The respiratory epithelium is characterized by a remarkable capacity to robustly respond to injury and regenerate (1). This response is mediated by region-specific stem or progenitor cells, such as airway basal stem cells and type II alveolar epithelial cells (AEC2s), but it is blunted in chronic and/or progressive lung disease, such as chronic obstructive pulmonary disease. Hence, a variety of approaches for lung epithelial regeneration are currently under investigation from enhanced recovery of functional human cadaveric lungs for transplantation (2) to pluripotent stem cell (PSC)-based replacement therapies (3).
Blastocyst complementation is another approach for the generation of functional tissues that has been gaining traction in the last decade. Initially described in the context of lymphoid development (4), blastocyst complementation is a particular case of tissue chimerism in which the injection of PSCs in an early embryo (blastocyst stage) results in the contribution of the donor cells to various tissues and organs postnatally (5). The deletion of transcription factors important for lineage formation in recipient embryos creates a tissue- or organ-specific niche that allows the formation of the respective parenchyma predominantly from injected PSCs. Since the first groundbreaking demonstration of solid organ generation (mouse pancreas in rats) (6), studies of intraspecies and interspecies blastocyst complementation have investigated questions of developmental potency and paved the road for future generation of xenogeneic (human) organs in large animal recipients. Overcoming the numerous technical hurdles involved in interspecies blastocyst complementation will open new avenues for precision-medicine studies in chimeric animal models and curative organ-replacement therapies.
In this issue of the Journal, Wen and colleagues (pp. 471–483) describe the highly efficient derivation of foregut epithelia (lung and thyroid) in mice by intraspecies blastocyst complementation of Nkx2-1−/− embryos (7). Deleting the transcription factor Nkx2-1 (NK2 homeobox 1), previously known as Ttf-1, to vacate the thyroid and lung niches is a judicious choice given its important role in the development of the forebrain, thyroid, and respiratory system (8). Nkx2-1+ lung primordial progenitors give rise to all lung epithelial lineages (9), and Nkx2-1 embryonic deletion results in severe lung defects, such as tracheoesophageal fistula and hypoplastic, cyst-like lungs lacking most epithelial cell types (10).
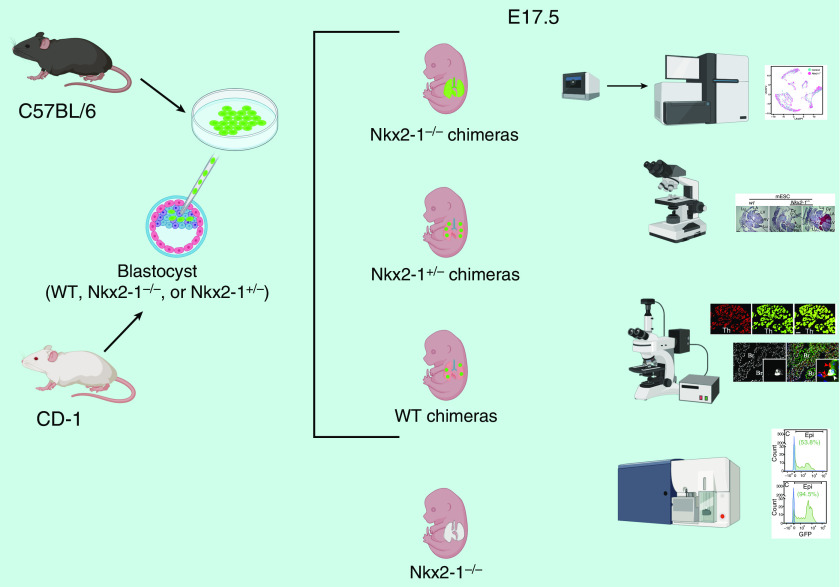
In the present study, blastocyst complementation was performed by injecting GFP (green fluorescent protein)-labeled mouse embryonic stem cells (ESCs) in wild-type, Nkx2-1+/−, and Nkx2-1−/− embryos (Figure 1). Although noncomplemented Nkx2-1−/− embryos displayed the aforementioned defects, Nkx2-1−/− chimeras exhibited restored lung morphogenesis and epithelial cell organization, with GFP-labeled type I alveolar epithelial cells and AEC2s lining the alveolar space and ciliated and club cells lining the conducting airways. Goblet and basal cells were also present, indicating that donor ESCs were able to contribute to major lung epithelial lineages. Morphometric and histological analysis was reinforced by single-cell RNA sequencing and flow cytometry, with the overall data strongly indicating that 1) the vast majority of lung epithelial cells in Nkx2-1−/− chimeras were derived from donor ESCs and 2) the donor cells were indistinguishable from endogenous cells (in Nkx2-1+/− chimeras) in terms of cell distribution, lineage-specific gene and protein expression, and ultrastructural features, such as AEC2 lamellar bodies. Interestingly, blastocyst complementation also fully restored the thyrocyte compartment of the thyroid gland, which is absent in Nkx2-1−/− embryos because of apoptosis of early thyroid progenitors (11). On the other hand, defects in dorsoventral patterning in the trachea and esophagus were not reversed, and the contribution of donor ESCs to forebrain-derived structures, such as diencephalon and corpus striatum, was minimal. Either or both of the latter observations may explain the inability of Nkx2-1−/− chimeras to survive postnatally.
