Abstract
Rationale: Aberrant lung remodeling in idiopathic pulmonary fibrosis (IPF) is characterized by elevated MMP9 (matrix metalloproteinase 9) expression, but the precise role of this matrix metalloproteinase in this disease has yet to be fully elucidated.
Objectives: To evaluate antifibrotic effects of MMP9 inhibition on IPF.
Methods: Quantitative genomic, proteomic, and functional analyses both in vitro and in vivo were used to determine MMP9 expression in IPF cells and the effects of MMP9 inhibition on profibrotic mechanisms.
Measurements and Main Results: In the present study, we demonstrate that MMP9 expression was increased in airway basal cell (ABC)-like cells from IPF lungs compared with ABC cells from normal lungs. The inhibition of MMP9 activity with an anti-MMP9 antibody, andecaliximab, blocked TGF-β1 (transforming growth factor β1)-induced Smad2 phosphorylation. However, in a subset of cells from patients with IPF, TGF-β1 activation in their ABC-like cells was unaffected or enhanced by MMP9 blockade (i.e., nonresponders). Further analysis of nonresponder ABC-like cells treated with andecaliximab revealed an association with type 1 IFN expression, and the addition of IFNα to these cells modulated both MMP9 expression and TGF-β1 activation. Finally, the inhibition of MMP9 ameliorated pulmonary fibrosis induced by responder lung cells but not a nonresponder in a humanized immunodeficient mouse model of IPF.
Conclusions: Together, these data demonstrate that MMP9 regulates the activation of ABC-like cells in IPF and that targeting this MMP might be beneficial to a subset of patients with IPF who show sufficient expression of type 1 IFNs.
Keywords: idiopathic pulmonary fibrosis, airway basal cells, metalloproteinase 9, andecaliximab, type I IFNs
At a Glance Commentary
Scientific Knowledge on the Subject
The antifibrotic effects of andecaliximab (a monoclonal antibody directed against human MMP9 [matrix metalloproteinase 9]) in in vitro and in vivo translational models of idiopathic pulmonary fibrosis were associated with the level of expression of type 1 IFNs.
What This Study Adds to the Field
Targeting effects of mAb against MMP9 in in vitro and in vivo models of idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a lung-scarring disease that typically manifests in adults (1). Although the availability of U.S. Food and Drug Administration–approved drug therapies has extended the median survival of patients with IPF (2), patients continue to experience symptoms that negatively impact quality of life (3). Disease triggers in IPF are unknown, but growing evidence suggests that recurring lung injury drives aberrant lung epithelial activation, leading to the production of mediators that activate myofibroblasts, which deposit excess extracellular matrix (1).
Airway basal cells (ABCs) are progenitor cells that self-renew or differentiate into basal, ciliated, and nonciliated cells within the healthy airway (4, 5). ABCs are located exclusively in the airway, but these cells have been located in the interstitial areas in IPF and hence are referred to as “ABC-like” cells (6). Recently, the expression of ABC-associated genes in cells recovered via BAL was associated with accelerated mortality in IPF (7, 8). We recently described an ABC-like cell population in IPF that is positive for CCR10 (CC chemokine receptor-10), highly expresses profibrotic transcripts, and directly induces lung remodeling in a humanized severe combined immunodeficiency (SCID) mouse model of IPF (9).
MMPs (matrix metalloproteinases) are a family of endopeptidases that regulate extracellular matrix remodeling, activation of latent growth factors, tissue repair, leukocyte behavior, and more (10). MMP9 contributes to the development of fibrosis in some experimental settings (11–14), but its role is less clear in IPF. Increased circulating MMP9 has been associated with a higher composite physiologic index, indicating worse prognosis in IPF (15), and MMP9 has been shown to be a top candidate for distinguishing patients with IPF from control subjects (16). An MMP9 gene polymorphism has been linked to efficacy of immunosuppression in IPF and to combined pulmonary fibrosis and emphysema (17, 18). Moreover, MMP9 promotes TGF-β1 production and activation by airway epithelial cells (19–21). Consequently, therapies aimed at MMP9 might reduce aberrant lung remodeling, thereby providing a novel clinical strategy in IPF.
Herein, we hypothesized that targeting MMP9 in IPF would ameliorate fibrosis. We demonstrate that IPF ABC-like cells express significantly greater MMP9 than normal ABCs, whereas other cells from IPF lungs expressed similar concentrations of MMP9 compared with equivalent cells from normal lung samples. The role of MMP9 in IPF ABC-like cells was examined both in vitro and in vivo using andecaliximab (AB0041; Gilead Sciences), a recombinant chimeric IgG4 monoclonal antibody that targets both latent pro-MMP9 and activated MMP9. Andecaliximab’s antifibrotic effect was associated with a reduction in TGF-β activation in a subset of patients with IPF, and this response was also associated with a prominent type 1 IFN signature in ABC-like cells. Together, our findings highlight a personalized antifibrotic response during the targeting of MMP9 in IPF, and we identify a type 1 IFN signature that distinguishes nonresponders from responders to aMMP9 (anti-MMP9) mAb (monoclonal antibody) therapy. Some of the results of these studies have been previously reported in the form of an abstract (22).
Methods
Study Approval
The institutional review boards at the University of Michigan Medical Center (HUM 00004350) and Cedars-Sinai Medical Center (PRO 00035396 and PRO 00034067) approved all experiments with primary human tissue. Informed consent was obtained from all patients before inclusion in the studies described herein. Cedars-Sinai Medical Center Department of Comparative Medicine approved all mouse studies described herein. All studies were performed in accordance with all relevant guidelines and regulations. COMET-IPF (Correlating Outcomes with Biochemical Markers to Estimate Time-Progression in Idiopathic Pulmonary Fibrosis) participants were diagnosed and recruited as previously described using American Thoracic Society and European Respiratory Society criteria (23). Progression-free survival, the primary combination endpoint for COMET-IPF, was defined as the time from study enrollment to death, acute exacerbation, lung transplant, or relative change in FVC ≥10% or DlCO ≥15%. Peripheral blood mononuclear cells were isolated, RNA-extracted, reverse transcribed to complementary DNA, and applied on a microarray chip. Patients with low and high MMP9 low and high were stratified by the lowest tertile for “MMP9 low” versus the highest two tertiles for “MMP9 high,” and the percentage of survival was calculated according to progression-free survival. The demographics for normal subjects and patients with IPF whose cells were used for the studies described in this report are provided in Table E1 in the online supplement.
