To the Editor:
Right atrial (RA) pacing to increase heart rate (HR) is a therapeutic strategy in acute right ventricular (RV) infarction, resulting in decrease in RA pressure and increases in Q̇ and systemic blood pressure (1, 2). In addition, RA pacing has been shown to increase RV contractility and Q̇ after cardiac surgery (3). However, the acute hemodynamic impact of RA pacing in patients with pulmonary arterial hypertension (PAH) has not been studied. In the current study, we report the hemodynamic response to RA pacing in a cohort of patients with PAH. Some results of this study were previously reported as an abstract presentation (4).
We performed a prospective, single-center study in subjects (age ≥18 yr) referred for right heart catheterization (RHC) for known or suspected PAH between January 2013 and April 2016. The research protocol was approved by the Johns Hopkins Medical Institutional Review Board. Our protocol has been described previously (5). Briefly, subjects underwent cardiac magnetic resonance imaging for RV volume calibration within 5 hours before standard RHC. After RHC, a 5F pressure–volume (PV) catheter (SPC-570–2; Millar Instruments) was inserted. Steady-state PV loops were generated, followed by PV loop repetitions during varying preload reduction. Slope of the end-systolic PV points (end-systolic elastance [Ees]) was calculated and applied to the resting PV loop to determine volume at zero pressure. Effective arterial elastance (Ea) was calculated from end-systolic pressure divided by stroke volume (SV). RV–pulmonary arterial (RV–PA) coupling was calculated by the ratio of Ees:Ea.
Next, a bipolar pacing wire (2.8F, D98500H; Edwards or 4F, 401994; St. Jude’s Medical) was positioned in the right atrium. The pacing rate was set to ∼80–90 beats per minute (bpm), a minimum of 5 bpm above resting HR. PV data were recorded at intervals of 20 bpm up to 120–139 bpm. High-fidelity RV pressure tracings were analyzed for contractile force (change in maximal pressure generated over time [dP/dtmax]), dP/dtmax normalized to the instantaneous pressure (IP) developed (dP/dtmax/IP), and RV end-diastolic volume (EDV) (dP/dtmax/EDV). Q̇ and SV were calculated from conductance catheter volume measurements. Only subjects paced at all intervals were included in this analysis.
Continuous variables are presented as means ± SD. Continuous variables were compared by repeated-measures ANOVA. Assessment of baseline hemodynamic and morphologic modifiers of Q̇ was performed by the use of general linear model repeated-measures covariate analysis. The Greenhouse-Geiser correction was applied to P values if sphericity was violated. A two-tailed P value of less than 0.05 was considered statistically significant. All statistical tests were performed on SPSS version 25.0 (IBM SPSS Statistics).
Of the 32 subjects enrolled, we excluded nine without PAH (pulmonary vascular resistance <3 Wood units or pulmonary artery wedge pressure >15 mm Hg) and seven without data at all pacing intervals. A majority of subjects were receiving PAH therapies at enrollment. The cohort had a mean pulmonary vascular resistance of 6.6 ± 4.2 Wood units with Q̇ of 4.8 ± 1.1 L/min, NTpro-BNP of 403 ± 483 pg/ml, baseline Ees of 0.84 ± 0.47, and mean RV–PA coupling ratio (Ees:Ea) of 1.06 ± 0.60.
