Abstract
OBJECTIVE
This study aimed to assess the safety and efficacy of MR-guided stereotactic laser ablation (SLA) therapy in the treatment of pediatric brain tumors.
METHODS
Data from 17 North American centers were retrospectively reviewed. Clinical, technical, and radiographic data for pediatric patients treated with SLA for a diagnosis of brain tumor from 2008 to 2016 were collected and analyzed.
RESULTS
A total of 86 patients (mean age 12.2 ± 4.5 years) with 76 low-grade (I or II) and 10 high-grade (III or IV) tumors were included. Tumor location included lobar (38.4%), deep (45.3%), and cerebellar (16.3%) compartments. The mean follow-up time was 24 months (median 18 months, range 3–72 months). At the last follow-up, the volume of SLA-treated tumors had decreased in 80.6% of patients with follow-up data. Patients with high-grade tumors were more likely to have an unchanged or larger tumor size after SLA treatment than those with low-grade tumors (OR 7.49, p = 0.0364). Subsequent surgery and adjuvant treatment were not required after SLA treatment in 90.4% and 86.7% of patients, respectively. Patients with high-grade tumors were more likely to receive subsequent surgery (OR 2.25, p = 0.4957) and adjuvant treatment (OR 3.77, p = 0.1711) after SLA therapy, without reaching significance. A total of 29 acute complications in 23 patients were reported and included malpositioned catheters (n = 3), intracranial hemorrhages (n = 2), transient neurological deficits (n = 11), permanent neurological deficits (n = 5), symptomatic perilesional edema (n = 2), hydrocephalus (n = 4), and death (n = 2). On long-term follow-up, 3 patients were reported to have worsened neuropsychological test results. Pre-SLA tumor volume, tumor location, number of laser trajectories, and number of lesions created did not result in a significantly increased risk of complications; however, the odds of complications increased by 14% (OR 1.14, p = 0.0159) with every 1-cm3 increase in the volume of the lesion created.
CONCLUSIONS
SLA is an effective, minimally invasive treatment option for pediatric brain tumors, although it is not without risks. Limiting the volume of the generated thermal lesion may help decrease the incidence of complications.
Keywords: magnetic resonance–guided stereotactic laser ablation, SLA, laser interstitial thermal therapy, LITT, minimally invasive technique, pediatric brain tumors, oncology
NEUROSURGICAL advances have focused on the development of minimally invasive approaches in an effort to increase treatment options for difficult-to-access lesions that may be located in surgically challenging locations due to anatomy or functional reasons. Stereotactic thermal ablation technologies relying on radiofrequency ablation or laser-induced thermal therapy were initially promising but slow to gain popularity due to the lack of precision of the thermal source and lack of real-time feedback during tissue damage, and thus there was high potential for damage to critical surrounding structures.1–7 More recent developments have led to significant improvements with the incorporation of image guidance and real-time feedback during the ablation process.5,8 Two FDA-cleared technologies are currently available that combine MRI guidance and laser-induced thermal therapy: the Visualase system (Medtronic)3 and the NeuroBlate system (Monteris Medical).9,10
These stereotactic laser ablation (SLA) systems allow real-time MR-guided monitoring of the ablation process and control over energy delivery to targets and critical surrounding structures. Since the introduction of these systems into neurosurgery, studies utilizing them have focused mostly on the management of unresectable tumors and metastatic tumors in the adult population.1,11,12 It was not until 2012 that Curry et al. first reported the successful use of MR-guided SLA therapy for the treatment of epileptic foci in a series of 5 pediatric patients.13 Tovar-Spinoza et al. reported the case of a 3-year-old patient with medically refractory gelastic seizures and severe behavioral issues secondary to a hypothalamic hamartoma that was successfully treated with MR-guided SLA therapy and resulted in resolution of seizures and behavioral improvement at his 6-month follow-up.8 While these initial reports showed an acceptable safety profile in its application to childhood epilepsy, with all patients showing improvement in seizure control and no major complications, they had major limitations of small sample numbers and short-term follow-up (2–13 months).8,13 In 2016, Tovar-Spinoza and Choi published a series of 12 tumors in 11 pediatric patients (mean age 10.3 years, range 4–17 years) treated with the Visualase thermal laser system.14 In that series, tumor histologies included pilocytic astrocytomas, ependymoma, recurrent medulloblastoma, choroid plexus xanthogranuloma, subependymal cell giant astrocytoma, and ganglioma; volumetric analysis revealed progressive cytoreductive tumor effect on follow-up evaluations (mean follow-up time 24.5 months, range 12–35 months), and transient neurological deficits were seen in 2 of the 11 patients. While that series was focused only on pediatric brain tumors, it still suffered from having a small heterogeneous sample with short to medium follow-up.
