Abstract
Introduction
There is a paucity of literature surrounding the in-hospital mortality and associated risk factors among coronavirus disease 2019 (COVID-19) affected patient populations in our geographical area, northern New Jersey.
Material and methods
A retrospective observational cohort study was performed in a tertiary care academic medical center with two locations in Paterson and Wayne serving Passaic County in northern New Jersey. The study included all 900 patients with a positive reverse transcriptase-polymerase chain reaction (RT-PCR) nasopharyngeal swab sample for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) viral test. We determined the in-hospital 75-day mortality of patients treated in the intensive care unit (ICU) compared to the medical-surgical floor unit.
Results
Overall in-hospital 75-day mortality was 40.7% (n = 367). The ICU group had a 77.1% (n = 237) mortality and the floor group a 21.9% (n = 130) mortality. The ICU group of patients had a higher incidence of cardiac injury, acute renal injury, liver failure, vasopressor use and the elevation of serum markers: ferritin, lactate dehydrogenase, interleukin 6 (IL-6), D-dimer, procalcitonin, and C-reactive protein compared to the floor group. Multiple logistic regression analyses revealed that age > 65 years, elevated IL6, acute renal injury, cardiac injury, and invasive mechanical ventilation were risk factors associated with mortality.
Conclusions
Age > 65 years, elevated IL6, acute renal injury, cardiac injury, and invasive mechanical ventilation were risk factors associated with mortality in our COVID-19 patients.
Keywords: COVID-19, mortality, hospitalization, intensive care unit
Introduction
The first cluster of cases of COVID-19 was described in China in December 2019 [1, 2]. Since then, COVID-19 has spread to unprecedented levels globally, causing significant morbidity and mortality. As of August 18, 2020, 22,046,135 cases have been reported globally [1]. Although initial cases were reported in Washington state on January 20th, 2020 [1], the first large-scale outbreak in the US, causing a heavy toll on lives and healthcare resources, was in New York and New Jersey. As of August 18, 2020, the United States of America had reported 169,870 deaths related to COVID-19 [2]. Since the beginning, COVID-19 has posed a myriad of challenges to the scientific community in understanding the transmission, pathophysiology, natural course of the disease, and response to interventions. As we learn more about the virus, we have been adapting and employing newer strategies to improve patient outcomes and contain its spread. With a lack of information on this aptly named “novel coronavirus”, we have to depend on large-scale analyses of COVID-19 patients who we have treated in the past few months to guide us in their optimal management.
Our study aims to describe demographics, presenting symptoms, laboratory features, clinical course, and outcomes and to explore risk factors associated with the death of all COVID-19 patients admitted to our hospital between March 15, 2020, and April 16, 2020, both in the intensive care unit (ICU) and on the regular floors.
Material and methods
This was a retrospective observational study of adult patients with laboratory-confirmed COVID-19 admitted to the St Joseph Healthcare System hospitals, between March 15, 2020, and April 16, 2020. The study proposal was approved by the Institutional Review Board (EX#2020-17). Waiver of consent was obtained as this was a retrospective study without interventions.
This study was conducted at St. Joseph’s Healthcare System, which comprises St. Joseph’s University Medical Center (SJUMC) and St. Joseph’s Wayne Medical Center (SJWMC) serving the population of Passaic County in northern New Jersey. SJUMC is a 698-bed academic tertiary medical center with 28 medical ICU beds. With the rapid influx of patients during this pandemic, the ICU capacity was increased to 86 beds. SJWMC has 16 ICU beds, and an extra unit with 12 ICU beds was added.
We retrospectively identified and enrolled all adult patients over the age of 18 years admitted to the hospital within the timeframe of 03/15/2020 to 04/16/2020, and with a laboratory-confirmed diagnosis of COVID-19. Enrolled patients were further followed until June 30, 2020 to record primary outcomes.
