
Epicardial fat tissue (EFT) is the visceral fat surrounding the heart that is found to be related to obesity, metabolic syndrome, and cardiovascular diseases [6]. Recently EFT was defined as a metabolically active organ that releases both anti-atherogenic (adiponectin) and atherogenic (TNF-α, MCP-1) bioactive molecules, which are associated with endothelial dysfunction [7]. Previous studies have shown that echocardiographic EFT measurement can be an indirect way to demonstrate endothelial dysfunction and predict high cardio-metabolic risk and should be a therapeutic target [8].
Endocan, also known as endothelial cell-specific molecule-1 (ESM-1), is a soluble form of dermatan sulphate, which is specific for the vascular endothelium [9]. The role of endocan in vascular protection and regulation of inflammation induced endothelial dysfunction is recently striking. It exerts its vasoprotective effect through inhibiting the adhesion and migration of leucocytes across the endothelium and promoting vasodilation. It interacts with lymphocyte function associated antigen-1 (LFA-1) on the lymphocytes, thus blocking the binding of intercellular adhesion molecules ICAM-1, ICAM-2, ICAM-3 [10]. It is suggested that this pathway play a key role in early atherogenesis [11].
Therefore, we evaluated endocan level and EFT thickness in a representative sample of patients with metabolic syndrome and assessed the correlation between serum endocan concentration and EFT by two-dimensional transthoracic echocardiography.
Material and methods
Subjects
This cross-sectional study included 44 subjects with metabolic syndrome and 26 healthy controls who were admitted to our internal medicine outpatient policlinic. Criteria used for the diagnosis of metabolic syndrome were those recommended by the National Cholesterol Education Program. The Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults – Adult Treatment Panel III (NCEP-ATP III), which is defined by the presence of at least three of these components: 1) increased waist circumference (> 102 cm for men, > 88 cm for women); 2) elevated triglycerides (≥ 150 mg/dl) or use of triglyceride-lowering drugs; 3) low HDL cholesterol (< 40 mg/dl in men, < 50 mg/dl in women); 4) hypertension (≥ 130/ ≥ 85 mm Hg) or use of antihypertensive drugs; and 5) fasting glucose (≥ 110 mg/dl) or use of antidiabetic drugs [12]. Exclusion criteria were the presence of heart and systemic diseases: ischemic heart disease, congenital heart disease, heart valve disease, neoplastic, inflammatory, and infectious diseases.
This study was performed according to the guidelines of the Declaration of Helsinki, and it was approved by the ethics review committee of Istanbul Gaziosmanpasa Taksim Education and Research Hospital. Written informed consent was obtained from all patients prior to study enrollment.
Physical and laboratory measurements
The collection of anthropometric data (weight, height, waist circumference) and measurement of systemic blood pressure were obtained by physical examination according to standard procedures. The body mass index (BMI) was calculated by dividing weight (kg) by height (m2). Waist circumferences were measured in the horizontal plane midway between the lowest rib and iliac crest. Resting systolic and diastolic BP was measured three times at 1-min intervals using a standard mercury sphygmomanometer after a 5-min rest. The average of the second and the third readings were used in the analyses. Fasting venous blood samples were collected in the morning after at least 8 h of fasting. Serum cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) were measured by enzymatic colorimetric methods with commercially available kits (COBAS 311, Roche Diagnostics GmbH, Mannheim, Germany), and low-density lipoprotein cholesterol C (LDL-C) was calculated according to the Friedewald formula. Serum glucose measures were determined enzymatically using the hexokinase method (Roche Diagnostics GmbH, Mannheim, Germany). Blood HbA1c was determined with a COBAS 311 analyzer using the particle-enhanced immunoturbidimetric method (Roche Diagnostics, Mannheim, Germany). Final results were expressed as percent HbA1c of the total Hb according to the protocol of the Diabetes Control and Complications Trial/National Glycohemoglobin Standardization Program (DCCT/NGSP). The particle-enhanced immunoturbidimetric method with a Behring Nephelometer BN-100 (Behring Diagnostic, Frankfurt, Germany) was used to measure C reactive protein (CRP). The sensitivity of the test was 0.1 mg/l. Serum endocan levels were measured with a Sunred ELISA kit (Sunred Biological Technologies Human ECSM1/ENDOCAN ELISA Kit, Catalog No: 201-12-1978, China). The intra- and inter-assay variabilities of the ELISA kit were 5.1% and 6.1% respectively. The minimum detectable dose of endocan was 31.2 pg/ml.