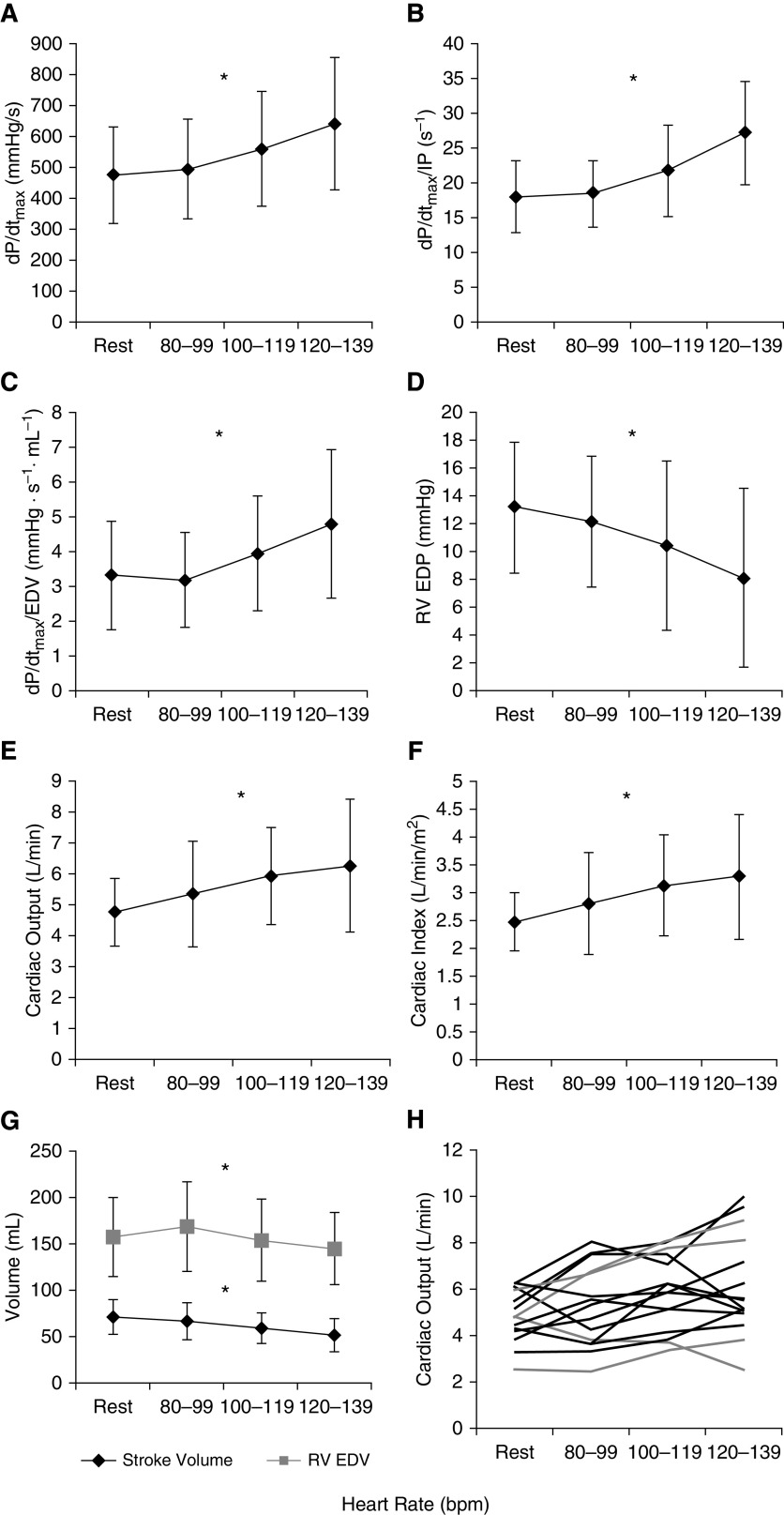
Figure 1 illustrates RV hemodynamics at rest and the three paced HR intervals. As HR increased, RV dP/dtmax, dP/dtmax/IP, and dP/dtmax/EDV increased, and RV end-diastolic pressure (EDP) and EDV decreased (Table 1; P < 0.001 for all). Although SV declined significantly (71 ± 19, 66 ± 20, 59 ± 17, and 51 ± 18 ml, respectively; P < 0.001), there was a significant increase in Q̇ (4.8 ± 1.1, 5.3 ± 1.7, 5.9 ± 1.6, and 6.3 ± 2.1 L/min, respectively; P = 0.02) and cardiac index (P = 0.01; Table 1). PAH diagnosis and RV hemodynamics, including baseline RV–PA coupling, did not modify the relationship between Q̇ and RA pacing rate as covariates (P > 0.05; Table 1). Including subjects without PAH (n = 7) did not significantly alter findings from the main cohort (data not shown).
Figure 1.