There is currently a paucity of pediatric-specific data regarding the use of this nascent technology for brain tumors. Quantifying the risk of complications remains difficult due to the lack of data beyond small case series. There are no commonly accepted indications or guidelines regarding patient selection in children. This study aimed to assess the current usage, efficacy, and safety profile of MR-guided SLA therapy in the treatment of brain tumors in pediatric patients. Data were pooled from multiple centers with the intent of gaining a snapshot of current usage patterns, outcomes, and complications.
Methods
Data from 17 North American centers were retrospectively reviewed. Clinical, technical, and radiographic data for pediatric patients treated with SLA for a diagnosis of brain tumor from 2008 to 2016 were collected and analyzed.
Ethics Review and Approval
This study was evaluated and approved by our local IRB. A waiver of consent was approved by our IRB given the minimal risk associated with this retrospective chart review study without experimental practices or the need for patients’ identifiable information. IRB approval was obtained for all participating centers.
Population
Both male and female patients, from infancy up to 21 years of age, with a diagnosis of brain tumors (primary and/or metastatic) who were treated via MR-guided SLA therapy at any of the participating centers between 2008 and 2016 and had a minimum follow-up time of 3 months were included. Patients older than 21 years of age and patients with a diagnosis other than brain tumor were excluded. Additionally, patients who otherwise met all other inclusion criteria but did not have a minimum follow-up of 3 months were similarly excluded from our data analysis.
Data Collection
Data collection was focused on demographics, SLA system used, stereotactic system used, diagnosis (i.e., primary vs metastatic brain tumor), pathology, history of prior cranial surgeries, goal of surgery (ablation vs disconnection), location and size of the target of interest (TOI), number of laser probe passes/trajectories, number and size of lesions created, total anesthesia time, day of discharge, routine versus required ICU stay, 30-day readmission, complications, interventions for complications, steroid use, post-SLA follow-up time, volume of SLA-treated tumor at most recent follow-up, time to maximal response, post-SLA cranial surgery, and post-SLA adjuvant treatment use.
Definitions
“Procedure” refers to a single anesthesia/day, may include multiple TOIs and lesions, where the TOI is a contiguous volume of tissue that was to be ablated. “Lesion” is defined a contiguous volume of tissue that was ablated, which may or may not exactly equal a TOI. “Trajectory” means a single track through the brain with the laser catheter. Multiple lesions could be created along a single trajectory, or a single lesion could require multiple trajectories. “Burn” refers to the delivery of energy via laser to a unique location. A single lesion may require multiple burns along a single trajectory.
TOI Categorization per Location
The TOI locations were categorized as lobar, deep, or cerebellar. Targets in the frontal, parietal, temporal, and/or occipital lobes were categorized as lobar; targets in cerebellar locations were categorized as cerebellar; and targets in basal ganglia, hypothalamus, thalamus, and periventricular locations were categorized as deep.
Available MR-Guided Laser Ablation Systems and Operative Technique
Two FDA-cleared technologies were utilized by participating centers in this series: the Visualase system3 and the NeuroBlate system.9,10 The operative techniques performed with these two systems have been previously described.2,3,5,8,15,16
Statistical Analysis
The study included 86 patients with a brain tumor who were treated with SLA. The probability of having a smaller or unchanged tumor size over a 72-month period was evaluated using survival and Kaplan-Meier curve analyses. Logistic regression was performed to analyze various outcomes such as tumor size after the SLA treatment, the need for a subsequent surgery after SLA treatment, and the need for an adjuvant treatment after the SLA treatment. Patient characteristics were compared across complications (yes vs no) using the t-test for continuous variables and the chi-square test for categorical variables. Adjusted analysis on complications was conducted using logistic regression.
Results
Population Characteristics
A total of 86 patients (mean age 12.2 ± 4.5 years [± SD]) with 76 low-grade (I or II) and 10 high-grade (III or IV) tumors were included. Tumor location included lobar (38.4%), deep (45.3%), and cerebellar (16.3%) compartments, where the deep compartment included basal ganglia, hypothalamus, thalamus, and periventricular locations (Table 1). The mean follow-up time was 24 months (median 18 months, range 3–72 months).
TABLE 1.
