The Challenge
Evolutionary toxicology focuses on the drivers, mechanisms, and outcomes of pollution-driven genetic differentiation among populations. The focal questions address the types of chemical contamination acting as selective pressures; the genetics, epigenetics, and demography of impacted populations; as well as fitness costs and cross-resistances that may follow rapid adaptation. In this field, researchers incorporate tools from environmental chemistry, conservation genetics, population biology, and toxicology to understand the health and stability of impacted populations.
Recent studies in evolutionary toxicology have illustrated diverse cases of population-wide adaptation to contamination (killifish, Hyalella, mosquitofish). Adaptation, by definition, is achieved through the localized loss of some individuals and genotypes and, thus, provides singular evidence of losses in biodiversity. Chemical regulations are generally supported by assessments that predict, largely through laboratory studies, the risks associated with chemical exposures. Evolutionary toxicology complements this approach by providing direct evidence of population and community impacts. Recent interest by the Society of Environmental Toxicology and Chemistry working group EVOGENERATE (Evolutionary and Multigenerational Effects of Chemicals) has launched discussions about the utility of evolutionary toxicology studies to inform chemical regulation. To further this discussion, one representative from each of 3 sectors, academia, government, and industry, was asked to provide opinions on the following questions:
The considerable number of adaptive events reported in recent years suggests that current risk-assessment methods may not be exhaustive. What is risk assessment missing by not incorporating evolutionary toxicology endpoints to inform the regulation of toxic compounds?
Changes in the US Toxic Substances Control Act call for attention in 2 major aspects of predictive toxicology: organizational frameworks (e.g., adverse outcomes pathway) and fast screening methods (e.g., Omics, Tox21). Can evolutionary toxicology contribute to these advancements?
What needs to be done to make an evolutionary approach more accessible and useful to chemical regulation?
In Response: One perspective from Academia
Discussions on how evolutionary toxicology should be treated in regulations and Ecological Risk Assessment (ERA) goes back to at least the 1980s. While some initially argued that evidence of increased tolerance in field exposed organisms suggested that regulations should be relaxed (as mentioned in (Klerks and Weis 1987)), it is now strongly argued that the presence of adapted organisms is itself evidence of ecological impairment (Klerks 2002). Early discussions emphasized that ERAs need to consider adaptations to pollution especially because adapted populations will likely “confuse” monitoring efforts giving the false impression that an area is free of the ecological impact of contamination (Klerks and Weis 1987; Morgan et al. 2007). However, risk assessment has had difficulty considering responses that reflect the dynamic nature of ecosystems (Rohr et al. 2016), and evolutionary responses are one such example. Despite potential difficulties, consideration of evolutionary events within conservation management is seen as essential (Ashley et al. 2003); and likewise, we will likely set misdirected priorities for environmental protection if ERA fails to consider evolution.
The Importance of Considering Evolution in ERA
In considering a role for evolutionary toxicology within ERA, it is important to highlight that adaptation to pollution harbors impacts that will not be realized using traditional monitoring tools. Most cases of adaptation to pollution involve evolutionary rescue, where an advantageous genetic adaptation allows the population to persist in the presence of an extreme selective pressure, in this case a pollutant exposure (Bell and Gonzalez 2009; Bell 2013). These adapted populations may undergo bottlenecks following evolutionary rescue, leading to decreased genetic diversity and increased fitness costs (Bickham et al. 2000). Bickham et al. (2000) argue that after a population bottleneck, some populations may rebound quickly, but recovery of genetic diversity may not occur for several generations (Morgan et al. 2007). Several studies have shown decreased tolerance to other stressors in natural adapted populations (Meyer and Di Giulio 2003; Janssens et al. 2014; Heim et al. 2018), which may be attributable to energetic costs associated with the adaptation or reduced genetic variation in the population (Oziolor et al. 2016b; Lindberg et al. 2017). It has been further argued that the genetic diversity lost through adaptation to pollution can “erode evolutionary potential,” making species less able to confront novel environmental challenges (Laroche et al. 2002). However, decreased genetic diversity is not universal in studies of evolutionary rescue because the severity of genetic diversity loss depends on the nature of the selective sweep, the presence or absence of gene flow, and the initial size of the population (Hoffmann and Willi 2008). For example, in one experimental evolution study, heterozygosity provided a selective advantage against metal exposure, which preserved genetic diversity even after the population experienced a bottleneck (Hochmuth et al. 2015).
In addition to hidden impacts to the adapted populations, adaptations may have indirect effects to the community through the food web. For example, bioaccumulation in resistant organisms can result in higher levels of trophic transfer of a contaminant to prey (Amiard-Triquet 2011), as shown with pyrethroid-resistant Hyalella azteca (Muggelberg et al. 2017; Figure 1). Without an evolutionary toxicology approach, the impacts to genetic diversity, population fitness, and bioaccumulation would be overlooked in a traditional ERA.
Figure 1.

Ecological costs of resistance to pyrethroid insecticides. (A, B) Resistant Hyalella azteca from both laboratory and field-collected populations are more sensitive to temperature and salinity stress compared with their nonresistant counterparts. (C) Because of their increased tolerance, resistant H. azteca are able to bioconcentrate pyrethroids orders of magnitude higher than nonresistant H. azteca. These concentrated insecticides are transferred to fish predators, increasing the potential for trophic transfer in the food web. Figures were designed using data originally published in Muggleberg et al. (2017) and Heim et al. (2018), with permission from the authors. LC50 = median lethal concentration.
Although evolutionary responses to pollution may be indicative of ecological impairment, the value of evolutionary toxicology in retrospective ERA rests in whether it can provide sensitive indicators of effect. For example, in an analysis of 3 sites where adaptation was assessed, Klerks (2002) concluded that adaptation appears to be rare even when community-evel effects are quite pronounced. This led to the conclusion that measuring community-level endpoints (e.g., species richness) is “easier” and “more efficient” than biomonitoring for adaptation (Klerks 2002). However, recent studies suggest that adaptation to pollution may be common in some species that appear to have a high potential to adapt (Reid et al. 2016; Major et al. 2018). Two recent meta-analyses sought to determine the sensitivity of adaptive events by investigating the levels of contamination required to cause adaptation. Whereas one study concluded that adaptation to Cd only occurred at concentrations above regulatory values (De Coninck et al. 2014), the other study demonstrated that adaptation to polyaromatic hydrocarbons was found at sites where sediment levels were below benchmark concentrations, suggesting that the benchmark was not protective (Oziolor et al. 2016a).