(A–C) With an increasing right atrial pacing rate, there was an increase in contractile force rate (change in maximal pressure generated over time [dP/dtmax]; P < 0.05) (A) even when normalized to instantaneous pressure (dP/dtmax/instantaneous pressure; P < 0.05) (B) and right ventricular end-diastolic volume (EDV) (dP/dtmax/EDV; P < 0.05) (C). (D) Right ventricular end-diastolic pressure decreased (P < 0.05). (E–G) Cardiac output (E) and index increased (F) (P < 0.05) above resting state even though stroke volume and EDV decreased (P < 0.05) (G). (H) Changes in cardiac output for each individual subject. *P < 0.05. bpm = beats per minute; EDP = end-diastolic pressure; IP = instantaneous pressure; RV = right ventricular.
Table 1.
RV Hemodynamics and Effect of Baseline Modifiers with RA Pacing
| Subjects with PAH (n = 16) | Resting HR | Paced 80–99 bpm | Paced 100–119 bpm | Paced 120–139 bpm | P Value |
|---|---|---|---|---|---|
| HR, bpm | 71 ± 10 | 82 ± 9 | 102 ± 7 | 123 ± 5 | <0.001 |
| Stroke volume, ml | 71 ± 19 | 66 ± 20 | 59 ± 17 | 51 ± 18 | <0.001 |
| Cardiac output, L/min | 4.8 ± 1.1 | 5.3 ± 1.7 | 5.9 ± 1.6 | 6.3 ± 2.1 | 0.02 |
| Cardiac index, L/min/m2 | 2.7 ± 0.5 | 2.8 ± 0.9 | 3.1 ± 0.9 | 3.3 ± 1.1 | 0.01 |
| dP/dtmax, mm Hg/s | 474 ± 155 | 494 ± 162 | 560 ± 185 | 640 ± 214 | <0.001 |
| dP/dtmax/IP, s−1 | 18.1 ± 5.2 | 18.4 ± 4.7 | 21.8 ± 6.6 | 27.1 ± 7.5 | <0.001 |
| dP/dtmax/EDV, mm Hg/s/ml | 3.3 ± 1.6 | 3.2 ± 1.4 | 3.9 ± 1.6 | 4.8 ± 2.1 | <0.001 |
| RV EDV, ml | 157 ± 42 | 168 ± 49 | 154 ± 44 | 145 ± 40 | <0.001 |
| RV EDP, mm Hg | 13.1 ± 4.7 | 12.1 ± 4.7 | 10.4 ± 6.1 | 8.1 ± 6.4 | <0.001 |
| |
|||||
|---|---|---|---|---|---|
| Analysis of Baseline Parameters for Effect Modification of Relationship of Cardiac Output with RA Pacing | P Value | ||||
| Baseline RV EDV |
0.27 | ||||
| Baseline RV stroke volume |
0.37 | ||||
| Baseline RA pressure |
0.25 | ||||
| Baseline RV EDP |
0.18 | ||||
| Baseline cardiac output |
0.67 | ||||
| Baseline pulmonary vascular resistance |
0.89 | ||||
| Baseline Ees:Ea |
0.84 | ||||
| Diagnosis (SSc vs. IPAH) | 0.95 | ||||
Definition of abbreviations: bpm = beats per minute; dP/dtmax = contractile force rate or change in pressure generated over time; Ea = arterial elastance; EDP = end-diastolic pressure; EDV = end-diastolic volume; Ees = end-systolic elastance; HR = heart rate; IP = instantaneous pressure; IPAH = idiopathic pulmonary arterial hypertension; RA = right atrial; RV = right ventricular; SSc = systemic sclerosis.
Data are shown as means ± SD.
We believe this study to be the first to report the effect of acute RA pacing on RV hemodynamics, including the influence of baseline RV–PA coupling, in patients with PAH. We demonstrate that acute RA pacing to increase HR increases RV contractility, lowers RV EDP, and produces a significant increase in Q̇ despite a decrease in SV. Neither baseline RV function nor morphology clearly modified this relationship. In exploratory analysis, subjects with low Ees:Ea (<1.0) showed a stronger response in Q̇ to pacing. The small sample size, however, increased stochasticity of this analysis, and this observation should only be considered hypothesis generating.
Studying subjects after coronary artery bypass surgery, Lancon and colleagues found higher RA pacing rates increased RV contractility and cardiac index. Our study found similar results to include those with PAH and RV dysfunction, as over half of our cohort had a baseline RV–PA coupling ratio of <1.0 and elevated RV EDP (3). Our group previously demonstrated a reduced RV force frequency response in subjects with systemic sclerosis versus those with idiopathic PAH (5). In the current study, subjects with Ees:Ea <1.0 predominately had systemic sclerosis, and this would suggest the increase in Q̇ is not solely mediated by increased contractility.