Patient characteristics
| Value | |
|---|---|
| No. of patients | 86 |
| Sex | |
| Female | 39 (45.3) |
| Male | 47 (54.7) |
| Age, yrs | |
| Median (range) | 13 (1–21) |
| Mean | 12.2 |
| Brain tumor type | |
| Primary | 84 (97.7) |
| Metastatic | 2 (2.3) |
| Pathology diagnosis | |
| Biopsy proven | 64 (74.4) |
| Presumed | 22 (25.6) |
| Tumor grade | |
| Low (I or II) | 76 |
| High (III or IV) | 10 |
| Pre-SLA cranial surgery | |
| Related to TOI | 28 (32.6) |
| Not related to TOI | 13 (15.1) |
| Tumor location | |
| Frontal lobe | 11 (12.8) |
| Temporal lobe (neocortical) | 3 (3.5) |
| Temporal lobe (mesial) | 10 (11.6) |
| Parietal lobe | 6 (7) |
| Occipital lobe | 3 (3.5) |
| Thalamus | 10 (11.6) |
| Hypothalamus | 6 (7.0) |
| Basal ganglia | 6 (7.0) |
| Periventricular | 17 (19.8) |
| Cerebellar | 14 (16.3) |
| SLA system used | |
| Visualase | 67 (78) |
| NeuroBlate | 19 (22) |
| Targeting system used | |
| Frameless | 22 (25.6) |
| Framed | 43 (50) |
| Robotic | 21 (24.4) |
| Pre-SLA neurological symptom or deficit | |
| Yes | 50 (58.1) |
| No | 36 (41.9) |
| Presenting symptom post-SLA (n = 77) | |
| Stable | 45 (58.4) |
| Improved | 28 (36.4) |
| Worsened | 4 (5.2) |
Values represent the number of patients (%) unless stated otherwise. Percent-ages are based on 86 patients unless noted otherwise.
Treatment Efficacy: Tumor Volume and Tumor Grade
Of the 86 patients included in the analysis, 72 had data available for tumor volume at the latest follow-up. For outcome analysis on post-SLA treated tumor volumes, these were divided into decreased compared with stable/increased volume. At the last follow-up, the volume of SLA-treated tumors had decreased in 80.6% of patients, remained unchanged in 12.5% of patients, and increased in 6.9% of patients. Of the 76 low-grade tumors, 65 had post-SLA volume data available at the latest follow-up. Of these, 54 (83.1%) showed a smaller volume at the latest follow-up, whereas 11 (16.9%) were stable or increased in size. Of the 10 high-grade tumors, 7 had data on tumor volume at the latest follow-up. Of these, 4 (57.1%) showed a decrease in volume, and 3 (42.9%) were stable or increased in size at the last follow-up (Table 2).
TABLE 2.
Tumor grade and associated descriptive variables
| Low- vs High-Grade Tumors | p Value | |||
|---|---|---|---|---|
| Total | High Grade | Low Grade | ||
| No. of patients | 86 | 10 | 76 | |
| Mean age at op, yrs | 12.2 ± 4.5 | 11.7 ± 6.0 | 12.3 ± 4.4 | 0.702* |
| Sex | 0.501†‡ | |||
| Female | 39 (45.3) | 6 (60.0) | 33 (43.4) | |
| Male | 47 (54.7) | 4 (40.0) | 43 (56.6) | |
| Location | 0.490†‡ | |||
| Cerebellar | 14 (16.3) | 2 (20.0) | 12 (15.8) | |
| Deep | 39 (45.3) | 6 (60.0) | 33 (43.4) | |
| Lobar | 33 (38.4) | 2 (20.0) | 31 (40.8) | |
| Mean pre-SLA tumor vol, cm3 | 8.0 ± 14.0 | 7.6 ± 10.3 | 8.1 ± 14.4 | 0.936* |
| Mean lesion vol, cm3 | 8.2 ± 9.1 | 12.1 ± 15.0 | 7.7 ± 8.1 | 0.167* |
| Post-SLA tumor vol (n = 72) | 0.128†‡ | |||
| Decreased | 58 (80.6) | 4 (57.1) | 54 (83.1) | |
| Unchanged/increased | 14 (19.4) | 3 (42.9) | 11 (16.9) | |
| Complications | >0.99†‡ | |||
| No | 63 (73.3) | 7 (70.0) | 56 (73.7) | |
| Yes | 23 (26.7) | 3 (30.0) | 20 (26.3) | |
Values represent the number of patients (%) unless stated otherwise. Mean values are reported as mean ± SD.
t-test.
Chi-square test.
Exact test.