Pretrained personnel manually reviewed the electronic medical record (Cerner Power Chart, Cerner Corporation) of the study group to obtain demographics, past medical history, presenting clinical features, laboratory results, ICU admission status, radiological interpretations, records of interventions, and records of medication administration.
The data were collected using a secure and detailed online questionnaire with required variables to collect data, and a standard definition was used to maintain homogeneity. The questions were formulated based on the clinical patterns and new evidence that was observed and published. Organ injury was recorded in the data based on predefined standard definitions with laboratory and other criteria. The classification published by the National Institutes of Health (NIH) was used to divide patients according to the severity of illness [2].
Testing was done using BioGX SARS-CoV-2 Reagents for the BD MAX System made by Becton, Dickinson, and Company 7 Loveton Circle, Sparks, Maryland-21152 USA. This is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in nasopharyngeal and oropharyngeal swab samples from patients who meet COVID-19 clinical and/or epidemiological criteria.
The primary outcome evaluated was the 75-day in-hospital mortality rate for various patient groups admitted to different levels of care during the principal COVID-19 admission. Secondary outcomes studied were the need for ventilation, vasopressor use, and organ failure and the factors associated with mortality. We analyzed the baseline characteristics and compared them between patient groups.
Statistical analysis
Categorical variables were expressed as counts and percentage of the total population n (%), while continuous variables were reported as medians (IQR). All baseline variables were compared between the ICU and floor groups. The χ2 or Fisher’s exact t-test or the Mann-Whitney U test was used to compare groups as appropriate. Based on emerging evidence, we selected a few variables and ran the univariate analysis to determine their effect on the final outcome of death. A multiple logistic regression analysis model was then developed using the significant variables to give us the most efficient model that determines the independent risk factors for mortality. All statistical tests were two-tailed, and statistical significance was defined as p < 0.05. Analyses were performed using version 3.5.2 of the R programming language.
Results
A total of 900 patients with COVID-19 were admitted to the hospital during this study period and 307 (34.1%) of these patients were admitted to the ICU while 593 (65.9%) were managed on the floors. Of the 900 patients, 717 (79.7%) were older than 50 years, and in this group, 53.4% (n = 383) were in the age group of 50–69 years. A similar trend is seen in the distribution of patients between the ICU and the floors. The median age of admitted patients was 64 years.
There were more male patients (58.8%, n = 529) in the study group, and this majority was noted in both the ICU (66.3%, n = 203) and floor groups (54.9%, n = 326). 38.4% (n = 203) of the total male patients required admission to the ICU while only 27.8% (n = 103) of the female patients required admission to the ICU. The majority of the patients were of Hispanic (46.4%, n = 418) ethnicity, followed by Caucasian (25%, n = 225) and African American (19.4%, n = 175). Both ICU and floor patients displayed a similar pattern of distribution, as noted in Table I.
Table I.