In some cases, the presence of adaptive populations may not reflect current conditions at a particular site because the selective pressure may have occurred in the past or resistant individuals may have migrated from the impacted area (Klerks 2002). However, this also provides a potential opportunity for detecting intermittent exposures that cause high mortality. For example, a major source of pesticide-related mortality occurs in California following rain events, and these intermittent exposures are thought to be responsible for the insecticide resistance that has evolved in the crustacean H. azteca (Major et al. 2018). Overall, evolutionary toxicology provides us with novel information about exposure events and sheds light on hidden impacts that deserve consideration within ERAs.
Evolutionary Toxicology’s Role in the Twenty-First-Century Vision of ERA
Over the past decade the landscape of ERA has been changing. For example, the “Tox21” paradigm (National Research Council 2007) calls for replacing traditional toxicity testing with mechanistic studies and high-throughput assays that are able to predict effects at higher levels of biological organization. In particular, the adverse outcome pathway (AOP) has been proposed as a framework to link molecular events that can be tested in a high-throughput manner to adverse outcomes that are relevant to ecological health (Ankley et al. 2010). These efforts facilitate the construction of novel AOPs; and in the prioritization of sites for ecological assessment using high-throughput assays, evolutionary toxicology has an essential role in this new vision for prospective ERA.
Since the organization framework of the AOP was first described, emphasis has been placed on developing AOPs and especially on uncovering the initial molecular events responsible for causing toxicity. In evolutionary toxicology, adaptations often result from mutations in genes with critical roles in the toxicity or metabolism of a chemical. Both targeted approaches based on proposed mechanisms of action and top-down, genomic approaches can help identify genes under selection in adapted populations (Oziolor et al. 2017). Understanding these genetic changes can inform key events within AOPs and possibly allow for the prediction of ecoevolutionary responses (Kramer et al. 2011). In addition, applying transcriptomics to adapted populations can reveal altered pathways and functional consequences of evolution or acclimation events (Oziolor et al. 2017), essentially defining the trade-offs necessary for them to survive in the presence of pollution.
Evolutionary toxicology will also support the transition of ERA toward high-throughput screening by providing methods to monitor population health and evolutionary events. High-throughput tools including targeted genetic assays can identify impaired areas and the sources of impairment. For example, molecular assays designed to detect pesticide resistance mutations in H. azteca assisted in characterizing the causal agent in a river consisting of a complex mixture of contaminants (Weston et al. 2018). Applying the same molecular assay in a survey that linked land use to the presence of resistance organisms, urban land use was found to be a strong predictor of resistance, suggesting that residential pesticide applications were causing ecological impairment across large geographic scales (Major 2018). In cases where the molecular target of adaptation is not known, ‘omic methods including restriction site–associated DNA sequencing and genome resequencing can detect evolutionary events and the potential consequences to these populations (Oziolor et al. 2017). Monitoring adaptive responses in wild populations can also uncover populations under threat of collapse and those that may not be able to adapt (Hansen et al. 2012).
Evolution As a Predictable Adverse Outcome
A final consideration within the “Tox21” paradigm of ERA is whether evolutionary rescue can be predicted to the extent that it could be incorporated as an adverse outcome in an AOP. For some chemicals with a very specific mode of action (i.e., pesticides), evolution in target species is “expected” within only a few years (Feyereisen et al. 2015). Laboratory selection experiments that demonstrate increased tolerance in populations after only a few generations also suggest that evolution is common and predictable (Xie and Klerks 2003; Ward and Robinson 2005). Evolutionary rescue studies have identified demographic and environmental factors that appear important, if not necessary, for adaptation. Adaptation is often detected in cases where pollutants are present at concentrations that cause acute mortality (i.e., pesticide resistance); however, experimental studies suggest that gradual change or chronic exposure is also likely to result in evolutionary rescue (Lindsey et al. 2013), and examples of chronic, sublethal exposures resulting in adaptation are now being discovered (e.g., adaptation to endocrine-disrupting chemicals in killifish [Cotter et al. 2016]). Because selection often acts on standing genetic variation, the population size must be large and contain high levels of genetic diversity to ensure that there are enough individuals with adaptive genotypes to survive, reproduce, and restore a population following evolutionary rescue (Bell 2013). Species with high fecundity and a short generation time will be able to quickly reestablish large populations, making evolutionary rescue more likely. Overall, our understanding of when evolutionary rescue is likely to occur is still limited, but surveys for adaptation monitoring and the integration of ‘omics methods into population studies will provide further examples and insights into when evolutionary toxicology occurs and perhaps provide predictability in the future.
We argue that inclusion of evolutionary toxicology in ERA is critical and that additional research is needed to better understand the predictability of adaptation and potential hidden impacts to populations and communities. Ultimately, this work will help provide better-defined adverse outcomes at the population and community levels, significantly improving the ecological relevance of AOPs. Because the transformation of ERA occurs toward more predictive and high-throughputs methods, evolutionary toxicology should play a prominent role. Application of evolutionary toxicology to ERA will help refocus efforts from time-consuming acute and chronic toxicity assays toward direct investigations of population health.
In Response: One perspective from government
How Evolutionary Toxicology Contributed to a Retrospective Ecological Risk Assessment
Chemical-specific regulatory guidelines are designed to protect the loss of our most sensitive or vulnerable biological receptors under worst-case exposure scenarios. Site-specific applications, regulatory or remediation goals are designed to protect the loss of representative or critical biological receptors to preserve ecological structure and function. Ecological risk assessments (ERAs) that support guidelines and goals are typically informed by chemical- and species-specific toxip data that link laboratory exposures to losses of individuals and from which ecological risks or outcomes are inferred. It is my opinion that an evolutionary toxicology perspective, which focuses on how chemical exposures alter genotype frequencies, provides information complementary to a traditional toxicological approach and directly relevant to ecological outcomes. Together, these perspectives broaden our understanding of the basis for variation within and among species in their vulnerability to stressors, which strengthens the scientific basis for the management of chemicals in the environment.
Although the temporal scale of evolution has been thought of as geological, there is ample evidence for contemporary evolution, which focuses on alterations in the temporal dynamics of genotypes (populations and species) in response to human-mediated stressors (e.g., Reznick et al. 2019). There are also many examples of evolutionary toxicology, which restricts this focus to genetic alterations related to chemical exposures. The following example is particularly well developed in that it extends from field observation to the discovery of new toxicologically important genes and takes place over several decades and across several research groups.