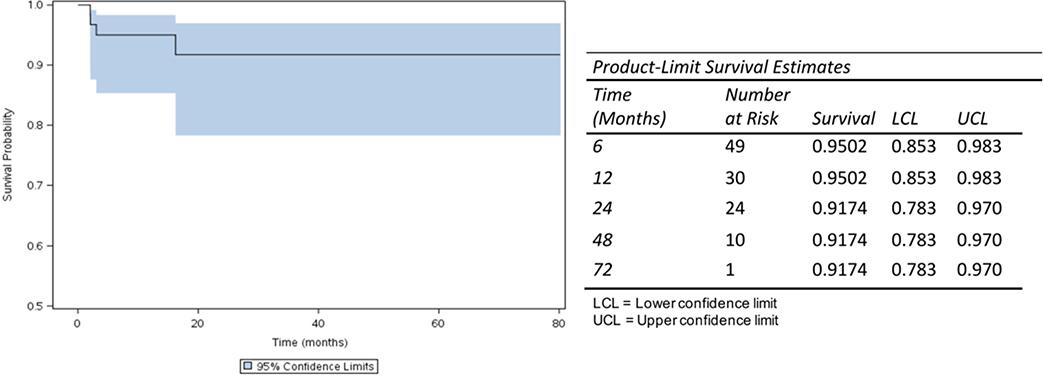
Progression-free survival after SLA treatment was 92% at 72 months (Fig. 1). Patients with high-grade tumors were more likely to have an unchanged or larger tumor volume after SLA treatment than patients with low-grade tumors (OR 7.49, p = 0.0364; Table 3).
FIG. 1.
Kaplan-Meier curve showing a progression-free survival of 92% at 72 months after SLA treatment. Figure is available in color online only.
TABLE 3.
Binary logistic regression to predict the effect of variables on treatment efficacy, need for subsequent surgery, and need for adjuvant therapy
| OR | 95% Wald Confidence Limits | p Value | ||
|---|---|---|---|---|
| Effect on treatment efficacy (decreased vs stable or increased tumor size) | ||||
| Location (cerebellar vs deep) | 0.52 | 0.04 | 6.12 | 0.6076 |
| Location (lobar vs deep) | 2.33 | 0.54 | 10.07 | 0.2542 |
| Tumor type (high vs low grade) | 7.49 | 1.13 | 49.45 | 0.0364 |
| Tumor vol | 1.06 | 0.98 | 1.14 | 0.1178 |
| No. of trajectories | 1.34 | 0.23 | 7.74 | 0.7386 |
| No. of lesions | 0.89 | 0.57 | 1.38 | 0.6185 |
| Lesion vol | 0.98 | 0.89 | 1.08 | 0.7344 |
| Effect of variables on subsequent surgery | ||||
| Location (cerebellar vs deep) | 1.48 | 0.12 | 17.22 | 0.7532 |
| Location (lobar vs deep) | 1.47 | 0.24 | 8.73 | 0.6720 |
| Tumor type (high vs low grade) | 2.25 | 0.21 | 23.32 | 0.4957 |
| Tumor vol | 1.02 | 0.98 | 1.06 | 0.3262 |
| Effect of variables on adjuvant treatment | ||||
| Tumor type (high vs low grade) | 3.77 | 0.56 | 25.28 | 0.1711 |
| Tumor vol | 1.05 | 0.99 | 1.11 | 0.0632 |
Boldface type indicates statistical significance.
Treatment Efficacy: Subsequent Surgery and Adjuvant Therapy
Subsequent surgery after SLA treatment was not required in 90.4% of patients, and adjuvant treatment after SLA treatment was not required in 86.7% of patients. Patients with high-grade tumors were more likely to need subsequent surgery (OR 2.25, p = 0.4957) and adjuvant treatment after SLA therapy (OR 3.77, p = 0.1711), without reaching significance (Table 3).
Complications
A total of 29 acute complications in 23 patients were reported, including malpositioned catheters (n = 3), intracranial hemorrhages (ICHs, n = 2), transient neurological deficits (n = 11), permanent neurological deficits (n = 5), symptomatic perilesional edema (n = 2), hydrocephalus (n = 4), and death (n = 2) (Table 4). On long-term follow-up, 3 patients were reported to experience worsened neuropsychological test results.
TABLE 4.
Incidence of short-term complications
| Complication | Occurrence |
|---|---|
| Malposition of laser probe | 3 |
| Symptomatic perilesional edema | 2 |
| Symptomatic ICH | 1 |
| Asymptomatic ICH ≥ 10 mm | 1 |
| Transient neurological deficit | 11 |
| Permanent neurological deficit | 5 |
| CSF leak | 0 |
| Hydrocephalus | 4 |
| Superficial wound dehiscence | 0 |
| Wound infection | 0 |
| Death | 2 |
| Total | 29* |
Short-term complications = those occurring within 30 days from surgery.