Baseline characteristics and demographics of adult inpatients admitted for COVID-19 at St. Joseph’s Healthcare System
| Baseline characteristics | All patients (n = 900) | ICU (n = 307) | Floor (n = 593) | P-value |
|---|---|---|---|---|
| Age [years]: | ||||
| 18–29 | 19 (2.1%) | 4 (1.3%) | 15 (2.5%) | 0.5382 |
| 30–49 | 164 (18.2%) | 49 (16%) | 115 (19.3%) | 0.5786 |
| 50–69 | 383 (42.5%) | 137 (44.6%) | 246 (41.4%) | 0.73 |
| 70–89 | 314 (34.9%) | 115 (37.4%) | 199 (33.5%) | 0.6432 |
| > 90 | 20 (2.2%) | 2 (0.6%) | 18 (3%) | 0.00005471 |
| Sex: | ||||
| Male | 529 (58.7%) | 203 (66.1%) | 326 (55%) | 0.3131 |
| Female | 371 (41.2%) | 104 (33.9%) | 267 (45%) | 0.2114 |
| Race: | ||||
| African American | 175 (19.4%) | 42 (13.7%) | 133 (22.4%) | 0.1476 |
| Hispanic | 418 (46.4%) | 150 (48.9%) | 268 (45.2%) | 0.7029 |
| Caucasian | 225 (25%) | 82 (26.7%) | 143 (24.1%) | 0.7153 |
| Middle eastern | 14 (1.5%) | 6 (1.9%) | 8 (1.3%) | 0.7373 |
| Asian | 26 (2.8%) | 10 (3.2%) | 16 (2.7%) | 0.8369 |
| Unknown | 42 (4.6%) | 17 (5.5%) | 25 (4.2%) | 0.6985 |
| BMI [kg/m2]: | ||||
| 24.9 and below | 189 (21%) | 57 (18.6%) | 132 (22.2%) | 0.573 |
| Overweight – 25–29.9 | 358 (39.7%) | 116 (37.8%) | 242 (40.8%) | 0.7351 |
| Obese – 30–39.9 | 292 (32.4%) | 105 (34.2%) | 187 (31.5%) | 0.7201 |
| Extreme obesity – Greater than 40 | 61 (6.7%) | 29 (9.4%) | 32 (5.4%) | 0.2985 |
| Comorbidities: | ||||
| Asthma | 63 (7%) | 16 (5.2%) | 47 (7.9%) | 0.4557 |
| Chronic obstructive lung disease | 52 (5.7%) | 19 (6.1%) | 33 (5.5%) | 0.8602 |
| Congestive heart failure | 73 (8.1%) | 25 (8.1%) | 48 (8.1%) | 1 |
| Diabetes | 344 (38.2%) | 127 (41.3%) | 217 (36.6%) | 0.5944 |
| Hypertension | 518 (57.5%) | 177 (57.6%) | 341 (57.5%) | 0.9926 |
| Obstructive sleep apnea | 18 (2%) | 7 (2.3%) | 11 (1.8%) | 0.805 |
| Chronic kidney disease/ESRD on HD | 91 (10.1%) | 21 (6.9%) | 70 (11.8%) | 0.2572 |
| Organ transplant | 7 (0.7%) | 3 (1%) | 4 (0.7%) | 0.818 |
| Liver disease | 10 (1.1%) | 4 (1.3%) | 6 (1%) | 0.8432 |
| Signs and symptoms: | ||||
| Cough | 625 (69.4%) | 192 (62.5%) | 433 (73%) | 0.367 |
| Fever | 538 (59.7%) | 164 (53.4%) | 374 (63%) | 0.3736 |
| Shortness of breath | 660 (73.3%) | 246 (80.1%) | 414 (69.8%) | 0.4002 |
| Diarrhea | 160 (17.7%) | 41 (13.3%) | 119 (20%) | 0.2456 |
| Myalgia | 263 (29.2%) | 85 (27.7%) | 178 (30%) | 0.7621 |
| Loss of taste or smell | 11 (1.2%) | 2 (0.6%) | 9 (1.5%) | 0.5346 |
| Markers: | Median (IQR) | Median (IQR) | Median (IQR) | |
| CRP [mg/l] | 190.9 (150.05) | 270 (143) | 163 (124) | 0.00001 |
| Ferritin [ng/ml] | 884 (1208.25) | 1321 (1832) | 733 (1063) | 0.00001 |
| LDH [U/l] | 476 (363) | 677 (425) | 391 (235) | 0.00001 |
| D dimer [µg/ml] | 3.28 (10.63) | 9.9 (16.4) | 1.99 (3.56) | 0.02179 |
| Procalcitonin [ng/ml] | 0.68 (3.55) | 2.1 (7.64) | 0.3 (1.04) | 0.2453 |
| IL-6 [pg/ml] | 67.75 (88.2) | 109 (136.3) | 52.6 (63.7) | 0.00001 |
| Organ failure: | ||||
| Acute kidney Injury | 315 (35%) | 193 (61%) | 122 (39%) | 1.00E-05 |
| Acute liver enzyme elevation | 199 (22.1%) | 110 (55%) | 89 (45%) | 0.1366 |
| Vasopressor use | 204 (22.6%) | 203 (100%) | 1 (0%) | 2.20E-16 |
| Troponin elevation (0.05 ng/ml) | 344 (38.2%) | 185 (54%) | 159 (46%) | 0.161 |