In a real-world example of a site-specific approach, an ERA was developed to support remediation goals for a polychlorinated biphenyl (PCB)-contaminated marine Superfund site (New Bedford Harbor, MA, USA; Munns et al. 1997). This ERA was informed by laboratory studies using a reference (uncontaminated site) population of indigenous fish, Atlantic killifish (Fundulus heteroclitus) exposed to the most toxic PCB congeners, classified as dioxin-like chemicals (DLCs). Population models integrating these laboratory results were used to make projections showing that killifish populations could not persist under the intense PCB exposures of New Bedford Harbor (Figure 2; Munns et al. 1997; Nacci et al. 2002). Yet, New Bedford Harbor supports a large, thriving population of this species (C. Oviatt, University of Rhode Island, Graduate School of Oceanography, Narragansett, RI, USA, unpublished data). Did the ERA come to the wrong conclusion that New Bedford Harbor is a toxic environment?
Figure 2:
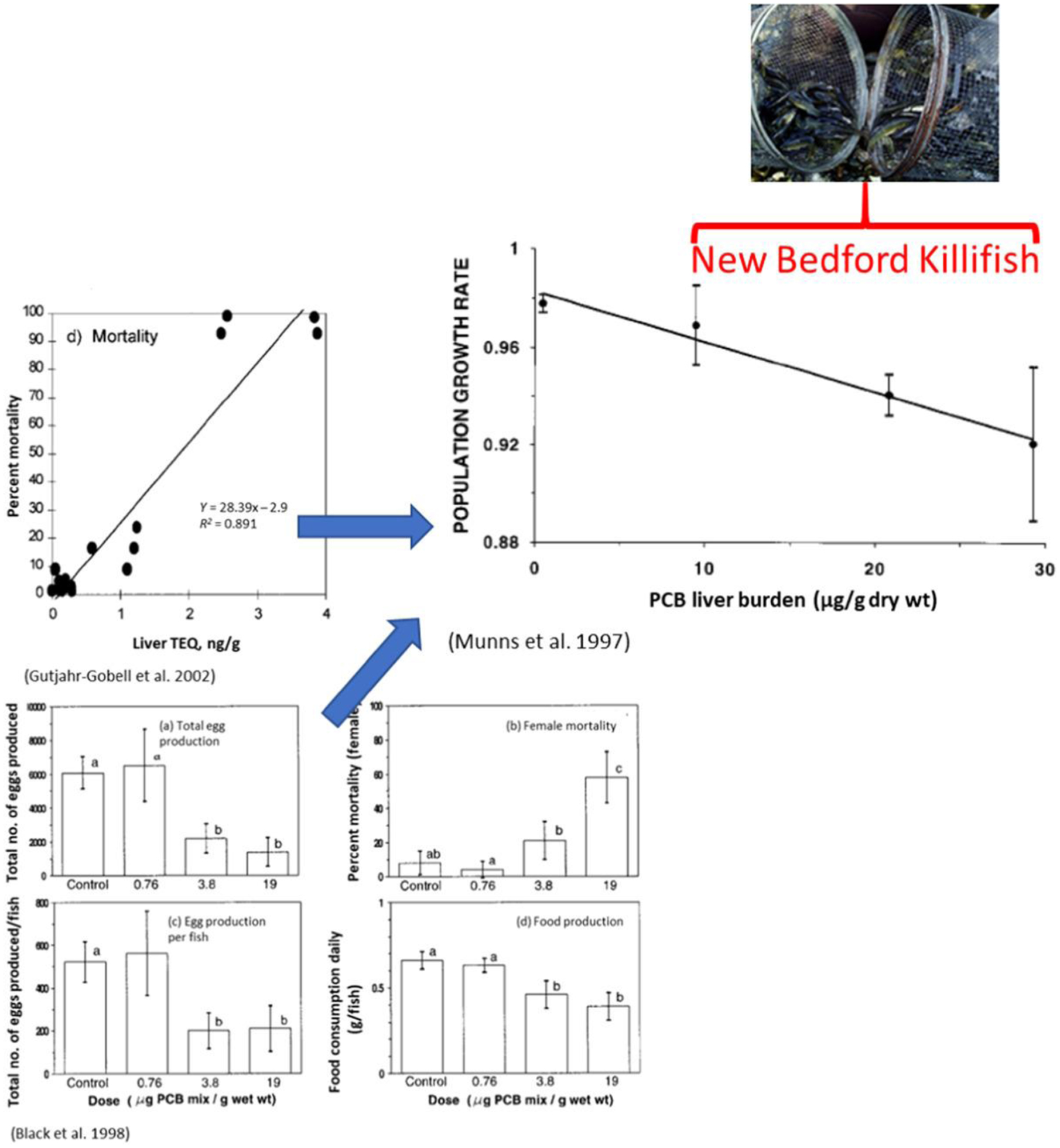
Laboratory studies of Atlantic killifish exposed to dioxin-like chemicals (DLCs) supported the prediction that sites with toxic levels of polychlorinated biphenyls (PCBs), which include DLC congeners, should not sustain populations of this fish species (inset; adapted from Nacci et al. 2002 and references therein). TEQ = toxic equivalency (a weighted quantity based on toxip relative to “dioxin”); TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
No, all evidence suggests that the ERA was conducted reasonably and appropriately and used pertinent information. Furthermore, other evidence suggests that New Bedford Harbor sediment PCB concentrations in comparison to residential, urban, and other industrialized US sites are extreme, that is, greatly exceeding sediment quality guidelines for PCBs. In fact, even sediment concentrations of PCBs orders of magnitude lower than New Bedford Harbor are associated with poor environmental quality (Long et al. 1995; Nelson et al. 1996). The contradiction between laboratory and field evidence was reconciled by further research focused on the Atlantic killifish of New Bedford Harbor.
Killifish as a native and sensitive species was an appropriate focal species for the New Bedford Harbor ERA. However, research by several groups has documented that the New Bedford Harbor killifish population has “evolved tolerance” as an adaptive response to a highly polluted environment and is among the most tolerant fish ever tested (Figure 3) (Van Veld and Nacci 2008). Further, this evolved tolerance provides direct causal evidence that a single class of chemicals, DLCs, has acted as a strong selective (evolutionary) agent, altering the genetic structure and diversity of New Bedford Harbor killifish (e.g., Proestou et al. 2014). But does this knowledge of evolutionary toxicology change our perception of site quality? Not if our goals for environmental quality are more stringent than “survival only of the fittest.”