A total of 29 acute or short-term complications in 23 patients were reported.
One of the patient deaths was directly related to the procedure, where targeting of a posterior fossa tumor resulted in a cerebellar hemorrhage with acute hydrocephalus and death despite a decompressive craniectomy and external ventricular drain placement. The second patient death was not directly related to the procedure but rather to a spontaneous posterior fossa hemorrhage from a known cerebellar lesion in a patient with a metastatic malignant nerve sheath tumor while targeting a right thalamic lesion.
In the cases with reported malpositioned catheters, 2 were frame-based targeting systems and 1 was robotic. The pre-SLA tumor volume, tumor location, number of laser trajectories, or number of lesions created did not result in a significantly increased risk of complications; however, the odds of complications increased by 14% (OR 1.14, p = 0.0159) with every 1-cm3 increase in the volume of the lesion created (Tables 5 and 6). Given the relatively low occurrence within each type of complication, the effect of the volume of the lesion created or the location of the tumor on the specific type of complication was not analyzed.
TABLE 5.
Short-term complications and associated descriptive variables
| Total | Complications | p Value | ||
|---|---|---|---|---|
| No | Yes | |||
| No. of patients | 86 | 63 | 23 | |
| Mean age at op, yrs | 12.2 ± 4.5 | 11.6 ± 4.5 | 14.0 ± 4.2 | 0.026 * |
| Sex | 0.780† | |||
| Female | 39 (45.3) | 28 (44.4) | 11 (47.8) | |
| Male | 47 (54.7) | 35 (55.6) | 12 (52.2) | |
| Compartment | 0.948†‡ | |||
| Cerebellar | 14 (16.3) | 10 (15.9) | 4 (17.4) | |
| Deep | 39 (45.3) | 28 (44.4) | 11 (47.8) | |
| Lobar | 33 (38.4) | 25 (39.7) | 8 (34.8) | |
| Tumor grade | >0.99†‡ | |||
| High (III or IV) | 10 (11.6) | 7 (11.1) | 3 (13.0) | |
| Low (I or II) | 76 (88.4) | 56 (88.9) | 20 (87.0) | |
| Mean pre-SLA tumor vol, cm3 | 8.0 ± 14.0 | 8.1 ± 15.4 | 7.7 ± 8.8 | 0.907* |
| Mean treated lesion vol, cm3 | 8.2 ± 9.1 | 6.7 ± 7.7 | 12.6 ± 11.4 | 0.009 * |
| No. of laser trajectories | 0.277†‡ | |||
| 1 | 70 (81.4) | 51 (81.0) | 19 (82.6) | |
| 2 | 15 (17.4) | 12 (19.0) | 3 (13.0) | |
| 3 | 1 (1.2) | 0 (0.0) | 1 (4.3) | |
| Mean no. of lesions created | 2.3 ± 1.8 | 2.3 ± 1.9 | 2.0 ± 1.6 | 0.432* |
| Preop steroid use | 0.029 † ‡ | |||
| No | 70 (81.4) | 55 (87.3) | 15 (65.2) | |
| Yes | 16 (18.6) | 8 (12.7) | 8 (34.8) | |
| Steroid use on day of op | 86 (100.0) | 63 (100.0) | 23 (100.0) | NA |
| Planned routine postop steroid use | >0.99†‡ | |||
| No | 6 (7.0) | 4 (6.3) | 2 (8.7) | |
| Yes | 80 (93.0) | 59 (93.7) | 21 (91.3) | |
Short-term complications = those occurring within 30 days from surgery. Boldface type indicates statistical significance.
t-test.
Chi-square test.
Exact test.
TABLE 6.
Binary logistic regression to predict the effects of different variables on complications
| Variable | OR | 95% Wald Confidence Limits | p Value | |
|---|---|---|---|---|
| Age | 1.13 | 0.96 | 1.32 | 0.1411 |
| Tumor location (cerebellar vs deep) | 2.24 | 0.41 | 12.18 | 0.3525 |
| Tumor location (lobar vs deep) | 0.95 | 0.23 | 3.93 | 0.9427 |
| Tumor vol | 0.97 | 0.89 | 1.05 | 0.4343 |
| No. of trajectories | 0.91 | 0.22 | 3.84 | 0.8957 |
| No. of lesions | 0.70 | 0.46 | 1.07 | 0.1011 |
| Lesion vol | 1.14 | 1.03 | 1.27 | 0.0159 |
| Preop steroid use (yes vs no) | 2.34 | 0.56 | 9.77 | 0.2450 |
| Planned-routine postop steroid use (yes vs no) | 0.71 | 0.08 | 6.24 | 0.7540 |
Boldface type indicates statistical significance.