ICU – intensive care unit, IQR – interquartile range, BMI – body mass index calculated as weight divided by height in meters squared, CRP – C-reactive protein in mg/l (to convert to nm/l, multiply by 9.524), LDH – lactate dehydrogenase in U/l (microkatals per liter, multiply by 0.0167), IL-6 – interleukin 6, D dimer in µg/ml (to convert to µm/l, multiply by 5.476), ferritin in ng/ml (pmol/l, multiply by 2.247), troponin in ng/ml (to convert troponin to micrograms per liter, multiply by 1; ESD on HD = end-stage renal disease on hemodialysis. P-value is the comparison between ICU and floor groups of patients. Cardiac injury defined as elevation of troponin above 0.05 ng/ml, renal injury defined as acute increase in serum creatinine > 0.3 mg/dl above baseline or new end stage renal disease, shock defined as vasopressor use, liver failure defined as elevation of liver function tests three times the upper limit of normal.
Hypertension was present in 57.6% (n = 518), obesity (body mass index greater than 30 kg/m2) in 39.2% (n = 353), diabetes in 38.2% (n = 344), chronic kidney disease/end stage renal disease in 10.1% (n = 91), congestive heart failure in 8.1% (n = 73), bronchial asthma in 7% (n = 63), and chronic obstructive pulmonary disease in 5.8% (n = 52). No major difference was noted in any of the comorbidities when comparing between patients in the ICU and floor groups (Table I).
The most common presenting symptom was shortness of breath (73.3%, n = 660), which was followed by cough (69.4%, n = 625). Shortness of breath was the most common presentation for ICU patients (n = 245, 79.8% of total ICU admissions) while cough was the most common presenting symptom for the patients on the floors (73.2%, n = 434).
Inflammatory markers including C-reactive protein (CRP), interleukin-6 (IL-6), lactic dehydrogenase (LDH), ferritin and D dimer were analyzed, and all of these inflammatory markers were significantly elevated in the ICU patients when compared to the floor patients (Table I).
Cardiac injury with elevated troponin (> 0.05 ng/ml) was present in 51.2% (n = 461) and renal failure in 35.1% (n = 316). Two hundred fifty-seven (28.5%) patients required invasive mechanical ventilation (IMV). One hundred forty-five (16.1%) patients were managed on BiPAP/AVAPS. Three hundred twenty-three (35.9%) patients were on high flow nasal cannula (HFNC).
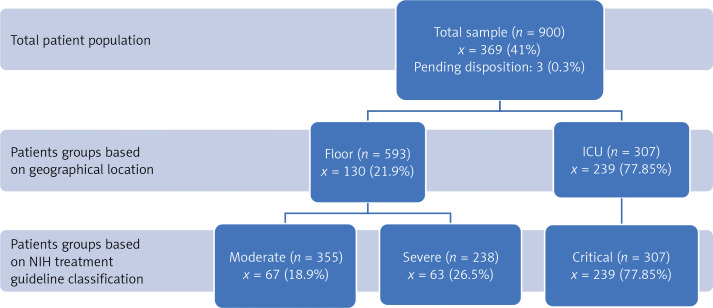
The primary outcomes of death and discharge from the hospital were recorded with the initial data collection, and then a follow-up was done at 75 days to record outcomes. Of the total of 900 patients, 530 (58.9%) were discharged from the hospital, 367 (40.7%) died and 3 (0.3%) patients remained in the hospital at the end of the study. The mortality of the ICU group was 77.85% (239), which includes 11.4% (35) patients who were transitioned to comfort care. Mortality of the floor group was 21.9% (130) including 6% (35) patients who were transitioned to comfort care measures. We did a secondary analysis of our patient-based severity criteria published by the National Institutes of Health (NIH classification) [6] which revealed that the mortality in the critical group is highest (77.85%, 239) as compared to the moderate (18.9%, 67) and severe (26.5%, 63) groups (Figure 1).