Figure 3:

Varying early–life stage sensitivity to “dioxin” (2,3,7,8-Tetrachlorodibenzo-p-dioxin [TCDD]) among tested fish species (by common names), lake trout (LT), brook trout (BT), rainbow trout (RT), fathead minnow (FM), common chub (CC), lake herring (LH), Japanese medaka (JM), white sucker (WS), northern pike (NP), zebrafish (ZF), and 2 genetically distinct populations of Atlantic killifish (AK and AK-R), from a relatively uncontaminated habitat or a highly polychlorinated biphenyl-contaminated habitat where fish have evolved pollution resistance (adapted from Van Veld and Nacci 2008). AK = Atlantic killifish from a relatively uncontaminated habitat; AK-R = Atlantic killifish from a highly polychlorinated biphenyl-contaminated habitat where fish have evolved pollution resistance; LCegg50 = median lethal concentration in eggs.
This example from a highly polluted site also led us to discover that evolutionary toxicology is not limited to extreme environments. In fact, adaptively evolved killifish populations have been found where sediment PCB concentrations are above effects range median concentrations (Figure 4), empirically derived guidelines describing >50% of sites assessed (Nacci et al. 2010). This suggests that a large geographic area, including many urban and other moderately human-impacted coastal sites, likely supports examples of evolutionary toxicology. Thus, the extreme and long-range ecological impact of pollutants such as DLCs has been strengthened by the recognition that such pollutants have influenced the evolutionary trajectory of Atlantic killifish (Reid et al. 2016) and the closely related Gulf killifish (Oziolor et al. 2019), and probably their ecological communities, in estuaries of the US Atlantic coast.
Fig. 4.

Dozens of populations of Atlantic killifish resident at sites along the US Atlantic coast (left map) have been shown in laboratory tests to vary in their sensitivity adaptively to the concentrations of toxic pollutants in their native locations (shown by proxy as sediment polychlorinated biphenyls [PCBs], central panel), which ranges below sediment guidelines (Long et al. 1995) for good (green vertical) to poor (red vertical) environmental quality. Adapted from Nacci et al. (2010). ER-L = effects range-low; ER-M = effects range-median; LC20 = 20% lethal concentration.
How Evolutionary Toxicology Can Contribute to Predictive ERAs
By classifying pollutants according to mechanisms of biological action (outcomes at the individual level), we gain the ability to extrapolate and predict biological outcomes across chemicals and species. Comparative genomics and targeted gene editing provide tools to predict and test species sensitivities. Cross-species comparisons at genetic targets using tools such as the US Environmental Protection Agency’s Sequence Alignment to Predict Across Species Susceptibility (https://seqapass.epa.gov/seqapass/) provide a powerful approach to reveal the genetic basis underlying responses to toxic chemicals and predict species sensitivities to mechanistically related chemical classes. Yet, predictive accuracy is predicated on our knowledge of the genetic bases for toxic action, which can be informed by both laboratory mechanistic work and evolutionary toxicology studies.
Knowledge about the demonstrated basis for DLC toxip, the subject of decades of research, has been enhanced by the development and application of genomic tools exploring this “(un)natural experiment” i.e., multiple unrelated killifish populations that have evolved DLC tolerance over a contemporary timescale (e.g., Whitehead et al. 2010, 2012, 2017; Nacci et al. 2016). The intraspecific genetic variation associated with DLC tolerance in killifish has provided a truly unique opportunity to reveal complex patterns of genes associated with response to a single particularly toxic class of chemicals. The tools for these explorations required the efforts of a large scientific community to produce recently available killifish genomic resources, including gene models for F. heteroclitus (MDI Biological Laboratory 2012) and the first killifish genetic map (Waits et al. 2016).
In this case, complementary laboratory and field studies have provided an unusually complete picture of adaptation to modern urban pollution. Specifically, quantitative trait locus (QTL) analysis provided a quantitative assessment of the genetic basis for DLC tolerance in New Bedford Harbor killifish (Nacci et al. 2016). Consistent with mechanistic knowledge of DLC toxip in fish and other vertebrates, the aryl hydrocarbon receptor 2 region accounted for 17% of trait variation; however, QTLs on independent linkage groups and their interactions have even greater explanatory power (44%; Figure 5). These findings contributed to the discovery of genes not previously known to be toxicologically important and further suggested the importance of genetic interaction. This interpretation was reinforced by field samples from multiple killifish populations characterized as DLC-tolerant and residing in distant urban estuaries contaminated with unique mixtures of pollutants, where signals of selection were found for several aryl hydrocarbon receptor pathway genes (Reid et al. 2016). Thus, these discovery approaches complement targeted approaches designed to test the association of pollution tolerance and known mechanistic targets (e.g., Proestou et al. 2014).
Fig. 5:

Representation of toxicological mechanism of dioxin-like chemicals (structure shown) acting through the aryl hydrocarbon receptor (AHR) signal transduction network, which includes AHR paralogs, heat shock proteins, AHR-interacting protein, AHR nuclear translocator, and the cochaperone p23, to affect production of indicator proteins including CYP1A. Components shown in red were identified as quantitative trait loci for pollution tolerance in Atlantic killifish. Adapted from Nacci et al. (2016). Ahrx = AHR paralog; Aip = AHR-interacting protein; Arnt = AHR nuclear translocator; DRE = dioxin response element; HSP = heat shock protein.
These “real-world” evolutionary studies provide mechanistic knowledge complementing reductionist approaches that support the development of adverse outcome networks as well as novel screening tests that could become components in comprehensive batteries of screening tests used to assess chemical risks.
What Would Make an Evolutionary Approach More Accessible and Useful to Chemical Regulation?
It is widely recognized that a complexity of information is required to understand comprehensively toxicological responses to chemical exposures. Yet the daunting array of chemicals discharged into the environment requires some form of screening to direct resources to most damaging chemicals. Thus, a tiered basis is often used to prioritize chemicals using a broad array of (relatively) inexpensive and rapid tests; only chemicals that fail such tests are subjected to further, more intensive characterization efforts. Furthermore, these more resource-intensive efforts, rather than taking a top-down approach, are focused on our knowledge or expectations of toxicological or ecological mechanisms or biological receptors of concern.