Isolation of Mixed Cells from Normal and IPF Explants
Normal and IPF lung explants were acquired from consenting donors. A total of 30 primary isolated lung cells and differentiated epithelial cell lines (i.e., 14 normal and 16 IPF lines) were examined, as detailed in Table E1. Fresh explant cells were isolated as previously described (24). Please see detailed isolation methodology in the online supplement.
Culturing Normal and IPF Lung Explant–derived Airway Epithelial Cells
A tissue culture technique was used to expand human basal and/or basal-like cells from primary epithelial progenitors present in IPF lung explant or normal lung donor samples. Briefly, normal and IPF lung epithelial cells were generated by culturing normal or IPF explant cell suspensions using conditional reprogramming culture conditions (conditionally reprogrammed cell medium [CRC-M]) or in PneumaCult-Ex Plus medium (STEMCELL Technologies) containing 10 μM Y27632 (PEB-M; STEMCELL Technologies) as previously described (9). Please see detailed culturing methodology on the online supplement. In experiments for assessment of TGF-β activation, cells were pretreated with active TGF-β1 (20 ng/ml; R&D Systems) for 24 hours, washed, and treated with human aMMP9 (andecaliximab, 50 μg/ml; Gilead Sciences) or IgG4 (50 μg/ml; Eureka Therapeutics; Biolegend) for an additional 24 hours.
Humanized Mouse Model of Pulmonary Fibrosis
Six- to eight-week-old female pathogen-free, nonobese diabetic Cg-PrkdcSCID IL2rgTm1wil Szi (NSG) mice were purchased from Jackson Laboratories and housed in Cedars-Sinai Medical Center’s high-isolation mouse room. NSG mice were allowed a minimum of 1 week to acclimate, and then each mouse received nothing (i.e., naive or nonhumanized) or approximately 1 × 106 IPF cells isolated from fresh explanted lung samples. Mice were treated twice a week intraperitoneally with either IgG4 or aMMP9 mAbs (both human [i.e., andecaliximab; AB0041; Gilead Sciences] and murine MMP9-specific [AB0046; Gilead Sciences] mAbs) (25) at 20 mg/kg. Treatment began at Day 0 (preventative regimen) or Day 35 (therapeutic regimen) after the intravenous injection of IPF lung cells. Sixty-three days after IPF cell injection and IgG or aMMP9 mAb treatment, BAL fluid and serum were collected for protein analysis, the superior and middle lobes were collected for biochemical hydroxyproline quantification, the inferior lobe and spleens were collected for flow cytometric analysis, the postcaval lobe was collected for quantitative PCR analysis and phosphorylated SMAD2 quantification, and the left lung was collected for histological analysis from each NSG mouse.
Statistics
Analyses were performed using GraphPad Prism (GraphPad Software V7.0c). Data are means ± SD and were assessed for significance by unpaired t test or by nonparametric Mann-Whitney U test as specified on figure legends. Values of P ≤ 0.05 were considered significant.
Results
IPF Lung Epithelial Cells Express Significantly Greater Concentrations of MMP9 Compared with Normal Lung Epithelial Cells
To address the identity of cells expressing MMP9 in IPF, we examined MMP9 transcript expression in mixed cell suspensions from explanted IPF and normal donor lung samples. IPF cells expressed significantly greater concentrations of MMP9 transcript compared with similar cell suspensions from lungs of normal donors (Figure 1A). As shown in Figure 1B, MMP9 protein was highly expressed in IPF lung tissue sections compared with in normal lung tissue sections. In addition when stratified into low-MMP9 and high-MMP9 transcript groups, the latter group of circulating peripheral blood mononuclear cells from patients with IPF (enrolled via the COMET study) (23) was associated with event-free survival in IPF (Figure E1A).
Figure 1.

MMP9 (matrix metalloproteinase 9) expression is elevated on EpCAM+ (epithelial cell adhesion molecule–positive) epithelial cells from patients with idiopathic pulmonary fibrosis (IPF). (A) MMP9 gene expression on normal (n = 5) and IPF (n = 5) lung cellular suspensions cells. (B, left) Representative MMP9 expression in biopsies from patients with IPF and normal donor lung tissue. (B, right) IgG control staining for the same areas. Top and bottom scale bars, 100 μm; middle scale bars, 200 μm. (C–H) Normal and IPF lung cellular suspensions were stained with anti-CD45 (cluster of differentiation 45), anti-EpCAM, and anti-MMP9 antibodies. Depicted are (C and D) dot plots, (E and G) average percentage of MMP9+ cells within EpCAM+ (E) and CD45+ (G) cells, and (F and H) geometric mean fluorescence intensities of MMP9 fluorescence staining on EpCAM+ (F) and CD45+ (H) cells. Data are means ± SDs. P values are as indicated or *P ≤ 0.05 and ***P ≤ 0.001 compared with normal lung samples or cell types when a Mann-Whitney test (A) or an unpaired t test (E–H) was applied. APC/Fire750 = allophycocyanin/Fire750; BV421 = brilliant violet 421; FITC = fluorescein isothiocyanate; FSC-A = forward scatter area; GMFI = geometric mean fluorescence intensity.
Mixed cell suspensions from normal and IPF lungs were stained with fluorescently conjugated antibodies directed against CD45 (cluster of differentiation 45), EpCAM (epithelial cell adhesion molecule), and MMP9 and analyzed by flow cytometry (Figures 1C–1H). Intracellular MMP9 was found in epithelial and immune cells (Figures 1E and 1G), confirming that both cell compartments are sources of MMP9 in IPF lungs. However, a significant increase in the percentage of MMP9+ cells was observed only within the epithelial (EpCAM+CD45−MMP9+) population, but no differences in cellular expression were observed in the leukocyte (EpCAM−CD45+MMP9+) populations between IPF and normal lung explants (Figures 1C and 1D; quantified in Figures 1E and 1G). Consistently, the geometric mean fluorescence intensity of MMP9 was elevated in EpCAM+CD45− cells but not in EpCAM−CD45+ cells from IPF samples compared with normal samples (Figures 1F and 1H, respectively). Because of our recent identification of a profibrotic CCR10+ epithelial population in IPF (9), we examined CCR10 expression in MMP9+ subpopulations and confirmed that MMP9 expressing cells also express CCR10 (Figure E1B), highlighting the potential pathogenic role of these cells. Together, these results indicate that MMP9 expression is elevated in IPF lungs and that MMP9-expressing epithelial cells are potentially pathogenic and more abundant in IPF lungs compared with normal lungs.