Figure 1.
Graphical depiction of distribution of patients between the two major lines of service including ICU and regular medical surgical floors with the distributions. The classification of severity groups was based on NIH (National Institutes of Health) COVID-19 (coronavirus disease 19) treatment guidelines. Primary outcome of in-hospital 75-day mortality among patient groups
ICU – intensive care unit, n is the total number of patients in each group, x is the number of deaths (percentage of total population in the respective group). *Patients divided into ICU and floor care groups based on their geographical location during inpatient stay.
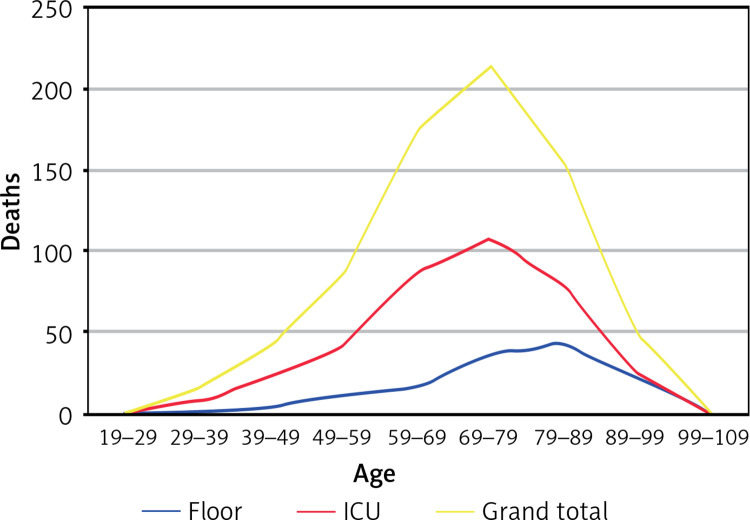
Figure 2 shows deaths based on the placement of patients on location of admission as regular medical surgical floors vs. ICU.
Figure 2.
Incidence of death age groups and different patient groups. . Deaths shown based on the placement of patients on location of admission as regular medical surgical floors vs. ICU
A model was developed to evaluate the effects of independent covariates against the outcome of death. Covariates were age, gender, comorbidities, D-dimer, ferritin, CRP, LDH, procalcitonin, IL-6, acute renal injury, liver failure, vasopressor use, cardiac injury or ventilator use. Age > 65 years, elevated IL-6, IMV, acute renal injury, and cardiac injury were significant risk factors associated with death (Table II).
Table II.
Multiple logistic regression analysis to evaluate risk factors associated with outcome of mortality
| Covariates | Logistic regression coefficients (95% CI) | P-value | Multiple logistic regression coefficients (95% CI) | P-value |
|---|---|---|---|---|
| Age above 65 years | 1.23 (0.95–1.51) | < 0.00001 | 1.68 (0.98–2.38) | < 0.00001 |
| Vent use | 2.62 (2.25–2.97) | < 0.00001 | 2.52 (1.81–3.22) | < 0.00001 |
| Renal injury | –12.37 (–1077.8 – 1053.1) | 0.982 | 1.81 (1.72–2.45) | < 0.00001 |
| Cardiac injury above 0.05 | 1.77 (1.42–2.11) | < 0.00001 | 1.36 (0.67–1.99) | < 0.00001 |
| IL-6 | 0.0025 (0.001–0.004) | 0.000151 | 0.0013 (0.0006–0.002) | 0.000484 |
Discussion
New Jersey was one of the hardest hit states during the pandemic, with 14,086 COVID-19 related deaths at the time of our study [3]. The study period was undoubtedly the peak phase when local healthcare systems experienced a surge in admissions and hospital resources were stretched significantly. In terms of scale, at the peak, on April 13, 2020, SJUMC had 334 hospitalized COVID-19 patients, of whom 114 were on IMV.