Thus, can we consider decision frameworks where additional resources are selectively directed to chemicals or exposures with demonstrated or predicted potential for long-term effects (i.e., evolutionary outcomes)? For example, it is hypothesized that evolutionary change may be most probable (and detectable) when exposures produce consistent, strong directional selection for most tolerant genotypes (Hendry et al. 2011). By definition, persistent bioaccumulative and toxic chemicals have high potential as evolutionary toxicants. However, it should also be noted that even chemicals that are not highly physiochemically persistent in the environment can produce consistent selective pressure through their constant exposures via continuous deposition in the environment, or “pseudopersistence.” We also recognize species attributes that provide the fodder for adaptation (i.e., large populations with high standing genetic variation and short generation time). It is in such species, like Atlantic killifish, where we are more likely to detect adapted populations that provide opportunities to explore mechanisms of “evolutionary toxip” in the real world (e.g., Reid et al. 2016).
Can next-generation products be developed that are “greener” by evolutionary standards? Perhaps there is much that has already been learned in the design of products whose desired outcome has been circumvented by evolution. Specifically, understanding the ecological and toxicological drivers of evolved resistance to antibiotics and pesticides has informed the design of products with properties like limited persistence and complex mechanisms of action (e.g., Hendry et al. 2011). Recognition of the potential for long-range evolutionary impacts might also be used to guide the design of alternative “replacement” industrial and commercial products with reduced environmental risks (e.g., Panacek et al. 2018).
In Response: One perspective from industry
How Evolutionary Toxicology Can Contribute to Ecological Risk Assessment
Ecological risk assessment (ERA) is used in many applications pertinent to industry: assessing risk of new or existing chemicals, environmental impact assessments, effluent discharge monitoring, establishing clean-up levels for contaminated sites, and so on. (Society of Environmental Toxicology and Chemistry 2018). Current standardized ecotoxicological testing protocols focus on endpoints like lethality, reproduction, and growth in laboratory populations; and they are meant to be conservative and, thus, protective in outcome. However, these simplified systems do not allow for environmental realism; and, depending on the test, the endpoints may be insufficiently descriptive of meaningful scalable or longitudinal outcomes. Chronic toxip studies allow for a longitudinal assessment, but subtle toxic effects beyond apical endpoints are often challenging to detect with standard ecotoxicological methods (e.g., behavioral, teratogenic, and genetic effects).
Evolutionary toxicology offers a different perspective on a population-based hazard endpoint over a longer time frame than typically considered for standard ecotoxicology testing. Impacts are the result of long-term chronic or repeated point exposures, with outcomes observed after multiple generations. Changes in the genetic variation of a population attributable to a selective or mutagenic pressure are a different type of endpoint that is not currently being addressed or monitored. The relatively young field of evolutionary toxicology sits at the interface between toxicology, genetics, and ecology, with different terminologies (e.g., evolutionary genomics, microevolution) used in different communities of practice. Although this does make it difficult for the nonpractitioner to appreciate the breadth of the field, there are consistent themes around the types of evolutionary processes (mutation, changes in genetic diversity, change of gene flow and dispersion, selection) that impact the health of a population, community, and ecosystem (Medina et al. 2007; Bickham 2011). One major hurdle for their incorporation into existing regulatory systems is deciding how evolutionary toxicology data, like changes in genetic diversity, could be quantified and interpreted.
There are several endpoints that can be used as indicators of change in populations. Pollutant-induced community tolerance (PICT) looks at community changes attributable to elimination of sensitive species and can act as an indicator of chemical exposure (Blanck 2002). Another method is to look at genetic erosion, or the loss of genetic diversity within a species attributable to chemical exposure (Fasola et al. 2015). This approach suggests that there are several negative impacts that can be used as markers of genetic erosion in amphibians, namely genetic-fitness correlations, reduced pathogen tolerance, reduced cotolerance to other stressors, loss of phenotypic and environmental plastip, and reduced fitness. In addition to looking for overt phenotypic indicators of evolutionary change, there are several omics-based techniques that allow one to pinpoint genetic differences resulting in evolutionary differences (Oziolor et al. 2017).
In standard ecotoxip testing, the effects being measured are clearly negative (e.g., death, loss of growth, reduced reproduction). The link between individual death, reduced growth, and fewer offspring is easy to correlate to a negative impact on a population. The answer is not as clear in evolutionary toxicology. Would all impacts on genetic diversity be considered negative? Would ecological resilience be a better endpoint than change in diversity (Bundschuh et al. 2017)? In addition, establishing causation between chemical exposure and the endpoint will require careful study design. One study showed that, although levels of species richness in a pollution-impacted community were consistent, there were still marked differences in ecosystem function (Spaak et al. 2017). Another showed that even when a population survives, its ecological function may be altered (Medina et al. 2007). In these cases, PICT can be used to reinforce causal arguments (Tlili et al. 2016).
Without considering evolutionary toxicology, chemical risk assessments potentially miss aspects of population and community health that cannot otherwise be predicted or explained. As yet, it does not appear that the state of the science is sufficiently mature where evolutionary toxicology can be easily incorporated into different types of risk assessments. Instead, it could be used for selected applications, such as looking at endangered species or at-risk species. Interpretation of monitoring data may also need to be tempered by an understanding of how species could be adapting to contaminants. There may also be certain chemical applications where assessment of ecological change is needed, such as pesticide use where a nontarget species is of concern. In these circumstances, it would also be necessary to account for differing chemical susceptibilities in the laboratory population (Barata et al. 2002) and any ecological interactions that may change how that population would respond to the chemical in the environment (Gabsi et al. 2014). Without these considerations, the environmental realism that is desired is undermined.
How Evolutionary Toxicology Can Advance Predictive Toxicology
Regulatory ecotoxicology, as seen in the US Environmental Protection Agency and the Toxic Substances Control Act, is improving in part by promoting the use of new approach methodologies (NAMs) such as high-throughput screening and adverse outcome pathways (AOPs; US Environmental Protection Agency 2018). Adverse outcome pathways are a construct to link chemical exposure to specific events, leading to a negative effect or adverse outcome (Carusi et al. 2018). The general idea is to have more targeted and yet quicker, cheaper tests that have greater predictive power. The benefits of NAMs is the ability to reduce vertebrate use, obtain more data, and reduce cost compared to traditional methods, all of which make NAMs attractive screening methods. The methodologies that have been established for evolutionary toxicology do not lend themselves to screening purposes. The very nature of looking at evolutionary impacts requires endpoints lasting for several generations, which necessarily increases the time frames, costs of the testing, and potential use of animals. However, the techniques used in evolutionary toxicology assessments are also ideally predictive of population and community impacts (Pelletier and Coltman 2018).