Measurement of epicardial fat tissue by echocardiography
Two-dimensional transthoracic echocardiography was performed by an experienced cardiologist. Three measurements of the two-dimensional parasternal long axis were performed. The measurement of EFT thickness was done using the DICOM system. The free wall of the right ventricle was measured using the papillary muscle, at end-diastole from the parasternal long-axis views of 3 cardiac cycles using the aorta annulus for anatomic reference. We measured the thickest point of the EFT and the mean value of the EFT thickness was calculated [13].
Statistical analysis
Frequency, ratio, mean, minimum, maximum, and standard deviation values were used in the descriptive statistics to determine continuous variables. Student’s t test was used for comparison of two independent and normally distributed variables. The Mann-Whitney U test was performed for comparison of independent and non-normally distributed variables. The χ2 test and Fisher’s exact test were performed to determine differences between categorical variables. Multiple linear logistic regression analysis was performed to determine the effect levels of the parameters. Spearman’s correlation tests were used for the correlation analysis. Statistical significance was assessed at p < 0.05. Statistical analysis were performed using MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba, Ostend, Belgium; 2013).
Results
Characteristics of the study population
A total of 70 subjects were recruited to this study (mean age: 55.7 ±11.43 years, 34.1% male, 65.9 % female), while volunteers were recruited as normal controls (mean age: 52.69 ±8.1 years, 42.3% male, 57.7% female). Table I displays the baseline characteristics of the participants. Metabolic syndrome patients demonstrated significantly higher BMI (p < 0.001), waist circumference (p < 0.001), systolic blood pressure (p = 0.001), fasting blood glucose (p < 0.001), HbA1c (p < 0.001), triglycerides (p < 0.001), CRP (p < 0.001), and EFT thickness (p = 0.042), compared to the control group. The serum endocan levels were significantly lower in patients with metabolic syndrome compared to the healthy controls (120.71 ±90.17 pg/ml vs. 414.59 ±277.57, p < 0.001) (Table I). Multiple linear regression analysis was performed in order to determine the factors associated with serum endocan levels. The model reveals that metabolic syndrome parameters explain the changes of serum endocan levels up to 28.7%. Among the criteria of metabolic syndrome waist circumference is mostly strongly associated with serum endocan (β = –0.499, p = 0.21) (Table II).
Table I.
Clinical and biochemical characteristics of the patients in the study
| Parameter | Metabolic syndrome (n = 44) Mean ± SD/n (%) |
Control group (n = 26) Mean ± SD/n (%) |
P-value |
|---|---|---|---|
| Age [years] | 54.05 ±9.09 | 52.5 ±7.98 | 0.41 |
| Gender: | |||
| Male | 15 (34.1%) | 11 (42.3%) | |
| Female | 29 (65.9%) | 15 (57.7%) | |
| BMI [kg/m2] | 34.02 ±6.98 | 25.77 ±3.38 | < 0.001** |
| Waist circumference [cm] | 111.9 ±13.36 | 86.19 ±11.31 | < 0.001** |
| Systolic pressure [mm Hg] | 134.89 ±21.58 | 119.23 ±9.81 | 0.001** |
| Diastolic pressure [mm Hg] | 75.34 ±12.96 | 70.96 ±8.13 | 0.176 |
| Fasting blood glucose [mg/dl] | 168.89 ±93.2 | 90.96 ±11.12 | < 0.001** |
| HbA1c (%) | 8.18 ±2.74 | 5.52 ±0.44 | < 0.001** |
| Triglyceride [mg/dl] | 222.57 ±167.99 | 112.92 ±54.09 | < 0.001** |
| Total cholesterol [mg/dl] | 219.11 ±46.74 | 206.77 ±38.36 | 0.213 |
| LDL [mg/dl] | 130.7 ±37.08 | 130.19 ±32.23 | 0.953 |
| HDL [mg/dl] | 47.2 ±9.3 | 53.96 ±13.94 | 0.114 |
| CRP [mg/l] | 5.75 ±3.57 | 2.46 ±2.22 | < 0.001** |
| Epicardial fat tissue [mm] | 6.3 ±2.2 | 5.2 ±1.1 | 0.043* |
Mann-Whitney U, Student t, Fisher’s exact,
Statistical significance: *p < 0.05, **p < 0.01,
BMI – body mass index, LDL – low-density lipoprotein, HDL – high-density lipoprotein, CRP – C-reactive protein.