TGF-β1 Is a Potent Inducer of MMP9 in IPF
A critical role for TGF-β1 signaling in the development of lung fibrosis is widely accepted (26). In the present study, we used ingenuity pathway analysis (IPA) to identify major upstream regulators and canonical pathways in IPF lung explants, lung biopsies, and cultured EpCAM+ ABC-like cells (9) compared with normal lung samples and cells. Not surprisingly, TGF-β1 was the most highly upregulated transcript in IPF explants (Figure 2A) and surgical lung biopsies (Figure 2B) compared with normal lung samples. Likewise, IPF ABC-like cells exhibited a marked increase in upstream transcriptional regulators (including TGF-β1; Figure 2C) and activated canonical pathways (Figure 2D) compared with normal ABC-like cells. Because normal and IPF ABC-like cells can also be derived and maintained in conditioned medium from senescent fibroblasts (i.e., CRC-M) (9), we explored the effect of CRC-M in the absence and presence of exogenous TGF-β1 on these cells. IPA for upstream regulators and canonical pathways further revealed IPF ABC-like cells exposed to CRC-M showed markedly fewer differences in upstream regulators (Figure E2A) and activated canonical pathways (Figure E2B) compared with normal ABC-like cells also exposed to CRC-M. These findings indicated that the senescent environment reduced the differences between normal and IPF ABC-like cells. Pro- and mature MMP9 were expressed by ABC-like cells (Figure E2C); specifically, when these cells were maintained in PEB-M (i.e., a nonsenescent environment), exogenous treatment with active TGF-β1 increased MMP9 transcript concentrations from approximately 20- to 1,000-fold (Figure E2D), but MMP9 transcript concentrations only increased from approximately 9- to 50-fold in IPF ABC-like cells maintained in CRC-M culture conditions (Figure E2E). Together, these results indicated that the TGF-β1 pathway is active in IPF ABC-like cells and that this growth factor dynamically regulates MMP9 expression in cultured IPF epithelial cells regardless of the culture conditions of these cells.
Figure 2.

TGF-β1 (transforming growth factor β1) is a potent inducer of MMP9 (matrix metalloproteinase 9) transcript expression. (A and B) Upstream regulators from publicly available datasets (GSE24206) comparing idiopathic pulmonary fibrosis (IPF) lung explants (A) or IPF surgical lung biopsies (B) with normal lung tissues. (C and D) Upstream regulators (C) and canonical pathways (D) from cultured epithelial cells previously derived in PneumaCult-Ex Plus medium from normal and IPF lung cellular suspensions. RNA from normal and IPF samples was extracted and subjected to RNA sequencing. Normalized FPKM values and EDGE significant transcripts were calculated using CLC workbench genomics and imported into ingenuity pathway analysis (Qiagen). Activation z-score cutoffs are depicted as a dotted line on each graph. FPKM = fragments per kilobase of transcript per million mapped reads; SLB = surgical lung biopsy.
Targeting MMP9 Modulates TGF-β1 Signaling in Epithelial Cells from a Subset of Patients with IPF
Because of the interplay between MMP9 and TGF-β1 in other cell types (19, 21), we determined the effect of andecaliximab (also referred to as aMMP9) on cultured IPF ABC-like cells. SMAD2 phosphorylation was quantified in ABC-like cells by immunoblotting after treatment with active recombinant TGF-β1 in the presence of IgG or aMMP9. Although IPF cells constitutively express higher concentrations of TGF-β1 than normal cells, the addition of active exogenous TGF-β1 was necessary to induce a positive feedback cascade or amplify the endogenous activation of TGF-β1, thereby allowing for the immunodetection of phosphorylated SMAD2. Surprisingly, the blockade of MMP9 produced two reproducible responses in the IPF cell lines in this study. First, ABC-like cell lines had reduced phosphorylated SMAD2 (pSMAD2) after treatment with aMMP9, and these cells were labeled as “R” or “responders” (Figure 3A; the quantification of Western blots is shown in Figures 3B and 3C). Second, other IPF ABC-like cell lines exhibited an increase in pSMAD2 expression after aMMP9 and TGF-β1 treatment, and these cells were labeled as “NR” or “nonresponders” (Figure 3A; the quantification of Western blots is shown in Figures 3B and 3C). Furthermore, treatment with aMMP9 had an effect on MMP9 gene expression. In ABC-like cells from responders, MMP9 blockade effectively abolished MMP9 transcript expression, whereas in ABC-like cells from nonresponders, MMP9 blockade markedly increased MMP9 transcription compared with IgG treatment (Figure 3D). Thus, compared with the appropriate IgG control, aMMP9 mAb treatment in ABC-like cells either increased MMP9 mRNA concentrations and SMAD2 phosphorylation together or decreased the expression of both factors, and these two analyses were used to identify responders and nonresponders to aMMP9 mAb treatment (Figure E3A).
Figure 3.