The median age of the patients in this cohort was 64 years, which was similar to the other large-scale studies from Italy [3], New York City [3], Seattle [3], and the United Kingdom [3] and higher than the study from China, where the reported median age was 47 years [3]. Males were predominant in all these studies. Our data indicate that the death rate is higher in the older age group (Figure 2). Higher age groups were also noted to have increased risk of developing ARDS (acute respiratory distress syndrome), requiring ICU care and IMV.
Racial and ethnicity distribution demonstrated a predominance of Hispanic patients, with a total of 418 (46.4%) patients. A similar trend existed across both ICU and floor patients. The Caucasian population in Passaic County is about 40.6%, and the Hispanic population is approximately 39.9% of the total population [1]. However, the number of Hispanic patients admitted to the hospital with COVID-19 (46.4%, n = 418) was almost twice as large as the number of Caucasian patients (25%, n = 225) in our study. Further studies are required to analyze the reasons behind these disparities. However, we did not see any significant mortality difference across various ethnicities as in many recent studies including a study by Gross et al. [4].
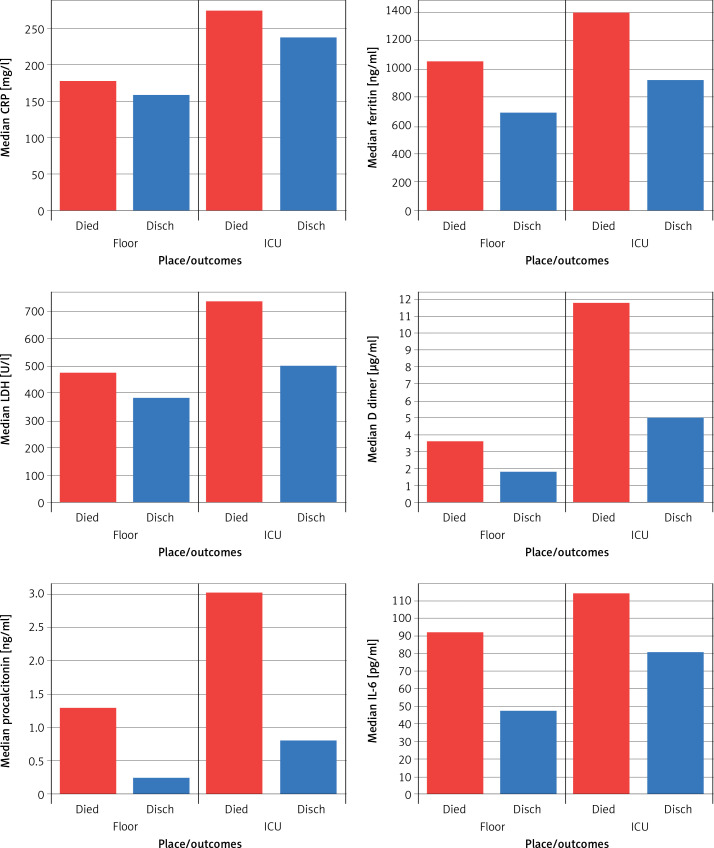
Hypertension was not associated with a poor outcome. However, Lippi et al. [5] and Huang et al. [6] showed that hypertension was associated with an increased risk of severe or fatal COVID-19 pneumonia. Inflammatory markers were significantly elevated in the ICU and also associated with mortality (Figure 3).
Figure 3.
Trends of inflammatory markers among patient groups (ICU, floor) and survivors vs. non-survivors. Graphical comparison of inflammatory markers among patient groups in the ICU and the floors and between survivors and non-survivors. The values were compared amongst their median values
CRP – C-reactive protein, IL-6 – interleukin-6, LDH – lactate dehydrogenase.