The advent of ‘omics-based ecotoxicological studies (Oziolor et al. 2017) and the use of AOPs to identify key events can provide more sensitive and meaningful endpoints. If well-defined adverse outcomes can be linked to evolutionary toxicology processes, the process by which the genetic changes occur can be defined as an AOP. Tests designed to capture events in such an AOP would address concerns about demonstrating causation in complex ecological systems but could also be more predictive of potential changes in genetic diversity.
What Would Make Evolutionary Approaches More Accessible and Useful to Chemical Regulation?
As more is understood about the impact of chemicals on the environment, new hazards and new exposures are raised that need to be considered (e.g., endocrine disruption). The long-term impact of chemicals examined in evolutionary toxicology is a prime example of a potential hazard that has not been adequately identified and is not being addressed. When considering the types of ERAs mentioned at the beginning of this section (assessing risk of new or existing chemicals, environmental impact assessments, effluent discharge monitoring, establishing clean-up levels for contaminated sites, etc.), there are certain applications where looking for chemically mediated evolutionary events is more pertinent and feasible. Evolutionary toxicology assessments on new or existing chemicals would be unreasonably costly and even unnecessary. On the other hand, monitoring of a “canary” set of genes or population in a location of interest would provide valuable information regarding the levels and impacts of chemicals in that scenario. It would also be useful to compare impacts of alternative chemicals and to be aware of their long-term implications. For contaminated sites, the presence of evolved organisms can be an indicator of an environmental impact, although the establishment of clean-up levels dependent on recovery of genetic diversity may not be practical.
There are no global standards for ERA, although the Organisation for Economic Co-operation and Development works with its member nations to come up with standardized testing protocols for chemical hazard assessment and a policy of mutual acceptance of data (MAD). To ensure MAD, the test methods themselves should be practical, valid, useful in prognoses, and standardized (Organisation for Economic Co-operation and Development 1998). Methods developed for evolutionary toxicology assessment would need to stand up to the same criteria. Following are some questions to consider when deciding how to integrate evolutionary toxicology into ERAs for regulatory purposes:
Can we define criteria for the characteristics or types of organisms that would make good harbingers of evolutionary risk? This question has been mostly answered in that one would need a certain population size, rate of reproduction, and genetic variability between organisms (Whitehead et al. 2017). However, it would be useful to be more prescriptive so that site-appropriate organisms can be identified.
How can AOPs be used to identify genes/gene families for monitoring?
For a single chemical, how can a dose at which selective pressure is exerted be established? How does this compare to the level of acute or chronic toxip?
Can we more clearly understand the impact of changes in genetic diversity, including recovery time? And how does this differ between types of chemicals?
How do these assessments change when looking at chemical mixtures?
Is a change in genetic diversity acceptable and, if so, in what kind of environments? This requires a judgment call. For example, the evolution of biodegradation pathways is encouraged in microorganisms—is this acceptable if other microbial functions are lost?
Evolutionary toxicology offers expanded insight on the effects of chemicals in the environment, beyond established ecotoxicological endpoints. The information generated in this relatively new field does not fit neatly into existing risk-assessment paradigms. This does not diminish its importance but rather is a gauntlet thrown to incorporate evolutionary toxicology in ERA. As the science of ecotoxicology is trending toward suborganismal test methods (in vitro, ‘omics, etc.) and faster throughput, this is the perfect time to place emphasis on the end goal of chemicals regulation and to provide population/ecosystem insight.
Unified Conclusion
The representatives of the 3 branches of the Society of Environmental Toxicology and Chemistry are unequivocal: evolutionary toxicology has the potential to inform and influence ecological risk assessment. What novel contribution does this field make? Time. Evolutionary toxicology truly measures impacts of pollutants on populations over time. In a world of in vitro to in vivo extrapolation and high-throughput toxicology attempting to build greener and safer chemicals, evolutionary toxicology provides long-term perspective on the mechanisms by which chemicals may pose a risk to the populations around us. It also provides a unique window into the past, allowing us to detect signatures of pollution exposure that may have occurred years ago. The study of evolutionary toxicology has already taught us important lessons about the mechanisms of organismal resilience in dire and extremely altered environments, and it can surely inform us about risk.
References
- Amiard-Triquet C 2011. Pollution tolerance: From fundamental biological mechanisms to ecological consequences In Amiard-Triquet C, Rainbow PS, Romeo M, eds, Tolerance to Environmental Contaminants. CRC, Boca Raton, FL, USA, pp 1–23. [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. [DOI] [PubMed] [Google Scholar]
- Ashley MV, Willson MF, Pergams OR, O’Dowd DJ, Gende SM, Brown JS. 2003. Evolutionarily enlightened management. Biol Conserv 111:115–123. [Google Scholar]
- Barata C, Baird DJ, Soares AMVM. 2002. Determining genetic variability in the distribution of sensitivities to toxic stress among and within field populations of Daphnia magna. Environ Sci Technol 36:3045–3049. [DOI] [PubMed] [Google Scholar]
- Bickham JW. 2011. The four cornerstones of evolutionary toxicology. Eco-toxicology 20:497–502. [DOI] [PubMed] [Google Scholar]
- Blanck H 2002. A critical review of procedures and approaches used for assessing pollution-induced community tolerance (PICT) in biotic communities. Hum Ecol Risk Assess 8:1003–1034. [Google Scholar]