Immunoneutralization of MMP9 (matrix metalloproteinase 9) modulates TGF-β (transforming growth factor β) signaling in cultured EpCAM+ (epithelial cell adhesion molecule–positive) epithelial cells from a subset of patients with idiopathic pulmonary fibrosis (IPF). (A–D) IPF epithelial cells were prestimulated with TGF-β (20 ng/ml) for 24 hours and then treated with IgG (50 μg/ml) or andecaliximab (aMMP9 [anti-MMP9]; 50 μg/ml) for an additional 24 hours. (A) Representative blots for pSMAD2, SMAD2, and β-tubulin. (B and C) Western blot analysis of pSMAD2/β-tubulin (B) and pSMAD2/SMAD2 (C) relative protein expression. IPF cell lines were stratified according to the response to aMMP9 in nonresponders (n = 3) and responders (n = 4). (D) MMP9 gene expression of IPF nonresponders (n = 3) and responders (n = 4) to aMMP9 mAb (i.e., andecaliximab). *P ≤ 0.05 when a Mann-Whitney test was applied. (E–H) Ingenuity analysis of IPF epithelial cells from nonresponder and responder cell lines treated with IgG or aMMP9 for 24 hours. RNA was extracted from cells and subject to RNA sequencing analysis. (E) Ingenuity canonical pathway analysis of responder versus nonresponder cells treated with aMMP9. Data shown are the proportion of transcripts from the transcriptomic analysis that are annotated in the ingenuity canonical pathway. The percentage of transcripts that are downregulated or upregulated in each canonical pathway are depicted in green or red, respectively. Canonical pathway analysis cutoff was −log(P value) of 1.5. (F and G) Upstream regulators of IgG and aMMP9-treated responder versus nonresponder cells (F) and upstream regulators present within the IFNA2 family (G). Upstream analysis was performed according to activation z-score, and regulators were sorted from higher to lower scores. (H) Fold change of unique markers in cell lines from both the responder and nonresponder groups treated with aMMP9 mAb. NR = nonresponder; pSMAD2 = phosphorylated SMAD2; R = responder.
To examine whether there were transcriptional differences between responders and nonresponders to aMMP9 treatment that might account for differential responses, cells from both groups were subjected to RNA sequencing and IPA. As shown in Figure 3E, IFN signaling (which includes 13 of 36 upregulated IFN-related genes) was the most differentially active canonical pathway in responders treated with aMMP9 compared with similarly treated nonresponders. Upstream regulator analysis revealed that IFNA2 was the most highly expressed transcript in responders, regardless of treatment (Figure 3F). Further analyses of upstream regulators within the IFNA2 family are depicted in Figure 3G, and again, the expression of many of these regulators were higher in responders than in nonresponders. Validation of the type I IFN signature was performed by quantitative PCR, and these data are summarized in Figure E3B. Finally, we identified which unique transcripts were upregulated and downregulated in aMMP9-treated responders compared with nonresponders, which included mucin-related genes, such as MUC4 and MUC20, and many zinc finger family members (Figure 3H). In agreement with the SMAD2 phosphorylation analysis, aMMP9-treated responder ABC-like cells had reduced TGFBI, a TGF-β1 inducible gene (Figure 3H). Together, these data suggest that the efficacy of aMMP9 antibody treatment aimed at reducing TGF-β1 signaling in ABC-like cells was supported by the presence of IFN-related transcripts in these same cells.
IFNα2, IRF-7, and IFN-induced Chemokines Are Increased in IPF Lung Samples and Cultured Epithelial Cells in Responders
To confirm the observation that IFNα2 was increased in IPF ABC-like cells from responders, immunohistochemical analysis was performed on IPF lung tissue sections. Immunostaining showed increased IFNα2 protein in airways and mucous-containing areas of lung honeycombing (Figure 4A, top right panel) in the responder group of patients with IPF compared with the nonresponder group of patients (Figure 4A, top left panel). Increased IRF-7 protein concentrations were also detected in cultured epithelial cells from responders compared with those from nonresponders (Figure 4B; quantified in Figure 4C). Next, IFN-inducible and IFN-inhibited chemokines were quantified in epithelial cell cultures maintained either in a nonsenescent or senescent environment. Regardless of the tissue culture environment, CXCL10 (CXC motif chemokine ligand 10) and CXCL11 (CXC motif chemokine ligand 11) were both significantly increased in cell-free supernatants from cultures containing responder cells compared with those containing nonresponder cells (Figures 4D and 4E). In contrast, in the same cultures, two CXC chemokines that are negatively regulated by IFN were elevated in cell-free supernatants from nonresponders compared with those from responders (Figures 4D and 4E). Together, these results suggest that differences in the concentrations of IFN, IFN regulatory factor, and IFN-responsive chemokines differed between patients with IPF, particularly when these patients were demarcated by responsiveness to aMMP9 treatment in vitro.
Figure 4.

IFNα2 and IFN-induced chemokines are increased in idiopathic pulmonary fibrosis (IPF) responders (R) to anti-MMP9 (matrix metalloproteinase 9) (aMMP9) monoclonal antibody treatment. (A) Representative immunohistochemical staining of IFNα2 expression in an IPF nonresponder (NR) (top) and responder (bottom) explanted lung sample. IgG control staining for the same areas are shown (top right corner of each image). Scale bars, 100 μm. (B–E) IPF epithelial cells were prestimulated with TGF-β (transforming growth factor β) (20 ng/ml) for 24 hours and then treated with IgG (50 μg/ml) or andecaliximab (aMMP9; 50 μg/ml) for an additional 24 hours. (B and C) Representative electropherogram peaks for IFR-7 (top) and β-tubulin (bottom) expression (B) and relative protein quantification in NR (n = 2) and R (n = 2) airway basal cell (ABC)-like cells grouped regardless of antibody treatment and analyzed using Compass for Simple Western (Protein Simple) (C). (D and E) ABC-like cell lines (n = 3–4) were cultured in PneumaCult-Ex Plus medium (D) or conditionally reprogrammed cell medium (E), and CXCL10 (CXC motif chemokine ligand 10), CXCL11, CXCL2, and CXCL5 were measured in cell-free supernatants using Bio-Plex Multiplex Immunoassay System (pro-human chemokine 40-plex panel; BioRad). Data are means ± SDs. *P ≤ 0.05 compared with NR ABC-like cell lines when a Mann-Whitney test was applied. IRF-7 = IFN regulatory factor 7; MW = molecular weight.