Table II.
Multiple linear regression analysis of the serum endocan and metabolic syndrome related parameters
| Parameter | β | P-value |
|---|---|---|
| EAT thickness | 0.07 | 0.547 |
| Systolic blood pressure | –0.021 | 0.860 |
| Waist circumference | –0.499 | 0.021* |
| BMI | 0.051 | 0.799 |
| Fasting blood glucose | –0.182 | 0.136 |
| Triglycerides | –0.047 | 0.710 |
| HDL-c | 0.013 | 0.913 |
Statistical significance: *p < 0.05, **p < 0.01,
BMI – body mass index, EFT – epicardial fat tissue, HDL-c – high-density lipoprotein cholesterol.
Correlation analysis between EFT thickness and clinical indicators
The mean value of EFT thickness in metabolic syndrome patients was 6.3 mm. The mean EFT thickness value of healthy subject was 5.2 mm (Table I). The p-value for the difference between the two groups was 0.043, which was statistically significant. To determine the association between EFT thickness and other parameters, Spearman correlation analysis was performed. As shown in Table III, a positive correlation with EFT thickness was observed with age (r = 0.397, p = 0.008) and weight (r = 0.308, p = 0.010). No significant correlations were found between EFT thickness and systolic blood pressure (r = –0.069, p = 0.657), diastolic blood pressure (r = 0.118, p = 0.446), waist circumference (r = 0.212, p = 0.167), BMI (r = 0.150, p = 0.330), fasting glucose (r = 0.072, p = 0.641), triglycerides (r = 0.202, p = 0.188), HDL-c (r = –0.227, p = 0.139), or endocan (r = –0.021, p = 0.893) (Table III).
Table III.
Correlation between epicardial adipose tissue thicknesses and characteristics of patients with metabolic syndrome
| Parameter | Value | |
|---|---|---|
| Age [years] | r | 0.397 |
| p | 0.008* | |
| SBP [mm Hg] | r | –0.069 |
| p | 0.657 | |
| DBP [mm Hg] | r | 0.118 |
| p | 0.446 | |
| Weight [kg] | r | 0.308 |
| p | 0.042* | |
| WC [cm] | r | 0.212 |
| p | 0.167 | |
| BMI [kg/m2] | r | 0.150 |
| p | 0.330 | |
| Fasting glucose [mg/dl] | r | 0.072 |
| p | 0.641 | |
| TG [mg/dl] | r | 0.202 |
| p | 0.188 | |
| HDL-c [mg/dl] | r | –0.227 |
| p | 0.139 | |
| Endocan [pg/ml] | r | –0.021 |
| p | 0.893 |
BMI – body mass index, WC – waist circumference, SBP – systolic blood pressure, DBP – diastolic blood pressure, TG – triglycerides, HDL-c – high-density lipoprotein cholesterol.
Statistical significance p < 0.05*, p < 0.001**. Spearman’s rho.
Discussion
In the present study, for the first time serum endocan levels and its associations with EFT thickness were studied in metabolic syndrome patients compared with healthy controls as a marker of endothelial damage in this patient group.
Metabolic syndrome patients are more susceptible to cardiovascular complications than the general population. Possible mechanisms are visceral obesity, glucose metabolism alterations and endothelial dysfunction [14, 15]. Clinical determinants of endothelial dysfunction recently have been focused on anticipating atherosclerotic and cardiovascular outcomes of metabolic diseases. Assessment of endothelial cell function is based mainly on measurement of vasoactive substances released by or that interact with vascular endothelium [16]. For this purpose, several markers of endothelial damage and inflammation are being investigated such as lipoprotein associated phospholipase A2 [17], 13-hydroxyoctadecadienoic acid (13-HODE) [18], CD40 receptor/CD40 ligand interaction [19], LOX-1 [20] and circulating endothelial progenitor cells [21].