- Bundschuh M, Schulz R, Schäfer RB, Allen CR, Angeler DG. 2017. Resilience in ecotoxicology: Toward a multiple equilibrium concept. Environ Toxicol Chem 36:2574–2580. [DOI] [PubMed] [Google Scholar]
- Bell G 2013. Evolutionary rescue and the limits of adaptation. Philos Trans RSoc B Biol Sci 368:20120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol Lett 12:942–948. [DOI] [PubMed] [Google Scholar]
- Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R. 2000. Effects of chemical contaminants on genetic diversity in natural populations: Im-plications for biomonitoring and ecotoxicology. Mutat Res 463:33–51. [DOI] [PubMed] [Google Scholar]
- Carusi A, Davies MR, De Grandis G, Escher BI, Hodges G, Leung KMY, Whelan M, Willett C, Ankley GT. 2018. Harvesting the promise of AOPs: An assessment and recommendations. Sci Total Environ 628–629:1542–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter KA, Nacci D, Champlin D, Yeo AT, Gilmore TD, Callard GV. 2016. Adaptive significance of ERαsplice variants in killifish (Fundulus heteroclitus) resident in an estrogenic environment. Endocrinology 157:2294–2308. [DOI] [PubMed] [Google Scholar]
- De Coninck DI, Janssen CR, De Schamphelaere KA. 2014. An approach to assess the regulatory relevance of microevolutionary effects in eco-logical risk assessment of chemicals: A case study with cadmium. Environ Toxicol Chem 33:453–457. [DOI] [PubMed] [Google Scholar]
- Fasola E, Ribeiro R, Lopes I. 2015. Microevolution due to pollution in amphibians: A review on the genetic erosion hypothesis. Environ Pollut 204:181–190. [DOI] [PubMed] [Google Scholar]
- Feyereisen R, Dermauw W, Van Leeuwen T. 2015. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol 121:61–77. [DOI] [PubMed] [Google Scholar]
- Gabsi F, Schäffer A, Preuss TG. 2014. Predicting the sensitivity of populations from individual exposure to chemicals: The role of ecological interactions. Environ Toxicol Chem 33:1449–1457. [DOI] [PubMed] [Google Scholar]
- Hansen MM, Olivieri I, Waller DM, Nielsen EE, GeM Working Group. 2012. Monitoring adaptive genetic responses to environmental change. MolEcol 21:1311–1329. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT, Heino M, Day T, Smith TB, Fitt G, Bergstrom CT, Oakeshott J, Jorgensen PS, Zalucki MP, Gilchrist G, Southerton S, Sih A, Strauss S, Denison RF, Carroll SP. 2011. Evolutionary principles and their practical application. Evol Appl 4:159–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim JR, Weston DP, Major K, Poynton H, Huff Hartz KE, Lydy MJ. 2018. Are there fitness costs of adaptive pyrethroid resistance in the amphipod, Hyalella azteca? Environ Pollut 235:39–46. [DOI] [PubMed] [Google Scholar]
- Hochmuth JD, De Meester L, Pereira CM, Janssen CR, De Schamphelaere KA. 2015. Rapid adaptation of a Daphnia magna population to metal stress is associated with heterozygote excess. Environ Sci Technol 49:9298–9307. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Willi Y. 2008. Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432. [DOI] [PubMed] [Google Scholar]
- Janssens L, Dinh Van K, Debecker S, Bervoets L, Stoks R. 2014. Local adaptation and the potential effects of a contaminant on predator avoidance and antipredator responses under global warming: A space-for-time substitution approach. Evol Appl 7:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerks PL. 2002. Adaptation, ecological impacts, and risk assessment: In-sights from research at Foundry Cove, Bayou Trepagnier, and Pass Fourchon. Hum Ecol Risk Assess 8:971–982. [Google Scholar]
- Klerks PL, Weis JS. 1987. Genetic adaptation to heavy metals in aquatic organisms: A review. Environ Pollut 45:173–205. [DOI] [PubMed] [Google Scholar]
- Kramer VJ, Etterson MA, Hecker M, Murphy CA, Roesijadi G, Spade DJ, Spromberg JA, Wang M, Ankley GT. 2011. Adverse outcome pathways and ecological risk assessment: Bridging to population-level effects. Environ Toxicol Chem 30:64–76. [DOI] [PubMed] [Google Scholar]
- Laroche J, Quiniou L, Juhel G, Auffret M, Moraga D. 2002. Genetic and physiological responses of flounder (Platichthysflesus) populations to chemical contamination in estuaries. Environ Toxicol Chem 21:2705–2712. [PubMed] [Google Scholar]
- Long ER, Macdonald DD, Smith SL, Calder FD. 1995. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97. [Google Scholar]
- Lindberg CD, Jayasundara N, Kozal JS, Leuthner TC, Di Giulio RT. 2017. Resistance to polycyclic aromatic hydrocarbon toxicity and associated bioenergetic consequences in a population of Fundulus heteroclitus. Ecotoxicology 26:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey HA, Gallie J, Taylor S, Kerr B. 2013. Evolutionary rescue from extinction is contingent on a lower rate of environmental change. Nature 494:463–467. [DOI] [PubMed] [Google Scholar]
- Major K 2018. Pesticide resistance in a nontarget amphipod, Hyalellaazteca: Prevalence, gerographic extent, and prediction of impacted populations. PhD thesis. University of Massachusetts, Boston, MA, USA. [Google Scholar]
- MDI Biological Laboratory. 2012. Welcome to the Fundulus genomics portal. Bar Harbor, ME, USA: [cited 2019 September 13]. Available from: https://my.mdibl.org/display/FGP/Home [Google Scholar]
- Munns WR, Black DE, Gleason TR, Salomon K, Bengtson D, Gutjahr-Gobell R. 1997. Evaluation of the effects of dioxin and PCBs on Fundulus heteroclitus populations using a modeling approach. Environ Toxicol Chem 16:1074–1081. [Google Scholar]
- Medina MH, Correa JA, Barata C. 2007. Microevolution due to pollution: Possible consequences for ecosystem responses to toxic stress. Chemosphere 67:2105–2114. [DOI] [PubMed] [Google Scholar]
- Major KM, Weston DP, Lydy MJ, Wellborn GA, Poynton HC. 2018. Unintentional exposure to terrestrial pesticides drives widespread and predictable evolution of resistance in freshwater crustaceans. Evol Appl 11:748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. 2003. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl 13:490–503. [Google Scholar]
- Morgan AJ, Kille P, Stürzenbaum SR. 2007. Microevolution and ecotoxicology of metals in invertebrates. Environ Sci Technol 41:1085–1096. [DOI] [PubMed] [Google Scholar]
- Muggelberg LL, Huff Hartz KE, Nutile SA, Harwood AD, Heim JR, Derby AP, Weston DP, Lydy MJ. 2017. Do pyrethroid-resistant Hyalella azteca have greater bioaccumulation potential compared to nonresistant populations? Implications for bioaccumulation in fish. Environ Pollut 220:375–382. [DOI] [PubMed] [Google Scholar]