The Addition of Exogenous IFNα2 to Cultured ABC-like Cells Reverses Nonresponsiveness to aMMP9 Treatment
To address the hypothesis that IFNα2 was necessary for responsiveness to a MMP9 mAb treatment, nonresponder ABC-like lines were treated with IgG or aMMP9 in the absence or presence of exogenous IFNα2. The addition of exogenous IFNα2 alone was sufficient to reverse the nonresponsiveness of these cells to aMMP9, as observed by a statistically significant reduction in SMAD2 phosphorylation compared with the vehicle-treated group (Figure 5A; quantified in Figures 5B and 5C). Further validation of these findings was observed in transcript profiles in these cells in the absence or presence of IFNα2 ± IgG or aMMP9 mAb. As expected, the inhibition of MMP9 in the absence of exogenous IFNα2 was associated with increased transcript expression of many profibrotic mediators, including MMP9, TGFB, ACTA2, VIM, PDGFRA, and CCL2 (Figure 5D). In contrast, when IFNα2 was present in the cell cultures, aMMP9 treatment abrogated the increase in transcript expression of many of these same transcripts (Figure 5D). Together, these data demonstrate that IFNα2 is necessary for responsiveness to aMMP9 mAb treatment in ABC-like cells.
Figure 5.
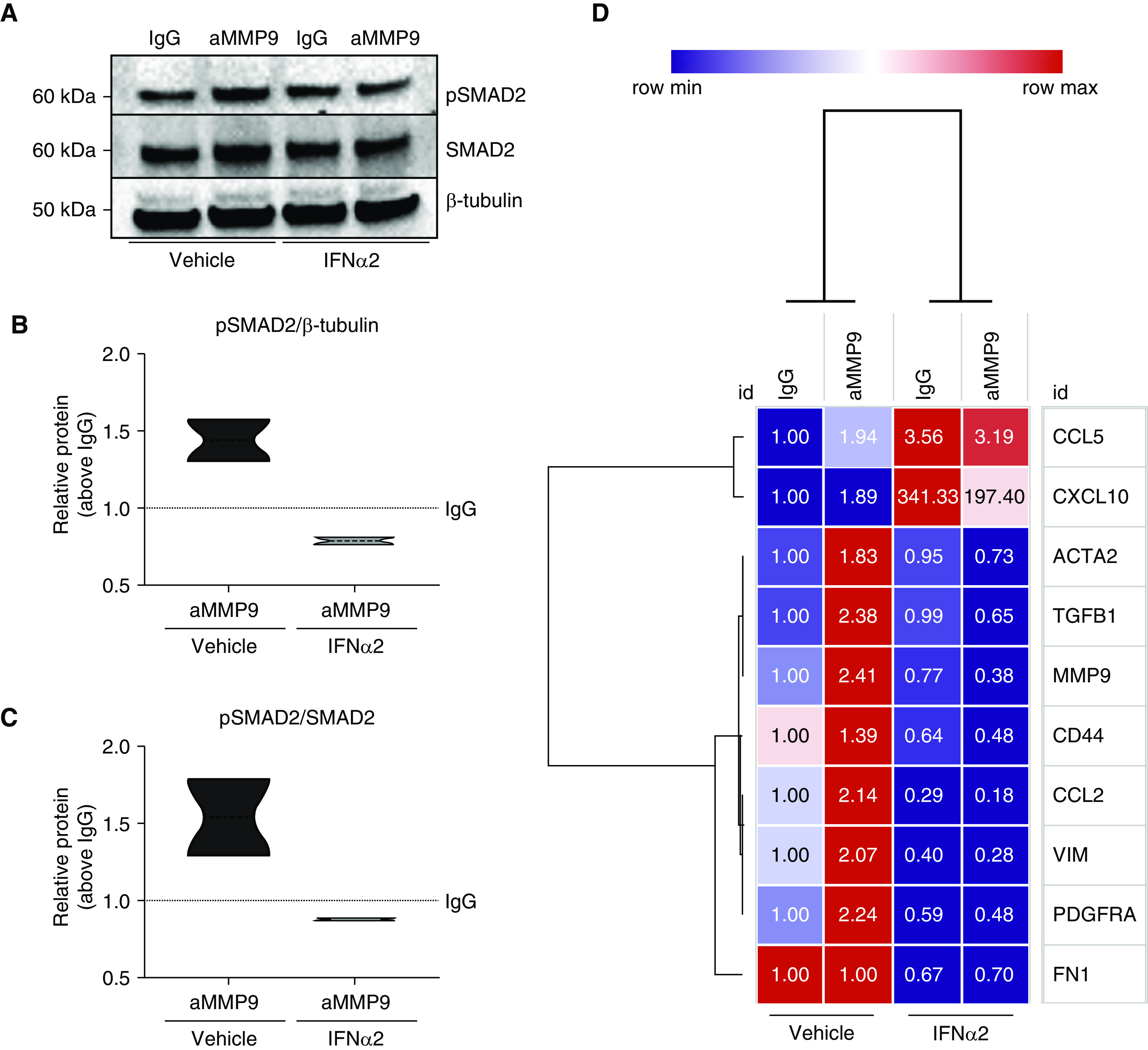
Exogenous IFNα2 added to cultured airway basal cell–like epithelial cells converts nonresponders into responders to anti-MMP9 (matrix metalloproteinase 9) mAb treatment. (A–D) Nonresponder idiopathic pulmonary fibrosis airway basal cell–like cells were activated with TGF-β (transforming growth factor β) (20 ng/ml) for 24 hours and then treated with IgG or andecaliximab (anti-MMP9) with or without the addition of recombinant IFNα2 (1,000 U/ml) for another 24 hours. (A) Representative blots for pSMAD2 (phosphorylated SMAD2), SMAD2, and β-tubulin. (B and C) Western blot analysis of pSMAD2/β-tubulin (B) and pSMAD2/SMAD2 (C) relative protein expression (n = 2). (D) Heatmap depicting gene expression alterations following addition of IFNα2 to cultures of idiopathic pulmonary fibrosis nonresponder cell lines. aMMP9 = anti-MMP9.
Targeting MMP9 in Vivo Ameliorates Fibrosis in the Responder Subset of Patients with IPF
To explore the in vivo antifibrotic effects of aMMP9 antibody treatment, we used a well-characterized humanized nonobese diabetic, SCID, IL-2 receptor γ (NSG) mouse model of IPF (24). In this IPF model, mixed explant lung cell suspensions from a nonresponder patient and a responder patient (based on studies with cultured ABC-like cells shown above) were intravenously injected into separate groups of NSG mice. The percentages of MMP9+ cells in explant lung suspensions are shown in Figure E4A, and we confirmed that MMP9 was present in the human cell preparations before injection into SCID mice. Thirty-five days later, mice were treated with IgG or anti-human (i.e., andecaliximab; Gilead Sciences) + anti-mouse MMP9 mAb (Gilead Sciences) from Day 35 to Day 63 after the injection of IPF cells. We have observed that injected human IPF cells promote the upregulation of murine MMP9 gene expression in the lungs of SCID mice, which peaks at 35 days after injection (Figure E4B), so a mixture of both anti-human and anti-mouse mAbs (25) was administered to the aMMP9-treated groups to remove any confounding effect of mouse MMP9 in our analysis.