Endocan is a soluble proteoglycan which is expressed by endothelial cells mainly in the lung, liver and kidney, and recently was found to be on malignant cells [22]. It is suggested that synthesis and secretion of endocan are primarily regulated by tumor necrosis factor (TNF), interleukin-1 and lipopolysaccharides [23]. Endocan is suggested as a marker for vascular pathogenesis [24]. We observed lower serum endocan levels in patients with metabolic syndrome compared to the healthy controls (Figure 1). Our results differ from the results obtained in the previous studies with endocan which demonstrated high endocan levels in cardiovascular disease, neoplasms, and sepsis [25]. In a recent meta-analysis, the highest levels of serum endocan were reported in patients with known cardiovascular disease (CVD), which suggests a close association with CVD [26]. On the other hand, our study is the first which investigates serum endocan in metabolic syndrome patients in association with EFT thickness. Our results seem contradictory to the literature; indeed our metabolic syndrome patients do not have a previous cardiovascular disease history, which may explain the low levels of endocan in the patient group. On the other hand, we do not have enough data about the drug and serum endocan relationship that may interfere with endocan serum concentration.
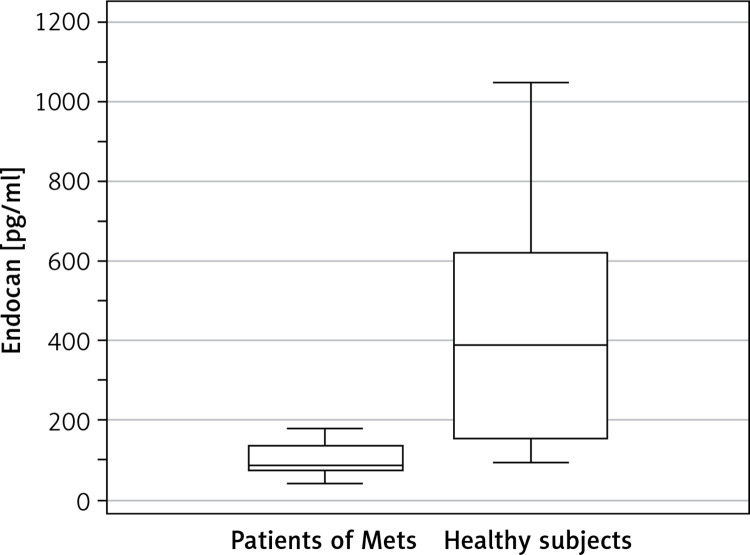
Figure 1.
Serum endocan levels according to the presence of metabolic syndrome (Mets). Mean serum endocan concentration in patients of Mets was 120.71 ±90.17 pg/ml. Mean serum endocan concentration in healthy subjects was 414.59 ±277.57 pg/ml (p < 0.001)
It was demonstrated that endocan, previously called endothelial cell specific molecule-1 (ESM-1), is also present in adipose tissue; however, ESM-1 adipocyte gene expression and circulating plasma levels are not correlated. Moreover, in the same study, Janke et al. found lower serum endocan levels in overweight and obese women when compared to lean women, similar to our results in metabolic syndrome patients. They hypothesized that due to loss of a vasoprotective factor, obesity could be associated with decreased ESM-1 formation in adipose tissue and decreased endocan levels in plasma [27]. In the same manner we found that waist circumference is the factor most strongly associated with serum endocan among metabolic syndrome criteria. Increased waist circumference is found to be associated with low serum endocan levels, which supports the findings of Janke et al. Other data from a recent study revealed low serum endocan levels in metabolic syndrome patients with hepatosteatosis [28]. According to our results and previous data, we can speculate that sites other than adipose tissue may dominate endocan serum levels.
Epicardial fat tissue is a specialized type of visceral fat tissue located between the myocardium and pericardium that shares coronary blood supply. It is related directly to cardiovascular disease development under pathological circumstances through triggering vasocrine or paracrine secretion of proinflammatory cytokines [6]. EFT thickness is found to be associated with pro-atherogenic biomarkers such as leptin, angiotensin II and nitric oxide, which results in endothelial damage through generation of reactive oxygen radicals [29]. Moreover, EFT can exert a mechanical effect on coronary arteries that leads to expansion of the coronary arteries with atherosclerotic lesions [30].