- National Research Council. 2007. Toxicity testing in the 21st century: Avision and a strategy. National Academies Press, Washington, DC. [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S. 2010. Adaptation of the estuarine fish Fundulus heteroclitus (Atlantic killifish) to polychlorinated biphenyls (PCBs). Estuaries Coast 33:853–864. [Google Scholar]
- Nacci D, Gleason T, Gutjahr-Gobell R, Huber M, Munns WR Jr. 2002. Effects of chronic stress on wildlife populations: A population modeling approach and case study In Newman MC, ed, Coastal and Estuarine Risk Assessment. CRC Press/Lewis Publishers, Washington, DC. [Google Scholar]
- Nacci D, Proestou D, Champlin D, Martinson J, Waits ER. 2016. Genetic basis for rapidly evolved tolerance in the wild: Adaptation to toxic pollutants by an estuarine fish species. Mol Ecol 25:5467–5482. [DOI] [PubMed] [Google Scholar]
- Nelson W, Bergen B, Benyi S, Morrison G, Voyer R, Strobel C, Rego S, Thursby G, Pesch C. 1996. New Bedford harbor long-term monitoring assessment report: Baseline sampling. EPA/600/R-96/097. US Environmental Protection Agency, Narragansett, RI. [Google Scholar]
- Oziolor EM, Reid NM, Yair S, Lee KM, VerPloeg SG, Bruns PC, Shaw JR, Whitehead A, Matson CW. 2019. Adaptive introgression enables evolutionary rescue from extreme environmental pollution. Science 364:455–457. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development. 1998. Detailed review paper on aquatic testing methods for pesticides and industrial chemicals. Series on Testing and Assessment, No. 69. NV/MC/CHEM(98)19/PART1. Paris, France. [Google Scholar]
- Oziolor EM, Bickham JW, Matson CW. 2017. Evolutionary toxicology in anomics world. Evol Appl 10:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oziolor EM, De Schamphelaere K, Matson CW. 2016a. Evolutionary toxicology: Meta-analysis of evolutionary events in response to chemical stressors. Ecotoxicology 25:1858–1866. [DOI] [PubMed] [Google Scholar]
- Oziolor EM, Dubansky B, Burggren WW, Matson CW. 2016b. Cross-resistance in Gulf killifish (Fundulus grandis) populations resistant todioxin-like compounds. Aquat Toxicol 175:222–231. [DOI] [PubMed] [Google Scholar]
- Panacek A, Kvitek L, Smekalova M, Vecerova R, Kolar M, Roderova M, Dycka F, Sebela M, Prucek R, Tomanec O, Zboril R. 2018. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol 13:65–71. [DOI] [PubMed] [Google Scholar]
- Proestou DA, Flight P, Champlin D, Nacci D. 2014. Targeted approach to identify genetic loci associated with evolved dioxin tolerance in Atlantic killifish (Fundulus heteroclitus). BMC Evol Biol 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier F, Coltman DW. 2018. Will human influences on evolutionary dynamics in the wild pervade the Anthropocene? BMC Biol 16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid NM, Proestou DA, Clark BW, Warren WC, Colbourne JK, Shaw JR, Karchner SI, Hahn ME, Nacci D, Oleksiak MF. 2016. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wildfish. Science 354:1305–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN, Losos J, Travis J. 2019. From low to high gear: There has been a paradigm shift in our understanding of evolution. Ecol Lett 22:233–244. [DOI] [PubMed] [Google Scholar]
- Reid NM, Proestou DA, Clark BW, Warren WC, Colbourne JK, Shaw JR, Karchner SI, Hahn ME, Nacci D, Oleksiak MF. 2016. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wildfish. Science 354:1305–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Salice CJ, Nisbet RM. 2016. The pros and cons of ecological risk assessment based on data from different levels of biological organization. Crit Rev Toxicol 46:756–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veld PA, Nacci D. 2008. Toxicity resistance In RT Di Giulio, DE Hinton, eds, The Toxicology of Fishes. CRC, Boca Raton, FL, USA, pp 597–641. [Google Scholar]
- Society of Environmental Toxicology and Chemistry. 2018. Environmental risk assessment of chemicals. Technical issue paper. Pensacola, FL, USA. [Google Scholar]
- Spaak JW, Baert JM, Baird DJ, Eisenhauer N, Maltby L, Pomati F, Radchuk V, Rohr JR, Van den Brink PJ, De Laender F. 2017. Shifts of community composition and population density substantially affect ecosystem function despite invariant richness. Ecol Lett 20:1315–1324. [DOI] [PubMed] [Google Scholar]
- Tlili A, Berard A, Blanck H, Bouchez A, Cássio F, Eriksson KM, Morin S, Montuelle B, Navarro E, Pascoal C, Pesce S, Schmitt-Jansen M, Behra R. 2016. Pollution-induced community tolerance (PICT): Towards an eco-logically relevant risk assessment of chemicals in aquatic systems. Freshw Biol 61:2141–2151. [Google Scholar]
- US Environmental Protection Agency. 2018. Strategic plan to promote the development and implementation of alternative test methods within the TSCA program. Washington, DC. [Google Scholar]
- Whitehead A, Clark BW, Reid NM, Hahn ME, Nacci D. 2017. When evolution is the solution to pollution: Key principles, and lessons from rapid repeated adaptation of killifish (Fundulus heteroclitus) populations. EvolAppl 10:762–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TJ, Robinson WE. 2005. Evolution of cadmium resistance in Daphniamagna. Environ Toxicol Chem 24:2341–2349. [DOI] [PubMed] [Google Scholar]
- Waits ER, Martinson J, Rinner B, Morris S, Proestou D, Champlin D, Nacci D. 2016. Genetic linkage map and comparative genome analysis for the Atlantic killifish, Fundulus heteroclitus. Open J Genet 6:10. [Google Scholar]
- Whitehead A, Clark BW, Reid NM, Hahn ME, Nacci D. 2017. When evolution is the solution to pollution: Key principles, and lessons from rapid repeated adaptation of killifish (Fundulus heteroclitus) populations. EvolAppl 10:762–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A, Pilcher W, Champlin D, Nacci D. 2012. Common mechanism underlies repeated evolution of extreme pollution tolerance. Proc BiolSci 279:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A, Triant DA, Champlin D, Nacci D. 2010. Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Mol Ecol 19:5186–5203. [DOI] [PubMed] [Google Scholar]
- Weston DP, Poynton HC, Major KM, Wellborn GA, Lydy MJ, Moschet C,Connon RE. 2018. Using mutations for pesticide resistance to identify the cause of toxicity in environmental samples. Environ Sci Technol 52:859–867. [DOI] [PubMed] [Google Scholar]
- Xie L, Klerks PL. 2003. Responses to selection for cadmium resistance in the least killifish, Heterandria formosa. Environ Toxicol Chem 22:313–320. [PubMed] [Google Scholar]