At Day 63 after IPF cell injection, NSG mice had increased whole-lung hydroxyproline content compared with naive control NSG mice (Figure 6A). The aMMP9 treatment was efficacious only in the NSG group that received IPF cells from a responder patient (Figure 6A). To evaluate other effects of aMMP9 treatment in this humanized mouse model of IPF, murine SP-C (surfactant protein C) was measured in BAL as a surrogate of type 2 epithelial cell activity. SP-C was decreased in the nonresponder group but increased in the responder group after aMMP9 treatment (Figure 6B). SMAD2 phosphorylation was measured in NSG mouse lung homogenates and was found to be decreased in the responder group treated with aMMP9 mAb compared with in the similarly treated nonresponder group (Figure 6C). Finally, NSG mice that received responder cells expressed higher CXCL10 compared with NSG mice that received nonresponder cells (Figure E4C).
Figure 6.

Targeting MMP9 (matrix metalloproteinase 9) ameliorates fibrosis in a humanized nonobese diabetic, severe combined immunodeficiency, IL-2 receptor γ (NSG) mouse model of idiopathic pulmonary fibrosis (IPF) initiated with the intravenous injection of lung cells from a responder (R) to anti-MMP9 (aMMP9) in vitro. (A–G) Cellular suspensions of IPF lung explants from a nonresponder patient and a responder patient were injected intravenously into NSG mice and allowed to engraft for 35 days. At that time, humanized NSG mice were treated intraperitoneally with IgG (20 mg/kg) and aMMP9 (20 mg/kg) twice a week from Day 35 to Day 63, after which the mice were killed, and their lungs were analyzed for remodeling. (A) The average hydroxyproline content in the superior and middle lobes of NSG mouse lungs. (B) SP-C (surfactant protein C) quantification in BAL fluid by ELISA. (C) Phospho-SMAD2 relative quantification on lung homogenates using a PathScan sandwich ELISA kit. (D) Flow cytometric histograms. (E) The respective percentages of humanized NSG mouse lung suspensions for MMP9 and human CD45 (cluster of differentiation 45), human EpCAM (epithelial cell adhesion molecule), and human CCR10 (CC chemokine receptor 10). (F) Representative Masson’s trichrome staining taken at ×20 magnification of NSG mouse lungs at Day 63 after IPF cell administration. Scale bars, 200 μm. (G) Correlation analyses of hydroxyproline concentration and number of MMP9+ cells in IgG-treated and aMMP9-treated NSG mouse lungs at Day 63 after IPF cell administration from a nonresponder or a responder explanted lung. Data are means ± SEMs; n = 4–5/group. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 when a Mann-Whitney test was applied. FITC = fluorescein isothiocyanate; Hu = human; Hyp = hydroxyproline; NR = nonresponder; PE = phycoerythrin; pSMAD2 = phosphorylated SMAD2.
Other effects of aMMP9 treatment in both groups of humanized NSG mice were observed with flow cytometric analysis of whole lung samples. Specifically, humanized NSG mice that received cells from a responder patient exhibited significantly fewer MMP9+ cells, including MMP9+CCR10+ and MMP9+CD45−EpCAM+CCR10+ subpopulations (Figure 6D; quantified in Figure 6E). In humanized NSG mice that received cells from a nonresponder patient and were treated with aMMP9, MMP9+ subpopulations were unchanged or increased (i.e., MMP9+CD45+CCR10+ cells) compared with the appropriate IgG control group (Figures 6D and 6E). Representative lung sections from all treatment groups are shown in Figure 6F. Nonresponder humanized NSG mice treated with aMMP9 exhibited consistent lung remodeling and foci of alveolar wall thickening that were similar to IgG-treated nonresponder humanized NSG mice. In contrast, NSG mice humanized with mixed lung cells from a responder exhibited less fibrosis after the inhibition of MMP9 compared with IgG-treated NSG mice humanized with cells from the same patient (Figure 6F). The percentage of MMP9+ cells in this group correlated positively with hydroxyproline concentrations after aMMP9 treatment in the nonresponder humanized NSG mouse group, but a negative correlation was observed in the responder humanized NSG mouse group (Figure 6G). In a separate experiment in which NSG mice were humanized with lung cells from a nonresponder patient with IPF, statistically significant positive correlations were observed between mmp9 transcript expression and the lung concentrations of hydroxyproline and the expression of Acta2, Col1a1, Tgfb, Fn1, Pdgfra, and Pdgfrb in the aMMP9-treated group (Figures E4D and E4E). Together, these results demonstrate the antifibrotic efficacy of aMMP9 antibody in a humanized NSG mouse model of IPF, and the efficacy of this therapy in vivo reflected the findings described in vitro pertaining to the effects of andecaliximab on SMAD2 activation and MMP9 expression.
Discussion
The key mechanisms leading to the unrelenting fibrotic process that ultimately robs patients of vital lung capacity in IPF remain poorly understood. Lung ABCs are critical for airway lung homeostasis (4), but recent studies indicate that ABCs or ABC-like cells appear to contribute to pulmonary fibrosis (7, 8, 27). Because MMP9 regulates the generation of TGF-β1 (21), MMP is significantly elevated in IPF versus normal EpCAM+ ABC-like cells (present study) and exogenous TGF-β1 upregulated MMP9 expression in IPF ABC-like cells (present study), we addressed the role of MMP9 in the activation in IPF and normal ABC-like cells. To explore the role of MMP9 in these cells, we employed andecaliximab, a monoclonal antibody developed at Gilead, which selectively binds to allosteric site of MMP9 inhibiting its activity (25, 28). Cultured IPF ABC-like cell lines were identified that either responded (i.e., responders) or failed to respond (i.e., nonresponders) to aMMP9 treatment based on the phosphorylation status of SMAD2 in these cells. Targeting MMP9 in responder ABC-like lines was associated with the downregulation of MMP9, whereas the opposite was observed in nonresponder ABC-like lines. The effect of andecaliximab on these cells was dependent on the endogenous expression of type 1 IFN. In addition, blocking MMP9 activity affected the development of fibrosis in a humanized NSG mouse model of IPF. Whereas aMMP9 mAbs effectively blocked fibrosis in in vivo model elicited using responder IPF cells, this mAb had no effect on the fibrotic response elicited by nonresponder IPF cells. Thus, MMP9 has a profibrotic role, but targeting MMP9 reduced fibrosis in a subset of patients with IPF.