Discussion
There are currently no other large-volume studies looking at the use of SLA in pediatric brain tumors. Most of the existing data regarding the use of SLA in brain tumors involve adult populations. A recent retrospective study by Patel et al. reported outcomes for 102 patients treated with the Visualase thermal therapy system, and, although it is among the largest series reported to date, it had a heterogeneous population with ages ranging from 1 to 85 years (mean age 53 years) and variable pathologies, with 10 epilepsy patients, 87 intracranial tumor patients (50 primary brain tumors, 37 recurrent metastases/radiation necrosis), and 5 chronic pain syndrome patients.17 This report did not specify the number of pediatric patients included in the series and was also limited by their short-term follow-up period of approximately 1 month. Kamath et al. reported another large retrospective series, where 133 intracranial lesions in 120 patients were treated using the NeuroBlate MR-guided interstitial laser ablation system.18 Similarly, their series had a heterogeneous population with an average age of 52 years (range 5–79 years) and diverse pathologies, including glioblastomas, other gliomas, metastases, epileptic foci, and radionecrosis.
In these studies, SLA was typically used as a second-line therapy for persistent or recurrent brain metastasis after stereotactic radiotherapy19–22 or small-volume high-grade gliomas (HGGs).22–25 The efficacy of SLA in these studies was relatively comparable to open surgery for tumors with smaller volumes. It is difficult to extrapolate these results to the pediatric population given the predominance of metastatic disease, HGGs, and prior radiation therapy. The current study represents the largest series evaluating outcomes of SLA for the treatment of brain tumors in children. We sought to evaluate the following primary goals of this study: 1) the current use of this technology throughout various institutions in the United States, 2) its efficacy and early outcomes, and 3) complications and safety profile to better guide decision-making and patient counseling.
Retrospective data collected from the 17 participating North American centers identified 86 pediatric patients (ages 1–21 years old) who underwent SLA for a diagnosis of brain tumor. The Visualase system3 and the NeuroBlate system 9,10 were used in 78% and 22% of cases, respectively. Frame-based targeting systems were more commonly utilized (50%), followed by frameless (25.6%) and robotic (24.4%) systems. The majority of tumors treated were primary CNS tumors (98% primary vs 2% metastatic), and 32.6% of patients had a history of prior cranial surgery related to the TOI. Not surprisingly, the tumors treated in this population were predominantly low-grade tumors (88.4% grade I or II vs 11.6% grade III or IV). However, it is important to note that the diagnosis of the tumor pathology was biopsy proven in only 74.4% of cases, whereas in 25.6% the diagnosis was presumed based on imaging findings, medical history (e.g., history of neurofibromatosis, tuberous sclerosis), and/or tissue confirmation from previous surgeries. This brings to light a limitation of the SLA approach as it relates to pathologic tissue sampling for diagnosis. While this approach allows the user to perform a needle biopsy at the time of the ablation, limited samples of pathologic tissue are obtained, which can often times be nondiagnostic. Additionally, speculations have been raised that blood or air generated at the time of biopsy could potentially interfere with the thermography sequences.
Of the 76 low-grade tumors, 54 (83.1%) showed a smaller volume at the latest follow-up, whereas 11 (16.9%) were stable or increased in size. In contrast, of the 10 high-grade tumors, 4 (57.1%) showed a decrease in volume at last follow-up, whereas 3 (42.9%) were stable or increased in size. These results suggest that SLA is an effective treatment for pediatric brain tumors, with greater efficacy seen in low-grade lesions.
Overall, 26.7% (n = 23) of the patients in this series experienced acute complications. These included 2 (2.3%) ICHs (1 [1.2%] symptomatic and 1 [1.2%] asymptomatic), 11 (12.8%) transient neurological deficits, 5 (5.8%) permanent neurological deficits, and 2 (2.3%) patient deaths (Table 4). Only 1 of the 2 deaths in this series was directly relatable to SLA treatment (a cerebellar hemorrhage leading to acute hydrocephalus that was refractory to placement of an external ventricular drain and decompressive surgery). These results are comparable to those in adult SLA series. In a small series of adult patients with low-volume, newly diagnosed HGGs, 4 of 24 patients (17%) experienced permanent neurological deficits following SLA.25 In a larger study of recurrent HGGs undergoing SLA, 7 of 63 patients (12%) had permanent neurological deficits. In both of these studies, as in the current series, a large proportion of tumors were in deep or eloquent locations. The nature of the advantages and disadvantages of SLA leads to a selection bias of smaller lesions that are less accessible with open surgery, thus making it difficult to compare complication rates with those of conventional open surgery.