Prior work in normal subjects and those with left ventricular (LV) hypertrophy showed atrial pacing was associated with increased LV contractility without significant change in Q̇ (6). On the other hand, in a rat model with chronic pulmonary hypertension, RV pacing significantly increased RV dP/dtmax by “mechanical” resynchronization of RV–LV interaction during diastole (7). We hypothesize that pacing-induced reduction of RV EDP and RV EDV in subjects with RV dysfunction may improve RV–LV interaction, optimizing LV filling and increasing Q̇. Our findings may have implications for other forms of RV failure, including after LV assist device insertion in which a pacing strategy to increase HR has been used clinically.
Conclusions from our study are limited by the relatively small sample size. This may have restricted our ability to detect clinical or hemodynamic factors that predict response to acute RA pacing. Furthermore, we did not compare against healthy control subjects nor acutely decompensated subjects with tachycardia. Whether the observed increase in Q̇ is sustainable with longer-term or chronic pacing is also unknown. Given some variability in Q̇ response, real-time hemodynamic monitoring should likely be employed if this strategy is attempted clinically. Pharmacologic therapies might alternatively provide similar HR increase without the potential arrhythmia risk attributable to pacing. Despite these limitations, our data suggest that acute RA pacing may be an acute therapeutic strategy for RV failure and warrants further study.
Supplementary Material
Footnotes
Supported by grants from NHLBI of the NIH (K23-HL146889-01, S.H.; R01-HL114910-05, D.A.K., P.M.H., and R.J.T.).
Author Contributions: J.S.K.: data analysis and manuscript drafting. B.A.H.: data collection and manuscript drafting. P.J.L.: data analysis, statistical support, and manuscript drafting. S.C.M.: data collection and manuscript drafting. T.M.K.: data analysis, data collection, and manuscript drafting. R.D.: data collection and manuscript drafting. P.M.H.: data analysis, funding, and manuscript drafting. D.A.K.: data collection, funding, data analysis, and manuscript drafting. S.H.: data collection, data analysis, and manuscript drafting. R.J.T.: lead investigator, data collection, funding, data analysis, and manuscript drafting. All authors gave final approval of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202006-2278LE on October 7, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Vrouchos GT, Kiulpalis A, Trullakis GA, Stasinos PG, Ellinkakis SG, Koumatzias NC, et al. High-rate cardiac pacing increases blood pressure and decreases right atrial pressure in patients with hemodynamic significant acute right ventricular myocardial infarction and bradyarrhythmia. Clin Cardiol. 1997;20:41–46. doi: 10.1002/clc.4960200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topol EJ, Goldschlager N, Ports TA, Dicarlo LA, Jr, Schiller NB, Botvinick EH, et al. Hemodynamic benefit of atrial pacing in right ventricular myocardial infarction. Ann Intern Med. 1982;96:594–597. doi: 10.7326/0003-4819-96-5-594. [DOI] [PubMed] [Google Scholar]
- 3.Lançon JP, Pillet M, Gabrielle F, Fayolle JL, Tatou E. Effects of atrial pacing on right ventricular contractility after coronary artery surgery. J Cardiothorac Vasc Anesth. 1994;8:536–540. [PubMed] [Google Scholar]
- 4.Khural JS, Houston BA, Leary PJ, Mathai SC, Kolb TM, Damico R, et al. Right atrial pacing to improve acute hemodynamics in pulmonary arterial hypertension [abstract]. Presented at the ISHLT Virtual Meeting 2020. July 22, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133:2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CP, Ting CT, Lawrence W, Maughan WL, Chang MS, Kass DA. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy: systolic versus diastolic determinants. Circulation. 1993;88:1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- 7.Handoko ML, Lamberts RR, Redout EM, de Man FS, Boer C, Simonides WS, et al. Right ventricular pacing improves right heart function in experimental pulmonary arterial hypertension: a study in the isolated heart. Am J Physiol Heart Circ Physiol. 2009;297:H1752–H1759. doi: 10.1152/ajpheart.00555.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.