Cardiac injury was the second most common organ injury noted after respiratory failure. It is postulated that COVID19 infection related myocardial injury usually occurs especially due to non-ischemic myocardial processes, including hypoxia, sepsis, systemic inflammation, pulmonary thromboembolism, cardiac adrenergic hyperstimulation during cytokine storm syndrome, and myocarditis [4]. Reliance on echocardiographic evaluation and perhaps point of care critical care ultrasound studies should be considered to look for cardiac contractility and wall motion abnormalities [4].
Renal insufficiency is another common complication in a COVID-19 affected patient population. There are multiple reasons as to this development. There are increased levels of ACE2 receptors in kidney tissues, leading to direct cellular injury with viral entry [4]. An older age group, patients with underlying comorbidities, and hemodynamic alterations due to systemic challenges with infection are also factors.
During the time frame of the study, patients were admitted only if they were hypoxic, and other patients with no major physiological or laboratory derangements were discharged home with self-quarantine and outpatient follow-up. Thus, almost all patients admitted to the hospital required some form of supplemental oxygen. At the beginning of the pandemic, the frequency and choice of non-invasive ventilation (NIV) were constrained, since it was discouraged by multiple societies because of the aerosolization risk [4]. In spite of this, 468 (52%) of the total cohort were on NIV (HFNC and BIPAP/AVAPS) and 182 (38%) of them eventually required IMV. A total of 275 (28.5%) patients required IMV in our study population, and the rate is similar to other studies [4]. The pathology of ARDS specific to this viral infection is unclear [4]. Some very common features were thrombotic microangiopathy, pulmonary thromboses, late fibrosis among other typical features of ARDS [4]. Due to overwhelmingly increased ventilator requirements, single mode and emergency use ventilators were used when adequate full-fledged ventilators were not available. This was a problem that critical care physicians encountered not just in our hospital but across the world. Individual hospital policy for ventilator triage and allocation should be set up in case emergency arises.
This pandemic brought into sharp focus some ethical dilemmas surrounding respiratory failure and mechanical ventilation of elderly patient groups [4]. Our palliative care team provided invaluable support helping us and families navigate through this period of fear and uncertainty. 7.9% (n = 71) of the study population group elected to pursue only comfort care measures when the clinical course deteriorated.
This study concerns one of the largest samples of hospitalized COVID-19 patients including the critically ill in northern New Jersey. The follow-up on the cohort of our patients was complete. This gives a better insight of in-hospital outcomes as compared to other studies. Our study population includes different ethnicities, and our healthcare system is a community-based academic center.
Limitations of our study include the fact that it is an observational study during a pandemic. Since data entry was done initially, and subsequent follow-up was done only for outcomes, some variables may have changed significantly over the clinical course of patients.
In conclusion, among patients hospitalized with COVID-19 illness, older male patients were more common. Approximately one-third of hospitalized patients were critically ill and required IMV. Mortality was significantly higher in patients requiring ICU level of care and IMV.
Acknowledgments
We wish to thank Stacy Lee4, Robin Craven4, Gabriella Messina4, Amy DeSilva4, Jerry Doffe4, Lama Chaddad5, and Anthony Baccay6 for their assistance in performing this study.
4St. George’s University – School of Medicine, University Centre Grenada, West Indies, Grenada
5William Paterson University, Wayne, New Jersey
6St. Joseph’s University Medical Center, Paterson, New Jersey
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Holshue ML, Debolt C, Lindquist S, et al. First Case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [August 18, 2020]
- 3.atUSA https://datausa.io/profile/geo/passaic-county-nj#about Accessed [August 18,2020]
- 4.Chu Q, Correa R, Henry TL, et al. Reallocating ventilators during the coronavirus disease 2019 pandemic: is it ethical? Surgery. 2020;168:388–91. doi: 10.1016/j.surg.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
- 6. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/