Tumor location did not appear to contribute to an increased risk of complications in our series. This may be a result of the selected subgroup analysis, which focused on lobar, deep, and cerebellar locations, where deep locations included structures such as basal ganglia, hypothalamus, thalamus, and periventricular locations. It is possible that a more detailed subset analysis of these structures may reveal different outcomes, as these are locations that stand out as higher-risk areas for surgical intervention of any kind.
Our data collection was too crude to look at location risk with regard to proximity to eloquent brain. However, given that in our series the neurological deficits far exceeded the number of ICHs reported, one can infer that these deficits are mostly incurred due to proximity of eloquent brain to the lesion(s) created, associated swelling, direct damage, etc. This suggests that caution needs to be used in trusting the damage estimate and that increased training or experience may be needed when placing temperature monitors on adjacent eloquent tissue. Furthermore, while the pre-SLA tumor volume, number of laser trajectories, and number of lesions created did not result in a significantly increased risk of complications, the odds of complications increased by 14% with every 1-cm3 increase in the volume of the lesion created, suggesting that a greater number of smaller lesions may be safer or more beneficial than fewer larger lesions.
It is difficult to compare the results of SLA to those of traditional open surgery. For centers with the capacity for performing SLA, there tend to be two main reasons for doing so. One is based on tumor location. With open surgery, certain locations of the brain require collateral damage to normal brain from the surgical approach or are associated with higher risk to normal brain due to the risks of the open approach (e.g., thalamus, insula, mesial temporal lobe, hypothalamus). In these cases, SLA may offer a path of a lower risk with a similar amount of efficacy. The other reason to offer SLA is for the traditional benefits of minimally invasive surgical approaches, including less scarring, less postoperative pain, and a shorter hospital stay. Both of these reasons require an efficacy and risk profile that is comparable to results with open surgery for lesions in similar locations. With regard to efficacy, the data are encouraging but not conclusive. To know whether risk is comparable would require a case-matched cohort of similar lesions with regard to size and location or a randomized trial, neither of which is very feasible. In this study, the average number of tumor procedures was 5 per center (range 1–17). The complication rate for SLA will likely decrease, as individual centers and the neurosurgical community as a whole gain more experience. Nevertheless, the complication rate in this series is significant and should be kept in mind when deciding on the option of SLA. As has been seen in many surgical arenas, nascent minimally invasive techniques should not be assumed to be safer, an assumption almost always made by the lay public and too frequently by practitioners.
This study has several limitations. The absence of a control population makes comparison with traditional therapies difficult. There is an obvious selection bias for tumors that are sufficiently small but also for tumors that are more difficult to access or treat with other techniques. Only 74.4% cases were biopsy proven, while 25.6% cases were presumed diagnoses, and, although tumor grade was provided for all biopsy-proven lesions, the specific histopathology was not provided for all biopsy cases. The retrospective nature of this study does not allow for consistency between patients regarding radiographic follow-up. The follow-up time is limited (mean 24 months), given the slow-growing nature of low-grade tumors, making it difficult to draw conclusions regarding long-term efficacy.
Conclusions
SLA is an effective, minimally invasive treatment option for pediatric brain tumors, although it is not without risks. Our results suggest that limiting the volume of the generated thermal lesion may help decrease the incidence of complications. We hope that this study will help practitioners with decision-making and patient counseling. However, larger-volume studies with longer follow-up are necessary to further assess the safety profile and effectiveness of this technology in the treatment of various types of brain tumors in the pediatric population. To further evaluate technical learning curves and complication profiles, future steps will be focused on pooling data from the current study with data collected in the parallel study arm focused on the use of SLA therapy for the treatment of refractory epilepsy in pediatric patients.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, award no. UL1TR001436. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH.
We thank Danielle Buetow for her instrumental assistance with coordination and submission of required materials for IRB review, approval, and project continuation.
This article is dedicated to one of our contributors, Dr. Sanjiv Bhatia. Dr. Bhatia was a champion for children with neurosurgical conditions and a gifted, innovative surgeon. He is dearly missed.
ABBREVIATIONS
- HGG
high-grade glioma
- ICH
intracranial hemorrhage
- SLA
stereotactic laser ablation
- TOI
target of interest
Footnotes
Disclosures
Dr. Tovar-Spinoza: consultant for Monteris Inc. Dr. Perry: consultant for Encoded Therapeutics and Stoke Therapeutics, and honoraria for advisory board/speaking from Biocodex and Zogenix. Dr. Barnett: consultant for Monteris Medical Inc.