Visceral fat tissue plays an important role in the development of metabolic syndrome. Studies suggest that EFT thickness measurement is a reliable indicator of visceral obesity, which enables the use of EFT thickness in cardiovascular outcome prediction of metabolic syndrome patients [31, 32]. In the light of these data we investigated the associations between EFT thickness and clinical indicators of metabolic syndrome. EFT was thicker in subjects with metabolic syndrome than those without metabolic syndrome (6.3 vs. 5.2 mm, p = 0.043). EFT thickness can be assessed by echocardiography, cardiac magnetic resonance imaging (MRI) and multidetector computed tomography (MDCT) [33]. Recent studies demonstrated asymmetric distribution of EFT and hypothesized that the importance of the EFT thickness may vary according to the regions [34]. In a similar manner, Song et al. demonstrated that EFT thickness calculated by MDCT, especially in the left atrioventricular groove (LAVG), is associated with metabolic syndrome [13]. Different threshold values for EFT thickness are mentioned until today. In 2013 the cut-off point of EFT thickness for predicting metabolic syndrome in Venezuelan population was suggested as more than 5 mm [35]. Lacobellis et al. found that the thickness of the epicardial fat tissue on the right ventricle varies between 4 and 17.4 mm in obese patients [36]. Our echocardiographic measurements of EFT thicknesses are similar to most of the other studies in this field.
EFT thickness is found to be correlated with age and weight but not with BMI, waist circumference or other parameters of metabolic syndrome. There are various data about the EFT thickness and BMI association. Some of them suggest a strong relation between BMI and EFT thickness [37]. However, Lacobellis et al. suggested that EFT thickness is related to regional distribution of fat tissue more than BMI [36]. Similarly, in a recent study EFT thickness was found not to be related with BMI and suggested that EAT is more significantly related to systemic inflammation and subclinical atherosclerosis than BMI and waist circumference [37]. In contrast, Xhiong Chen et al. found lower BMI values associated with EAT thickness in a diabetic Chinese population, supporting the hypothesis that EFT thickness is not related to subcutaneous fat [38]. Actually, both individual and ethnic differences in distribution of adipose fat tissue may explain the confounding results in the literature.
The first limitation of our study is the relatively small sample size. The second one is the lack of data about the abdominal visceral fat tissue amount. Previous studies suggested that different distributions of ectopic fat tissue may be associated with various degrees of cardiometabolic risks [39, 40]. Third, precise measurement of EFT thickness with echocardiography is difficult due to anatomical variations, especially in obese patients, which can explain the different results in the literature.
In conclusion, serum endocan concentrations are low in metabolic syndrome patients. EFT thickness is higher in metabolic syndrome patients in comparison to healthy controls, which enables us to perform cardiovascular risk assessment. However, the lack of evidence of a correlation between serum endocan concentration and EFT thickness necessities further studies elucidating the role of serum endocan levels in cardiovascular risk management in metabolic syndrome patients.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Dommermuth R, Ewing K. Metabolic syndrome: systems thinking in hearth disease. Prim Care. 2018;45:109–29. doi: 10.1016/j.pop.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Whaley-Connell A, Sowers JR. Indices of obesity and cardio metabolic risk. Hypertension. 2011;58:991–3. doi: 10.1161/HYPERTENSIONAHA.111.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 5.Polovina MM, Potpara TS. Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med. 2014;126:38–53. doi: 10.3810/pgm.2014.03.2739. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity. 2008;16:887–92. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 9.Scherpereel A, Depontieu F, Grigoriu B, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–7. doi: 10.1097/01.ccm.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- 10.Béchard D, Scherpereel A, Hammad H, et al. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167:3099–106. doi: 10.4049/jimmunol.167.6.3099. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Fan J. Atherosclerosis and inflammation mononuclear cell recruitment and adhesion molecules with reference to the implication of ICAM-1/LFA-1 pathway in atherogenesis. Int J Cardiol. 1998;66:45–53. doi: 10.1016/s0167-5273(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 13.Song DK, Hong YS, Lee H, Oh JY, Sung YA, Kim Y. Increased epicardial adipose tissue thickness in type 2 diabetes mellitus and obesity. Diabetes Metabolism J. 2015;39:405–13. doi: 10.4093/dmj.2015.39.