RNA sequencing of normal and IPF ABC-like cells revealed that the type 1 IFN signaling pathway was the most significantly upregulated pathway in responder ABC-like cells compared with nonresponder cells. IFNα2 protein was also increased in both the lung tissue and cultured ABC-like cells from the responder group compared with those from the nonresponder group. Although type 1 IFNs have modulatory roles, including regulating MMP9 expression in immune cells (29, 30), it is not presently clear how these cytokines might be regulating MMP9 expression in lung epithelial cells. We observed key differences in the baseline generation of IFN-inducible non-ELR-CXC chemokines (absence of the ELR motif), CXCL10, and CXCL11, and ELR-CXC chemokines (presence of the ELR motif), CXCL2 (CXC motif chemokine ligand 2), and CXCL5, between responders and nonresponders, suggesting that this might be one mechanism. Responder cell lines expressed significantly more non-ELR-CXC chemokines, whereas the nonresponder lines expressed significantly more ELR-CXC proteins. It is interesting to note that non-ELR-CXC chemokines ameliorate pulmonary fibrosis (31), whereas ELR-CXC chemokines have been shown to contribute to the worsening of fibrotic processes (31). The most compelling evidence that the endogenous generation of type 1 IFN dictated responsiveness to aMMP9 was obtained in vitro. The addition of IFNα to nonresponder ABC-like cells restored responsiveness of these cells to aMMP9, as demonstrated by reduced pSMAD2 concentrations and profibrotic factors. Thus, these data demonstrate that the antifibrotic response to aMMP9 antibody treatment in IPF was dependent on type 1 IFN. In some functional comparisons, the n used was not higher than n = 3–4 per condition or n = 2 per condition or differentiating media on RNA sequencing when comparing IPF responders with nonresponders, which was due mostly to the technical feasibility in differentiating ABC-like cells and running different conditions and control samples (normal and IgG control samples) used for comparison with IPF cells treated with aMMP9. Note that for all in vitro functional analyses, a total of normal (n = 5) and IPF (n = 10) primary lung cells were differentiated into ABC-like cells. Once the IPF group was segregated into responders or nonresponders, n was reduced to n = 3–4 per group for functional assays (i.e., SMAD phosphorylation and transcript analysis). In the context of RNA sequencing, a total of 24 samples were sequenced among normal and IPF responder or nonresponder cell lines, segregated into PEB-M or CRC-M conditions and IgG or aMMP9 treatment. We also have encountered study limitations regarding some semiquantitative measurements on Western blotting and the need to expand ABC-like cells in vitro instead of using primary samples to have the ability to achieve optimal cell numbers to perform the necessary experiments.
In a humanized NSG mouse model of IPF, MMP9 blockade significantly reduced lung remodeling in NSG mice that received responder cells compared with NSG mice that received nonresponder cells. Most notably, pSMAD2 concentrations in the lungs of NSG mice humanized with responder cells were significantly lower than in humanized NSG mice that received nonresponder IPF cells. In addition, the reduction of fibrosis in these mice was associated with the presence of fewer human EpCAM+ epithelial cells expressing both MMP9 and CCR10. We have recently reported that CCR10+ epithelial populations promoted lung fibrosis after intravenous injection into NSG mice (9). However, because MMP9 was present on many lung cell types, we injected immune and nonimmune cells into NSG mice. Although the manner in which the human lung cells induce fibrosis in NSG mice is an active area of investigation, we observed that mouse SP-C concentrations were markedly diminished in NSG mice humanized with IPF cells, suggesting that human cells might target mouse type 2 epithelial cells. MMP9 neutralization in NSG mice humanized with responder cells restored mouse SP-C to concentrations comparable with naive NSG mice, whereas this treatment did not alter mouse SP-C concentrations in NSG mice humanized with nonresponder cells. Although the data obtained from the humanized NSG model of IPF confirmed our in vitro studies regarding differential responsiveness to aMMP9, we recognize that there limitations to this model because of differences in the magnitude of the human cell engraftment between groups of NSG mice and major gaps in our understanding as to the mechanism(s) through which human IPF cells remodel the NSG mouse lung. The fibrotic lung remodeling is clearly heterogenous in this model (as it is in other models), but we consistently note marked and significant changes in histological, biochemical, and molecular markers of fibrosis that provide confidence that this is a valid translational model of pulmonary fibrosis.
Conclusions
Therapeutic targeting approaches in IPF must account for heterogeneities in cell responsiveness in this patient population. In this study, the presence of a type 1 IFN signature determined responsiveness of ABC-like cells in vitro and predicted responsiveness to aMMP9 therapy with andecaliximab in a humanized NSG mouse model of IPF to (see Figure 7 for summary). Clearly, further studies are necessary to validate this ABC-like cell–based approach and to confirm that targeting MMP9 provides clinical benefit to a subset of patients with IPF who exhibit the type 1 IFN transcript signature described herein.
Figure 7.
Production of type I IFNs dictates responsiveness of andecaliximab by idiopathic pulmonary fibrosis airway basal cell–like cells. Idiopathic pulmonary fibrosis lungs are rich in TGF-β1 (transforming growth factor β1), a key growth factor in lung fibrosis that upregulates MMP9 (matrix metalloproteinase 9) transcript concentrations. In a type I IFN–rich environment (i.e., responders; right) that accordingly has increased concentrations of CXCL10 (CXC motif chemokine ligand 10) and CXCL11, blockade of MMP9 reduces TGF-β1 signaling and MMP9 gene expression, culminating in an antifibrotic effect of andecaliximab. In the absence of type 1 IFNs (nonresponders; left), treatment with andecaliximab either does not alter or increases TGF-β1 signaling and MMP9 gene expression, thereby contributing to maintenance of fibrotic processes. IPF = idiopathic pulmonary fibrosis.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Kathy McClinchey and McClinchey Histology Lab Inc. for their histological services.