Figure 1.
Schematic of methodology and major findings in the study by Wen and colleagues (7). E17.5 = Embryonic Day 17.5; WT = wild-type.
The current study, together with two recent studies that used similar strategies to vacate the pulmonary niche (12, 13), firmly establishes blastocyst complementation as a viable research strategy for lung developmental studies, disease modeling, and, in the long term, generation of xenogeneic, transplantable human lungs. One can envision future mechanistic studies of pathways with putative roles in lung epithelial specification or progenitor expansion and differentiation using a conditional gene ablation strategy within the anterior foregut endoderm (12). In addition, patient-specific induced PSCs (e.g., from patients with pulmonary arterial hypertension) can be used in the creation of interspecies chimeras for human disease modeling in laboratory animals.
Interspecies blastocyst complementation in the lung will bring about an entirely different host of ethical and scientific problems, especially in chimeras containing human-derived material (14). Salient questions of organ size control, efficiency of donor cell engraftment, and functionality of the chimeric organ will need to be systematically addressed for this approach to be successfully used in basic research and organ manufacturing. Most importantly, it is unlikely that Nkx2-1 deletion will be an ethically acceptable strategy in creation of animal–human chimeras, as Nkx2-1+ forebrain progenitors give rise to neurons within structures such as the striatum, cerebral cortex, and pituitary (15). A region-targeted approach, such as conceptus complementation, may be preferable in this context for the formation of human lungs or thyroid in such chimeras.
Overall, the study by Wen and colleagues provides compelling proof of principle as to the possibility of efficient lung and thyroid epithelial reconstitution after blastocyst complementation in mice. Future studies will most certainly delve into the complexities of interspecies chimeras and establish whether this approach will find its place in the armamentarium of lung regenerative medicine.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202009-3548ED on October 26, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hozain AE, O’Neill JD, Pinezich MR, Tipograf Y, Donocoff R, Cunningham KM, et al. Xenogeneic cross-circulation for extracorporeal recovery of injured human lungs. Nat Med. 2020;26:1102–1113. doi: 10.1038/s41591-020-0971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikonomou L, Wagner DE, Turner L, Weiss DJ. Translating basic research into safe and effective cell-based treatments for respiratory diseases. Ann Am Thorac Soc. 2019;16:657–668. doi: 10.1513/AnnalsATS.201812-890CME. [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suchy F, Nakauchi H. Lessons from interspecies mammalian chimeras. In: Schekman R, editor. Annual review of cell and developmental biology. Vol. 33. Palo Alto, CA: Annual Reviews; 2017. pp. 203–217. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 7. Wen B, Li E, Ustiyan V, Wang G, Guo M, Na C-L, et al. In vivo generation of lung and thyroid tissues from embryonic stem cells using blastocyst complementation. Am J Respir Crit Care Med. 2021;203:471–483. doi: 10.1164/rccm.201909-1836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 9. Ikonomou L, Herriges MJ, Lewandowski SL, Marsland R, III, Villacorta-Martin C, Caballero IS, et al. The in vivo genetic program of murine primordial lung epithelial progenitors. Nat Commun. 2020;11:635. doi: 10.1038/s41467-020-14348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 11. Kimura S, Ward JM, Minoo P. Thyroid-specific enhancer-binding protein/thyroid transcription factor 1 is not required for the initial specification of the thyroid and lung primordia. Biochimie. 1999;81:321–327. doi: 10.1016/s0300-9084(99)80077-7. [DOI] [PubMed] [Google Scholar]
- 12. Mori M, Furuhashi K, Danielsson JA, Hirata Y, Kakiuchi M, Lin CS, et al. Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells. Nat Med. 2019;25:1691–1698. doi: 10.1038/s41591-019-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitahara A, Ran Q, Oda K, Yasue A, Abe M, Ye X, et al. Generation of lungs by blastocyst complementation in apneumic Fgf10-deficient mice. Cell Rep. 2020;31:107626. doi: 10.1016/j.celrep.2020.107626. [DOI] [PubMed] [Google Scholar]
- 14.The Academy of Medical Sciences. Animals containing human material. London: The Academy of Medical Sciences; 2011. [Google Scholar]
- 15. Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.