References
- 1.Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–950. [DOI] [PubMed] [Google Scholar]
- 2.LaRiviere MJ, Gross RE. Stereotactic laser ablation for medically intractable epilepsy: the next generation of minimally invasive epilepsy surgery. Front Surg. 2016;3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medvid R, Ruiz A, Komotar RJ, et al. Current applications of MRI-guided laser interstitial thermal therapy in the treatment of brain neoplasms and epilepsy: a radiologic and neurosurgical overview. AJNR Am J Neuroradiol. 2015;36(11):1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North RY, Raskin JS, Curry DJ. MRI-guided laser interstitial thermal therapy for epilepsy. Neurosurg Clin N Am. 2017;28(4):545–557. [DOI] [PubMed] [Google Scholar]
- 5.Patel NV, Mian M, Stafford RJ, et al. Laser interstitial thermal therapy technology, physics of magnetic resonance imaging thermometry, and technical considerations for proper catheter placement during magnetic resonance imaging-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(suppl 1):S8–S16. [DOI] [PubMed] [Google Scholar]
- 6.Prince E, Hakimian S, Ko AL, et al. Laser interstitial thermal therapy for epilepsy. Curr Neurol Neurosci Rep. 2017;17(9):63. [DOI] [PubMed] [Google Scholar]
- 7.Rolston JD, Chang EF. Stereotactic laser ablation for hypothalamic hamartoma. Neurosurg Clin N Am. 2016;27(1):59–67. [DOI] [PubMed] [Google Scholar]
- 8.Tovar-Spinoza Z, Carter D, Ferrone D, et al. The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv Syst. 2013;29(11):2089–2094. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors—the NeuroBlate System. Expert Rev Med Devices. 2014;11(2):109–119. [DOI] [PubMed] [Google Scholar]
- 10.Rennert RC, Khan U, Bartek J Jr, et al. Laser Ablation of Abnormal Neurological Tissue Using Robotic Neuroblate System (LAANTERN): procedural safety and hospitalization. Neurosurgery. 2019;nyz141. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361–368. [DOI] [PubMed] [Google Scholar]
- 12.Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1)(suppl 1):ONS21–ONS29. [DOI] [PubMed] [Google Scholar]
- 13.Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24(4):408–414. [DOI] [PubMed] [Google Scholar]
- 14.Tovar-Spinoza Z, Choi H. Magnetic resonance-guided laser interstitial thermal therapy: report of a series of pediatric brain tumors. J Neurosurg Pediatr. 2016;17(6):723–733. [DOI] [PubMed] [Google Scholar]
- 15.Buckley R, Estronza-Ojeda S, Ojemann JG. Laser ablation in pediatric epilepsy. Neurosurg Clin N Am. 2016;27(1):69–78. [DOI] [PubMed] [Google Scholar]
- 16.Fayed I, Sacino MF, Gaillard WD, et al. MR-guided laser interstitial thermal therapy for medically refractory lesional epilepsy in pediatric patients: experience and outcomes. Pediatr Neurosurg. 2018;53(5):322–329. [DOI] [PubMed] [Google Scholar]
- 17.Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016;125(4):853–860. [DOI] [PubMed] [Google Scholar]
- 18.Kamath AA, Friedman DD, Hacker CD, et al. MRI-guided interstitial laser ablation for intracranial lesions: a large single-institution experience of 133 cases. Stereotact Funct Neurosurg. 2017;95(6):417–428. [DOI] [PubMed] [Google Scholar]
- 19.Alattar AA, Bartek J Jr, Chiang VL, et al. stereotactic laser ablation as treatment of brain metastases recurring after stereotactic radiosurgery: a systematic literature review. World Neurosurg. 2019;128:134–142. [DOI] [PubMed] [Google Scholar]
- 20.Ali MA, Carroll KT, Rennert RC, et al. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: a multiinstitutional experience. Neurosurg Focus. 2016;41(4):E11. [DOI] [PubMed] [Google Scholar]
- 21.Beechar VB, Prabhu SS, Bastos D, et al. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J Neurooncol. 2018;137(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaunzwa TL, Deng D, Leuthardt EC, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63. [DOI] [PubMed] [Google Scholar]
- 23.Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–811. [DOI] [PubMed] [Google Scholar]
- 24.Lee I, Kalkanis S, Hadjipanayis CG. Stereotactic laser interstitial thermal therapy for recurrent high-grade gliomas. Neurosurgery. 2016;79(suppl 1):S24–S34. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi AM, Sharma M, Beaumont TL, et al. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: a multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery. 2019;85(6):762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]