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park HE, Choi SY, Kim M. Association of epicardial fat with left ventricular diastolic function in subjects with metabolic syndrome: assessment using 2-dimensional echocardiography. BMC Cardiovasc Disord. 2014;14:3. doi: 10.1186/1471-2261-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamagata K. Do coffee polyphenols have a preventive action on metabolic syndrome associated endothelial dysfunctions? An assessment of the current evidence. Antioxidants. 2018;7:26. doi: 10.3390/antiox7020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 17.Dada N, Kim NW, Wolfert RL. Lp-PLA2: an emerging biomarker of coronary artery disease. Expert Rev Mol Diagn. 2002;2:17–22. doi: 10.1586/14737159.2.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Brister SJ, Buchanan MR. Effects of linoleic acid and/or marine fish oil supplements on vessel wall thromboresistance in patients undergoing cardiac surgery. Adv Exp Med Biol. 1997;423:275–8. doi: 10.1007/978-1-4899-1810-9_58. [DOI] [PubMed] [Google Scholar]
- 19.Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89:1092–103. doi: 10.1161/hh2401.101272. [DOI] [PubMed] [Google Scholar]
- 20.Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am Coll Cardiol. 2002;39:1429–35. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 22.Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006;72:136–45. doi: 10.1016/j.mvr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Sarrazin S, Adam E, Lyon M, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006;1765:25–37. doi: 10.1016/j.bbcan.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Turan T, Akyuz AR, Aykan AC, et al. Plasma endocan levels in patients with isolated coronary artery ectasia. Angiology. 2016;67:932–6. doi: 10.1177/0003319716637789. [DOI] [PubMed] [Google Scholar]
- 25.Wang XS, Yang W, Luo T. Serum endocan levels are correlated with the presence and severity of coronary artery disease in patients with hypertension. Genet Test Mol Biomarkers. 2015;19:124–7. doi: 10.1089/gtmb.2014.0274. [DOI] [PubMed] [Google Scholar]
- 26.Zhao T, Kecheng Y, Zhao X, et al. The higher serum endocan levels may be a risk factor for the onset of cardiovascular disease: a meta-analysis. Medicine. 2018;97:e13407. doi: 10.1097/MD.0000000000013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janke J, Engeli S, Gorzelniak K, et al. Adipose tissue and circulating endothelial cell specific molecule-1 in human obesity. Horm Metab Res. 2006;38:28–33. doi: 10.1055/s-2006-924973. [DOI] [PubMed] [Google Scholar]
- 28.Erman H, Beydogan E, Cetin SI, Boyuk B. Endocan: a biomarker for hepatosteatosis in patients with metabolic syndrome. Mediators Inflamm. 2020;2020:3534042. doi: 10.1155/2020/3534042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez A, Fortuño A, Gómez-Ambrosi J, Zalba G, Díez J, Frühbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–31. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- 30.Prati F, Arbustini E, Labellarte A, et al. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J. 2003;24:329–36. doi: 10.1016/s0195-668x(02)00426-8. [DOI] [PubMed] [Google Scholar]
- 31.Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 32.Kirac Utku I, Okuturlar Y, Demir E, et al. Relationship between epicardial adipose tissue thickness and vitamin D in patients with metabolic syndrome. Int J Clin Exp Med. 2015;8:5707–14. [PMC free article] [PubMed] [Google Scholar]
- 33.Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43:1651–4. doi: 10.1016/j.biocel.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Liang KW, Tsai IC, Lee WJ, et al. MRI measured epicardial adipose tissue thickness at the right AV groove differentiates inflammatory status in obese men with metabolic syndrome. Obesity. 2012;20:525–32. doi: 10.1038/oby.2011.155. [DOI] [PubMed] [Google Scholar]
- 35.Lima-Martínez MM, Paoli M, Donis JH, Odreman R, Torres C, Iacobellis G. Cut-off point of epicardial adipose tissue thickness for predicting metabolic syndrome in Venezuelan population. Endocrinol Nutr. 2013;60:570–6. doi: 10.1016/j.endonu.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–2. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 37.Erdogan T, Durakoglugil ME, Cetin M, et al. Epicardial tissue predicts carotid intima-media thickness independently of body mass index and waist circumference. Acta Cardiol Sin. 2019;35:32–41. doi: 10.6515/ACS.201901_35(1).20180628A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Wu W, Wang L, et al. Association between 25-hydroxyvitamin D and epicardial adipose tissue in chinese non-obese patients with type 2 diabetes. Med Sci Monit. 2017;23:4304–11. doi: 10.12659/MSM.904755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:837–41. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 40.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–76. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]