Footnotes
Supported by funds and reagents from Gilead Sciences, Cedars-Sinai Medical Center, and the NIH (R01HL123899 to C.M.H.).
Author Contributions: Conception and design: M.S.E., A.M.-V., V.S., and C.M.H. Acquisition of data: M.S.E., D.M.H., and A.L.C. Analysis and interpretation of data: M.S.E., D.M.H., D.L., J.O., F.J.M., I.N., and C.M.H. Drafting the manuscript and intellectual content: M.S.E., D.M.H., B.S., W.C.P., and C.M.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-1977OC on October 14, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis. Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. Marked deterioration in the quality of life of patients with idiopathic pulmonary fibrosis during the last two years of life. BMC Pulm Med. 2018;18:172. doi: 10.1186/s12890-018-0738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest. 2012;122:2724–2730. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chilosi M, Poletti V, Murer B, Lestani M, Cancellieri A, Montagna L, et al. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab Invest. 2002;82:1335–1345. doi: 10.1097/01.lab.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 7.Prasse A, Binder H, Schupp JC, Kayser G, Bargagli E, Jaeger B, et al. BAL cell gene expression is indicative of outcome and airway basal cell involvement in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:622–630. doi: 10.1164/rccm.201712-2551OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaykhiev R. Basal-like cells in the BAL fluid: an echo of regenerative crisis in idiopathic pulmonary fibrosis lungs. Am J Respir Crit Care Med. 2019;199:555–557. doi: 10.1164/rccm.201808-1557ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habiel DM, Espindola MS, Jones IC, Coelho AL, Stripp B, Hogaboam CM. CCR10+ epithelial cells from idiopathic pulmonary fibrosis lungs drive remodeling. JCI Insight. 2018;3:e122211. doi: 10.1172/jci.insight.122211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 11.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deatrick KB, Obi A, Luke CE, Elfline MA, Sood V, Upchurch GR, Jr, et al. Matrix metalloproteinase-9 deletion is associated with decreased mid-term vein wall fibrosis in experimental stasis DVT. Thromb Res. 2013;132:360–366. doi: 10.1016/j.thromres.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molyneaux PL, Willis-Owen SAG, Cox MJ, James P, Cowman S, Loebinger M, et al. Host-microbial interactions in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;195:1640–1650. doi: 10.1164/rccm.201607-1408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todd JL, Vinisko R, Liu Y, Neely ML, Overton R, Flaherty KR, et al. IPF-PRO Registry Investigators. Circulating matrix metalloproteinases and tissue metalloproteinase inhibitors in patients with idiopathic pulmonary fibrosis in the multicenter IPF-PRO Registry cohort. BMC Pulm Med. 2020;20:64. doi: 10.1186/s12890-020-1103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Bian W, Gu XH, Shen C. Genetic polymorphism in matrix metalloproteinase-9 and transforming growth factor-β1 and susceptibility to combined pulmonary fibrosis and emphysema in a Chinese population. Kaohsiung J Med Sci. 2017;33:124–129. doi: 10.1016/j.kjms.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HT, Fang SC, Wang CY, Wang W, Wu J, Wang C, et al. MMP-9 1562C>T gene polymorphism and efficacy of glucocorticoid therapy in idiopathic pulmonary fibrosis patients. Genet Test Mol Biomarkers. 2015;19:591–597. doi: 10.1089/gtmb.2015.0057. [DOI] [PubMed] [Google Scholar]
- 19.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 20.Dayer C, Stamenkovic I. Recruitment of matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-β (TGF-β) activation and fibroblast differentiation. J Biol Chem. 2015;290:13763–13778. doi: 10.1074/jbc.M114.622274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perng DW, Chang KT, Su KC, Wu YC, Chen CS, Hsu WH, et al. Matrix metalloprotease-9 induces transforming growth factor-β(1) production in airway epithelium via activation of epidermal growth factor receptors. Life Sci. 2011;89:204–212. doi: 10.1016/j.lfs.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Espindola M, Habiel D, Coelho A, Narayanan R, Jones I, Mikels-Vigdal AJ, et al. Evaluation of the role of anti-mmp9 in idiopathic pulmonary fibrosis [abstract] Am J Respir Crit Care Med. 2017;195:A7251. [Google Scholar]
- 23.Huang Y, Ma SF, Espindola MS, Vij R, Oldham JM, Huffnagle GB, et al. COMET-IPF Investigators. Microbes are associated with host innate immune response in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:208–219. doi: 10.1164/rccm.201607-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habiel DM, Espindola MS, Coelho AL, Hogaboam CM. Modeling idiopathic pulmonary fibrosis in humanized severe combined immunodeficient mice. Am J Pathol. 2018;188:891–903. doi: 10.1016/j.ajpath.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, et al. Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS One. 2015;10:e0127063. doi: 10.1371/journal.pone.0127063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol. 2015;309:L756–L767. doi: 10.1152/ajplung.00238.2015. [DOI] [PubMed] [Google Scholar]
- 27.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juric V, O’Sullivan C, Stefanutti E, Kovalenko M, Greenstein A, Barry-Hamilton V, et al. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS One. 2018;13:e0207255. doi: 10.1371/journal.pone.0207255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benveniste EN, Qin H. Type I interferons as anti-inflammatory mediators. Sci STKE. 2007;2007:pe70. doi: 10.1126/stke.4162007pe70. [DOI] [PubMed] [Google Scholar]
- 30.Khorooshi R, Owens T. Injury-induced type I IFN signaling regulates inflammatory responses in the central nervous system. J Immunol. 2010;185:1258–1264. doi: 10.4049/jimmunol.0901753. [DOI] [PubMed] [Google Scholar]
- 31.Strieter RM, Belperio JA, Burdick MD, Keane MP. CXC chemokines in angiogenesis relevant to chronic fibroproliferation. Curr Drug Targets Inflamm Allergy. 2005;4:23–26. doi: 10.2174/1568010053622902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





